Effects of Wood-Derived Biochar on Soil Respiration of a European Beech Forest Under Current Climate and Simulated Climate Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Soil Samples
2.2. Preparation of Experimental Treatments
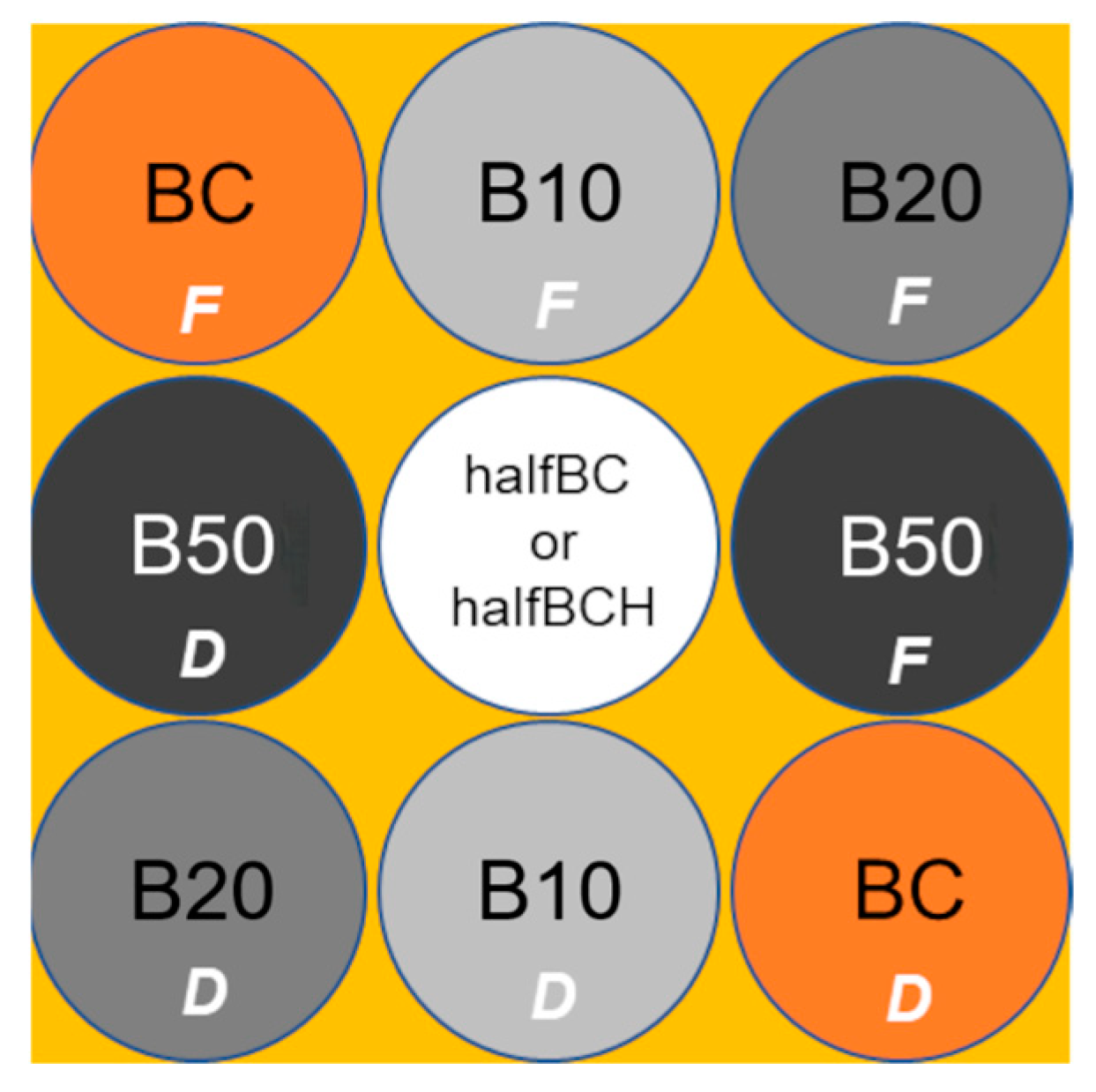
2.3. Experimental Design
2.4. Measurements of Soil Respiration
2.5. Measurements of Soil pH, Soil Temperature, and Soil Moisture
2.6. Data Analysis
3. Results
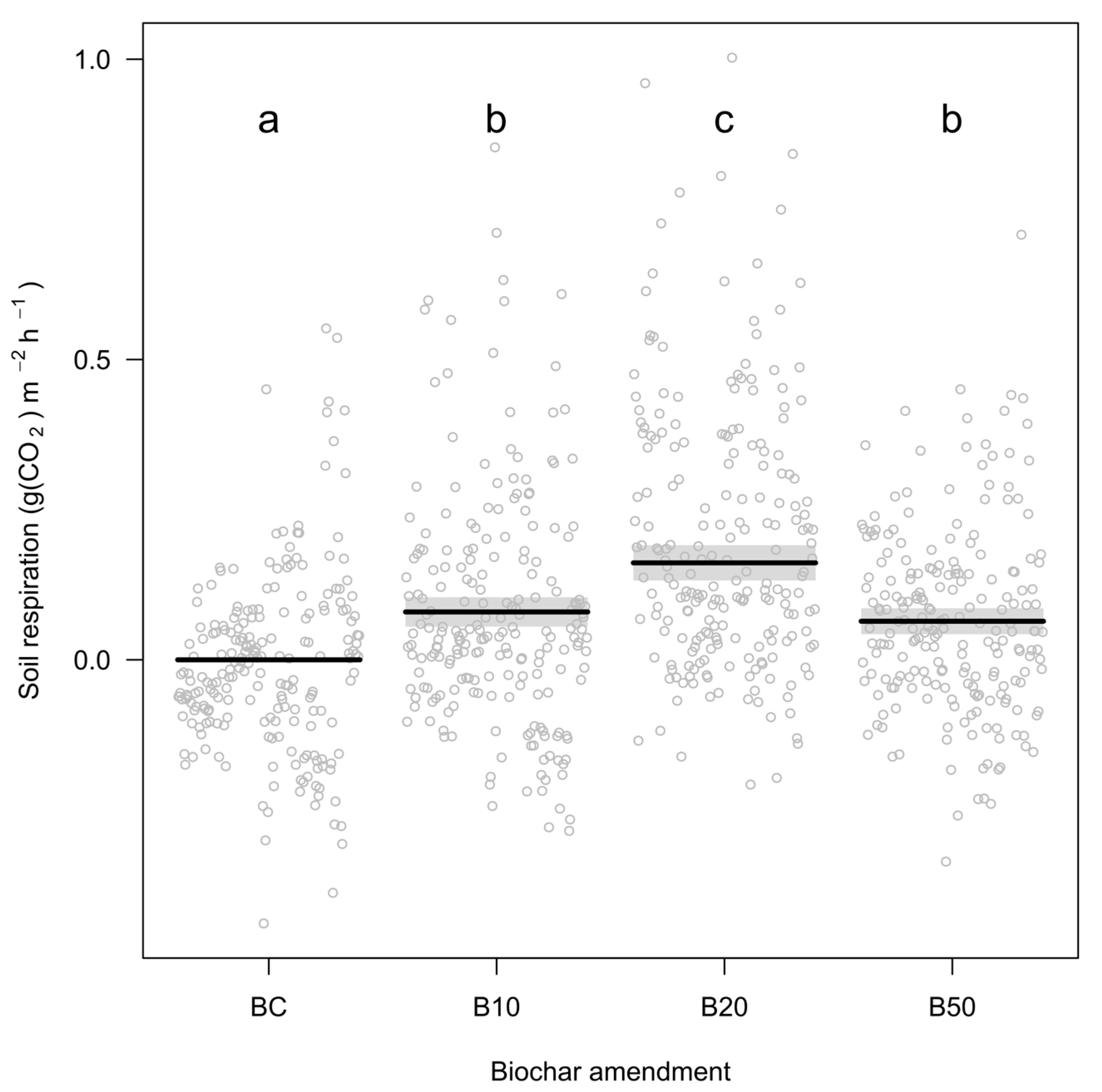
3.1. Effects of Biochar, Simulated Warming and Drought
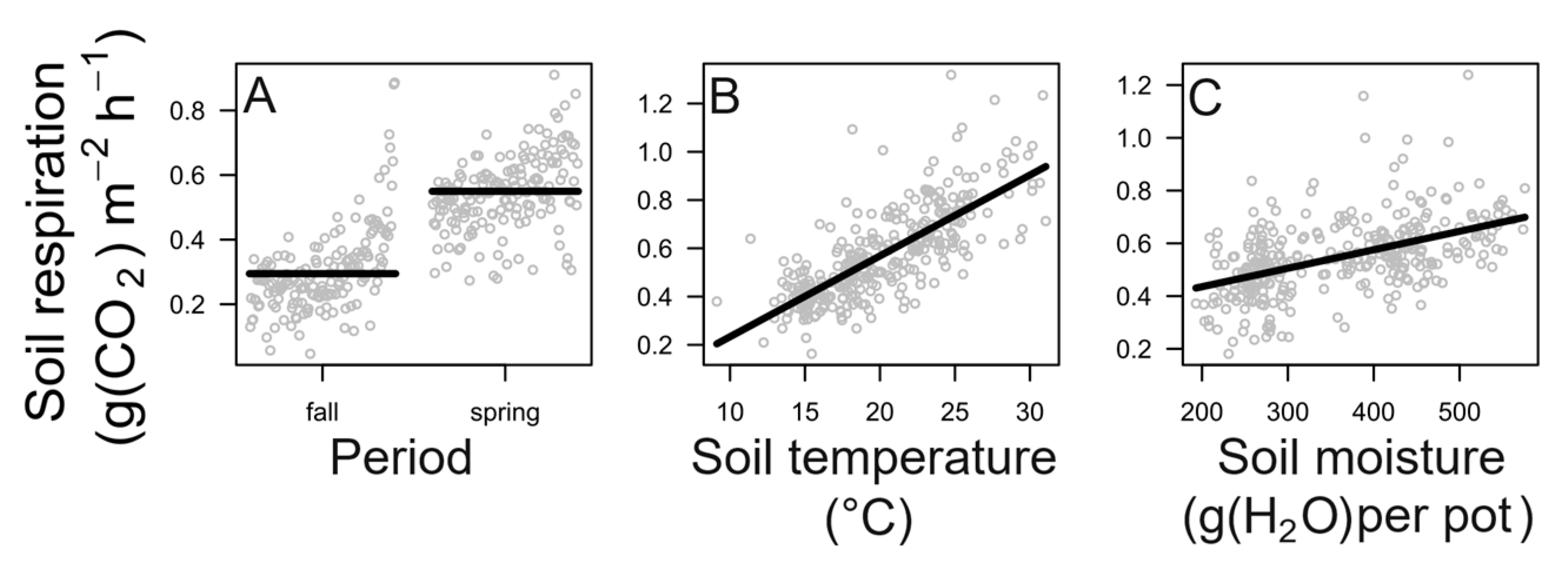
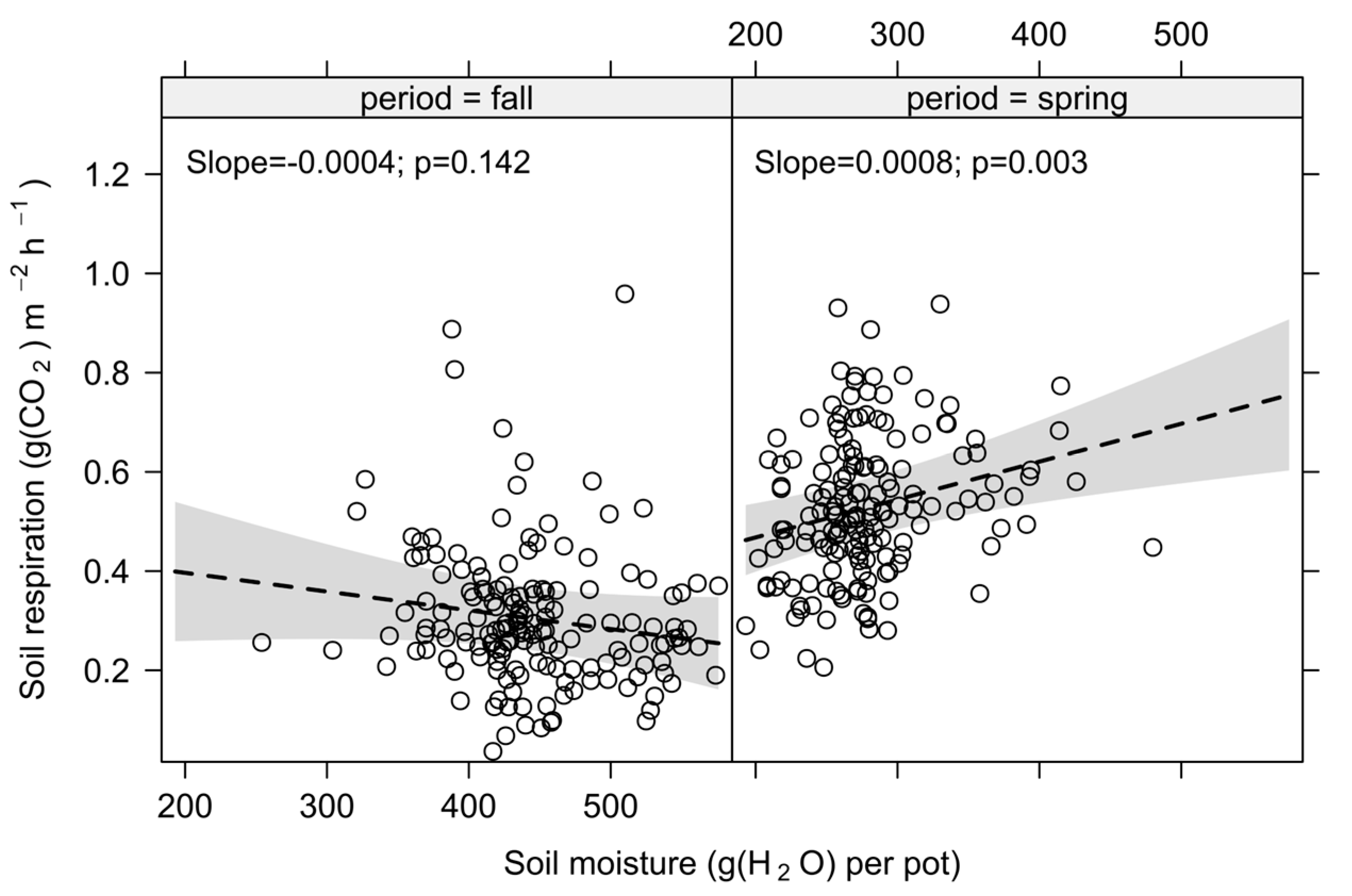
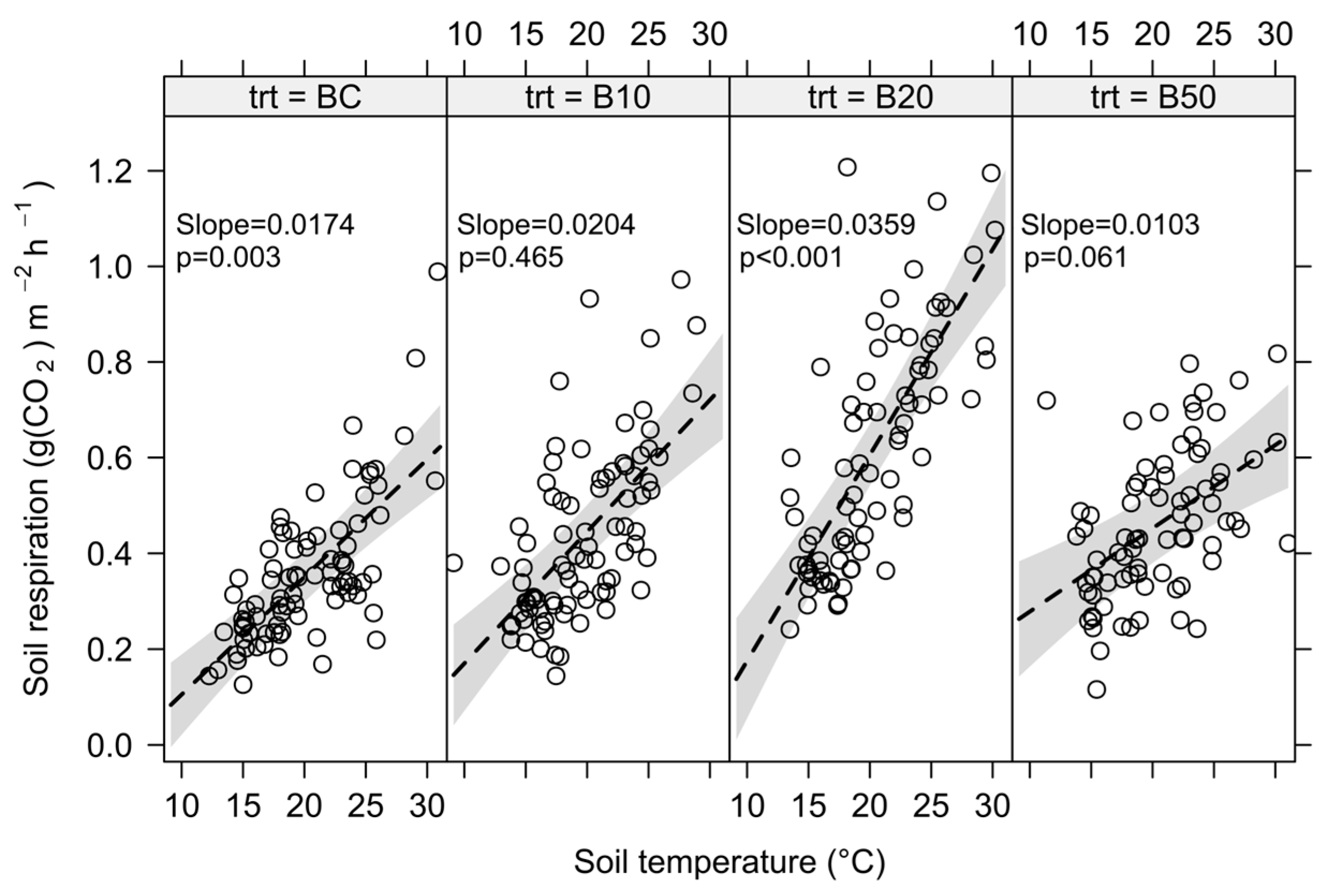
3.2. Effects of Season, Soil Temperature, Soil Moisture and Their Interaction
4. Discussion
4.1. Effects on Soil Respiration of Biochar, Simulated Warming and Drought
4.2. Effects on Soil Respiration of Season, Soil Temperature, Soil Moisture and Their Interaction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCH | Biochar |
| C | Carbon |
| CC | Climate change |
| OTC | Open Top Chamber |
| PAH | Polycyclic Aromatic Hydrocarbon |
| SOC | Soil Organic Carbon |
| SOM | Soil Organic Matter |
Appendix A
Appendix A.1
| Soil Characteristics | BC | B10 | B20 | B50 |
|---|---|---|---|---|
| Sand (%) | 45.2 | 37.5 | 28.9 | 4.6 |
| Silt (%) | 42.5 | 49.8 | 59.3 | 84.1 |
| Clay (%) | 12.3 | 12.6 | 11.6 | 10.8 |
| Total N (g/kg) | 4.2 | 6.62 | 6.7 | 10.6 |
Appendix A.2
| Season | Warming | BC | B10 | B20 | B50 |
|---|---|---|---|---|---|
| Fall | W | 19.2 ± 2.4 | - | 19.7 ± 6.2 | - |
| nW | 18.3 ± 0.5 | 21.1 ± 7.4 | 21.8 ± 6.6 | 23.3 ± 2.7 | |
| Spring | W | 23.8 ± 0.4 | - | 27.4 ± 1.2 | - |
| nW | 23.3 ± 0.8 | 29.0 ± 1.1 | 28.8 ± 0.8 | 32.7 ± 2.1 |
