Assessing Ash (Fraxinus excelsior L.) Dieback Dynamics in the Białowieża Forest, Poland, Using Bi-Temporal High-Resolution Remote Sensing Data
Abstract
1. Introduction
- To map the dynamics of ash mortality using bi-temporal high-resolution remote sensing data;
- To assess the influence of habitat and stand factors on the severity of ash mortality.
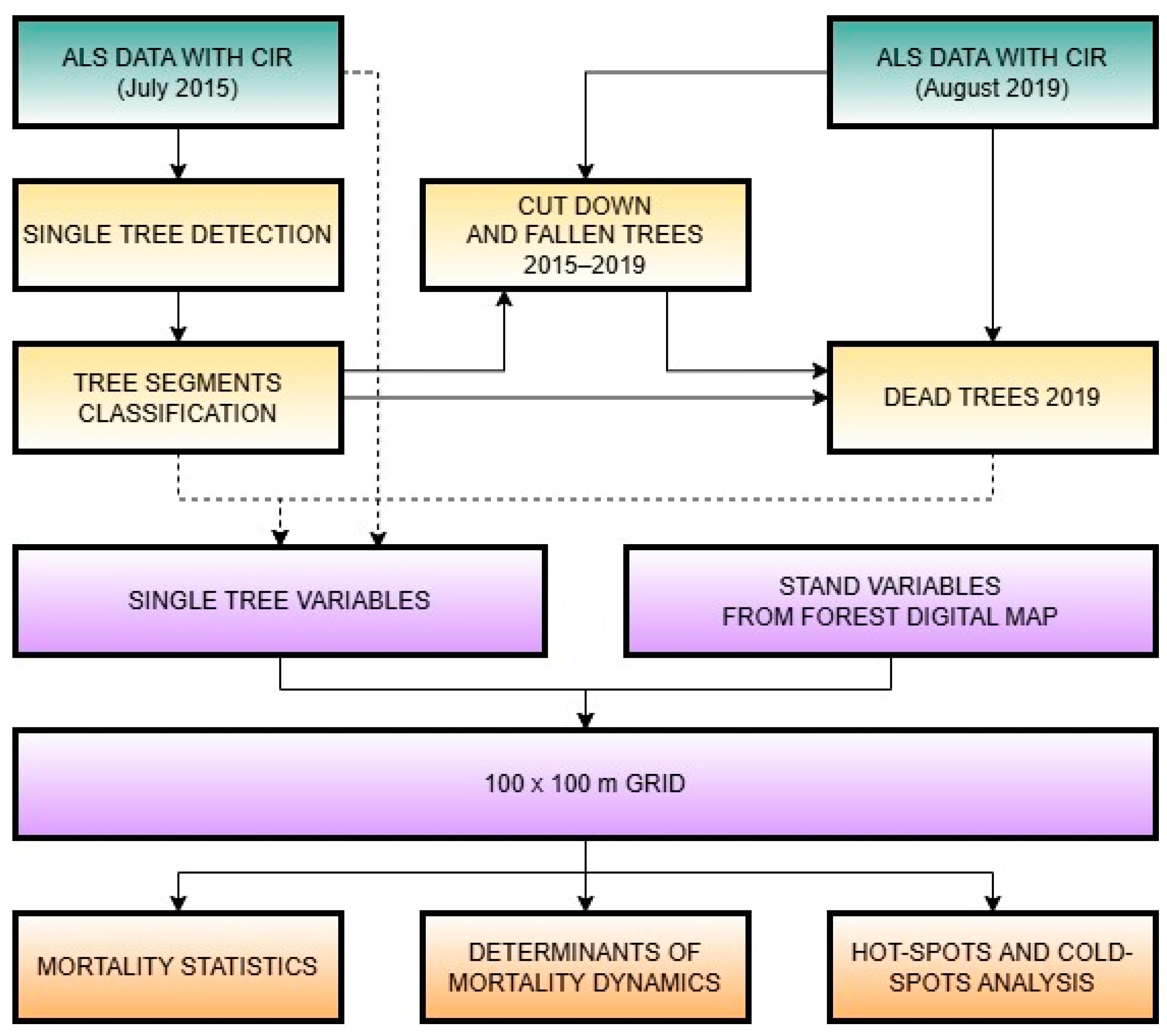
2. Materials and Methods
2.1. Study Area
2.2. Remote Sensing Datasets
2.2.1. Airborne Laser Scanning and CIR Imagery
