Biochar Addition Increased Soil Carbon Storage but Did Not Exacerbate Soil Carbon Emission in Young Subtropical Plantation Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
| Bulk Density (g/cm3) | Water Content (%) | pH | Total C (g/kg) | Total N (mg/g) | Total P (mg/g) | |
|---|---|---|---|---|---|---|
| Soil | 1.16 ± 0.05 | 39.30 ± 0.71 | 4.40 ± 0.02 | 23.53 ± 0.56 | 1.20 ± 0.12 | 0.27 ± 0.02 |
| Biochar | / | / | 8.13 ± 0.06 | 684.21 ± 0.11 | 4.28 ± 0.16 | 0.18 ± 0.04 |
2.3. Experimental Measurements
2.3.1. Soil CO2 Efflux
2.3.2. Measurement of Net Photosynthetic Rate and Plant Growth Traits
2.3.3. Measurement of Soil Carbon and Other Physiochemical Properties
2.4. Calculation and Statistical Analyses
3. Results
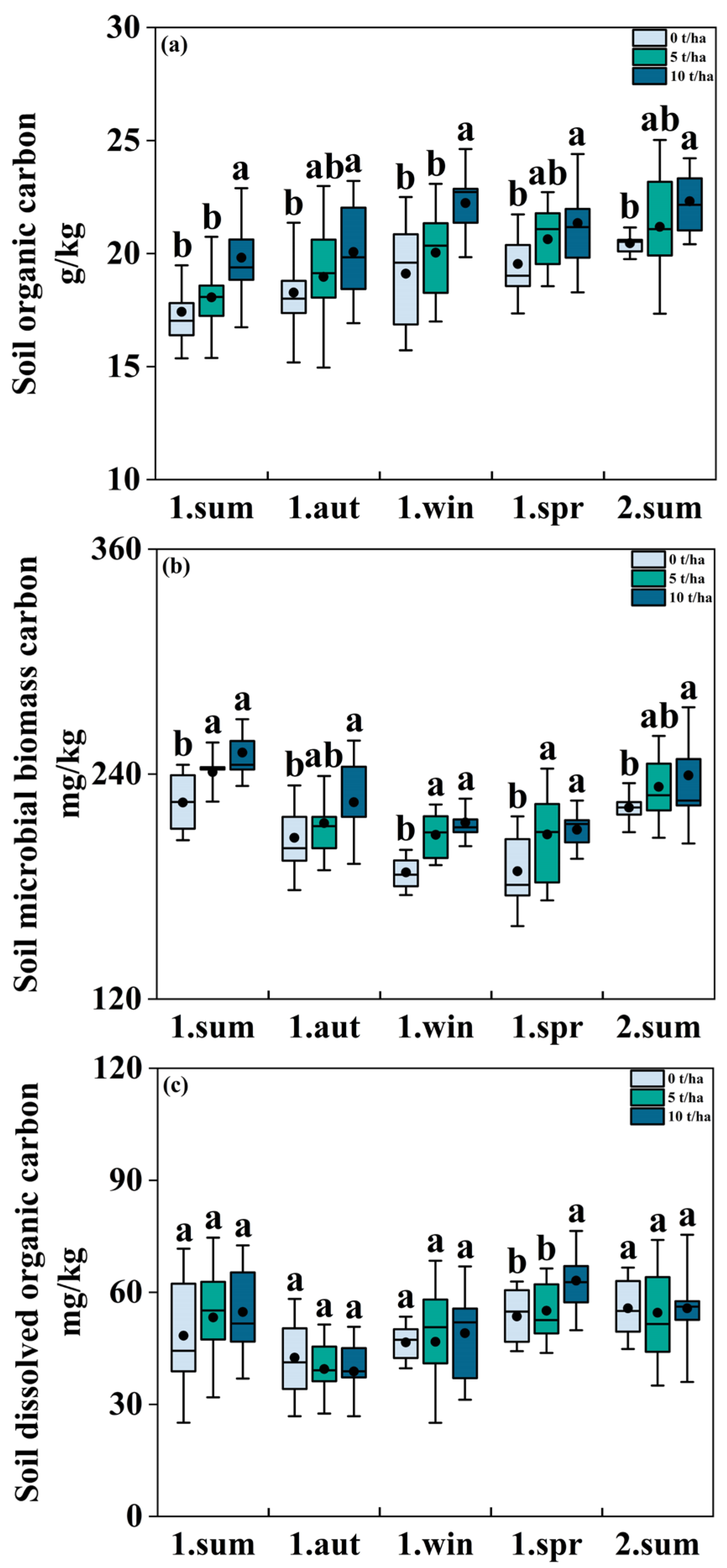
3.1. Effect of Biochar Addition on SOC, DOC and MBC
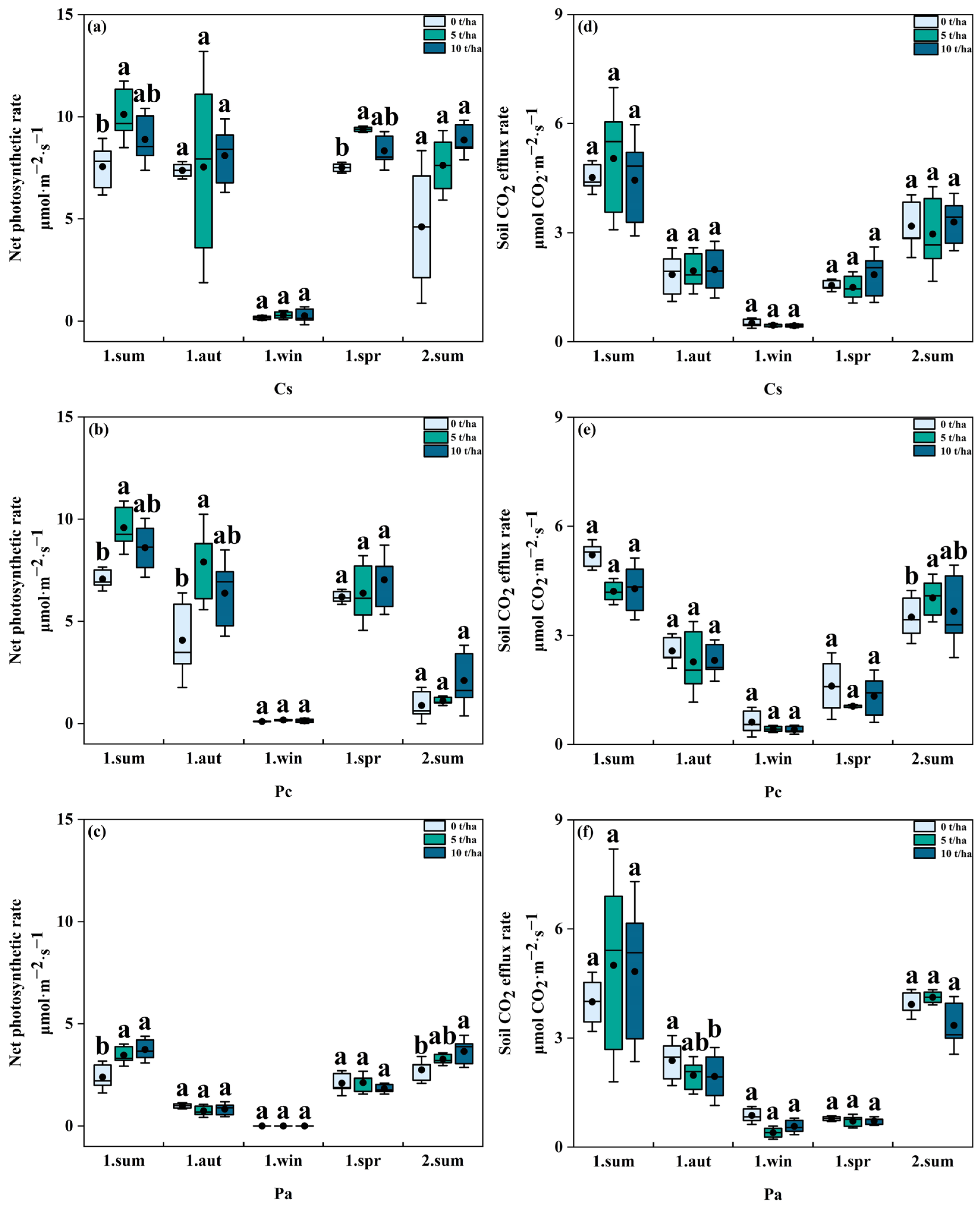
3.2. Effects of Biochar Addition on Soil CO2 Efflux Rate and Annual Cumulative Soil CO2 Efflux
| Plantations | Annual Cumulative Soil CO2 Efflux (g C·m−2·year−1) | Annual Growth of Basal Diameter (mm) | Annual Growth of Tree Height (cm) | Annual Crown Area Growth (cm2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 t/ha | 5 t/ha | 10 t/ha | 0 t/ha | 5 t/ha | 10 t/ha | 0 t/ha | 5 t/ha | 10 t/ha | 0 t/ha | 5 t/ha | 10 t/ha | |
| C. subavenium | 800.48 ± 41.59 Aa | 849.34 ± 99.15 Aa | 827.70 ± 70.80 Aa | 10.36 ± 0.79 Bb | 14.73 ± 0.36 ABa | 14.92 ± 2.07 Aab | 35.83 ± 0.48 Ab | 36.37 ± 0.02 Ab | 38.33 ± 4.48 Ab | 4051 ± 936 Ab | 3105 ± 311 Ab | 5074 ± 1342 Ab |
| P. chekiangensis | 949.67 ± 57.56 Aa | 755.44 ± 52.55 Aa | 790.02 ± 79.13 Aa | 10.40 ± 0.69 Bb | 15.79 ± 1.23 Aa | 12.81 ± 0.98 ABb | 28.83 ± 3.77 Ab | 24.50 ± 6.17 Ab | 25.72 ± 7.38 Ab | 4030 ± 263 Ab | 4787 ± 354 Ab | 4732 ± 1376 Ab |
| P. amabilis | 761.44 ± 61.66 Aa | 768.12 ± 144.45 Aa | 765.12 ± 127.40 Aa | 15.01 ± 0.28 Ba | 15.94 ± 0.82 ABa | 17.94 ± 0.75 Aa | 58.30 ± 8.17 Aa | 52.67 ± 0.87 Aa | 60.27 ± 5.29 Aa | 9003 ± 673 Aa | 9495 ± 1111 Aa | 9684 ± 889 Aa |
| All quadrats | 837.20 ± 39.49 A | 790.97 ± 54.81 A | 794.28 ± 48.73 A | 11.92 ± 0.83 B | 15.48 ± 0.48 A | 15.22 ± 1.02 A | 40.99 ± 5.15 A | 37.84 ± 4.46 A | 41.44 ± 5.83 A | 5695 ± 895 A | 5796 ± 1018 A | 6497 ± 1006 A |
| Association of Variable | Soil CO2 Efflux Rate (μmol CO2·m−2·s−1) | Basal Diameter (mm) | Tree Height (cm) | Crown Area (cm2) | Net Photosynthetic Rate (μmol CO2·m−2·s−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | F | p | F | p | F | p | F | p | F | |
| Biochar | 0.83 | 0.18 | 0.07 | 3.16 | 0.07 | 3.15 | <0.05 | 3.92 | <0.001 | 11.94 |
| Season | <0.001 | 265.96 | <0.001 | 169.02 | <0.001 | 30.35 | <0.001 | 60.97 | <0.001 | 212.84 |
| Species | 0.72 | 0.33 | <0.001 | 22.37 | <0.001 | 24.29 | 0.10 | 2.69 | <0.001 | 177.88 |
| Season × Biochar | 0.73 | 0.49 | 0.06 | 2.53 | 0.43 | 0.98 | 0.55 | 0.69 | <0.05 | 2.76 |
| Season × Species | 0.04 | 2.92 | <0.01 | 3.85 | <0.01 | 5.46 | <0.001 | 16.95 | <0.001 | 41.70 |
| Biochar × Species | 0.88 | 0.18 | 0.42 | 1.03 | 0.53 | 0.83 | 0.53 | 0.82 | 0.24 | 1.51 |
| Biochar × Species × Season | 0.37 | 1.14 | <0.05 | 2.08 | 0.65 | 0.74 | 0.68 | 0.64 | 0.06 | 1.99 |
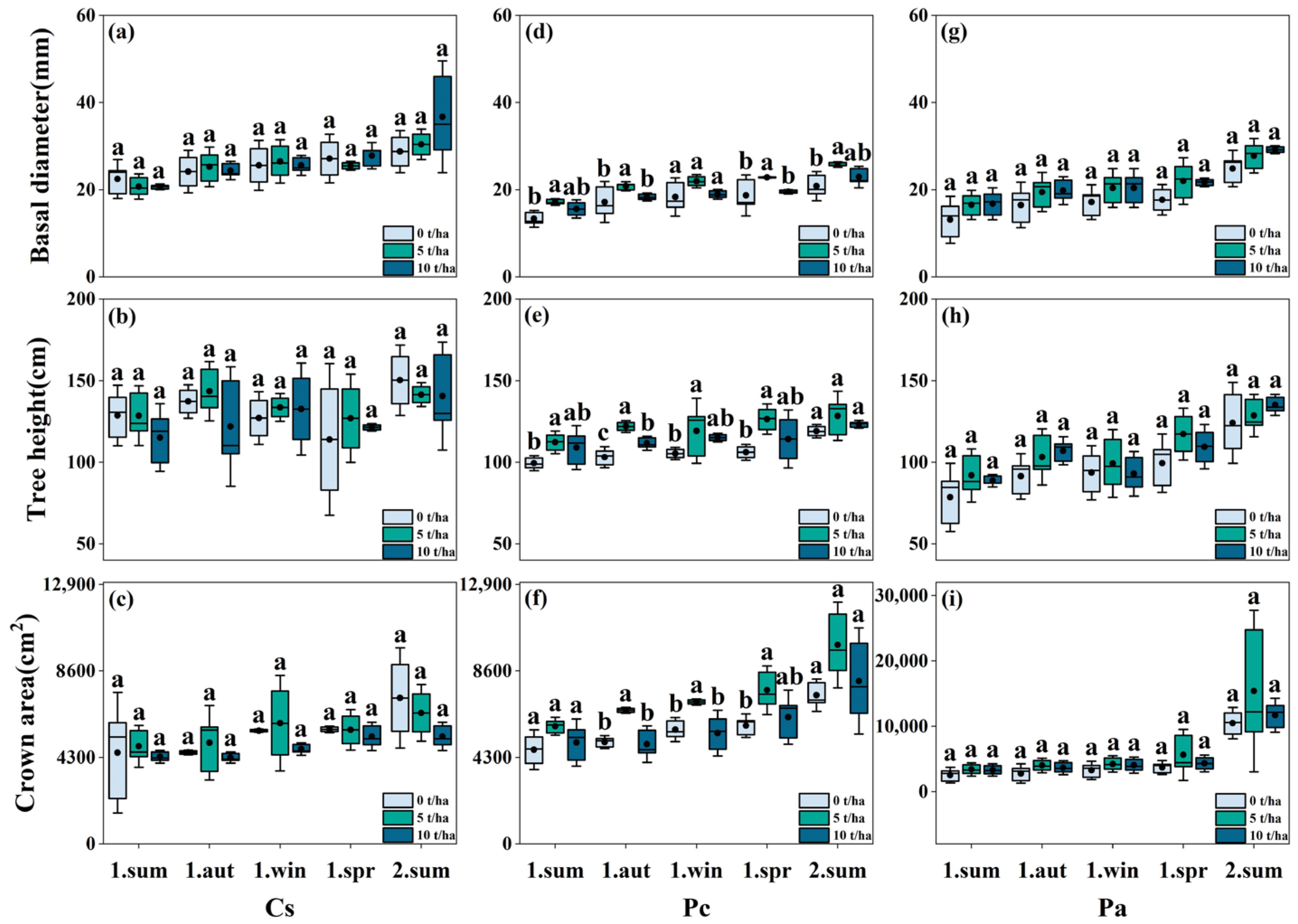
3.3. Effects of Biochar Addition on Plant Growth and Net Photosynthetic Rate
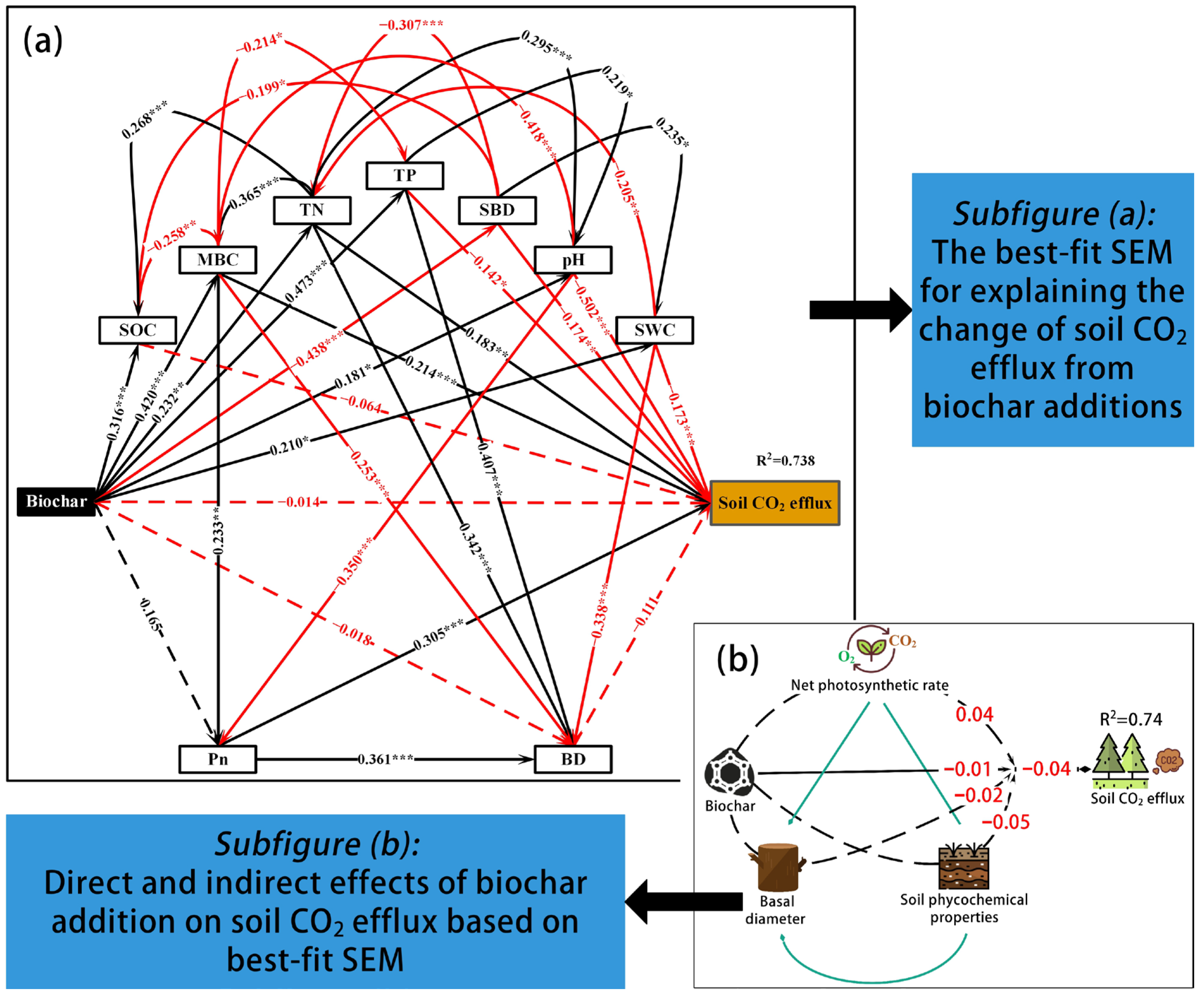
3.4. Direct and Indirect Effects of Biochar Addition on Soil CO2 Efflux
4. Discussion
4.1. Differential Responses of SOC, MBC, and DOC to Biochar Addition
