Physiological and Transcriptomic Analyses Reveal the Mechanisms of Ilex chinensis Response to Different Types of Simulated Acid Rain
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Treatment of Simulated Acid Rain
2.3. Determination of Height Growth Rate and Specific Leaf Weight
2.4. Determination of Photosynthetic Pigments and Photosynthetic Parameters
2.5. Determination of MDA and SOD
2.6. Determination of Nitrogen Metabolism-Related Products and Enzymes
2.7. Transcriptome Sequencing Analysis
2.8. Statistics
3. Results
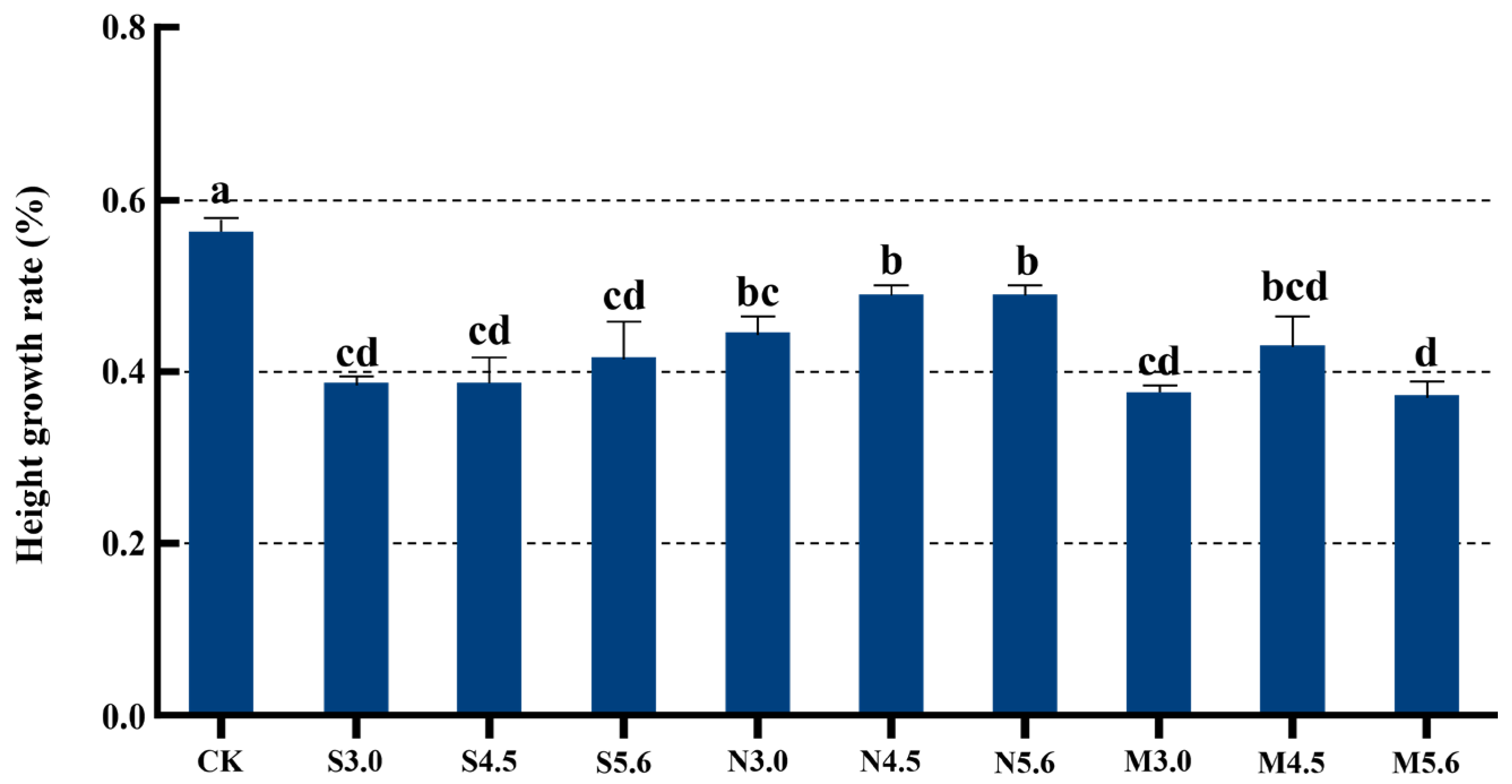
3.1. Growth Response of I. chinensis Under Acid Rain Stress
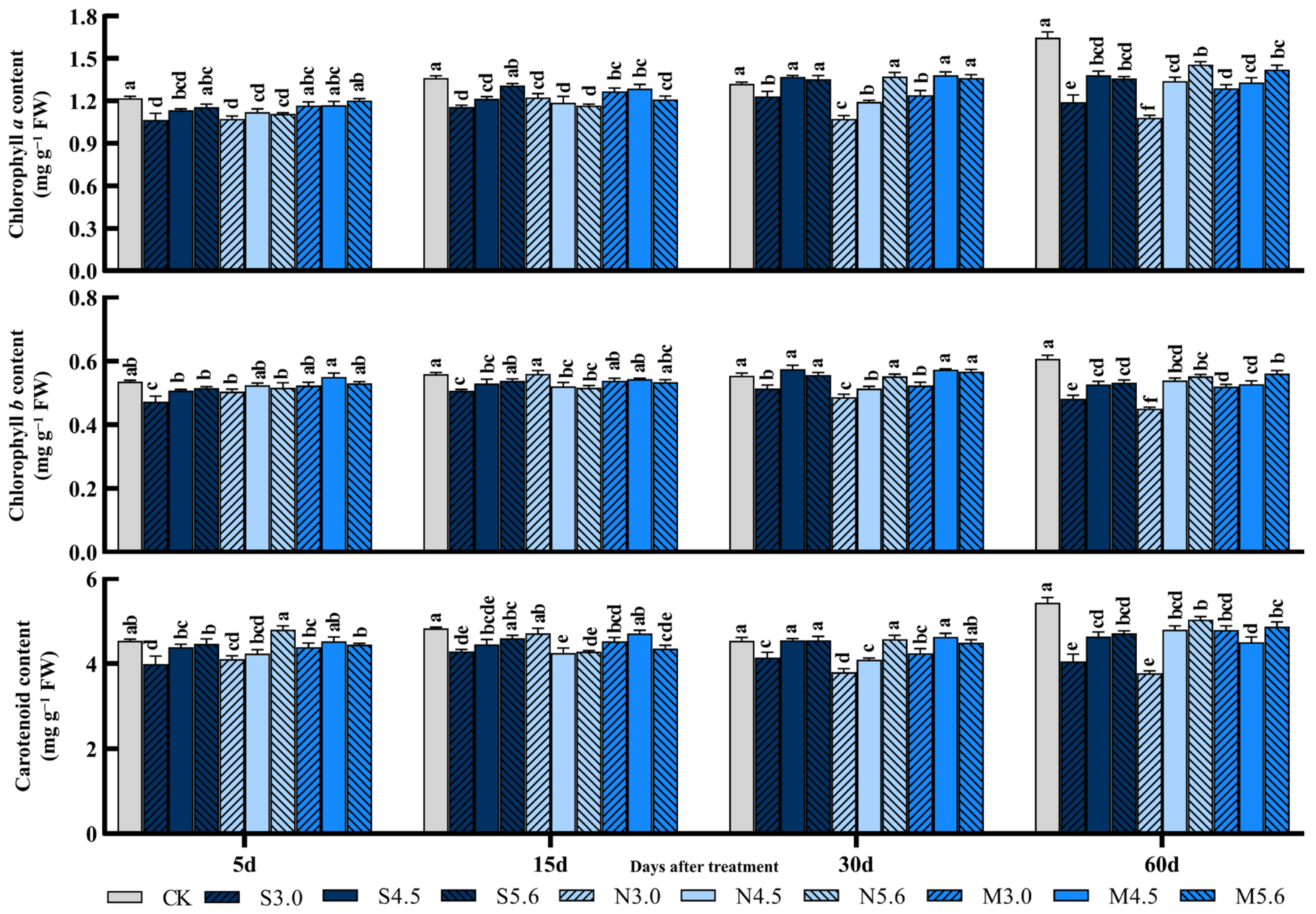
3.2. Photosynthetic Characteristics of I. chinensis Under Acid Rain Stress
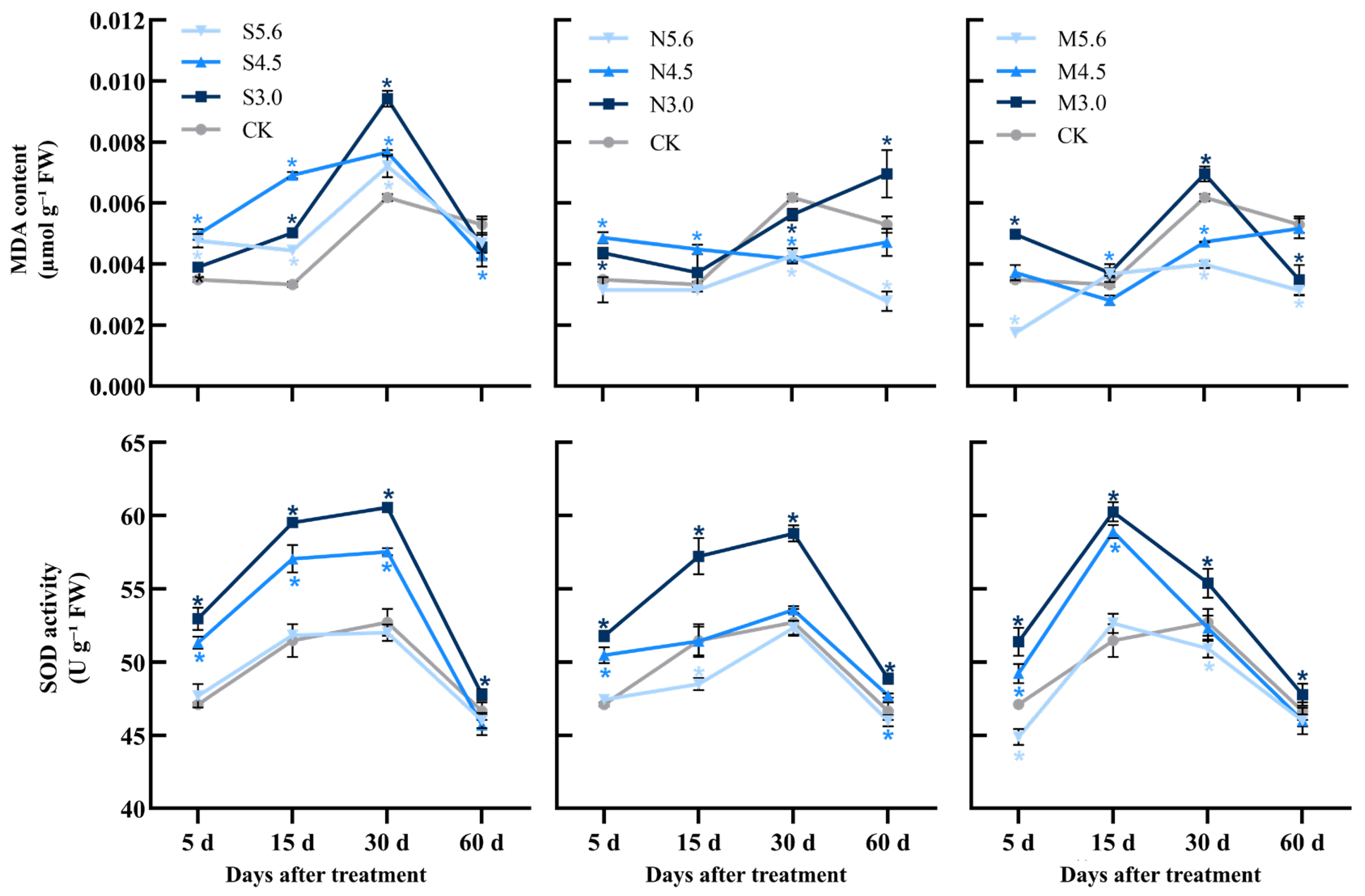
3.3. MDA Content and SOD Activity of I. chinensis Under Acid Rain Stress
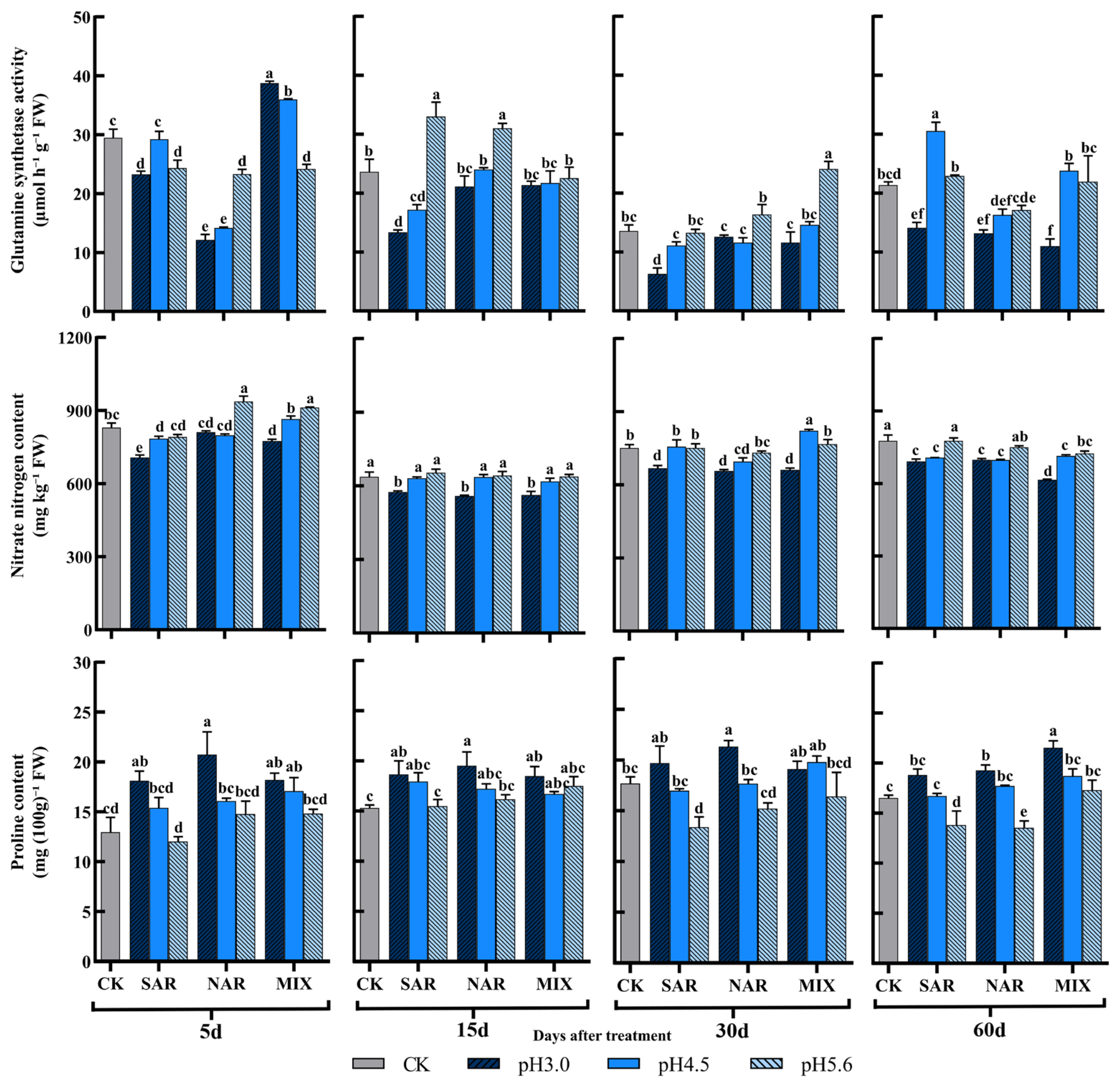
3.4. Effects of Acid Rain on I. chinensis of Enzymes and Products Related to Nitrogen Metabolism
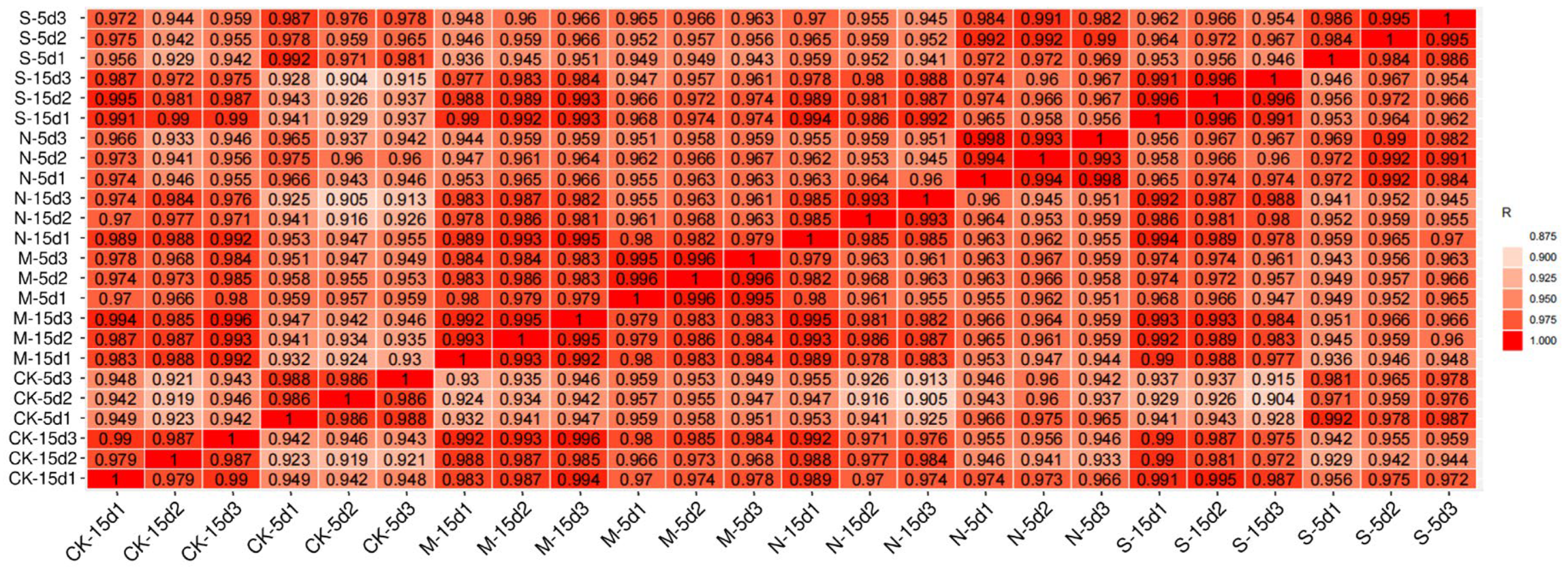
3.5. Illumina Sequencing and Correlation Between Samples
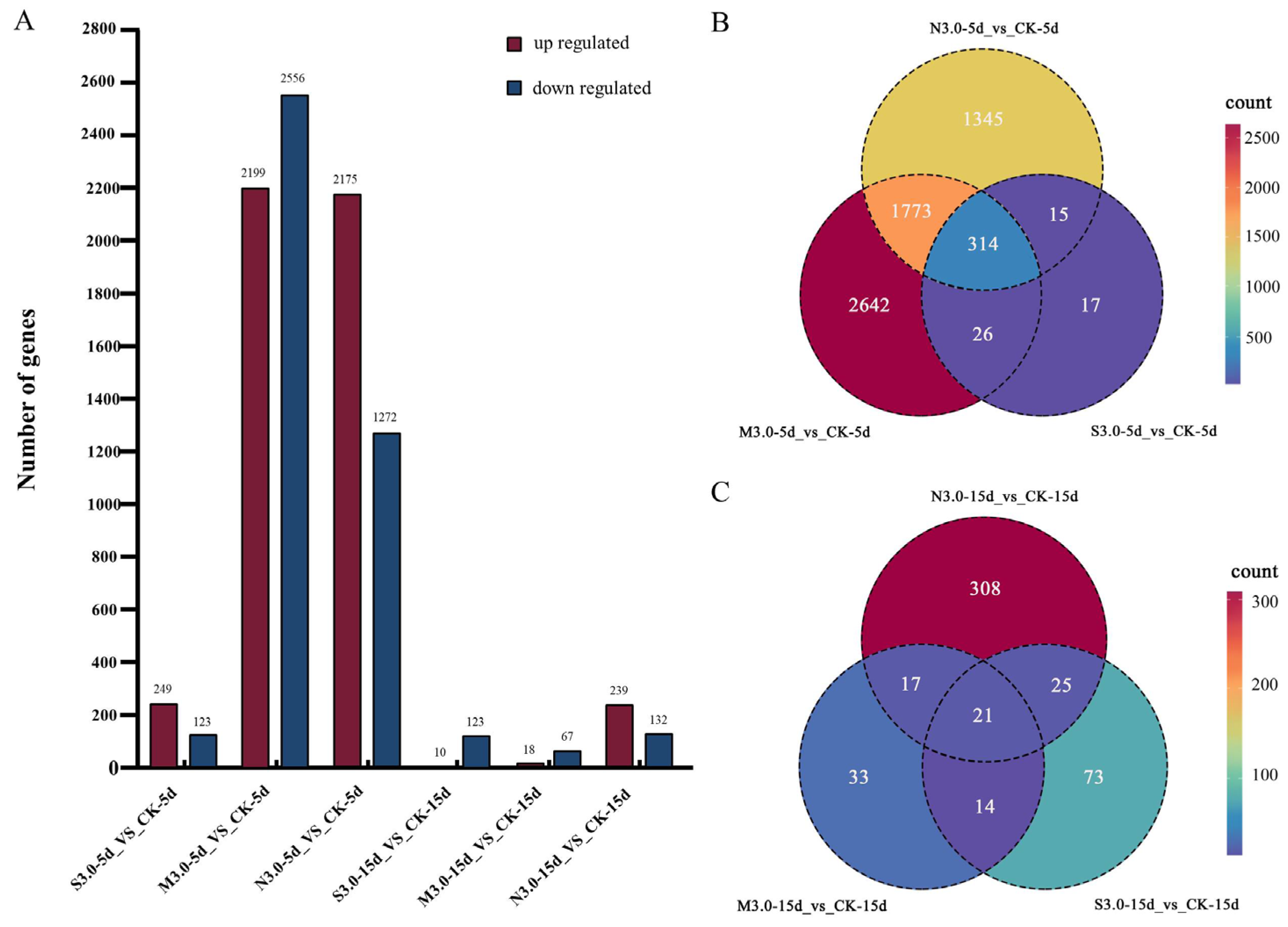
3.6. DEGs Identification
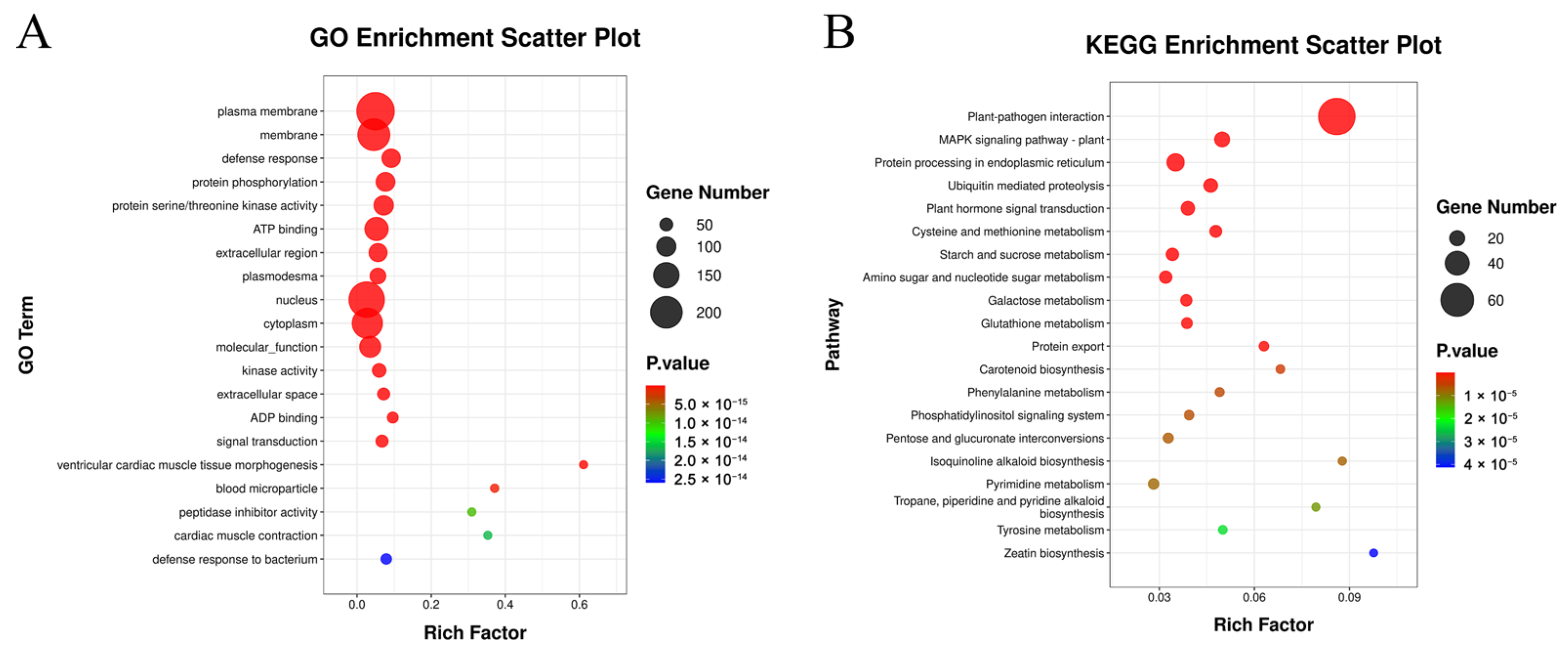
3.7. GO Enrichment Analysis of DEGs
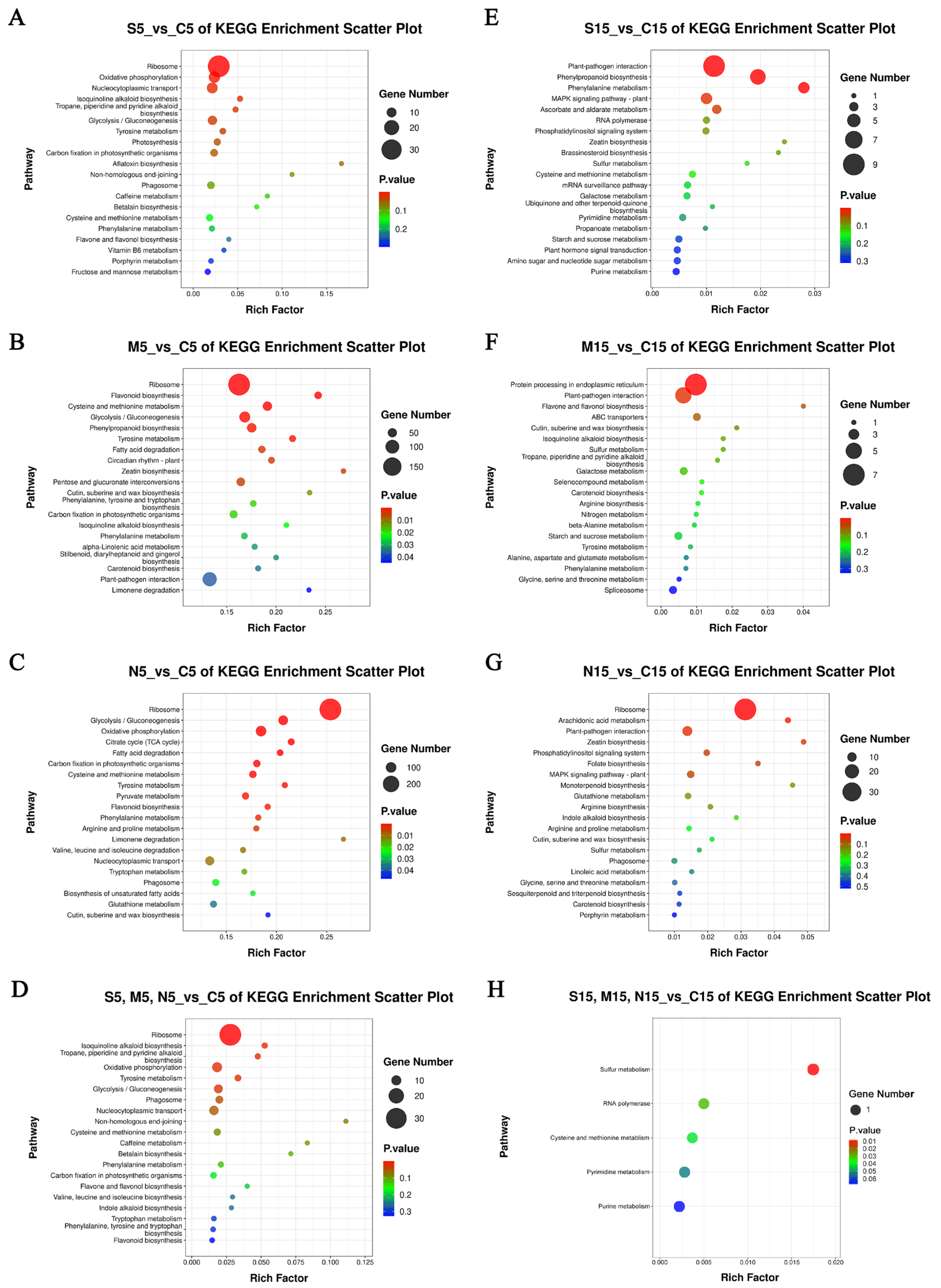
3.8. KEGG Enrichment Analysis of DEGs
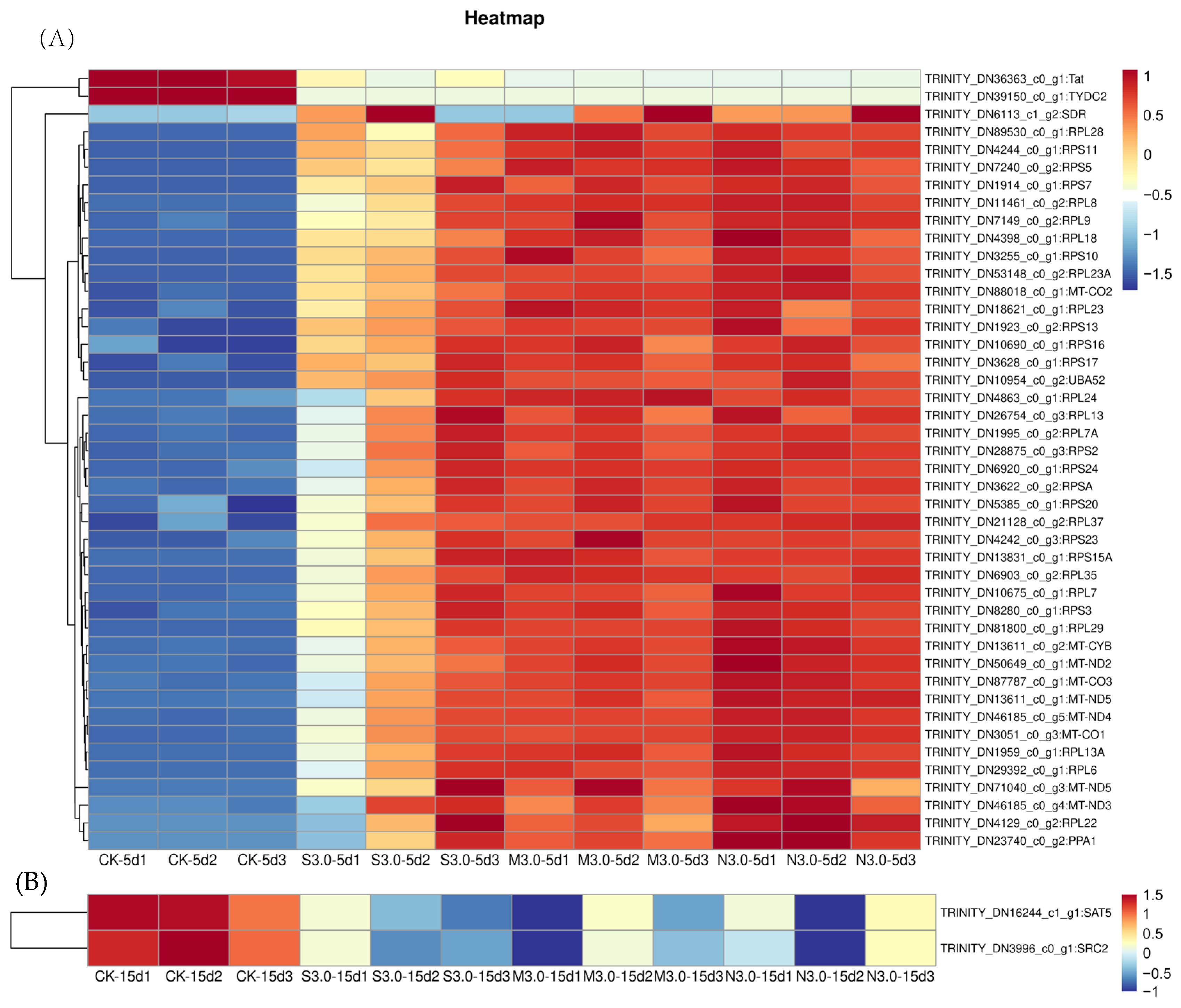
3.9. Candidate Genes Involved in the Response of I. chinensis to Acid Rain Stress
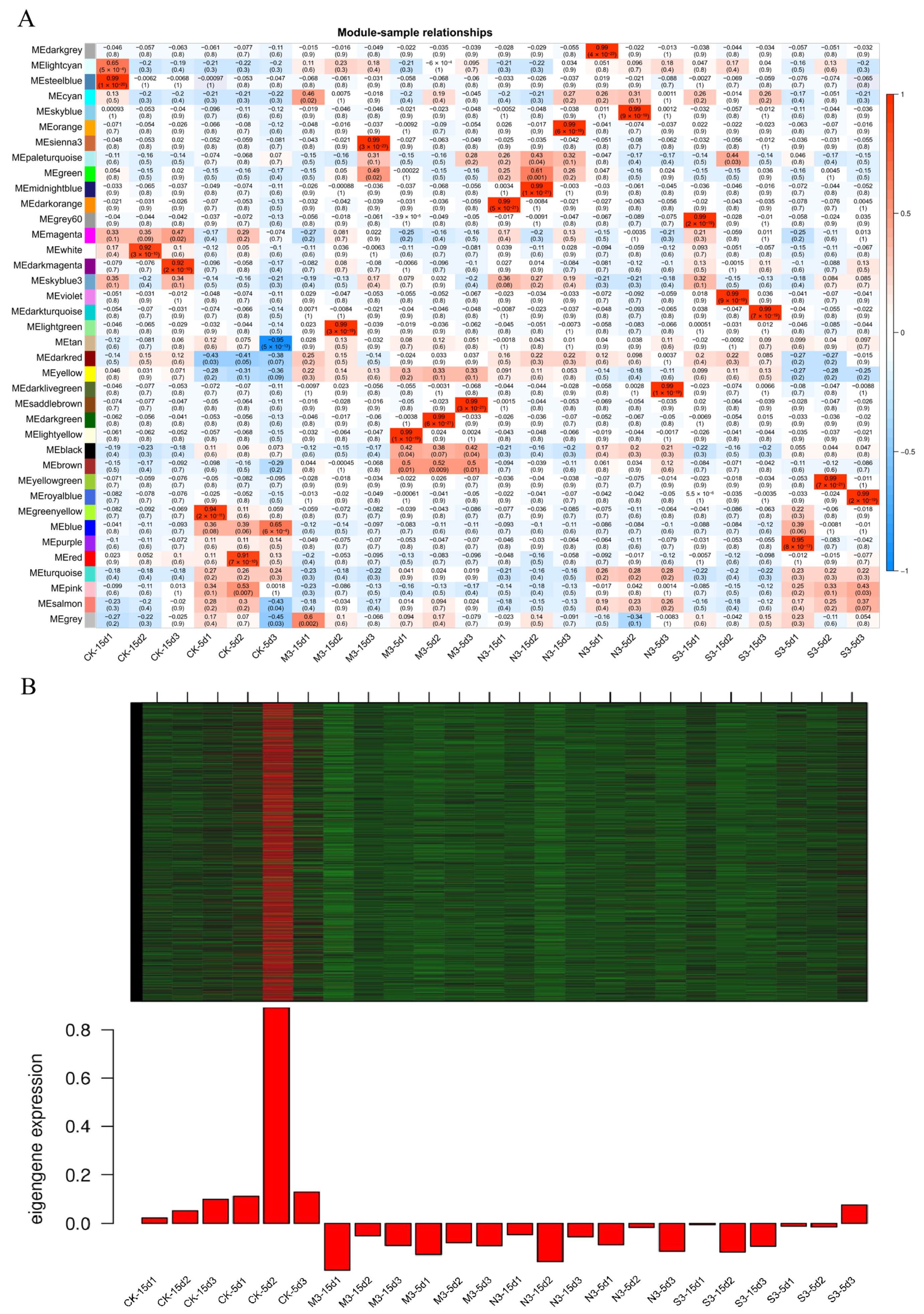
3.10. Screening of Core Genes Involved in Acid Rain Related to I. chinensis by WGCNA
4. Discussion
4.1. Physiological Response of I. chinensis Under Different Types of Acid Rain
4.2. Transcriptome Response of I. chinensis Under Different Types of Acid Rain
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, T.F. Introduction to Environmental Geoscience; China Environmental Science Press: Beijing, China, 1996. [Google Scholar]
- Larssen, T.; Seip, H.M.; Semb, A.; Mulder, J.; Muniz, I.P.; Vogt, R.D.; Lydersen, E.; Angell, V.; Dagang, T.; Eilertsen, O. Acid deposition and its effects in China: An overview. Environ. Sci. Policy 1999, 2, 9–24. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Li, H.; Yang, X.; Sun, L.; Wang, X.; Wang, T.; Wang, W. Organic acids in cloud water and rainwater at a mountain site in acid rain areas of South China. Environ. Sci. Pollut. Res. Int. 2016, 23, 9529–9539. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; An, J.; He, Y.; Zheng, J. An overview of emissions of SO2 and NOx and the long-range transport of oxidized sulfur and nitrogen pollutants in East Asia. J. Environ. Sci. 2016, 44, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Liu, X.; Zhang, J.C. Changing trends of acid rain types in the Yangtze River Delta region. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 45, 168–174. [Google Scholar]