2.2.2. RS Data Processing
2.3. Statistical Analyses
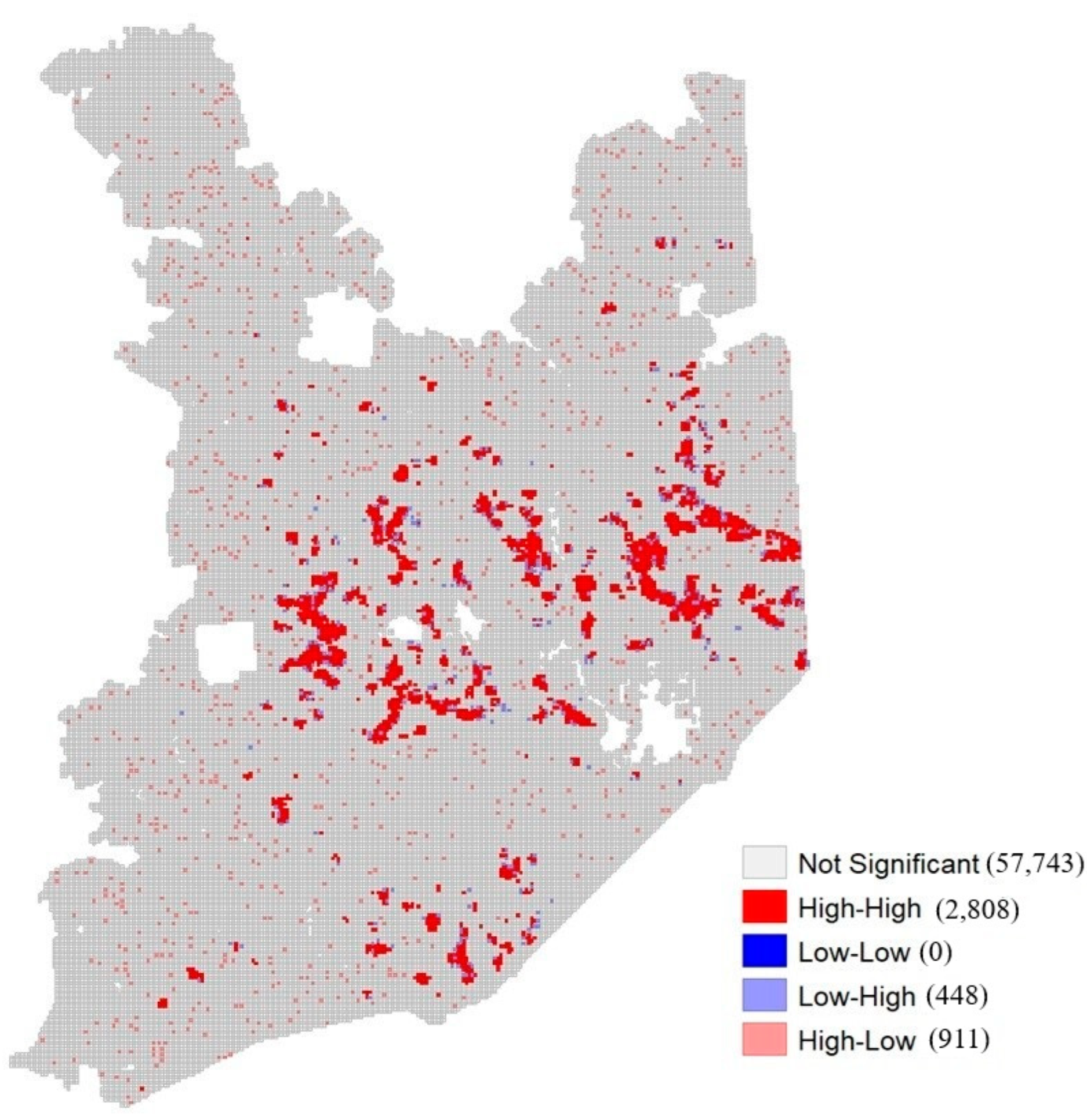
2.3.1. Hotspot Analysis
- Locations with no significant local autocorrelation (class 0);
- High–high locations with high values and similar neighbors (class 1);
- Low–low locations with low values and similar neighbors (class 2);
- Low–high locations with low values and high-value neighbors (class 3);
- High–low locations with high values and low-value neighbors (class 4).