Appendix B
Appendix B.1
Appendix B.2

References
- Oelkers, E.H.; Cole, D.R. Carbon Dioxide Sequestration A Solution to a Global Problem. Elements 2008, 4, 305–310. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Elsevier Academic Press: Amsterdam, The Netherlands, 2006; ISBN 978-0-12-088782-8. [Google Scholar]
- Georgiou, K.; Jackson, R.B.; Vindušková, O.; Abramoff, R.Z.; Ahlström, A.; Feng, W.; Harden, J.W.; Pellegrini, A.F.A.; Polley, H.W.; Soong, J.L.; et al. Global Stocks and Capacity of Mineral-Associated Soil Organic Carbon. Nat. Commun. 2022, 13, 3797. [Google Scholar] [CrossRef] [PubMed]
- Rustad, L.E.; Huntington, T.G.; Boone, R.D. Controls on Soil Respiration: Implications for Climate Change. Biogeochemistry 2000, 48, 1–6. [Google Scholar] [CrossRef]
- Lei, J.; Guo, X.; Zeng, Y.; Zhou, J.; Gao, Q.; Yang, Y. Temporal Changes in Global Soil Respiration since 1987. Nat. Commun. 2021, 12, 403. [Google Scholar] [CrossRef]
- Crowther, T.W.; Todd-Brown, K.E.O.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; Machmuller, M.B.; Snoek, B.L.; Fang, S.; Zhou, G.; Allison, S.D.; et al. Quantifying Global Soil Carbon Losses in Response to Warming. Nature 2016, 540, 104–108. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA) How Much Coal Is Left. Available online: https://www.eia.gov/energyexplained/coal/how-much-coal-is-left.php (accessed on 2 December 2023).
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C.; Luijkx, I.T.; Olsen, A.; Peters, G.P.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C Sequestration as a Biological Negative Emission Strategy. Front. Clim. 2019, 1, 482133. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Makarem, M.A.; Meshksar, M. Advances in Synthesis Gas: Methods, Technologies and Applications: Syngas Production and Preparation; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-323-91871-8. [Google Scholar]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-Char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strat. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Tomotsune, M.; Ando, M.; Tsukimori, Y.; Koizumi, H.; Yoshitake, S. Effects of the Application of Biochar to Plant Growth and Net Primary Production in an Oak Forest. Forests 2021, 12, 152. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the Stability of Biochar in Soils: Predictability of O:C Molar Ratios. Carbon. Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Smith, P. Soil Carbon Sequestration and Biochar as Negative Emission Technologies. Glob. Change Biol. 2016, 22, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Biochar and Soil Carbon Sequestration. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Soil Science Society of America: Madison, WI, USA, 2015; pp. 175–197. ISBN 978-0-89118-967-1. [Google Scholar]
- Matovic, D. Biochar as a Viable Carbon Sequestration Option: Global and Canadian Perspective. Energy 2011, 36, 2011–2016. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Laird, D.A.; Heaton, E.A.; Rathke, S.; Acharya, B.S. Soil Carbon Increased by Twice the Amount of Biochar Carbon Applied after 6 Years: Field Evidence of Negative Priming. GCB Bioenergy 2020, 12, 240–251. [Google Scholar] [CrossRef]
- Bruckman, V.J.; Pumpanen, J. Chapter 17—Biochar Use in Global Forests: Opportunities and Challenges. In Developments in Soil Science; Global Change and Forest Soils, Busse, M., Giardina, C.P., Morris, D.M., Page-Dumroese, D.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 36, pp. 427–453. [Google Scholar]