4.2. Plant Growth and Photosynthetic Productivity Is Influenced by Biochar Additions Species Type and Season
4.3. Biochar Addition Did Not Promote Soil CO2 Efflux while Increased Whole System Carbon Storage in Young Sub-Tropical Plantations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in climate change mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Ali, J.; Faridi, S.; Sardar, M. Carbonic anhydrase as a tool to mitigate global warming. Environ. Sci. Pollut. Res. 2023, 30, 83093–83112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yuan, J.; Yang, X.; Han, X.; Lan, Y.; Cao, D.; Sun, Q.; Cui, X.; Meng, J.; Chen, W. Responses of soil respiration and C sequestration efficiency to biochar amendment in maize field of Northeast China. Soil Tillage Res. 2022, 223, 105442. [Google Scholar] [CrossRef]
- Al-qaness, M.A.A.; Ewees, A.A.; Thanh, H.V.; AlRassas, A.M.; Dahou, A.; Elaziz, M.A. Predicting CO2 trapping in deep saline aquifers using optimized long short-term memory. Environ. Sci. Pollut. Res. 2023, 30, 33780–33794. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.A.; Montero, S.A.; Hernández Gómez, I.U.; Romero Montero, J.A.; Ellis, P.W.; Rodríguez-Ward, D.; Blanco Reyes, P.; Putz, F.E. Reduced-impact logging practices reduce forest disturbance and carbon emissions in community managed forests on the Yucatán Peninsula, Mexico. For. Ecol. Manag. 2019, 437, 396–410. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Biffi, S.; Chapman, P.J.; Grayson, R.P.; Ziv, G. Planting hedgerows: Biomass carbon sequestration and contribution towards net-zero targets. Sci. Total Environ. 2023, 892, 164482. [Google Scholar] [CrossRef] [PubMed]
- Boyero, L.; Pearson, R.G.; Gessner, M.O.; Barmuta, L.A.; Ferreira, V.; Graça, M.A.S.; Dudgeon, D.; Boulton, A.J.; Callisto, M.; Chauvet, E.; et al. A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration. Ecol. Lett. 2011, 14, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.B.; Malhi, Y.; Ewers, R.M.; Kho, L.K.; Teh, Y.A.; Both, S.; Burslem, D.F.R.P.; Majalap, N.; Nilus, R.; Huaraca Huasco, W.; et al. Tropical forests post-logging are a persistent net carbon source to the atmosphere. Proc. Natl. Acad. Sci. USA 2023, 120, e2214462120. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Wang, G.; Tian, J.; Li, W. Global patterns and edaphic-climatic controls of soil carbon decomposition kinetics predicted from incubation experiments. Nat. Commun. 2023, 14, 2171. [Google Scholar] [CrossRef] [PubMed]
- Chagas, J.K.M.; Figueiredo, C.C.d.; Ramos, M.L.G. Biochar increases soil carbon pools: Evidence from a global meta-analysis. J. Environ. Manag. 2022, 305, 114403. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.T.; Cowie, A.L.; Waters, C.M. Pyrolysis of invasive woody vegetation for energy and biochar has climate change mitigation potential. Sci. Total Environ. 2021, 770, 145278. [Google Scholar] [CrossRef] [PubMed]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015; pp. 89–110. [Google Scholar]
- Chen, G.; Ma, Y.; Xu, W.; Chen, Z.; Li, Z.; Zhou, J.; Yu, W. Remediation of cadmium-contaminated soil by micro-nano nitrogen-doped biochar and its mechanisms. Environ. Sci. Pollut. Res. 2023, 30, 48078–48087. [Google Scholar] [CrossRef]
- Dissanayake, D.K.R.P.L.; Dissanayaka, D.M.N.S.; Udumann, S.S.; Nuwarapaksha, T.D.; Atapattu, A.J.; Dissanayake, D.K.R.P.L.; Dissanayaka, D.M.N.S.; Udumann, S.S.; Nuwarapaksha, T.D.; Atapattu, A.J. Is biochar a promising soil amendment to enhance perennial crop yield and soil quality in the tropics? Technol. Agron. 2023, 3, 4. [Google Scholar] [CrossRef]