2.3.2. Boosted Regression Tree Analyses
3. Results
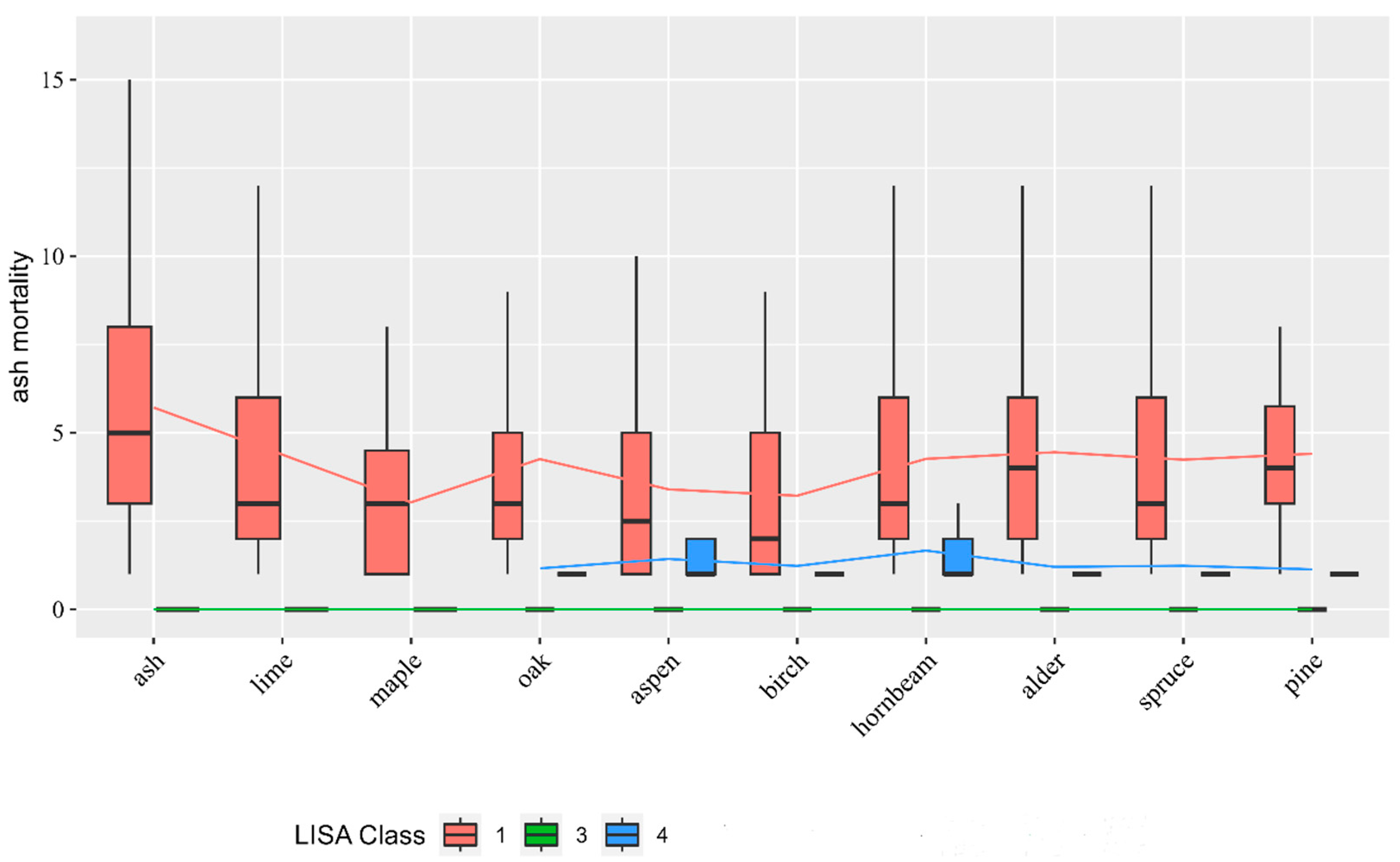
3.1. Ash Mortality
3.2. HotSpot Analysis
3.3. BRT Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anderegg, W.R.L.; Martinez-Vilalta, J.; Cailleret, M.; Camarero, J.J.; Ewers, B.E.; Galbraith, D.; Gessler, A.; Grote, R.; Huang, C.; Levick, S.R.; et al. When a Tree Dies in the Forest: Scaling Climate-Driven Tree Mortality to Ecosystem Water and Carbon Fluxes. Ecosystems 2016, 19, 1133–1147. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of Widespread Tree Mortality Triggered by Drought and Temperature Stress. Nat. Clim. Change 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Gross, A.; Hosoya, T.; Queloz, V. Population Structure of the Invasive Forest Pathogen Hymenoscyphus pseudoalbidus. Mol. Ecol. 2014, 23, 2943–2960. [Google Scholar] [CrossRef]
- Carroll, D.; Boa, E. Ash Dieback: From Asia to Europe. Plant Pathol. 2024, 73, 741–759. [Google Scholar] [CrossRef]
- Timmermann, V.; Børja, I.; Hietala, A.M.; Kirisits, T.; Solheim, H. Ash Dieback: Pathogen Spread and Diurnal Patterns of Ascospore Dispersal, with Special Emphasis on Norway. EPPO Bull. 2011, 41, 14–20. [Google Scholar] [CrossRef]
- Kowalski, T. O Zamieraniu Jesionów. Trybunał Leśnika 2001, 4, 6–7. [Google Scholar]
- Stocki, J. Przyczyny Zamierania Drzew w i Drzewostanów Jesionowych w Polsce. Głos Lasu 2001, 4, 17–19. [Google Scholar]
- Pautasso, M.; Aas, G.; Queloz, V.; Holdenrieder, O. European Ash (Fraxinus excelsior) Dieback—A Conservation Biology Challenge. Biol. Conserv. 2013, 158, 37–49. [Google Scholar] [CrossRef]
- Bakys, R.; Vasaitis, R.; Barklund, P.; Thomsen, I.M.; Stenlid, J. Occurrence and Pathogenicity of Fungi in Necrotic and Non-Symptomatic Shoots of Declining Common Ash (Fraxinus excelsior) in Sweden. Eur. J. For. Res. 2009, 128, 51–60. [Google Scholar] [CrossRef]
- Przybył, K. Fungi Associated with Necrotic Apical Parts of Fraxinus excelsior Shoots. For. Pathol. 2002, 32, 387–394. [Google Scholar] [CrossRef]
- Solheim, H.; Hietala, A.M. Spread of Ash Dieback in Norway. Balt. For. 2017, 23, 144–149. [Google Scholar]
- Clark, J.; Webber, J. The Ash Resource and the Response to Ash Dieback in Great Britain. In Dieback of European Ash (Fraxinus spp.): Consequences and Guidelines for Sustainable Management; Vasaitis, R., Enderle, R., Eds.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2017; pp. 228–237. [Google Scholar]
- McCracken, A.R.; Douglas, G.C.; Ryan, C.; Destefanis, M.; Cooke, L.R. Ash Dieback on the Island of Ireland. In Dieback of European Ash (Fraxinus spp.): Consequences and Guidelines for Sustainable Management; Vasaitis, R., Enderle, R., Eds.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2017; pp. 125–139. [Google Scholar]