- Conte, P.; Bertani, R.; Sgarbossa, P.; Bambina, P.; Schmidt, H.-P.; Raga, R.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P. Recent Developments in Understanding Biochar’s Physical–Chemistry. Agronomy 2021, 11, 615. [Google Scholar] [CrossRef]
- Sarauer, J.L.; Coleman, M.D. Biochar as a Growing Media Component for Containerized Production of Douglas-Fir. Can. J. For. Res. 2018, 48, 581–588. [Google Scholar] [CrossRef]
- Sohi, S.; Kuppens, T. Systems Integration for Biochar in European Forestry: Drivers and Strategies. In BIOCHAR: A Regional Supply Chain Approach in View of Climate Change Mitigation; Cambridge University Press: Cambridge, UK, 2016; pp. 70–95. ISBN 978-1-107-11709-9. [Google Scholar]
- Chagas, J.K.M.; de Figueiredo, C.C.; Ramos, M.L.G. Biochar Increases Soil Carbon Pools: Evidence from a Global Meta-Analysis. J. Environ. Manag. 2022, 305, 114403. [Google Scholar] [CrossRef]
- Thomas, S.C.; Gale, N. Biochar and Forest Restoration: A Review and Meta-Analysis of Tree Growth Responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Chen, J.; Müller, K.; Li, Y.; Fu, W.; Lin, Z.; Wang, H. Effects of Biochar Application in Forest Ecosystems on Soil Properties and Greenhouse Gas Emissions: A Review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Walkiewicz, A.; Kalinichenko, K.; Kubaczyński, A.; Brzezińska, M.; Bieganowski, A. Usage of Biochar for Mitigation of CO2 Emission and Enhancement of CH4 Consumption in Forest and Orchard Haplic Luvisol (Siltic) Soils. Appl. Soil Ecol. 2020, 156, 103711. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; Zhang, T.; Du, Z.; He, Y.; Wang, X.; Shao, J.; Cao, Y.; Xue, S.; Wang, H.; et al. Biochar Increased Soil Respiration in Temperate Forests but Had No Effects in Subtropical Forests. For. Ecol. Manag. 2017, 405, 339–349. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.-C.; Zackrisson, O. Fire-Derived Charcoal Causes Loss of Forest Humus. Science 2008, 320, 629. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar Addition Rate Influences Soil Microbial Abundance and Activity in Temperate Soils. Eur. J. Soil Sci. 2014, 65, 28–39. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.; Singh, B.P. Effect of Temperature on Biochar Priming Effects and Its Stability in Soils. Soil Biol. Biochem. 2015, 80, 136–145. [Google Scholar] [CrossRef]
- Ding, F.; Van Zwieten, L.; Zhang, W.; Weng, Z.; Shi, S.; Wang, J.; Meng, J. A Meta-Analysis and Critical Evaluation of Influencing Factors on Soil Carbon Priming Following Biochar Amendment. J. Soils Sediments 2018, 18, 1507–1517. [Google Scholar] [CrossRef]
- IPCC. IPCC Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar]
- Seidl, R.; Schelhaas, M.-J.; Rammer, W.; Verkerk, P.J. Increasing Forest Disturbances in Europe and Their Impact on Carbon Storage. Nat. Clim. Change 2014, 4, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest Disturbances under Climate Change. Nat. Clim. Change 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Alemu, B. The Role of Forest and Soil Carbon Sequestrations on Climate Change Mitigation. J. Environ. Earth Sci. 2014, 4, 98–111. [Google Scholar]
- Mo, L.; Zohner, C.M.; Reich, P.B.; Liang, J.; de Miguel, S.; Nabuurs, G.-J.; Renner, S.S.; van den Hoogen, J.; Araza, A.; Herold, M.; et al. Integrated Global Assessment of the Natural Forest Carbon Potential. Nature 2023, 624, 92–101. [Google Scholar] [CrossRef]