- Sun, S.; Liu, S.; Li, L.; Zhao, W. Components, acidification characteristics, and sources of atmospheric precipitation in Beijing from 1997 to 2020. Atmos. Environ. 2021, 266, 118707. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Wang, S.; Wang, C.; Yang, F.; Li, T. Chemical characteristics of long-term acid rain and its impact on lake water chemistry: A case study in Southwest China. J. Environ. Sci. 2024, 138, 121–131. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, R.G.; Zhao, Y.F.; Wang, P.; Lin, L.C.; Chen, J.Y.; Li, Y.; Wang, Z.L.; Wang, Q. Interannual variation characteristics of acid rain from 2006–2021 in China. Environ. Pollut. Control 2023, 45, 849–854. [Google Scholar] [CrossRef]
- Yoshihisa, K. Effects of simulated acid rain on Asian crops and garden plants. Air Pollut. Impacts Plants East Asia 2017, 32, 223–235. [Google Scholar]
- Shu, P.; Gong, X.; Du, Y.; Han, Y.; Jin, S.; Wang, Z.; Qian, P.; Li, X. Effects of Simulated Acid Rain on Photosynthesis in Pinus massoniana and Cunninghamia lanceolata in Terms of Prompt Fluorescence, Delayed Fluorescence, and Modulated Reflection at 820 nm. Plants 2024, 13, 622. [Google Scholar] [CrossRef]
- He, Y.; Zhang, S.; Sun, X.; Wang, T.; Dai, L.; Xie, Y. Response of photosynthetic characteristics of Pleioblastus fortunei to high frequent simulated acid rain. J. Nanjing For. Univ. 2016, 59, 49. [Google Scholar] [CrossRef]
- Liu, M.; Korpelainen, H.; Dong, L.; Yi, L. Physiological responses of Elaeocarpus glabripetalus seedlings exposed to simulated acid rain and cadmium. Ecotoxicol. Environ. Saf. 2019, 175, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Bezerril Fontenele, N.M.; Otoch, M.D.; Gomes-Rochette, N.F.; Sobreira, A.C.; Barreto, A.A.; de Oliveira, F.D.; Costa, J.H.; Borges, S.D.; do Nascimento, R.F.; Fernandes de Melo, D. Effect of lead on physiological and antioxidant responses in two Vigna unguiculata cultivars differing in Pb-accumulation. Chemosphere 2017, 176, 397–404. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, Y.; Chen, X.; Ye, Z.; Li, Y.; Li, W. The impact of acid rain on cadmium phytoremediation in sunflower (Helianthus annuus L.). Environ. Pollut. 2024, 340 Pt 2, 122778. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Meng, K.; Chen, X.; Zhang, J.E.; Xiang, H.M.; Zhong, J.W.; Shi, Z.J. Effects of Acid Rain on Yield, Quality and Physiological Characteristics of Lettuce and Brassica chinensis L. Ecol. Environ. Sci. 2023, 32, 1098–1107. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.; Zhong, Y.; Huang, J.; Fu, X.; Wang, L.; Teng, W. Growth and physiological response of an endangered tree, Horsfieldia hainanensis merr., to simulated sulfuric and nitric acid rain in southern China. Plant Physiol. Biochem. 2019, 144, 118–126. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Im, K.; Kim, K.; Lee, J.; Lee, K.; Park, J.A.; Lee, T.K.; Park, D.S.; Yang, J.S.; et al. Arabidopsis leaf necrosis caused by simulated acid rain is related to the salicylic acid signaling pathway. Plant Physiol. Biochem. 