- Mandal, S.; Sarkar, B.; Bolan, N.; Novak, J.; Ok, Y.S.; Van Zwieten, L.; Singh, B.P.; Kirkham, M.B.; Choppala, G.; Spokas, K.; et al. Designing advanced biochar products for maximizing greenhouse gas mitigation potential. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1367–1401. [Google Scholar] [CrossRef]
- Wei, B.; Peng, Y.; Lin, L.; Zhang, D.; Ma, L.; Jiang, L.; Li, Y.; He, T.; Wang, Z. Drivers of biochar-mediated improvement of soil water retention capacity based on soil texture: A meta-analysis. Geoderma 2023, 437, 116591. [Google Scholar] [CrossRef]
- Zhao, P.; Yu, J.; Zhang, X.; Ren, Z.; Li, M.; Han, S. Trifolium repens and biochar addition affecting soil nutrients and bacteria community. Environ. Sci. Pollut. Res. 2023, 30, 33927–33941. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef] [PubMed]
- Diacono, M.; Montemurro, F. Long-Term Effects of Organic Amendments on Soil Fertility. In Sustainable Agriculture Volume 2; Springer: Dordrecht, The Netherlands, 2011; pp. 761–786. [Google Scholar]
- Bakhshandeh, E.; Gholamhosseini, M.; Yaghoubian, Y.; Pirdashti, H. Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress. Plant Growth Regul. 2020, 90, 123–136. [Google Scholar] [CrossRef]
- Johnson, M.S.; Webster, C.; Jassal, R.S.; Hawthorne, I.; Black, T.A. Biochar influences on soil CO2 and CH4 fluxes in response to wetting and drying cycles for a forest soil. Sci. Rep. 2017, 7, 6780. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhou, X.; Zhang, T.; Du, Z.; He, Y.; Wang, X.; Shao, J.; Cao, Y.; Xue, S.; Wang, H.; et al. Biochar increased soil respiration in temperate forests but had no effects in subtropical forests. For. Ecol. Manag. 2017, 405, 339–349. [Google Scholar] [CrossRef]
- Tao, B.; Chen, Q.; Jiang, Y.; Zhang, B.; Yuan, H.; Wang, Y. Effect of particle sizes of biochar on CO2 emissions in a poplar plantation of ancient Yellow River channel, China. J. Environ. Manag. 2023, 345, 118721. [Google Scholar] [CrossRef] [PubMed]
- Grau-Andrés, R.; Pingree, M.R.A.; Öquist, M.G.; Wardle, D.A.; Nilsson, M.-C.; Gundale, M.J. Biochar increases tree biomass in a managed boreal forest, but does not alter N2O, CH4, and CO2 emissions. GCB Bioenergy 2021, 13, 1329–1342. [Google Scholar] [CrossRef]
- Cui, J.; Glatzel, S.; Bruckman, V.J.; Wang, B.; Lai, D.Y.F. Long-term effects of biochar application on greenhouse gas production and microbial community in temperate forest soils under increasing temperature. Sci. Total Environ. 2021, 767, 145021. [Google Scholar] [CrossRef] [PubMed]
- Sarauer, J.L.; Page-Dumroese, D.S.; Coleman, M.D. Soil greenhouse gas, carbon content, and tree growth response to biochar amendment in western United States forests. GCB Bioenergy 2019, 11, 660–671. [Google Scholar] [CrossRef]
- Gogoi, L.; Narzari, R.; Gogoi, N.; Borkotoki, B.; Kataki, R. Effect of Biochar on Soil Respiration from a Semi-evergreen, Moist Deciduous Forest Soil. Int. J. Geosynth. Ground Eng. 2020, 6, 26. [Google Scholar] [CrossRef]
- Zhang, R.; Qu, Z.; Liu, L.; Yang, W.; Wang, L.; Li, J.; Zhang, D. Soil Respiration and Organic Carbon Response to Biochar and Their Influencing Factors. Atmosphere 2022, 13, 2038. [Google Scholar] [CrossRef]