- Baral, H.-O.; Queloz, V.; Hosoya, T. Hymenoscyphus fraxineus, the Correct Scientific Name for the Fungus Causing Ash Dieback in Europe. IMA Fungus 2014, 5, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Moncada, B.; Hawksworth, D.L. Gone with the Wind: Sequencing Its Type Species Supports Inclusion of Cryptolechia in Gyalecta (Ostropales: Gyalectaceae). Lichenologist 2019, 51, 287–299. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Nowakowska, J.A.; Oszako, T. Phytophthora Species Isolated from Ash Stands in Białowieża Forest Nature Reserve. For. Pathol. 2016, 46, 660–662. [Google Scholar] [CrossRef]
- Cholewińska, O.; Keczyński, A.; Smerczyński, I.; Jaroszewicz, B. European Ash (Fraxinus excelsior L.) Dieback in a Core Area of Białowieża National Park. Natl. Park. Nat. Reserv. 2018, 37, 3–18. [Google Scholar]
- Matisone, I.; Matisons, R.; Laiviņš, M.; Gaitnieks, T. Statistics of Ash Dieback in Latvia. Silva Fenn. 2018, 52, 9901. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Thomsen, I.M.; Skovgaard, I.M.; Martinussen, T. Associations among Symptoms of Dieback in Even-Aged Stands of Ash (Fraxinus excelsior L.). For. Pathol. 2010, 40, 7–18. [Google Scholar] [CrossRef]
- Enderle, R.; Metzler, B.; Riemer, U.; Kändler, G. Ash Dieback on Sample Points of the National Forest Inventory in South-Western Germany. Forests 2018, 9, 25. [Google Scholar] [CrossRef]
- Pušpure, I.; Matisons, R.; Laiviņš, M.; Gaitnieks, T.; Jansons, J. Natural Regeneration of Common Ash in Young Stands in Latvia. Balt. For. 2017, 23, 209–217. [Google Scholar]
- Dobrowolska, D.; Hein, S.; Oosterbaan, A.; Wagner, S.; Clark, J.; Skovsgaard, J.P. A Review of European Ash (Fraxinus excelsior L.): Implications for Silviculture. For. Int. J. For. Res. 2011, 84, 133–148. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Latifi, H.; Stereńczak, K.; Modzelewska, A.; Lefsky, M.; Waser, L.T.; Straub, C.; Ghosh, A. Review of Studies on Tree Species Classification from Remotely Sensed Data. Remote Sens. Environ. 2016, 186, 64–87. [Google Scholar] [CrossRef]
- Hansen, M.C.; Loveland, T.R. A Review of Large Area Monitoring of Land Cover Change Using Landsat Data. Remote Sens. Environ. 2012, 122, 66–74. [Google Scholar] [CrossRef]
- Kamińska, A.; Lisiewicz, M.; Stereńczak, K. Single Tree Classification Using Multi-Temporal ALS Data and CIR Imagery in Mixed Old-Growth Forest in Poland. Remote Sens. 2021, 13, 5101. [Google Scholar] [CrossRef]
- Kamińska, A.; Lisiewicz, M.; Stereńczak, K.; Kraszewski, B.; Sadkowski, R. Species-Related Single Dead Tree Detection Using Multi-Temporal ALS Data and CIR Imagery. Remote Sens. Environ. 2018, 219, 31–43. [Google Scholar] [CrossRef]
