The Physical Properties of Surface Layer Thermally Modified Wood and Energy Consumption in the Preparation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SLTM and CoTM Processes
2.3. Mass Loss Analyses
2.4. Color Distribution Analyses
2.5. Water Absorption Test
2.6. Moisture Sorption Test
2.7. Energy Consumption
2.8. Statistical Analysis
3. Results and Discussion
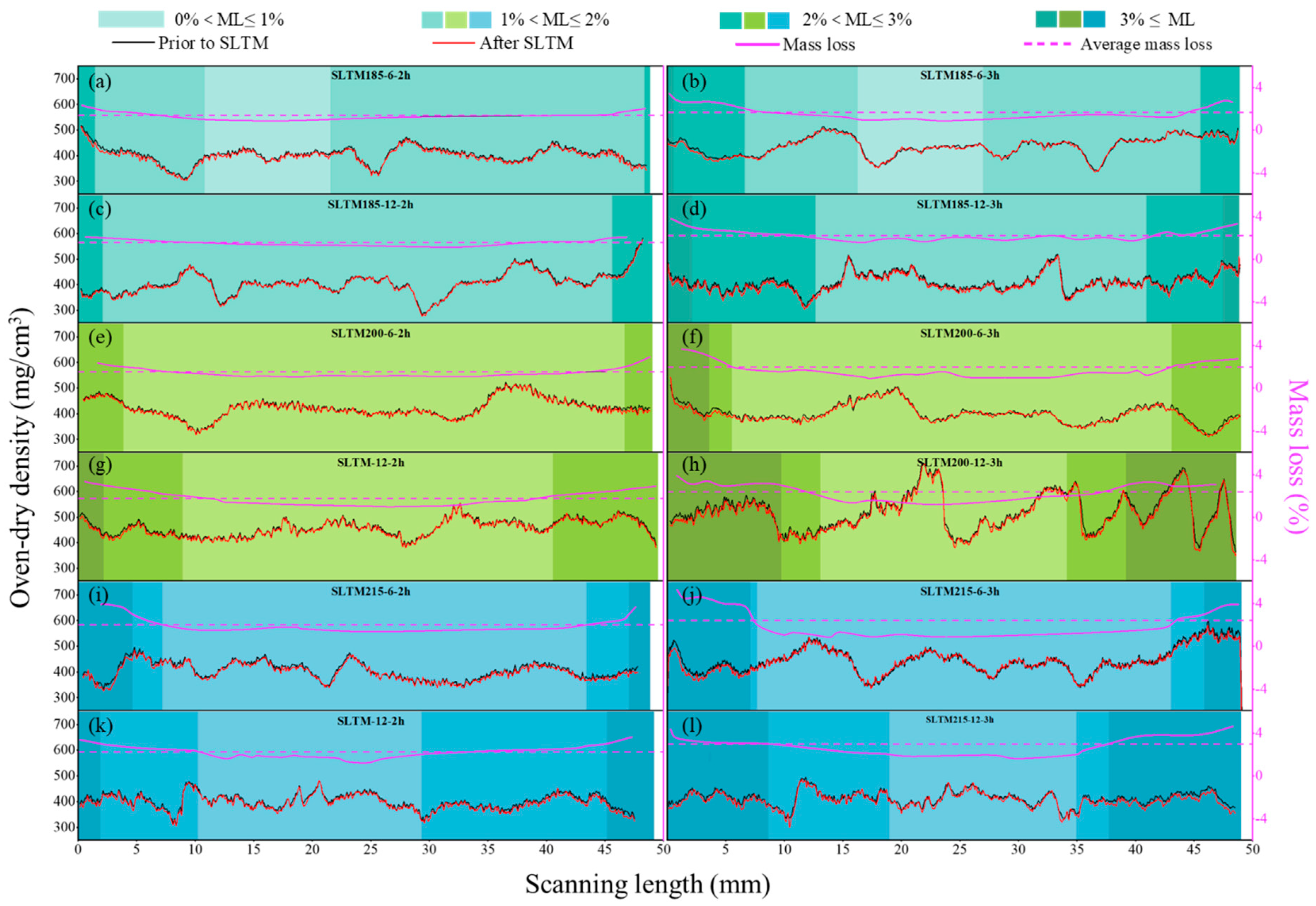
3.1. Mass Change
3.2. Color Changes
3.3. Water Absorption and Dimensional Stability
3.4. Moisture Sorption
3.5. Treatment Time and Energy Consumption
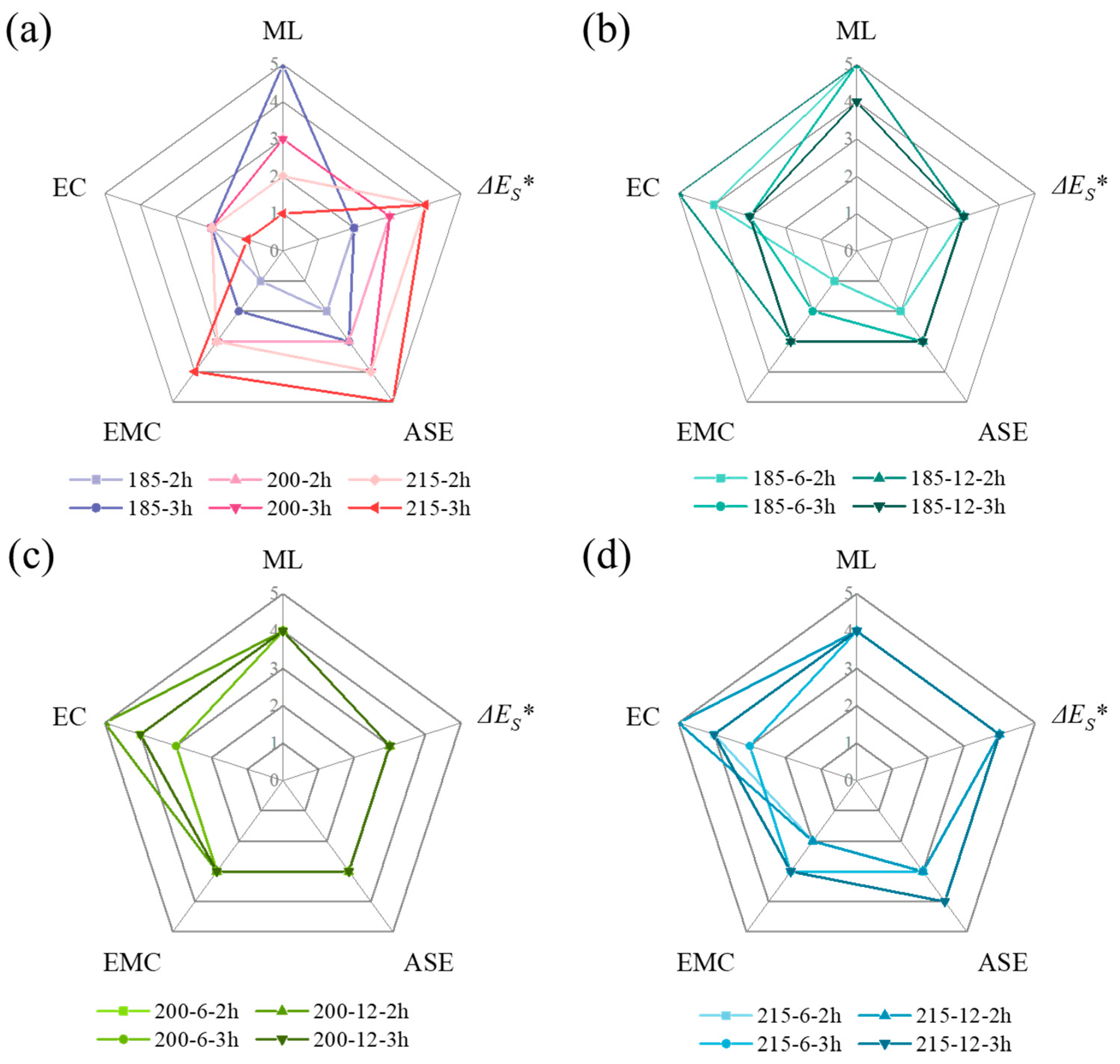
3.6. Evaluation of SLTM and CoTM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nepal, P.; Johnston, C.M.T.; Ganguly, I. Effects on Global Forests and Wood Product Markets of Increased Demand for Mass Timber. Sustainability 2021, 13, 13943. [Google Scholar] [CrossRef]
- Barbu, M.C.; Tudor, E.M. State of the Art of the Chinese Forestry, Wood Industry and Its Markets. Wood Mater. Sci. Eng. 2022, 17, 1030–1039. [Google Scholar] [CrossRef]
- McEwan, A.; Marchi, E.; Spinelli, R.; Brink, M. Past, Present and Future of Industrial Plantation Forestry and Implication on Future Timber Harvesting Technology. J. For. Res. 2020, 31, 339–351. [Google Scholar] [CrossRef]
- Qian, J.; Yue, K.; Li, X.; Pu, Y.; Chen, K.; Wu, P.; Zhang, Z. Strength Grading of Full-Scale Chinese Fast-Growing Poplar Wood for Structural Building Applications. Forests 2024, 15, 1602. [Google Scholar] [CrossRef]
- Julian, T.C.; Fukuda, H.; Novianto, D. The Influence of High-Temperature and -Pressure Treatment on Physical Properties of Albizia Falcataria Board. Forests 2022, 13, 239. [Google Scholar] [CrossRef]
- Liu, M.; Lyu, S.; Cai, L.; Peng, L.; Tang, J.; Huang, Z.; Lyu, J. Performance Improvement of Radiata Pine Wood by Combining Impregnation of Furfuryl Alcohol Resin and Densification for Making Fretboard Materials. Ind. Crop. Prod. 2021, 172, 114029. [Google Scholar] [CrossRef]
- Guo, D.; Yang, S.; Fu, F.; Guo, N.; Li, G.; Chu, F. Modification Mechanism of Plantation Wood via Grafting Epoxy Monomers onto Cell Walls. Wood Sci. Technol. 2022, 56, 813–831. [Google Scholar] [CrossRef]