- Prietzel, J.; Christophel, D. Organic Carbon Stocks in Forest Soils of the German Alps. Geoderma 2014, 221–222, 28–39. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Huang, P.; Li, Z.; Zhang, X. A New Concept for Modelling the Moisture Dependence of Heterotrophic Soil Respiration. Soil Biol. Biochem. 2023, 185, 109147. [Google Scholar] [CrossRef]
- Stillhard, J.; Hobi, M.L.; Brang, P.; Brändli, U.-B.; Korol, M.; Pokynchereda, V.; Abegg, M. Structural Changes in a Primeval Beech Forest at the Landscape Scale. For. Ecol. Manag. 2022, 504, 119836. [Google Scholar] [CrossRef]
- Vannini, A.; Carbognani, M.; Chiari, G.; Forte, T.G.W.; Rodolfi, M.; Ganino, T.; Petraglia, A. Biochar Effects on Early Decomposition of Standard Litter in a European Beech Forest (Northern Italy). Sci. Total Environ. 2023, 903, 166224. [Google Scholar] [CrossRef]
- Vannini, A.; Carbognani, M.; Chiari, G.; Forte, T.G.W.; Lumiero, F.; Malcevschi, A.; Rodolfi, M.; Ganino, T.; Petraglia, A. Effects of Wood-Derived Biochar on Germination, Physiology, and Growth of European Beech (Fagus sylvatica L.) and Turkey Oak (Quercus cerris L.). Plants 2022, 11, 3254. [Google Scholar] [CrossRef]
- Carbognani, M.; Tomaselli, M.; Petraglia, A. Different Temperature Perception in High-Elevation Plants: New Insight into Phenological Development and Implications for Climate Change in the Alpine Tundra. Oikos 2018, 127, 1014–1023. [Google Scholar] [CrossRef]
- Dext3r. Agenzia Prevenzione Ambiente Energia Emilia-Romagna. Available online: https://simc.arpae.it/dext3r/ (accessed on 15 June 2022).