2006, 44, 38–42. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.H.; Liu, T.W.; Wu, F.H.; Zheng, H.L. Photosynthetic and antioxidant responses of Liquidambar formosana and Schima superba seedlings to sulfuric-rich and nitric-rich simulated acid rain. Plant Physiol. Biochem. 2013, 64, 41–51. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Y.; Zhang, R.; Wang, X.; Yang, C. Comprehensive transcriptome profiling of soybean leaves in response to simulated acid rain. Ecotoxicol. Environ. Saf. 2018, 158, 18–27. [Google Scholar] [CrossRef]
- Liu, T.W.; Niu, L.; Fu, B.; Chen, J.; Wu, F.H.; Chen, J.; Wang, W.H.; Hu, W.J.; He, J.X.; Zheng, H.L. A transcriptomic study reveals differentially expressed genes and pathways respond to simulated acid rain in Arabidopsis thaliana. Genome 2013, 56, 49–60. [Google Scholar] [CrossRef]
- Xia, W.Q.; Li, R.F.; Liu, J.B.; Cui, B.S.; Hou, Q.; Sun, H.; Li, S. Triterpenoids from the leaves of Ilex chinensis. Phytochemistry 2018, 148, 113–121. [Google Scholar] [CrossRef]
- Li, R.F.; Xia, W.Q.; Liu, J.B.; Cui, B.S.; Hou, Q.; Sun, H.; Li, S. New triterpenoid saponins from the leaves of Ilex chinensis. Fitoterapia 2018, 131, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.P.; Li, P. Compounds from Leaf of Ilex purpurea Hassk. J. China Pharm. Univ. 2004, 35, 205–206. [Google Scholar]
- Xie, J.B.; Li, P. Studies on Phenolic Acids from Ilex purpurea Hassk. J. China Pharm. Univ. 2002, 33, 78–79. [Google Scholar]
- Yao, X.; Zhang, F.; Corlett, R.T. Utilization of the Hollies (Ilex L. spp.): A Review. Forests 2022, 13, 94. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, B.; Ma, J. Comparison of heavy metal accumulation ability in rainwater by 10 sponge city plant species. Environ. Sci. Pollut. Res. Int. 2019, 26, 26733–26747. [Google Scholar] [CrossRef]
- Li, Q.Y.; Wang, X.B.; Shen, D.F.; Zhang, J.H.; Wang, S.W. Comparison of Photosynthetic Characteristics Between Ilex×altaclerensis ‘Belgica Aurea’ and Ilex Chinensis Under Salt Stress. J. Mt. Agric. Biol. 2021, 40, 36–42. [Google Scholar] [CrossRef]
- Chen, J.S. Effect of Simulated Acid Rain on the Physiology, Growth Characteristics and Microbial Diversity of Sinocalycanthus chinensis Seedling; Shanghai Normal University: Shanghai, China, 2016. [Google Scholar]
- Li, B.F. Simulated Acid Rain on the Shape of Oak and Elaeocarpus decipiens Bald Disc and Optical Response Characteristics; Zhejiang A&F University: Hangzhou, China, 2013. [Google Scholar]
- Tao, X.T. Effects of Nitrogen Deposition and Mixed Acid Rain Stress on Photosynthetic and Water Physiology Characteristics of Typical Subtropical Trees; Zhejiang A&F University: Hangzhou, China, 2013. [Google Scholar]