- Vannini, A.; Carbognani, M.; Chiari, G.; Forte, T.a.G.W.; Rodolfi, M.; Ganino, T.; Petraglia, A. Biochar effects on early decomposition of standard litter in a European beech forest (northern Italy). Sci. Total Environ. 2023, 903, 166224. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lian, R.; Bai, M.; Bao, J.; Liu, Y.; Li, S.; Liang, C.; Qin, H.; Chen, J.; Xu, Q. Biochar co-application mitigated the stimulation of organic amendments on soil respiration by decreasing microbial activities in an infertile soil. Biol. Fertil. Soils 2021, 57, 793–807. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chang, S.X.; Yang, Y.; Fu, S.; Jiang, P.; Luo, Y.; Yang, M.; Chen, Z.; Hu, S.; et al. Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon-degrading microbial activity. Soil Biol. Biochem. 2018, 122, 173–185. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Roy, K.S.; Neogi, S.; Manna, M.C.; Adhya, T.K.; Rao, K.S.; Nayak, A.K. Influence of elevated carbon dioxide and temperature on belowground carbon allocation and enzyme activities in tropical flooded soil planted with rice. Environ. Monit. Assess. 2013, 185, 8659–8671. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cai, Q.; Zheng, N.; Li, Y.; Lin, C.; Li, Y. Forest Carbon Sink Evaluation—An Important Contribution for Carbon Neutrality. IOP Conf. Ser. Earth Environ. Sci. 2021, 811, 012009. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Chen, J.; Müller, K.; Li, Y.; Fu, W.; Lin, Z.; Wang, H. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Satdichanh, M.; Dossa, G.G.O.; Yan, K.; Tomlinson, K.W.; Barton, K.E.; Crow, S.E.; Winowiecki, L.; Vågen, T.-G.; Xu, J.; Harrison, R.D. Drivers of soil organic carbon stock during tropical forest succession. J. Ecol. 2023, 111, 1722–1734. [Google Scholar] [CrossRef]
- Yan, E.-R.; Wang, X.-H.; Huang, J.-J. Shifts in plant nutrient use strategies under secondary forest succession. Plant Soil 2006, 289, 187–197. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Wang, H.; Singh, B.P.; Hu, S.; Luo, Y.; Li, J.; Xiao, Y.; Cai, X.; Li, Y. Responses of soil greenhouse gas emissions to different application rates of biochar in a subtropical Chinese chestnut plantation. Agric. For. Meteorol. 2019, 271, 168–179. [Google Scholar] [CrossRef]
- Sun, J.; He, F.; Pan, Y.; Zhang, Z. Effects of pyrolysis temperature and residence time on physicochemical properties of different biochar types. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 12–22. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Li, J.; Lin, S.; Jia, X.; Zhang, Q.; Ye, J.; Wang, H.; Wu, Z. Study on the Effect of pH on Rhizosphere Soil Fertility and the Aroma Quality of Tea Trees and Their Interactions. Agriculture 2023, 13, 1739. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Li, J.; Lin, S.; Jia, X.; Zhang, Q.; Ye, J.; Wang, H.; Wu, Z. Carbon Sources Application Increase Wheat Yield and Soil Fertility. Commun. Soil Sci. Plant Anal. 2021, 52, 695–703. [Google Scholar]
- Li, W.; Ali, I.; Han, X.; Ullah, S.; Yang, M. Soil C, N, P, K and Enzymes Stoichiometry of an Endangered Tree Species, Parashorea chinensis of Different Stand Ages Unveiled Soil Nutrient Limitation Factors. Forests 2023, 14, 624. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Wang, Z.-L.; Zhang, W.; Guo, C.; Wen, X.; Liu, Y. Optimizing manual sampling schedule for estimating annual soil CO2 efflux in a young exotic pine plantation in subtropical China. Eur. J. Soil Biol. 2012, 52, 41–47. [Google Scholar] [CrossRef]
- Niu, J.; Feng, Z.; Zhang, W.; Zhao, P.; Wang, X. Non-Stomatal Limitation to Photosynthesis in Cinnamomum camphora Seedings Exposed to Elevated O3. PLoS ONE 2014, 9, e98572. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.; Offer, C.; Hansen, U. Induction of CO2-gas exchange and electron transport: Comparison of dynamic and steady-state responses in Fagus sylvatica leaves. Trees 2003, 17, 153–163. [Google Scholar] [CrossRef]