- Sosins, G.; Grinins, J.; Brazdausks, P.; Zicans, J. Water-Related Properties of Wood after Thermal Modification in Closed Process under Pressure in Nitrogen. Forests 2024, 15, 140. [Google Scholar] [CrossRef]
- Yue, K.; Li, X.; Jiao, X.; Fu, F.; Wu, P.; Song, X. Strength grading of Chinese poplar wood for structural use following thermal modification. Polym. Test. 2023, 123, 108032. [Google Scholar] [CrossRef]
- Mania, P.; Wróblewski, M.; Wójciak, A.; Roszyk, E.; Moliński, W. Hardness of Densified Wood in Relation to Changed Chemical Composition. Forests 2020, 11, 506. [Google Scholar] [CrossRef]
- Hill, C.; Altgen, M.; Rautkari, L. Thermal Modification of Wood—A Review: Chemical Changes and Hygroscopicity. J. Mater. Sci. 2021, 56, 6581–6614. [Google Scholar] [CrossRef]
- Mohareb, A.; Sirmah, P.; Pétrissans, M.; Gérardin, P. Effect of Heat Treatment Intensity on Wood Chemical Composition and Decay Durability of Pinus Patula. Eur. J. Wood Prod. 2012, 70, 519–524. [Google Scholar] [CrossRef]
- Rowell, R.M.; Ibach, R.E.; McSweeny, J.; Nilsson, T. Understanding Decay Resistance, Dimensional Stability and Strength Changes in Heat-Treated and Acetylated Wood. Wood Mater. Sci. Eng. 2009, 4, 14–22. [Google Scholar] [CrossRef]
- Ebner, D.; Stelzer, R.; Barbu, M.C. Study of Wooden Surface Carbonization Using the Traditional Japanese Yakisugi Technique. Pro Ligno 2019, 15, 278–283. [Google Scholar]
- Ebner, D.H.; Barbu, M.-C.; Klaushofer, J.; Čermák, P. Surface Modification of Spruce and Fir Sawn-Timber by Charring in the Traditional Japanese Method—Yakisugi. Polymers 2021, 13, 1662. [Google Scholar] [CrossRef]
- Sorčan, N. Wood Charring as a Finishing Technique. Master’s Thesis, Univerza v Ljubljani, Ljubljana, Slovenia, 2019. [Google Scholar]
- Kampe, A.; Pfriem, A. A Note on Artificial Weathering of Spruce (Picea abies) with a Carbonised Layer. Int. Wood Prod. J. 2018, 9, 86–89. [Google Scholar] [CrossRef]