- Wang, X.K. Principles and Techniques of Plant Physiology and Biochemistry Experiments, 2nd ed.; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Odiyi, B.; Bamidele, J. Effects of simulated acid rain on growth and yield of cassava Manihot esculenta (Crantz). J. Agric. Sci. 2013, 6, 28563. [Google Scholar] [CrossRef]
- Hu, L. The Effects of Subtropical Woody Plants Under UV-B Radiation Stress and Acid Rainforce; Zhejiang A&F University: Hangzhou, China, 2012. [Google Scholar]
- Li, Q.; Wang, F.; He, M.; Lu, X.M.; Wang, H.Q.; Shi, L. Effects of soil water stress on photosynthetic characteristic of Miscanthus sacchariflorus. Pratacultural Sci. 2013, 30, 1031–1035. [Google Scholar]
- Guo, H. The effect of cadmium stress on the photosynthetic characteristics and rhizosphere soil enzyme activity of wheat. Agric. Technol. 2022, 42, 14–17. [Google Scholar]
- Wu, Q.; Han, Y.N.; Gao, R.; Ma, S.; Zhao, H.Y. The Respondence of Morphology and Structure of Glycyrrhiza uralensis Seedling Under Salt Stress. Seed 2015, 34, 25–31+34. [Google Scholar] [CrossRef]
- Du, E.; Dong, D.; Zeng, X.; Sun, Z.; Jiang, X.; de Vries, W. Direct effect of acid rain on leaf chlorophyll content of terrestrial plants in China. Sci. Total Environ. 2017, 605–606, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Huang, X.; Liu, X. Stress effects of simulant acid rain on three woody plants. Environ. Sci. 2002, 23, 42–46. [Google Scholar]
- Odiyi, B.O.; Eniola, A.O. The Effect of Simulated Acid Rain on Plant Growth Component of Cowpea (Vigna unguiculata) L. Walps. Jordan J. Biol. Sci. 2015, 8, 51–54. [Google Scholar] [CrossRef]
- Pei, C.M. Response Mechanism of Three Plants to Simulated Acid Rain in the Three Gorges Reservoir Area; Beijing Forestry University: Beijing, China, 2019. [Google Scholar]
- Zhao, W.W.; Jiang, H.; Ma, Y.D. Photosynthesis and water use characteristics of Cinnamomum camphora seedlings with simulated acid rain. J. Zhejiang AF Univ. 2013, 30, 179–186. [Google Scholar] [CrossRef]
- Yao, Z.B.; Jiang, H.; Yu, S.Q.; Lu, M.J. Effects of simulated acid rain on water physiological characteristics of Myrica rubra seedlings. Chin. J. Appl. Ecol. 2011, 22, 1967–1974. [Google Scholar]
- Hu, H.; Hua, W.; Shen, A.; Zhou, H.; Sheng, L.; Lou, W.; Zhang, G. Photosynthetic rate and chlorophyll fluorescence of barley exposed to simulated acid rain. Environ. Sci. Pollut. Res. 2021, 28, 42776–42786. [Google Scholar] [CrossRef]
- Copolovici, L.; Adelina, B.A.N.; Ioana, F.A.U.R.; Copolovici, D. The influence of simulated acidic rain on plants volatile organic compounds emission and photosynthetic parameters. AgroLife Sci. J. 2017, 6, 73–76. [Google Scholar]
- Qiu, D.L.; Liu, X.H. Effects of simulated acid rain on chloroplast activity in Dimorcarpus longana Lour. Cv. wulongling leaves. Chin. J. Appl. Ecol. 2002, 13, 1559–1562. [Google Scholar] [PubMed]