- Pan, J.; Bai, Z.; Cao, Y.; Zhou, W.; Wang, J. Influence of soil physical properties and vegetation coverage at different slope aspects in a reclaimed dump. Environ. Sci. Pollut. Res. 2017, 24, 23953–23965. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Z.; Shi, L.; Zhou, Y.; Yang, M.; Cao, J.; Wu, S.; Lei, G. Effects of Different Grazing Intensities on Soil C, N, and P in an Alpine Meadow on the Qinghai—Tibetan Plateau, China. Int. J. Environ. Res. Public Health 2018, 15, 2584. [Google Scholar] [CrossRef] [PubMed]
- Babur, E.; Dindaroğlu, T.; Solaiman, Z.M.; Battaglia, M.L. Microbial respiration, microbial biomass and activity are highly sensitive to forest tree species and seasonal patterns in the Eastern Mediterranean Karst Ecosystems. Sci. Total Environ. 2021, 775, 145868. [Google Scholar] [CrossRef]
- Wander, M. Soil Organic Matter Fractions and Their Relevance to Soil Function. Soil Organic Matter in Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 2004; pp. 67–102. [Google Scholar]
- Kravchenko, A.N.; Guber, A.K.; Razavi, B.S.; Koestel, J.; Quigley, M.Y.; Robertson, G.P.; Kuzyakov, Y. Microbial spatial footprint as a driver of soil carbon stabilization. Nat. Commun. 2019, 10, 3121. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Wang, X.; Jiang, Y.; Yang, H.; Mai, S.; Xu, W.; Hou, E.; Huang, X.; Yang, Q.; Liu, W.; et al. Variations and influencing factors of soil organic carbon during the tropical forest succession from plantation to secondary and old–growth forest. Front. Ecol. Evol. 2023, 10, 2022. [Google Scholar] [CrossRef]
- Du, W.; Wang, D.; Wu, X.; Zhao, L.; Zang, S. Effects of Forest Types on SOC and DOC in the Permafrost Region of the Daxing’anling Mountains. Processes 2022, 10, 1293. [Google Scholar] [CrossRef]
- Yu, M.; Liang, S.; Dai, Z.; Li, Y.; Luo, Y.; Tang, C.; Xu, J. Plant material and its biochar differ in their effects on nitrogen mineralization and nitrification in a subtropical forest soil. Sci. Total Environ. 2021, 763, 143048. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Y.; Wu, Z.; Yan, X.; Gunina, A.; Kuzyakov, Y.; Xiong, Z. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation. J. Clean. Prod. 2020, 242, 118435. [Google Scholar] [CrossRef]
- Gao, Q.; Hasselquist, N.J.; Palmroth, S.; Zheng, Z.; You, W. Short-term response of soil respiration to nitrogen fertilization in a subtropical evergreen forest. Soil Biol. Biochem. 2014, 76, 297–300. [Google Scholar] [CrossRef]
- Lei, L.; Xiao, W.; Zeng, L.; Liu, C.; Zhu, J.; Li, M.-H. Phenological season-dependent temperature effects on soil respiration in a subtropical Pinus massoniana forest. Agric. For. Meteorol. 2022, 323, 109086. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, L.; Guan, D.; Chen, Y.; Motelica-Heino, M.; Peng, Y.; Lee, S.Y. The role of mangrove fine root production and decomposition on soil organic carbon component ratios. Ecol. Indic. 2021, 125, 107525. [Google Scholar] [CrossRef]