- Hasburgh, L.E.; Zelinka, S.L.; Bishell, A.B.; Kirker, G.T. Durability and Fire Performance of Charred Wood Siding (Shou Sugi Ban). Forests 2021, 12, 1262. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Hautamäki, S.; Lillqvist, K.; Segerholm, K.; Rautkari, L. Surface Modification of Solid Wood by Charring. J. Mater. Sci. 2017, 52, 6111–6119. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Turunen, H.; Čermák, P.; Hautamäki, S.; Rautkari, L. Sorption-Related Characteristics of Surface Charred Spruce Wood. Materials 2018, 11, 2083. [Google Scholar] [CrossRef]
- Šeda, V.; Machová, D.; Dohnal, J.; Dömény, J.; Zárybnická, L.; Oberle, A.; Vacenovská, V.; Čermák, P. Effect of One-Sided Surface Charring of Beech Wood on Density Profile and Surface Wettability. Appl. Sci. 2021, 11, 4086. [Google Scholar] [CrossRef]
- Čermák, P.; Dejmal, A.; Paschová, Z.; Kymäläinen, M.; Dömény, J.; Brabec, M.; Hess, D.; Rautkari, L. One-Sided Surface Charring of Beech Wood. J. Mater. Sci. 2019, 54, 9497–9506. [Google Scholar] [CrossRef]
- Ding, T.; Cheng, Y.; Jiang, T. Preparation and Characterization of Heat-Treated Douglas Fir Wood with Core–Shell Structure. Forests 2022, 13, 825. [Google Scholar] [CrossRef]
- Tang, J.; Zhan, T.; Li, Z.; Jiang, J.; Lyu, J. Optimization of Dimensional Stability and Mechanical Performance of Thermally Modified Wood Using Cyclic-Gradient Thermal Treatment. Constr. Build. Mater. 2025, 458, 139596. [Google Scholar] [CrossRef]
- Čermák, P.; Dömény, J.; Brabec, M.; Milch, J.; Baar, J.; Horáček, P.; Rademacher, P. Unevenly Distributed Thermal Treatment of Wood: Preliminary Study—Density Profiles. Eur. J. Wood Prod. 2016, 74, 629–631. [Google Scholar] [CrossRef]
- Yue, K.; Qian, J.; Wu, P.; Jiao, X.; Lu, D.; Song, X. Experimental Analysis of Thermally-Treated Chinese Poplar Wood with Focus on Structural Application. Ind. Crop. Prod. 2023, 197, 116612. [Google Scholar] [CrossRef]
- ISO/CIE 11664-6:2022; Colorimetry—Part 6: CIEDE2000 Colour-Difference Formula. International Organization for Standardization (ISO): Geneva, Switzerland; International Commission on Illumination (CIE): Vienna, Austria, 2022.