- Li, J.X.; Wang, W.P.; Hu, Z.J.; Shi, K. Effects of Simulated Acid Rain Conditions on Plant Photosynthesis and Disease Susceptibility in Tomato and Its Alleviation of Brassinosteroid. Sci. Agric. Sin. 2021, 54, 1728–1738. [Google Scholar] [CrossRef]
- Pan, R.C. Plant Physiology; Higher Education Press: Beijing, China, 2012. [Google Scholar]
- Zhang, Z.; Teng, Z.; Wang, N.; Zhang, M.; Sun, G.; Hu, Y.; Zhang, X. Responses of photosynthesis and antioxidants to simulated acid rain in mulberry seedlings. Physiol. Plant. 2021, 172, 188–200. [Google Scholar] [CrossRef]
- Song, L.Y.; Ke, Z.H.; Sun, L.L.; Peng, C.L. Effect of Simulated Acid Rain on Gas Exchanges of Three Compositae Invasive Plants. Chin. Bull. Bot. 2013, 48, 160–167. [Google Scholar]
- Xu, H.; Xie, C.; Wei, Y.K.; Huang, Y.B.; Lin, X.J. Comparison of tolerance between two salvia plants to stress of simulated acid rain and study of the physiological mechanisms. Asian J. Ecotoxicol. 2017, 12, 206–214. [Google Scholar] [CrossRef]
- Wang, C.C. Study on the Response of Physiological and Ecology Characteristics of Hosta Ventricosa to Combination of Cd and Simulated Acid Rain Stress; Sichuan Agricultural University: Ya’an, China, 2014. [Google Scholar]
- Liu, X.; Ma, S.; Jia, Z.; Ramzan, M.; Meng, M.; Wang, J.; Li, C.; Zhang, Y.; Zhang, J. Complex effects of different types of acid rain on root growth of Quercus acutissima and Cunninghamia lanceolata saplings. Ecol. Process. 2022, 11, 8. [Google Scholar] [CrossRef]
- Shaukat, S.S.; Khan, M.A. Growth and physiological responses of tomato (Lycopersicon Esculentum Mill.) to simulated acid rain. Pak. J. Bot. 2008, 40, 2427–2435. [Google Scholar]
- Ma, Y.J.; Liang, C.J. Regulation of exogenous calcium on plasma membrane compositions and calcium forms of rice roots under simulated acid rain stress. J. Agro-Environ. Sci. 2021, 40, 1159–1166. [Google Scholar] [CrossRef]
- Guo, X.J.; Wang, J.Y.; Ren, X.Q.; Chen, W.Y.; Tao, Q.W.; Deng, Y.; Liang, C.J. Response of antioxidant enzyme activities and isozyme patten in rice roots to acid rain stress. Environ. Chem. 2019, 38, 377–384. [Google Scholar] [CrossRef]
- Zhu, J.L.; Hou, R.Q.; Wang, Y.Q.; Wang, Y.J.; Yang, F.; Zhang, Y.X.; Zheng, Y.L.; Si, H.T. Response mechanism of plant seedling roots and soil to acid rain in Jinyun Mountain, Chongqing. Sci. Soil Water Conserv. 2023, 21, 121–130. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Estravis Barcala, M.; Mattera, M.G.; Soliani, C.; Bellora, N.; Opgenoorth, L.; Heer, K.; Arana, M.V. Molecular bases of responses to abiotic stress in trees. J. Exp. Bot. 2020, 71, 3765–3779. [Google Scholar] [CrossRef]
- Good, A.G.; Zaplachinski, S.T. The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus. Physiol. Plant. 1994, 90, 9–14. [Google Scholar] [CrossRef]