- Serie Generale n° 248 21/10/1999. Approvazione dei “Metodi ufficiali di analisi chimica del suolo”. Gazzetta Ufficiale della Repubblica Italiana, 21 October 1999.
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-155. Available online: https://CRAN.R-project.org/package=nlme (accessed on 16 October 2023).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2022. [Google Scholar]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of Microbial Communities to Biochar-Amended Soils: A Critical Review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Liu, Q.; He, X.; Wang, K.; Li, D. Biochar Drives Humus Formation during Composting by Regulating the Specialized Metabolic Features of Microbiome. Chem. Eng. J. 2023, 458, 141380. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Ouyang, L. Priming of Pyrogenic C (Biochar) Mineralization by Dissolved Organic Matter and Vice Versa. Soil Biol. Biochem. 2019, 130, 105–112. [Google Scholar] [CrossRef]
- Sagrilo, E.; Jeffery, S.; Hoffland, E.; Kuyper, T.W. Emission of CO2 from Biochar-Amended Soils and Implications for Soil Organic Carbon. GCB Bioenergy 2015, 7, 1294–1304. [Google Scholar] [CrossRef]
- Maestrini, B.; Nannipieri, P.; Abiven, S. A Meta-Analysis on Pyrogenic Organic Matter Induced Priming Effect. GCB Bioenergy 2015, 7, 577–590. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Lan, Y.; Meng, J.; Jiang, L.; Sun, Q.; Cao, D.; Sun, Y.; Chen, W. Labile Organic Carbon Fractions and Carbon Pool Management Index in a 3-Year Field Study with Biochar Amendment. J. Soils Sediments 2018, 18, 1569–1578. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar Increases Soil Microbial Biomass but Has Variable Effects on Microbial Diversity: A Meta-Analysis. Sci. Total Environ. 2020, 749, 141593. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Simpson, A.J.; Soong, R.; Simpson, M.J. Biochar Amendment Altered the Molecular-Level Composition of Native Soil Organic Matter in a Temperate Forest Soil. Environ. Chem. 2016, 13, 854–866. [Google Scholar] [CrossRef]
- Cross, A.; Sohi, S.P. The Priming Potential of Biochar Products in Relation to Labile Carbon Contents and Soil Organic Matter Status. Soil Biol. Biochem. 2011, 43, 2127–2134. [Google Scholar] [CrossRef]
- Jones, D.L.; Murphy, D.V.; Khalid, M.; Ahmad, W.; Edwards-Jones, G.; DeLuca, T.H. Short-Term Biochar-Induced Increase in Soil CO2 Release Is Both Biotically and Abiotically Mediated. Soil Biol. Biochem. 2011, 43, 1723–1731. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Laird, D.A. Quantification and Characterization of Chemically-and Thermally-Labile and Recalcitrant Biochar Fractions. Chemosphere 2018, 194, 247–255. [Google Scholar] [CrossRef]
- Pathy, A.; Ray, J.; Paramasivan, B. Biochar Amendments and Its Impact on Soil Biota for Sustainable Agriculture. Biochar 2020, 2, 287–305. [Google Scholar] [CrossRef]
- Lü, F.; Liu, Y.; Shao, L.; He, P. Powdered Biochar Doubled Microbial Growth in Anaerobic Digestion of Oil. Appl. Energy 2019, 247, 605–614. [Google Scholar] [CrossRef]
- Bruun, S.; Clauson-Kaas, S.; Bobuľská, L.; Thomsen, I.K. Carbon Dioxide Emissions from Biochar in Soil: Role of Clay, Microorganisms and Carbonates. Eur. J. Soil Sci. 2014, 65, 52–59. [Google Scholar] [CrossRef]
- Wu, D.; Senbayram, M.; Zang, H.; Ugurlar, F.; Aydemir, S.; Brüggemann, N.; Kuzyakov, Y.; Bol, R.; Blagodatskaya, E. Effect of Biochar Origin and Soil pH on Greenhouse Gas Emissions from Sandy and Clay Soils. Appl. Soil. Ecol. 2018, 129, 121–127. [Google Scholar] [CrossRef]
- Novara, A.; Armstrong, A.; Gristina, L.; Semple, K.T.; Quinton, J.N. Effects of Soil Compaction, Rain Exposure and Their Interaction on Soil Carbon Dioxide Emission. Earth Surf. Process. Landf. 2012, 37, 994–999. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Wang, Y.; Lu, H.; He, L.; Yang, S. Negative Priming Effect of Three Kinds of Biochar on the Mineralization of Native Soil Organic Carbon. Land Degrad. Dev. 2018, 29, 3985–3994. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Ro, K.S.; Díaz, F.J. Biochar and Earthworms Working in Tandem: Research Opportunities for Soil Bioremediation. Sci. Total Environ. 2019, 688, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting Soil pH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Buss, W.; Hilber, I.; Graham, M.C.; Mašek, O. Composition of PAHs in Biochar and Implications for Biochar Production. ACS Sustain. Chem. Eng. 2022, 10, 6755–6765. [Google Scholar] [CrossRef]