- 13061-8:2021; Test Methods for Physical and Mechanical Properties of Small Clear Wood Specimens—Part 8: Determination of Swelling. Standardization Administration of China: Beijing, China, 2021.
- Wang, B.; He, Z.; Wang, Z.; Yi, S. Effects of Zinc Chloride Impregnation Treatment on Dimensional Stability and Energy Consumption of Heat-Treated Mongolian Scots Pine. J. Beijing For. Univ. 2024, 46, 123–131. [Google Scholar]
- Park, Y.; Han, Y.; Park, J.-H.; Chang, Y.-S.; Yang, S.-Y.; Chung, H.; Yeo, H. Evaluation of the Energy Efficiency of Combined Drying and Heat Treatment by Superheated Steam. Dry. Technol. 2017, 35, 1460–1467. [Google Scholar] [CrossRef]
- Niu, H.; Wang, B.; Yang, Z.; Liu, X.; Liu, X.; Shi, W. Impact of Control Strategies on Energy Consumption in Cold Storage Facilities. Energy Build. 2024, 322, 114736. [Google Scholar] [CrossRef]
- Tian, S.; Shao, S.; Liu, B. Investigation on Transient Energy Consumption of Cold Storages: Modeling and a Case Study. Energy 2019, 180, 1–9. [Google Scholar] [CrossRef]
- Putra, A.S.; Garniwa, I.; Arilanggaaji, E.S.; Pradana, S.S. A Study on Carbon Cap and Trade Effect to Cost of Electricity in Accordance with the Merit Order of 300–400 MW Coal Power Plants. IOP Conf. Ser. Earth Environ. Sci. 2021, 880, 012049. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, R.K. Cost of CO2 Emission Mitigation and Its Decomposition: Evidence from Coal-Fired Thermal Power Sector in India. Empir. Econ. 2021, 61, 693–717. [Google Scholar] [CrossRef]
- Östberg, G.; Salmén, L.; Terlecki, J. Softening Temperature of Moist Wood Measured by Differential Scanning Calorimetry. Holzforschung 1990, 44, 223–225. [Google Scholar] [CrossRef]
- Rayirath, P.; Avramidis, S.; Mansfield, S.D. The Effect of Wood Drying on Crystallinity and Microfibril Angle in Black Spruce (Picea mariana). J. Wood Chem. Technol. 2008, 28, 167–179. [Google Scholar] [CrossRef]
- Shen, Y.; Gao, Z.; Hou, X.; Chen, Z.; Jiang, J.; Sun, J. Spectral and Thermal Analysis of Eucalyptus Wood Drying at Different Temperature and Methods. Dry. Technol. 2019, 38, 313–320. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions, 1st ed.; Verlag Kessel: Remagen, Germany, 2003; pp. 154–196. [Google Scholar]
- Jurczyková, T.; Šárovec, O.; Kačík, F.; Hájková, K.; Jurczyk, T.; Hrčka, R. Chromophores’ Contribution to Color Changes of Thermally Modified Tropical Wood Species. Polymers 2023, 15, 4000. [Google Scholar] [CrossRef]
- Sikora, A.; Kačík, F.; Gaff, M.; Vondrová, V.; Bubeníková, T.; Kubovský, I. Impact of Thermal Modification on Color and Chemical Changes of Spruce and Oak Wood. J. Wood Sci. 2018, 64, 406–416. [Google Scholar] [CrossRef]
- Yao, C.; Yongming, F.; Jianmin, G.; Houkun, L. Coloring Characteristics of in Situ Lignin during Heat Treatment. Wood Sci Technol. 2012, 46, 33–40. [Google Scholar] [CrossRef]
- González-Peña, M.M.; Hale, M.D.C. Colour in Thermally Modified Wood of Beech, Norway Spruce and Scots Pine. Part 1: Colour Evolution and Colour Changes. Holzforschung 2009, 63, 385–393. [Google Scholar] [CrossRef]