- Vincent, D.; Ergül, A.; Bohlman, M.C.; Tattersall, E.A.; Tillett, R.L.; Wheatley, M.D.; Woolsey, R.; Quilici, D.R.; Joets, J.; Schlauch, K.; et al. Proteomic analysis reveals differences between Vitis vinifera L. cv. Chardonnay and cv. Cabernet sauvignon and their responses to water deficit and salinity. J. Exp. Bot. 2007, 58, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Ullah, H.; Wang, X.; Liu, J.; Chen, B.; Jiang, P.; Lin, H.; Sunahara, G.I.; You, S.; Zhang, X.; et al. Integrated transcriptome and metabolome analysis reveals the mechanism of tolerance to manganese and cadmium toxicity in the Mn/Cd hyperaccumulator Celosia argentea Linn. J. Hazard. Mater. 2023, 443 Pt A, 130206. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, B.; Sahito, Z.A.; Chen, S.; Liang, Z. Transcriptome analysis reveals cadmium exposure enhanced the isoquinoline alkaloid biosynthesis and disease resistance in Coptis chinensis. Ecotoxicol. Environ. Saf. 2024, 271, 115940. [Google Scholar] [CrossRef] [PubMed]
- Hell, R. Molecular physiology of plant sulfur metabolism. Planta 1997, 202, 138–148. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J.A.; Wang, W.; Simon, M.; Wu, F.; Hu, W.; Chen, J.B.; Zheng, H. A combined proteomic and transcriptomic analysis on sulfur metabolism pathways of Arabidopsis thaliana under simulated acid rain. PLoS ONE 2014, 9, e90120. [Google Scholar] [CrossRef]
- Yi, X.Q. Effects of Simulated Acid Rain on Physiological Characteristics and Related Gene Expression of Tea Plants; Hunan Agricultural University: Changsha, China, 2019. [Google Scholar]
- Bao, Q.H. Physiological Characteristics and Mechanism Analysis of Transcriptome and Metabolome of Quinoa under Salt and Alkali; Inner Mongolia Agricultural University: Hohhot, China, 2023. [Google Scholar]
- Liang, L.; Ze, M.; Yang, J.; Xu, Q.; Du, C.; Hu, X.; Dong, M.; Zou, L.; Qi, T. Physiological and transcriptomic response reveals new insight into manganese tolerance of Celosia argentea Linn. J. Hazard. Mater. 2024, 465, 133079. [Google Scholar] [CrossRef]
- Khan, A.; Jie, Z.; Xiangjun, K.; Ullah, N.; Short, A.W.; Diao, Y.; Zhou, R.; Xiong, Y. Pre-treatment of melatonin rescues cotton seedlings from cadmium toxicity by regulating key physio-biochemical and molecular pathways. J. Hazard. Mater. 2022, 445, 130530. [Google Scholar] [CrossRef]
- Liu, J.; Yang, F.; Mao, S.; Li, S.; Lin, H.; Yan, X.; Lin, J. Advances in the physiological functions of plant lipids in response to stresses. Chin. J. Biotechnol. 2021, 37, 2658–2667. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, Z.; Chen, J.; Xie, M.; Huang, W.; Li, J.; Zhuang, C.; Liu, Z.; Zheng, S. SET DOMAIN GROUP 711-mediated H3K27me3 methylation of cytokinin metabolism genes regulates organ size in rice. Plant Physiol. 2024, 194, 2069–2085. [Google Scholar] [CrossRef]
- Bartwal, A.; Rakesh, B.; Bullet, M.; Lohani, P.; Guru, B.S.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Fan, Y.T.; Fan, R.N.; Zhang, H.; Li, B.Z. Biosynthesis and function of flavonol in plants: A review. Chin. Agric. Sci. Bull. 2020, 36, 95–101. [Google Scholar]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Howell, S.H. Endoplasmic Reticulum Protein Quality Control and Its Relationship to Environmental Stress Responses in Plants. Plant Cell 2010, 22, 2930–2942. [Google Scholar] [CrossRef]