- Kamińska, A.; Lisiewicz, M.; Kraszewski, B.; Stereńczak, K. Habitat and Stand Factors Related to Spatial Dynamics of Norway Spruce Dieback Driven by Ips typographus (L.) in the Białowieża Forest District. For. Ecol. Manag. 2020, 476, 118432. [Google Scholar] [CrossRef]
- Kamińska, A.; Lisiewicz, M.; Kraszewski, B.; Stereńczak, K. Mass Outbreaks and Factors Related to the Spatial Dynamics of Spruce Bark Beetle (Ips typographus) Dieback Considering Diverse Management Regimes in the Białowieża Forest. For. Ecol. Manag. 2021, 498, 119530. [Google Scholar] [CrossRef]
- Grodzki, W.; Starzyk, J.R.; Kosibowicz, M. Impact of Selected Stand Characteristics on the Occurrence of the Bark Beetle Ips typographus (L.) in the Beskid Żywiecki Mountains. For. Res. Pap. 2014, 75, 159–169. [Google Scholar] [CrossRef]
- Gašparović, M.; Pilaš, I.; Klobučar, D.; Gašparović, I. Monitoring Ash Dieback in Europe—An Unrevealed Perspective for Remote Sensing? Remote Sens. 2023, 15, 1178. [Google Scholar] [CrossRef]
- Polk, S.L.; Chan, A.H.Y.; Cui, K.; Plemmons, R.J.; Coomes, D.A.; Murphy, J.M. Unsupervised Detection of ASH Dieback Disease (Hymenoscyphus fraxineus) Using Diffusion-Based Hyperspectral Image Clustering. In Proceedings of the IGARSS 2022—2022 IEEE International Geoscience and Remote Sensing Symposium, Kuala Lumpur, Malaysia, 17–22 July 2022; pp. 2287–2290. [Google Scholar]
- Sproull, G.J.; Adamus, M.; Szewczyk, J.; Kersten, G.; Szwagrzyk, J. Fine-Scale Spruce Mortality Dynamics Driven by Bark Beetle Disturbance in Babia Góra National Park, Poland. Eur. J. For. Res. 2016, 135, 507–517. [Google Scholar] [CrossRef]
- Lisiewicz, M.; Kamińska, A.; Kraszewski, B.; Kuberski, Ł.; Pilch, K.; Stereńczak, K. Comprehensive Mapping of Individual Living and Dead Tree Species Using Leaf-On and Leaf-Off ALS and CIR Data in a Complex Temperate Forest. For. Int. J. For. Res. 2025, cpaf007. [Google Scholar] [CrossRef]
- Stereńczak, K.; Kraszewski, B.; Mielcarek, M.; Piasecka, Ż.; Lisiewicz, M.; Heurich, M. Mapping Individual Trees with Airborne Laser Scanning Data in an European Lowland Forest Using a Self-Calibration Algorithm. Int. J. Appl. Earth Obs. Geoinf. 2020, 93, 102191. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 1984. [Google Scholar]
- Kärvemo, S.; Van Boeckel, T.P.; Gilbert, M.; Grégoire, J.C.; Schroeder, M. Large-Scale Risk Mapping of an Eruptive Bark Beetle—Importance of Forest Susceptibility and Beetle Pressure. For. Ecol. Manag. 2014, 318, 158–166. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.r-project.org/ (accessed on 27 January 2025).