- Esteves, B.; Marques, A.V.; Domingos, I.; Pereira, H. Heat-Induced Colour Changes of Pine (Pinus pinaster) and Eucalypt (Eucalyptus globulus) Wood. Wood Sci. Technol. 2008, 42, 369–384. [Google Scholar] [CrossRef]
- Matsuo, M.; Yokoyama, M.; Umemura, K.; Gril, J.; Yano, K.; Kawai, S. Color Changes in Wood during Heating: Kinetic Analysis by Applying a Time-Temperature Superposition Method. Appl. Phys. A 2010, 99, 47–52. [Google Scholar] [CrossRef]
- Kačíková, D.; Kačík, F.; Čabalová, I.; Ďurkovič, J. Effects of Thermal Treatment on Chemical, Mechanical and Colour Traits in Norway Spruce Wood. Bioresour. Technol. 2013, 144, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Brischke, C.; Welzbacher, C.R.; Brandt, K.; Rapp, A.O. Quality Control of Thermally Modified Timber: Interrelationship between Heat Treatment Intensities and CIE L*a*b* Color Data on Homogenized Wood Samples. Holzforschung 2007, 61, 19–22. [Google Scholar] [CrossRef]
- Yang, T.-H.; Chang, F.-R.; Lin, C.-J.; Chang, F.-C. Effects of Temperature and Duration of Heat Treatment on the Physical, Surface, and Mechanical Properties of Japanese Cedar Wood. Bioresources 2016, 11, 3947–3963. [Google Scholar] [CrossRef]
- Kartal, S.N.; Hwang, W.-J.; Imamura, Y. Water Absorption of Boron-Treated and Heat-Modified Wood. J. Wood Sci. 2007, 53, 454–457. [Google Scholar] [CrossRef]
- Kekkonen, P.M.; Ylisassi, A.; Telkki, V.-V. Absorption of Water in Thermally Modified Pine Wood As Studied by Nuclear Magnetic Resonance. J. Phys. Chem. C 2014, 118, 2146–2153. [Google Scholar] [CrossRef]
- Scheiding, W.; Direske, M.; Zauer, M. Water Absorption of Untreated and Thermally Modified Sapwood and Heartwood of Pinus sylvestris L. Eur. J. Wood Prod. 2016, 74, 585–589. [Google Scholar] [CrossRef]
- Krackler, V.; Ammann, S.; Camathias, U.; Niemz, P. Investigation on Moisture Behaviour and Porosity of Thermally Modified Timber. Bauphysik 2011, 33, 374–381. [Google Scholar] [CrossRef]
- Zhou, F.; Fu, Z.; Gao, X.; Zhou, Y. Changes in the Wood-Water Interactions of Mahogany Wood Due to Heat Treatment. Holzforschung 2020, 74, 853–863. [Google Scholar] [CrossRef]
- Bessala, L.F.B.; Gao, J.; He, Z.; Wang, Z.; Yi, S. Effects of Heat Treatment on Color, Dimensional Stability, Hygroscopicity and Chemical Structure of Afrormosia and Newtonia Wood: A Comparative Study of Air and Palm Oil Medium. Polymers 2023, 15, 774. [Google Scholar] [CrossRef]
- Aytin, A.; Korkut, S. Effect of Thermal Treatment on the Swelling and Surface Roughness of Common Alder and Wych Elm Wood. J. For. Res. 2016, 27, 225–229. [Google Scholar] [CrossRef]
- Gunduz, G.; Aydemir, D.; Karakas, G. The Effects of Thermal Treatment on the Mechanical Properties of Wild Pear (Pyrus elaeagnifolia Pall.) Wood and Changes in Physical Properties. Mater. Des. 2009, 30, 4391–4395. [Google Scholar] [CrossRef]
- Fu, Z.; Zhou, Y.; Gao, X.; Liu, H.; Zhou, F. Changes of Water Related Properties in Radiata Pine Wood Due to Heat Treatment. Constr. Build. Mater. 2019, 227, 116692. [Google Scholar] [CrossRef]