- Nam, J.J.; Thomas, G.O.; Jaward, F.M.; Steinnes, E.; Gustafsson, O.; Jones, K.C. PAHs in Background Soils from Western Europe: Influence of Atmospheric Deposition and Soil Organic Matter. Chemosphere 2008, 70, 1596–1602. [Google Scholar] [CrossRef]
- Fabbri, D.; Rombolà, A.G.; Torri, C.; Spokas, K.A. Determination of Polycyclic Aromatic Hydrocarbons in Biochar and Biochar Amended Soil. J. Anal. Appl. Pyrolysis 2013, 103, 60–67. [Google Scholar] [CrossRef]
- Dutta, T.; Kwon, E.; Bhattacharya, S.S.; Jeon, B.H.; Deep, A.; Uchimiya, M.; Kim, K.-H. Polycyclic Aromatic Hydrocarbons and Volatile Organic Compounds in Biochar and Biochar-Amended Soil: A Review. GCB Bioenergy 2017, 9, 990–1004. [Google Scholar] [CrossRef]
- Sakshi; Singh, S.K.; Haritash, A.K. Polycyclic Aromatic Hydrocarbons: Soil Pollution and Remediation. Int. J. Environ. Sci. Technol. 2019, 16, 6489–6512. [Google Scholar] [CrossRef]
- Lee, M.; Nakane, K.; Nakatsubo, T.; Koizumi, H. Seasonal Changes in the Contribution of Root Respiration to Total Soil Respiration in a Cool-Temperate Deciduous Forest. Plant Soil 2003, 255, 311–318. [Google Scholar] [CrossRef]
- Janssens, I.A.; Pilegaard, K. Large Seasonal Changes in Q10 of Soil Respiration in a Beech Forest. Glob. Change Biol. 2003, 9, 911–918. [Google Scholar] [CrossRef]
- Cai, Y.; Nishimura, T.; Ida, H.; Hirota, M. Spatial Variation in Soil Respiration Is Determined by Forest Canopy Structure through Soil Water Content in a Mature Beech Forest. For. Ecol. Manag. 2021, 501, 119673. [Google Scholar] [CrossRef]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.J.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.-A.; Wookey, P.A.; Ågren, G.I.; Sebastià, M.-T.; et al. Temperature Sensitivity of Soil Respiration Rates Enhanced by Microbial Community Response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse Gas Emissions from Soils—A Review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Arands, R.R.; van der Sloot, H.A.; Kosson, D.S. Modeling of the Gaseous Diffusion Coefficient through Unsaturated Soil Systems. J. Contam. Hydrol. 1997, 29, 1–21. [Google Scholar] [CrossRef]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of Greenhouse Gases between Soil and Atmosphere: Interactions of Soil Physical Factors and Biological Processes. Eur. J. Soil Sci. 2018, 69, 10–20. [Google Scholar] [CrossRef]
- Miano, T.; Mondelli, D. Sostanza organica e carbonio organico. In Metodi di Analisi Chimica del Suolo; Associazione Italiana dei Laboratori Pubblici di Agrochimica, Claudio, C., Società Italiana Della Scienza del Suolo, Miano, T., Eds.; Pubblicità & Stampa: Modugno, Italy, 2015; pp. 253–254. ISBN 978-88-940679-0-3. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannini, A.; Tarasconi, D.; Pietropoli, F.; Forte, T.G.W.; Grillo, F.; Carbognani, M.; Petraglia, A. Effects of Wood-Derived Biochar on Soil Respiration of a European Beech Forest Under Current Climate and Simulated Climate Change. Forests 2025, 16, 474. https://doi.org/10.3390/f16030474
Vannini A, Tarasconi D, Pietropoli F, Forte TGW, Grillo F, Carbognani M, Petraglia A. Effects of Wood-Derived Biochar on Soil Respiration of a European Beech Forest Under Current Climate and Simulated Climate Change. Forests. 2025; 16(3):474. https://doi.org/10.3390/f16030474
Chicago/Turabian StyleVannini, Andrea, Debora Tarasconi, Federico Pietropoli, T’ai Gladys Whittingham Forte, Filippo Grillo, Michele Carbognani, and Alessandro Petraglia. 2025. "Effects of Wood-Derived Biochar on Soil Respiration of a European Beech Forest Under Current Climate and Simulated Climate Change" Forests 16, no. 3: 474. https://doi.org/10.3390/f16030474
APA StyleVannini, A., Tarasconi, D., Pietropoli, F., Forte, T. G. W., Grillo, F., Carbognani, M., & Petraglia, A. (2025). Effects of Wood-Derived Biochar on Soil Respiration of a European Beech Forest Under Current Climate and Simulated Climate Change. Forests, 16(3), 474. https://doi.org/10.3390/f16030474