- Li, L.; Wilson, C.B.; He, H.; Zhang, X.; Zhou, F.; Schaeffer, S.M. Physical, biochemical, and microbial controls on amino sugar accumulation in soils under long-term cover cropping and no-tillage farming. Soil Biol. Biochem. 2019, 135, 369–378. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Li, C.; Chen, Y.; Zheng, L.; Ding, D.; Shan, S. Synergistic mechanism of iron manganese supported biochar for arsenic remediation and enzyme activity in contaminated soil. J. Environ. Manag. 2023, 347, 119127. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Van Zwieten, L.; Singh, B.P.; Tavakkoli, E.; Joseph, S.; Macdonald, L.M.; Rose, T.J.; Rose, M.T.; Kimber, S.W.L.; Morris, S.; et al. Biochar built soil carbon over a decade by stabilizing rhizodeposits. Nat. Clim. Change 2017, 7, 371–376. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, Z.; Lou, Y.; He, X. A one-year short-term biochar application improved carbon accumulation in large macroaggregate fractions. CATENA 2015, 127, 26–31. [Google Scholar] [CrossRef]
- Page, K.L.; Dang, Y.P.; Dalal, R.C. The Ability of Conservation Agriculture to Conserve Soil Organic Carbon and the Subsequent Impact on Soil Physical, Chemical, and Biological Properties and Yield. Front. Sustain. Food Syst. 2020, 4, 26–31. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.; Choudhury, B.U.; Mishra, V.K.; Kundu, M.C.; Roy, A.; Mondal, T.; Lama, A.; Dhakre, D.S. Organic nutrient sources and biochar technology on microbial biomass carbon and soil enzyme activity in maize-black gram cropping system. Biomass Convers. Biorefinery 2023, 13, 9277–9287. [Google Scholar] [CrossRef]
- de Sousa, R.N.; Soares, M.B.; dos Santos, F.H.; Leite, C.N.; Mendes, K.F. Interaction Mechanisms between Biochar and Herbicides. In Interactions of Biochar and Herbicides in the Environment; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–52. [Google Scholar]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Deb, S.; Sahoo, S.S.; Sahoo, U.K. Soil microbial biomass carbon stock and its relation with climatic and other environmental factors in forest ecosystems: A review. Acta Ecol. Sin. 2023, 43, 933–945. [Google Scholar] [CrossRef]
- Lin, S.; Xu, Y.; Fu, X.; Zhang, Y. Soil carbon stocks in plantations and natural forests of the sub-tropics. Acta Ecol. Sin. 2019, 39, 478–486. [Google Scholar]
- Xu, H.; Huang, L.; Chen, J.; Zhou, H.; Wan, Y.; Qu, Q.; Wang, M.; Xue, S. Changes in soil microbial activity and their linkages with soil carbon under global warming. CATENA 2023, 232, 107419. [Google Scholar] [CrossRef]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Swapnil, P.; Harish; Marwal, A.; Kumar, S. Multifarious Responses of Forest Soil Microbial Community Toward Climate Change. Microb. Ecol. 2023, 86, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Y.; Liu, X.; Ma, X.; Meng, Y.; Li, X.; Quan, X.; Shan, J.; Zhao, W.; Wang, H. Comparing the Effects of Biochar and Straw Amendment on Soil Carbon Pools and Bacterial Community Structure in Degraded Soil. J. Soil Sci. Plant Nutr. 2020, 20, 751–760. [Google Scholar] [CrossRef]
- Dong, X.; Singh, B.P.; Li, G.; Lin, Q.; Zhao, X. Biochar has little effect on soil dissolved organic carbon pool 5 years after biochar application under field condition. Soil Use Manag. 2019, 35, 466–477. [Google Scholar] [CrossRef]
- Palviainen, M.; Aaltonen, H.; Laurén, A.; Köster, K.; Berninger, F.; Ojala, A.; Pumpanen, J. Biochar amendment increases tree growth in nutrient-poor, young Scots pine stands in Finland. For. Ecol. Manag. 2020, 474, 118362. [Google Scholar] [CrossRef]
- Thomas, S.C.; Halim, M.A.; Gale, N.V.; Sujeeun, L. Biochar enhancement of facilitation effects in agroforestry: Early growth and physiological responses in a maize-leucaena model system. Agrofor. Syst. 2019, 93, 2213–2225. [Google Scholar] [CrossRef]