- Ge, J.; Tao, J.; Zhao, J.; Wu, Z.; Zhang, H.; Gao, Y.; Tian, S.; Xie, R.; Xu, S.; Lu, L. Transcriptome analysis reveals candidate genes involved in multiple heavy metal tolerance in hyperaccumulator Sedum alfredii. Ecotoxicol. Environ. Saf. 2022, 241, 113795. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, K.; Tian, Y.; Han, K.; El-Kassaby, Y.A.; Yang, H.; Si, H.; Sun, Y.; Li, Y. Time-course transcriptomics analysis reveals key responses of populus to salt stress. Ind. Crops Prod. 2023, 194, 116278. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Sun, X.; Yuan, J.; Zhao, Z.; Gao, J.; Wen, X.; Tang, F.; Kang, M.; Abliz, B.; et al. Genome-Wide Identification of MDH Family Genes and Their Association with Salt Tolerance in Rice. Plants 2022, 11, 1498. [Google Scholar] [CrossRef]
- Luo, D.; Li, Z.; Mubeen, S.; Rehman, M.; Cao, S.; Wang, C.; Yue, J.; Pan, J.; Jin, G.; Li, R.; et al. Functional characterization of malate dehydrogenase, HcMDH1, gene in enhancing abiotic stress tolerance in kenaf (Hibiscus cannabinus L.). Chem. Biol. Technol. Agric 2024, 11, 141. [Google Scholar] [CrossRef]




| Treatment | SLW/mg m−2 | |||
|---|---|---|---|---|
| 5 d | 15 d | 30 d | 60 d | |
| CK | 8.0230 ± 0.0309 c | 8.2664 ± 0.0872 a | 8.7552 ± 0.0245 bc | 9.7098 ± 0.5341 b |
| S3.0 | 7.6032 ± 0.2891 d | 7.2457 ± 0.2204 e | 9.0182 ± 0.0744 b | 10.5027 ± 0.8263 ab |
| S4.5 | 8.7073 ± 0.0624 a | 8.1279 ± 0.0036 ab | 9.033 ± 0.0192 b | 9.8202 ± 0.0622 ab |
| S5.6 | 8.3215 ± 0.1469 b | 8.1576 ± 0.0271 a | 9.0679 ± 0.0611 b | 10.3534 ± 0.8959 ab |
| N3.0 | 7.9642 ± 0.0537 c | 7.5439 ± 0.1131 d | 8.6641 ± 0.2328 bc | 9.7163 ± 0.1171 b |
| N4.5 | 8.3181 ± 0.0149 b | 7.8641 ± 0.0699 bc | 8.7654 ± 0.5923 bc | 9.6618 ± 0.3497 b |
| N5.6 | 8.3914 ± 0.2087 b | 8.4001 ± 0.0693 a | 9.0527 ± 0.3745 b | 10.2540 ± 0.2215 ab |
| M3.0 | 7.8807 ± 0.1112 c | 7.8470 ± 0.1486 bc | 8.6875 ± 0.0179 bc | 10.6185 ± 0.2491 ab |
| M4.5 | 7.9008 ± 0.0971 c | 8.2883 ± 0.3902 a | 9.7912 ± 0.1075 a | 10.6955 ± 0.5969 a |
| M5.6 | 8.4384 ± 0.0734 b | 7.6049 ± 0.0643 cd | 8.4010 ± 0.2328 c | 10.2786 ± 0.365 ab |
| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Valid% | Q20% | GC% |
|---|---|---|---|---|---|---|---|
| CK-15 d-1 | 39,175,518 | 5.88 G | 37,593,562 | 5.50 G | 95.96 | 96.58 | 44.90 |
| CK-15 d-2 | 43,987,490 | 6.60 G | 41,948,868 | 6.18 G | 95.37 | 97.04 | 45.19 |
| CK-15 d-3 | 48,175,598 | 7.23 G | 46,221,760 | 6.79 G | 95.94 | 96.84 | 44.70 |
| CK-5 d-1 | 49,053,114 | 7.36 G | 47,079,842 | 6.88 G | 95.98 | 96.28 | 44.64 |
| CK-5 d-2 | 48,261,548 | 7.24 G | 46,324,214 | 6.76 G | 95.99 | 96.28 | 44.59 |