- Olarescu, M.C.; Campean, M.; Ispas, M.; Cosereanu, C. Effect of Thermal Treatment on Some Properties of Lime Wood. Eur. J. Wood Prod. 2014, 72, 559–562. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Zhao, L.; Lyu, J. Effect of Heat Treatment on Hygroscopicity of Chinese Fir (Cunninghamia lanceolata [Lamb.] Hook.). Wood. Forests 2024, 15, 630. [Google Scholar] [CrossRef]
- Guo, F.; Huang, R.; Lu, J.; Chen, Z.; Cao, Y. Evaluating the Effect of Heat Treating Temperature and Duration on Selected Wood Properties Using Comprehensive Cluster Analysis. J. Wood Sci. 2014, 60, 255–262. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Ramsay, J.; Laine, K.; Rautkari, L.; Hughes, M. Water Vapour Sorption Behaviour of Thermally Modified Wood. Int. Wood Prod. J. 2013, 4, 191–196. [Google Scholar] [CrossRef]
- Čermák, P.; Baar, J.; Dömény, J.; Výbohová, E.; Rousek, R.; Pařil, P.; Oberle, A.; Čabalová, I.; Hess, D.; Vodák, M.; et al. Wood-Water Interactions of Thermally Modified, Acetylated and Melamine Formaldehyde Resin Impregnated Beech Wood. Holzforschung 2022, 76, 437–450. [Google Scholar] [CrossRef]
- Simón, C.; Fernández, F.G.; Esteban, L.G.; Palacios, P.; Hosseinpourpia, R.; Mai, C. Comparison of the Saturated Salt and Dynamic Vapor Sorption Methods in Obtaining the Sorption Properties of Pinus pinea L. Eur. J. Wood Prod. 2017, 75, 919–926. [Google Scholar] [CrossRef]
- Olek, W.; Majka, J.; Czajkowski, Ł. Sorption Isotherms of Thermally Modified Wood. Holzforschung 2013, 67, 183–191. [Google Scholar] [CrossRef]
- Li, T.; Cheng, D.; Avramidis, S.; Wålinder, M.E.P.; Zhou, D. Response of Hygroscopicity to Heat Treatment and Its Relation to Durability of Thermally Modified Wood. Constr. Build. Mater. 2017, 144, 671–676. [Google Scholar] [CrossRef]
- Willems, W. The Water Vapor Sorption Mechanism and Its Hysteresis in Wood: The Water/Void Mixture Postulate. Wood Sci. Technol. 2014, 48, 499–518. [Google Scholar] [CrossRef]
- Hou, S.; Wang, J.; Yin, F.; Qi, C.; Mu, J. Moisture Sorption Isotherms and Hysteresis of Cellulose, Hemicelluloses and Lignin Isolated from Birch Wood and Their Effects on Wood Hygroscopicity. Wood Sci. Technol. 2022, 56, 1087–1102. [Google Scholar] [CrossRef]
- Čermák, P.; Horáček, P.; Rademacher, P. Measured Temperature and Moisture Profiles during Thermal Modification of Beech (Fagus sylvatica L.) and Spruce (Picea abies L. Karst.) Wood. Holzforschung 2014, 68, 175–183. [Google Scholar] [CrossRef]
- Torniainen, P.; Popescu, C.-M.; Jones, D.; Scharf, A.; Sandberg, D. Correlation of Studies between Colour, Structure and Mechanical Properties of Commercially Produced ThermoWood® Treated Norway Spruce and Scots Pine. Forests 2021, 12, 1165. [Google Scholar] [CrossRef]
- Pétrissans, A.; Lin, Y.-Y.; Nguyen, T.N.; Colin, B.; Quirino, R.L.; Rios-Teixeira, P.; Chen, W.-H.; Pétrissans, M. Influence of the Heating Rate on the Thermodegradation during the Mild Pyrolysis of the Wood. Wood Mater. Sci. Eng. 2023, 18, 412–421. [Google Scholar] [CrossRef]
- Chen, C.; Tu, D.; Zhao, X.; Zhou, Q.; Cherdchim, B.; Hu, C. Influence of Cooling Rate on the Physical Properties, Chemical Composition, and Mechanical Properties of Heat-Treated Rubberwood. Holzforschung 2020, 74, 1033–1042. [Google Scholar] [CrossRef]