- Anselin, L. Local Indicators of Spatial Association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, L.; Xu, W.; Ledwith, V. Use of Local Moran’s I and GIS to Identify Pollution Hotspots of Pb in Urban Soils of Galway, Ireland. Sci. Total Environ. 2008, 398, 212–221. [Google Scholar] [CrossRef]
- Anselin, L.; Syabri, I.; Kho, Y. GeoDa: An Introduction to Spatial Data Analysis. Geogr. Anal. 2006, 38, 5–22. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A Working Guide to Boosted Regression Trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Elith, J.; Leathwich, J. Boosted Regression Trees for Ecological Modelling and Prediction. 2017. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=3fa2b8826c881d732169995869b9d356c6996029 (accessed on 6 March 2025).
- Ridgeway, G. The Gbm Package. Generalized Boosted Regression Models. 2024. Available online: https://cran.r-project.org/web/packages/gbm/gbm.pdf (accessed on 6 March 2025).
- Gil, W.; Łukaszewicz, J.; Paluch, R.; Zachara, T. Jesion Wyniosły. Hodowla i Zagrożenia.; Gil, W., Ed.; PWRiL: Warsaw, Poland, 2010; ISBN 978-83-09-99019-2. [Google Scholar]
- Pacia, A.; Borowik, P.; Hsiang, T.; Marozau, A.; Matić, S.; Oszako, T. Ash Dieback in Forests and Rural Areas—History and Predictions. Forests 2023, 14, 2151. [Google Scholar] [CrossRef]
- Dahlsjö, C.A.L.; Malhi, Y. Unravelling a Hidden Synergy: How Pathogen-Climate Interactions Transform Habitat Hydrology and Affect Tree Growth. Sci. Total Environ. 2024, 954, 176325. [Google Scholar] [CrossRef]
- Jung, T.; Nechwatal, J. Phytophthora Gallica Sp. Nov., a New Species from Rhizosphere Soil of Declining Oak and Reed Stands in France and Germany. Mycol. Res. 2008, 112, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Paluch, R. Rate and Direction of Changes in Tree Species Composition of Natural Stands in Selected Forest Associations in the Białowieża Forest. For. Res. Pap. 2015, 75, 385–406. [Google Scholar] [CrossRef][Green Version]
- Skovsgaard, J.P.; Wilhelm, G.J.; Thomsen, I.M.; Metzler, B.; Kirisits, T.; Havrdová, L.; Enderle, R.; Dobrowolska, D.; Cleary, M.; Clark, J. Silvicultural Strategies for Fraxinus excelsior in Response to Dieback Caused by Hymenoscyphus fraxineus. For. Int. J. For. Res. 2017, 90, 455–472. [Google Scholar] [CrossRef]
- Bartelheimer, M.; Poschlod, P. Functional Characterizations of Ellenberg Indicator Values—A Review on Ecophysiological Determinants. Funct. Ecol. 2016, 30, 506–516. [Google Scholar] [CrossRef]
- Turczański, K.; Rutkowski, P.; Nowiński, M.; Zawieja, B. Health Status of European Ash (Fraxinus excelsior L.) in Relation to the Moisture of Selected Forest Sites. Sylwan 2020, 164, 133–141. [Google Scholar] [CrossRef]
- Turczański, K. Site Conditions for Decline of Ash Stands in Europe. Acta Sci. Pol. Silvarum Colendarum Ratio Et Ind. Lignaria 2020, 19, 67–73. [Google Scholar] [CrossRef]
- Boczoń, A.; Kowalska, A.; Ksepko, M.; Sokołowski, K. Climate Warming and Drought in the Bialowieza Forest from 1950–2015 and Their Impact on the Dieback of Norway Spruce Stands. Water 2018, 10, 1502. [Google Scholar] [CrossRef]