| CK-5 d-3 | 45,040,372 | 6.76 G | 43,204,992 | 6.31 G | 95.93 | 96.36 | 45.12 |
| S3.0-15 d-1 | 48,612,410 | 7.29 G | 46,554,660 | 6.84 G | 95.77 | 96.76 | 44.57 |
| S3.0-15 d-2 | 46,304,382 | 6.95 G | 44,252,666 | 6.50 G | 95.57 | 96.62 | 44.87 |
| S3.0-15 d-3 | 48,246,472 | 7.24 G | 45,979,116 | 6.75 G | 95.30 | 96.89 | 44.57 |
| S3.0-5 d-1 | 49,295,668 | 7.39 G | 47,374,138 | 6.92 G | 96.10 | 96.13 | 44.57 |
| S3.0-5 d-2 | 47,387,478 | 7.11 G | 45,526,294 | 6.69 G | 96.07 | 96.77 | 44.84 |
| S3.0-5 d-3 | 49,101,054 | 7.37 G | 46,693,738 | 6.85 G | 95.10 | 96.70 | 44.63 |
| N3.0-15 d-1 | 48,518,126 | 7.28 G | 46,620,444 | 6.86 G | 96.09 | 96.59 | 44.70 |
| N3.0-15 d-2 | 48,050,306 | 7.21 G | 46,205,706 | 6.79 G | 96.16 | 96.26 | 44.66 |
| N3.0-15 d-3 | 49,303,012 | 7.40 G | 47,192,052 | 6.93 G | 95.72 | 96.49 | 44.80 |
| N3.0-5 d-1 | 39,087,588 | 5.86 G | 36,970,130 | 5.41 G | 94.58 | 96.71 | 44.86 |
| N3.0-5 d-2 | 37,942,400 | 5.69 G | 35,654,304 | 5.23 G | 93.97 | 97.04 | 44.97 |
| N3.0-5 d-3 | 47,262,930 | 7.09 G | 44,912,056 | 6.59 G | 95.03 | 96.91 | 44.71 |
| M3.0-15 d-1 | 40,326,472 | 6.05 G | 38,642,716 | 5.68 G | 95.82 | 96.65 | 44.74 |
| M3.0-15 d-2 | 48,835,948 | 7.33 G | 46,741,698 | 6.87 G | 95.71 | 96.91 | 44.73 |
| M3.0-15 d-3 | 49,060,938 | 7.36 G | 47,284,246 | 6.95 G | 96.38 | 96.75 | 44.74 |
| M3.0-5 d-1 | 48,935,998 | 7.34 G | 46,751,994 | 6.86 G | 95.54 | 96.50 | 44.69 |
| M3.0-5 d-2 | 49,144,248 | 7.37 G | 46,818,268 | 6.88 G | 95.27 | 96.82 | 44.74 |
| M3.0-5 d-3 | 48,425,054 | 7.26 G | 46,464,284 | 6.83 G | 95.95 | 96.65 | 44.91 |
| DB | All | GO | KEGG | Pfam | Swissprot | EggNOG | NR | TF |
|---|---|---|---|---|---|---|---|---|
| Number | 80,166 | 34,862 | 13,240 | 30,729 | 29,101 | 38,161 | 36,495 | 1586 |
| Ratio (%) | 100 | 43.49 | 16.52 | 38.33 | 36.3 | 47.6 | 45.52 | 1.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, D.; Zhang, T.; Chen, Y.; Jiao, J.; Zheng, B. Physiological and Transcriptomic Analyses Reveal the Mechanisms of Ilex chinensis Response to Different Types of Simulated Acid Rain. Forests 2025, 16, 485. https://doi.org/10.3390/f16030485
Yan D, Zhang T, Chen Y, Jiao J, Zheng B. Physiological and Transcriptomic Analyses Reveal the Mechanisms of Ilex chinensis Response to Different Types of Simulated Acid Rain. Forests. 2025; 16(3):485. https://doi.org/10.3390/f16030485
Chicago/Turabian StyleYan, Daoliang, Tiantian Zhang, Yushuang Chen, Jiejie Jiao, and Bingsong Zheng. 2025. "Physiological and Transcriptomic Analyses Reveal the Mechanisms of Ilex chinensis Response to Different Types of Simulated Acid Rain" Forests 16, no. 3: 485. https://doi.org/10.3390/f16030485
APA StyleYan, D., Zhang, T., Chen, Y., Jiao, J., & Zheng, B. (2025). Physiological and Transcriptomic Analyses Reveal the Mechanisms of Ilex chinensis Response to Different Types of Simulated Acid Rain. Forests, 16(3), 485. https://doi.org/10.3390/f16030485