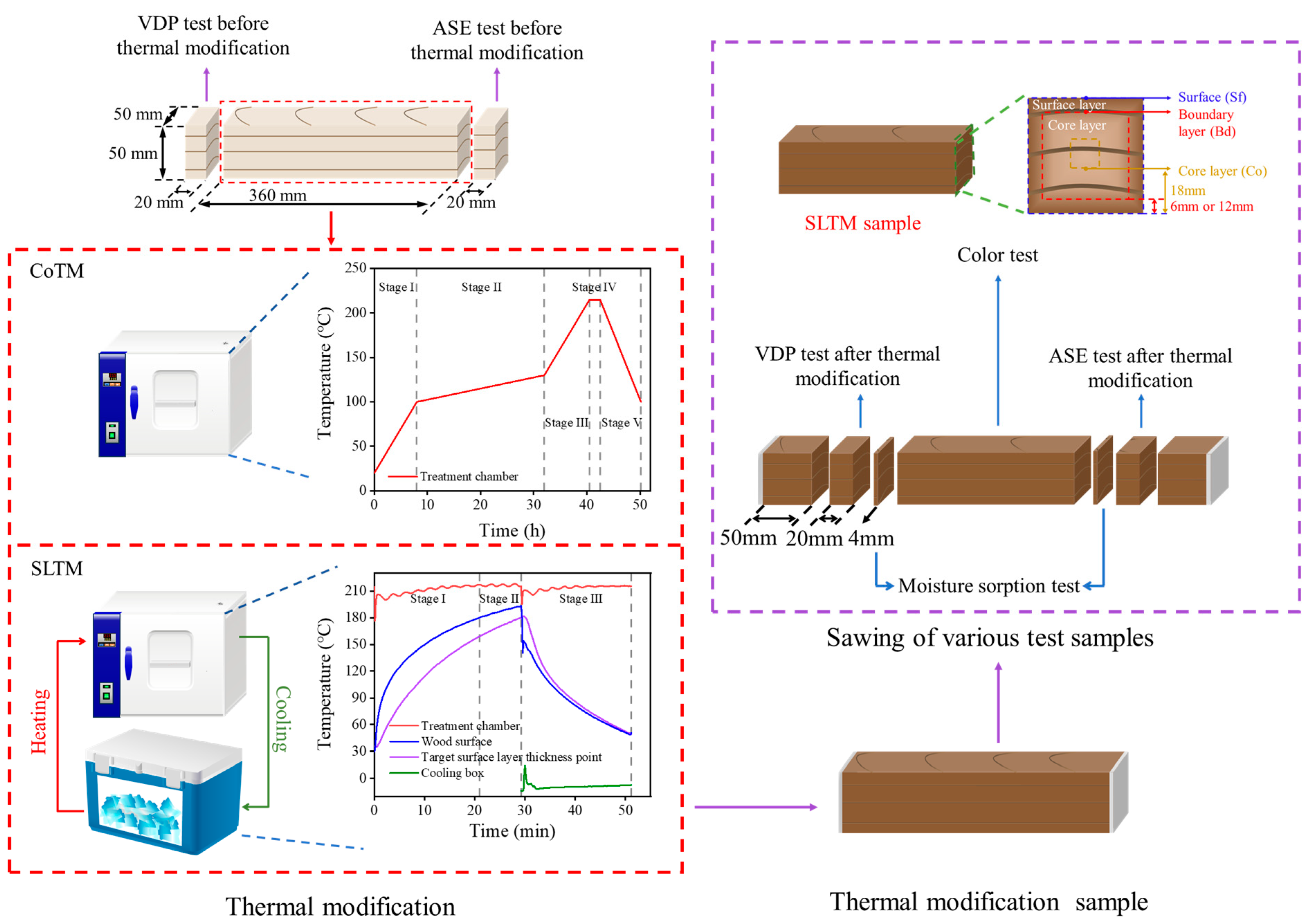




| Logs ID | Height Above Ground (m) | Growth Ring Region | Oven-Dry Density (g∙cm−3) | Dimensions of Samples (R × T × L, mm3) | Number of Samples for CoTM | Number of Samples for SLTM |
|---|---|---|---|---|---|---|
| P1 | 1.3–4 | 12–17 | 0.42 ± 0.02 | 50 × 50 × 360 | 14 | 24 |
| P2 | 1.3–4 | 13–19 | 0.45 ± 0.04 | 50 × 50 × 360 | 7 | 12 |
| Treatment Type | Temperature (°C) | SLT (mm) | Time (h) | SLA (mm) | MLSL (%) | ML (%) | PT with 0% < ML ≤ 1% (%) | PT with 1% < ML ≤ 2% (%) | PT with 2% < ML ≤ 3% (%) | PT with 3% ≤ ML (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| SLTM | 185 | 6 | 2 | 8.28 | 1.69 | 1.36 | 22.01 | 73.95 | 4.04 | 0 |
| 3 | 6.50 | 2.34 | 1.67 | 21.18 | 79.51 | 1.01 | 0 | |||
| 12 | 2 | 10.03 | 1.87 | 1.57 | 0 | 88.65 | 11.35 | 0 | ||
| 3 | 9.23 | 2.66 | 2.21 | 0 | 57.72 | 35.05 | 7.23 | |||
| 200 | 6 | 2 | 6.44 | 2.08 | 1.55 | 0 | 87.22 | 12.78 | 0 | |
| 3 | 5.84 | 2.74 | 1.96 | 0 | 76.58 | 16.07 | 7.35 | |||
| 12 | 2 | 10.54 | 2.38 | 1.78 | 0 | 63.79 | 31.80 | 4.41 | ||
| 3 | 11.64 | 3.08 | 2.38 | 0 | 43.31 | 17.30 | 39.39 | |||
| 215 | 6 | 2 | 6.35 | 2.95 | 2.00 | 0 | 74.00 | 12.74 | 13.26 | |
| 3 | 6.49 | 3.74 | 2.40 | 0 | 71.98 | 7.07 | 20.95 | |||
| 12 | 2 | 12.75 | 2.79 | 2.26 | 0 | 38.79 | 49.17 | 12.04 | ||
| 3 | 12.61 | 3.54 | 2.96 | 0 | 32.56 | 26.71 | 40.73 | |||
| CoTM | 185 | - | 2 | - | - | 1.38 | - | 100 | - | - |
| - | 3 | - | - | 1.84 | - | 100 | - | - | ||
| 200 | - | 2 | - | - | 4.24 | - | - | - | 100 | |
| - | 3 | - | - | 5.68 | - | - | - | 100 | ||
| 215 | - | 2 | - | - | 7.34 | - | - | - | 100 | |
| - | 3 | - | - | 9.69 | - | - | - | 100 |
| Temperature (°C) | Time (h) | Amax (%) | Duncan Grouping | ASE (%) | Duncan Grouping | Differential Swelling | Duncan Grouping |
|---|---|---|---|---|---|---|---|
| Untreated | 86.6 | A | - | - | 3.3 | A | |
| 185 | 2 | 81.6 | AB | 18.0 | A | 3.0 | A |
| 3 | 80.1 | ABC | 21.4 | A | 3.0 | A | |
| 200 | 2 | 74.3 | ABC | 37.9 | B | 2.9 | A |
| 3 | 74.4 | ABC | 43.9 | BC | 2.8 | A | |
| 215 | 2 | 65.2 | BC | 49.2 | BC | 2.8 | A |
| 3 | 62.9 | C | 54.8 | C | 2.6 | A |
| Temperature (°C) | SLT (mm) | Time (h) | Amax (%) | Duncan Grouping | ASE (%) | Duncan Grouping | Differential Swelling | Duncan Grouping |
|---|---|---|---|---|---|---|---|---|
| 185 | 6 | 2 | 84.5 | A | 17.2 | A | 3.3 | A |
| 3 | 83.8 | A | 23.5 | ABC | 3.2 | A | ||
| 12 | 2 | 80.7 | AB | 21.0 | AB | 3.1 | A | |
| 3 | 80.8 | AB | 25.0 | BC | 3.1 | A | ||
| 200 | 6 | 2 | 79.7 | AB | 22.6 | ABC | 3.1 | A |
| 3 | 78.9 | AB | 25.9 | BC | 3.1 | A | ||
| 12 | 2 | 78.8 | AB | 25.0 | BC | 3.0 | A | |
| 3 | 77.6 | AB | 28.7 | CD | 3.0 | A | ||
| 215 | 6 | 2 | 76.0 | AB | 26.7 | BC | 2.9 | A |
| 3 | 74.4 | AB | 33.3 | BE | 3.0 | A | ||
| 12 | 2 | 74.7 | AB | 38.7 | EF | 2.9 | A | |
| 3 | 72.5 | B | 43.3 | F | 2.9 | A |