- Husson, C.; Caël, O.; Grandjean, J.P.; Nageleisen, L.M.; Marçais, B. Occurrence of Hymenoscyphus pseudoalbidus on Infected Ash Logs. Plant Pathol. 2012, 61, 889–895. [Google Scholar] [CrossRef]
- Grosdidier, M.; Scordia, T.; Ioos, R.; Marçais, B. Landscape Epidemiology of Ash Dieback. J. Ecol. 2020, 108, 1789–1799. [Google Scholar] [CrossRef]
- Kjær, E.D.; McKinney, L.V.; Nielsen, L.R.; Hansen, L.N.; Hansen, J.K. Adaptive Potential of Ash (Fraxinus excelsior) Populations against the Novel Emerging Pathogen Hymenoscyphus pseudoalbidus. Evol. Appl. 2012, 5, 219–228. [Google Scholar] [CrossRef]
- Lygis, V.; Vasiliauskas, R.; Larsson, K.H.; Stenlid, J. Wood-Inhabiting Fungi in Stems of Fraxinus excelsior in Declining Ash Stands of Northern Lithuania, with Particular Reference to Armillaria Cepistipes. Scand. J. For. Res. 2005, 20, 337–346. [Google Scholar] [CrossRef]
- Madsen, C.L.; Kosawang, C.; Thomsen, I.M.; Hansen, L.N.; Nielsen, L.R.; Kjær, E.D. Combined Progress in Symptoms Caused by Hymenoscyphus fraxineus and Armillaria Species, and Corresponding Mortality in Young and Old Ash Trees. For. Ecol. Manag. 2021, 491, 119177. [Google Scholar] [CrossRef]
- Flynn, W.R.M.; Grieve, S.W.D.; Henshaw, A.J.; Owen, H.J.F.; Buggs, R.J.A.; Metheringham, C.L.; Plumb, W.J.; Stocks, J.J.; Lines, E.R. UAV-Derived Greenness and within-Crown Spatial Patterning Can Detect Ash Dieback in Individual Trees. Ecol. Solut. Evid. 2024, 5, e12343. [Google Scholar] [CrossRef]
- Waser, L.T.; Küchler, M.; Jütte, K.; Stampfer, T. Evaluating the Potential of Worldview-2 Data to Classify Tree Species and Different Levels of Ash Mortality. Remote Sens. 2014, 6, 4515–4545. [Google Scholar] [CrossRef]
- Chan, A.H.Y.; Barnes, C.; Swinfield, T.; Coomes, D.A. Monitoring Ash Dieback (Hymenoscyphus fraxineus) in British Forests Using Hyperspectral Remote Sensing. Remote Sens. Ecol. Conserv. 2021, 7, 306–320. [Google Scholar] [CrossRef]


| Variable Type | Variable | Description |
|---|---|---|
| tree | alive ash | Number of alive ash |
| other deciduous mortality | Number of deciduous without ash dying between 2015 and 2019 | |
| dead deciduous | Number of dead deciduous | |
| other alive deciduous | Number of alive deciduous without ash | |
| coniferous trees | Number of coniferous | |
| coniferous mortality | Number of coniferous without ash dying between 2015 and 2019 | |
| avg height | Mean height of trees | |
| cover all | Percent of a grid cell covered by tree crowns | |
| stand and habitat | dominant tree species | Dominant tree species |
| habitat type | Basic unit in the classification of forest habitats in Poland | |
| dominant age | Age of the dominant tree species | |
| topographic | aspect | The compass direction |
| slope | Rate of change of elevation | |
| tpi 500 | Topographic Position Index calculated with a 500 m radius of influence. | |
| area | district | Name of Forest District |
| reserve |
| Area Type | Number of Detected Alive Ash | Number of Detected Dead Ash | Percentage of Dead Ash |