- George, E.; Ventura, M.; Panzacchi, P.; Scandellari, F.; Tonon, G. Can hydrochar and pyrochar affect nitrogen uptake and biomass allocation in poplars? J. Plant Nutr. Soil Sci. 2017, 180, 178–186. [Google Scholar] [CrossRef]
- Hu, M.; Lehtonen, A.; Minunno, F.; Mäkelä, A. Age effect on tree structure and biomass allocation in Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies [L.] Karst.). Ann. For. Sci. 2020, 77, 90. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, K.; Xu, X.; Song, T.; Xu, Y.; Zeng, F. Biogeographical patterns of biomass allocation in leaves, stems and roots in China’s forests. Sci. Rep. 2015, 5, 15997. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Konôpka, B.; Pajtík, J.; Moravčík, M.; Lukac, M. Biomass partitioning and growth efficiency in four naturally regenerated forest tree species. Basic Appl. Ecol. 2010, 11, 234–243. [Google Scholar] [CrossRef]
- Tanazawa, Y.; Tomotsune, M.; Suzuki, T.; Koizumi, H.; Yoshitake, S. Photosynthetic response of young oaks to biochar amendment in field conditions over 3 years. J. For. Res. 2021, 26, 116–126. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.-W.; Huchzermeyer, B.; Gul, B.; Khan, M.A. Impact of a biochar or a biochar-compost mixture on water relation, nutrient uptake and photosynthesis of Phragmites karka. Pedosphere 2020, 30, 466–477. [Google Scholar] [CrossRef]
- Fracheboud, Y.; Luquez, V.; Björkén, L.; Sjödin, A.; Tuominen, H.; Jansson, S. The Control of Autumn Senescence in European Aspen. Plant Physiol. 2009, 149, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.; Xia, J.; Luo, Y. Nitrogen use strategy drives interspecific differences in plant photosynthetic CO2 acclimation. Glob. Change Biol. 2023, 29, 3667–3677. [Google Scholar] [CrossRef] [PubMed]
- Herrel, A.; Speck, T.; Rowe, N.P. Ecology and Biomechanics: A Mechanical Approach to the Ecology of Animals and Plants; CRC Press: Boca Raton, FL, USA, 2006; pp. 85–100. [Google Scholar]
- Buss, W.; Graham, M.C.; Shepherd, J.G.; Mašek, O. Risks and benefits of marginal biomass-derived biochars for plant growth. Sci. Total Environ. 2016, 569–570, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A. Rhizoctonia solani suppression and plant growth promotion in cucumber as affected by biochar pyrolysis temperature, feedstock and concentration. Soil Biol. Biochem. 2014, 69, 110–118. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Liqun, X.; Weiming, Z.; Di, W.; Yanyan, S.; Honggui, Z.; Wenqi, G.; Jun, M.; Chen, W. Heat Storage Capacity and Temporal-spatial Response in the Soil Temperature of Albic Soil Amended with Maize-derived Biochar for 2 Years. Soil Tillage Res. 2021, 205, 104762. [Google Scholar] [CrossRef]
- Gross, N.; Suding, K.N.; Lavorel, S.; Roumet, C. Complementarity as a mechanism of coexistence between functional groups of grasses. J. Ecol. 2007, 95, 1296–1305. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, B.; Li, C.; Gao, M.; Wang, Y.; Zhou, Z.; Yuan, H. Dissecting soil CO2 fluxes from a subtropical forest in China by integrating field measurements with a modeling approach. Geoderma 2011, 161, 88–94. [Google Scholar] [CrossRef]