| Treatment Type | Temperature (°C) | Target Surface Layer Thickness (mm) | Time (h) | E1 (kJ) | E2 (kJ) | E3 (kJ) | E4 (kJ) | E5 (kJ) | E6 (kJ) | E7 (kJ) | Etotal (kJ) | Consumed Electrical Energy (kW∙h) | CO2 Production (kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLTM | 185 | 6 | 2 | 144,672 | 1614 | 44,565 | 63,385 | 1,117,074 | 1,628,698 | 973,901 | 3,973,908 | 1104 | 1049 |
| 3 | 212,938 | 1614 | 44,565 | 63,385 | 1,755,401 | 1,737,032 | 1,011,917 | 4,826,851 | 1341 | 1274 | |||
| 12 | 2 | 112,887 | 1614 | 44,565 | 63,385 | 797,910 | 1,509,109 | 931,738 | 3,461,207 | 961 | 913 | ||
| 3 | 182,719 | 1614 | 44,565 | 63,385 | 1,436,237 | 1,964,683 | 1,092,442 | 4,785,644 | 1329 | 1263 | |||
| 200 | 6 | 2 | 174,909 | 1614 | 44,565 | 65,248 | 1,463,439 | 1,510,883 | 912,038 | 4,172,696 | 1159 | 1101 | |
| 3 | 241,616 | 1614 | 44,565 | 65,248 | 2,113,857 | 1,808,361 | 1,008,115 | 5,283,376 | 1468 | 1394 | |||
| 12 | 2 | 115,247 | 1614 | 44,565 | 65,248 | 813,022 | 1,351,204 | 860,544 | 3,251,443 | 903 | 858 | ||
| 3 | 150,950 | 1614 | 44,565 | 65,248 | 1,138,231 | 1,569,320 | 931,046 | 3,900,972 | 1084 | 1029 | |||
| 215 | 6 | 2 | 208,263 | 1614 | 44,565 | 67,109 | 1,823,038 | 1,498,720 | 891,302 | 4,534,610 | 1260 | 1197 | |
| 3 | 274,970 | 1614 | 44,565 | 67,109 | 2,485,961 | 1,836,157 | 991,872 | 5,702,247 | 1584 | 1505 | |||
| 12 | 2 | 117,618 | 1614 | 44,565 | 67,109 | 828,654 | 1,288,486 | 828,749 | 3,176,794 | 882 | 838 | ||
| 3 | 190,603 | 1614 | 44,565 | 67,109 | 1,491,577 | 1,650,179 | 936,576 | 4,382,222 | 1217 | 1156 | |||
| CoTM | 185 | - | 2 | 26,386 | 1614 | 49,450 | 55,467 | 11,896 | 2,999,016 | 2,413,440 | 5,557,269 | 1544 | 1467 |
| 3 | 26,386 | 1614 | 49,450 | 55,467 | 11,896 | 3,096,794 | 2,448,000 | 5,689,606 | 1580 | 1501 | |||
| 200 | - | 2 | 29,408 | 1614 | 49,450 | 56,072 | 14,918 | 3,284,943 | 2,499,840 | 5,936,245 | 1649 | 1567 | |
| 3 | 29,408 | 1614 | 49,450 | 56,072 | 14,918 | 3,391,610 | 2,534,400 | 6,077,472 | 1688 | 1604 | |||
| 215 | - | 2 | 32,535 | 1614 | 49,450 | 56,676 | 18,045 | 3,597,536 | 2,586,240 | 6,342,095 | 1762 | 1674 | |
| 3 | 32,535 | 1614 | 49,450 | 56,676 | 18,045 | 3,713,092 | 2,620,800 | 6,492,211 | 1803 | 1713 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Xu, Y.; He, J.; Peng, H.; Li, Z.; Jiang, J.; Zhan, T.; Lyu, J. The Physical Properties of Surface Layer Thermally Modified Wood and Energy Consumption in the Preparation Process. Forests 2025, 16, 427. https://doi.org/10.3390/f16030427
Tang J, Xu Y, He J, Peng H, Li Z, Jiang J, Zhan T, Lyu J. The Physical Properties of Surface Layer Thermally Modified Wood and Energy Consumption in the Preparation Process. Forests. 2025; 16(3):427. https://doi.org/10.3390/f16030427
Chicago/Turabian StyleTang, Jun, Yisheng Xu, Jinrong He, Hui Peng, Zhu Li, Jiali Jiang, Tianyi Zhan, and Jianxiong Lyu. 2025. "The Physical Properties of Surface Layer Thermally Modified Wood and Energy Consumption in the Preparation Process" Forests 16, no. 3: 427. https://doi.org/10.3390/f16030427
APA StyleTang, J., Xu, Y., He, J., Peng, H., Li, Z., Jiang, J., Zhan, T., & Lyu, J. (2025). The Physical Properties of Surface Layer Thermally Modified Wood and Energy Consumption in the Preparation Process. Forests, 16(3), 427. https://doi.org/10.3390/f16030427