|---|---|---|---|
| 2015 | 2015–2019 | 2015–2019 | |
| Białowieża F.D. | 38,292 | 12,088 | 31.57% |
| Browsk F.D. | 25,828 | 6092 | 23.59% |
| Hajnówka F.D. | 35,552 | 10,274 | 28.90% |
| Białowieża N.P. | 45,397 | 13,111 | 28.88% |
| Total | 145,069 | 41,565 | 28.65% |
| Factor | Whole Area | LISA Class | |||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 4 | ||
| number_of_dead_ash | 1.24 ± 2.07 | 0.92 ± 1.48 b | 4.76 ± 3.82 d | 0 ± 0 a | 1.2 ± 0.57 c |
| number of alive ash | 4.33 ± 4.64 | 3.65 ± 3.44 b | 12.24 ± 7.81 c | 3.98 ± 3.34 b | 2.25 ± 1.95 a |
| dead_deciduous | 9.86 ± 10.86 | 8.27 ± 9.19 b | 21.67 ± 18.0 c | 9.83 ± 10.12 b | 6.20 ± 7.34 a |
| other alive deciduous | 206.3 ± 80.0 | 205.6 ± 81.5 b | 220.3 ± 50.5 c | 233.0 ± 67.2 c | 175.3 ± 95.4 a |
| other_deciduous_mortality | 14.15 ± 13.69 | 13.67 ± 12.91 a, b | 19.31 ± 18.58 c | 11.28 ± 8.44 a | 15.04 ± 18.29 b |
| coniferous trees | 81.77 ± 83.80 | 85.06 ± 84.90 b | 36.45 ± 28.95 a | 47.90 ± 43.45 a | 130.1 ± 113.2 c |
| coniferous_mortality | 26.67 ± 37.59 | 27.85 ± 38.54 b | 12.85 ± 17.12 a | 15.51 ± 22.66 a | 36.03 ± 46.28 c |
| Predictor Type | Variable | Relative Contribution [%] |
|---|---|---|
| tree | alive ash | 79.44 [↑] |
| other deciduous mortality | 5.42 [↑] | |
| dead deciduous | 2.66 [↑] | |
| other alive deciduous | <1 | |
| coniferous trees | 1.51 | |
| coniferous mortality | <1 | |
| avg height | 1.59 | |
| cover all | <1 | |
| stand and habitat | dominant tree species | 2.28 |
| habitat type | <1 | |
| dominant age | <1 | |
| topographic | aspect | 1.26 |
| slope | 1.31 | |
| tpi 500 | <1 | |
| area | district | <1 |
| reserve | <1 | |
| training data correlation | 0.87 | |
| CV correlation | 0.82 | |
| standard error | 0.004 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska, A.; Lisiewicz, M.; Kraszewski, B.; Tkaczyk, M.; Stereńczak, K.; Wysocka-Fijorek, E. Assessing Ash (Fraxinus excelsior L.) Dieback Dynamics in the Białowieża Forest, Poland, Using Bi-Temporal High-Resolution Remote Sensing Data. Forests 2025, 16, 506. https://doi.org/10.3390/f16030506
Kamińska A, Lisiewicz M, Kraszewski B, Tkaczyk M, Stereńczak K, Wysocka-Fijorek E. Assessing Ash (Fraxinus excelsior L.) Dieback Dynamics in the Białowieża Forest, Poland, Using Bi-Temporal High-Resolution Remote Sensing Data. Forests. 2025; 16(3):506. https://doi.org/10.3390/f16030506
Chicago/Turabian StyleKamińska, Agnieszka, Maciej Lisiewicz, Bartłomiej Kraszewski, Miłosz Tkaczyk, Krzysztof Stereńczak, and Emilia Wysocka-Fijorek. 2025. "Assessing Ash (Fraxinus excelsior L.) Dieback Dynamics in the Białowieża Forest, Poland, Using Bi-Temporal High-Resolution Remote Sensing Data" Forests 16, no. 3: 506. https://doi.org/10.3390/f16030506
APA StyleKamińska, A., Lisiewicz, M., Kraszewski, B., Tkaczyk, M., Stereńczak, K., & Wysocka-Fijorek, E. (2025). Assessing Ash (Fraxinus excelsior L.) Dieback Dynamics in the Białowieża Forest, Poland, Using Bi-Temporal High-Resolution Remote Sensing Data. Forests, 16(3), 506. https://doi.org/10.3390/f16030506