- Song, X.; Yuan, H.; Kimberley, M.O.; Jiang, H.; Zhou, G.; Wang, H. Soil CO2 flux dynamics in the two main plantation forest types in subtropical China. Sci. Total Environ. 2013, 444, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Raich, J.W.; Tufekciogul, A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Hamada, Y.; Tanaka, T. Dynamics of carbon dioxide in soil profiles based on long-term field observation. Hydrol. Process. 2001, 15, 1829–1845. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Högberg, P.; Read, D.J. Towards a more plant physiological perspective on soil ecology. Trends Ecol. Evol. 2006, 21, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Cheng, W. Photosynthesis controls of CO2 efflux from maize rhizosphere. Plant Soil 2004, 263, 85–99. [Google Scholar] [CrossRef]
- Shi, P.; Qin, Y.; Liu, Q.; Zhu, T.; Li, Z.; Li, P.; Ren, Z.; Liu, Y.; Wang, F. Soil respiration and response of carbon source changes to vegetation restoration in the Loess Plateau, China. Sci. Total Environ. 2020, 707, 135507. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Adachi, M.; Bekku, Y.S.; Rashidah, W.; Okuda, T.; Koizumi, H. Differences in soil respiration between different tropical ecosystems. Appl. Soil Ecol. 2006, 34, 258–265. [Google Scholar] [CrossRef]
- Sun, D.; Meng, J.; Xu, E.G.; Chen, W. Microbial community structure and predicted bacterial metabolic functions in biochar pellets aged in soil after 34 months. Appl. Soil Ecol. 2016, 100, 135–143. [Google Scholar] [CrossRef]
- Fa, K.-Y.; Zhang, Y.-Q.; Wu, B.; Qin, S.-G.; Liu, Z.; She, W.-W. Patterns and possible mechanisms of soil CO2 uptake in sandy soil. Sci. Total Environ. 2016, 544, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Xu, Y.; Lu, X. Biochar phosphorus fertilizer effects on soil phosphorus availability. Chemosphere 2020, 244, 125471. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, L.; Nan, H.; Cao, Y.; Wang, H.; Kumar, T.V.; Wang, C. Phosphorus adsorption by functionalized biochar: A review. Environ. Chem. Lett. 2023, 21, 497–524. [Google Scholar] [CrossRef]
- Li, Q.; Song, X.; Chang, S.X.; Peng, C.; Xiao, W.; Zhang, J.; Xiang, W.; Li, Y.; Wang, W. Nitrogen depositions increase soil respiration and decrease temperature sensitivity in a Moso bamboo forest. Agric. For. Meteorol. 2019, 268, 48–54. [Google Scholar] [CrossRef]
- Green, J.K.; Berry, J.; Ciais, P.; Zhang, Y.; Gentine, P. Amazon rainforest photosynthesis increases in response to atmospheric dryness. Sci. Adv. 2020, 6, eabb7232. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Cheng, W. Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol. Biochem. 2001, 33, 1915–1925. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, N.; Sarkar, B.; Li, S.; Tian, Y.; Sha, L.; Gao, Y.; Luo, X.; Yang, X. Biochar Addition Increased Soil Carbon Storage but Did Not Exacerbate Soil Carbon Emission in Young Subtropical Plantation Forest. Forests 2024, 15, 917. https://doi.org/10.3390/f15060917
Sun N, Sarkar B, Li S, Tian Y, Sha L, Gao Y, Luo X, Yang X. Biochar Addition Increased Soil Carbon Storage but Did Not Exacerbate Soil Carbon Emission in Young Subtropical Plantation Forest. Forests. 2024; 15(6):917. https://doi.org/10.3390/f15060917
Chicago/Turabian StyleSun, Nan, Binoy Sarkar, Saiqiang Li, Youhua Tian, Longbing Sha, Yongchao Gao, Xu Luo, and Xiaodong Yang. 2024. "Biochar Addition Increased Soil Carbon Storage but Did Not Exacerbate Soil Carbon Emission in Young Subtropical Plantation Forest" Forests 15, no. 6: 917. https://doi.org/10.3390/f15060917
APA StyleSun, N., Sarkar, B., Li, S., Tian, Y., Sha, L., Gao, Y., Luo, X., & Yang, X. (2024). Biochar Addition Increased Soil Carbon Storage but Did Not Exacerbate Soil Carbon Emission in Young Subtropical Plantation Forest. Forests, 15(6), 917. https://doi.org/10.3390/f15060917






