Polyploid Advantage? Comparing Salt Stress Responses of Di- and Tetraploid Acacia senegal (L.) Willd. Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Stands with Respect to Salinity and pH
2.2. Plant Material for Controlled Salinity Study and Experimental Design
2.3. Measurement of Morphological and Physiological Changes in Response to Salt Stress
2.4. Data Analysis
- -
- Cytotype frequency in relation to site characteristics
- -
- Salt stress tolerance evaluation between ploidy levels
3. Results
3.1. Cytotype Occurrence in Relation to Site Characteristics
3.2. Performance of Diploid and Tetraploid Seedlings Under Salt Stress
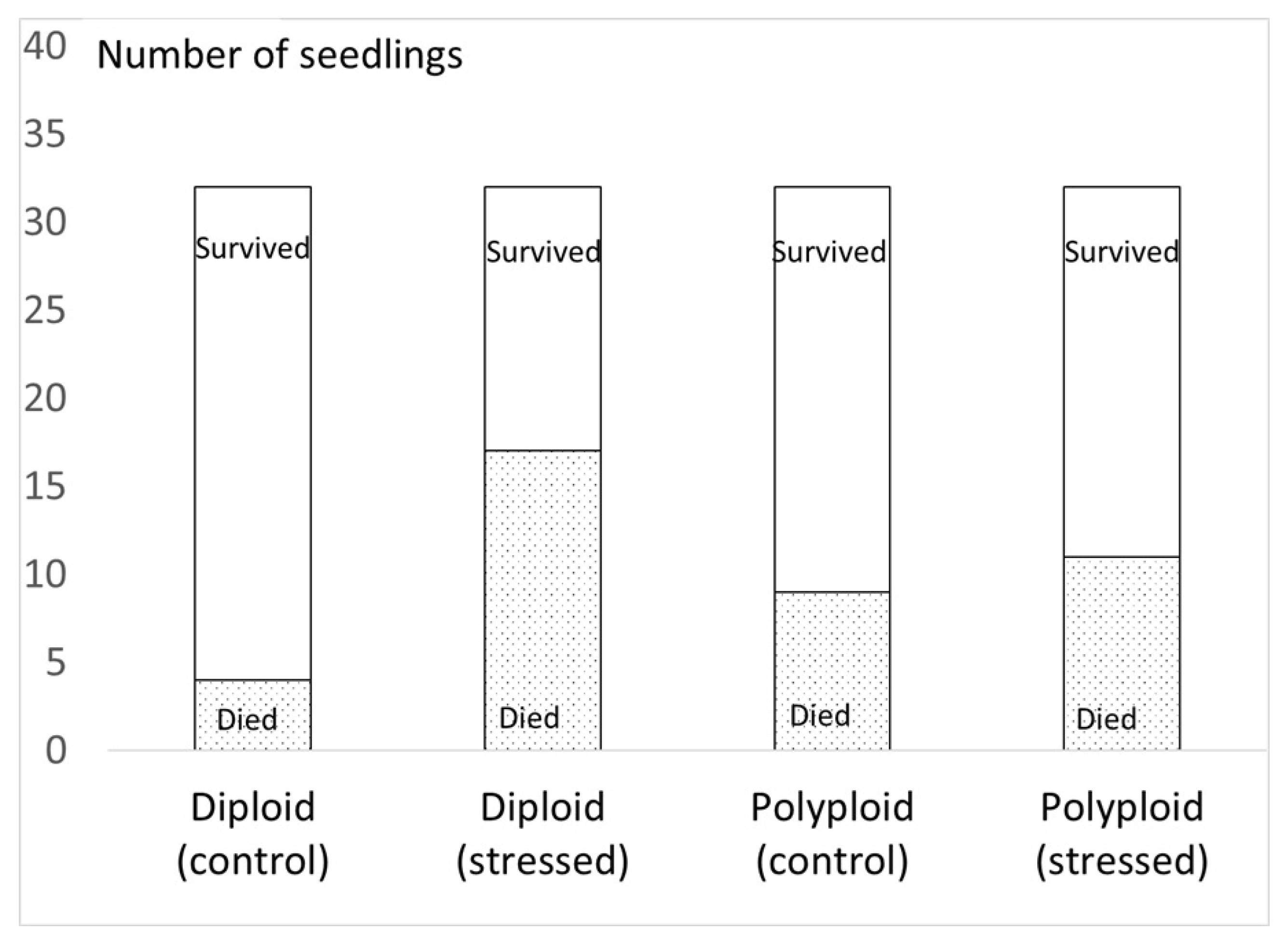
3.2.1. Survival
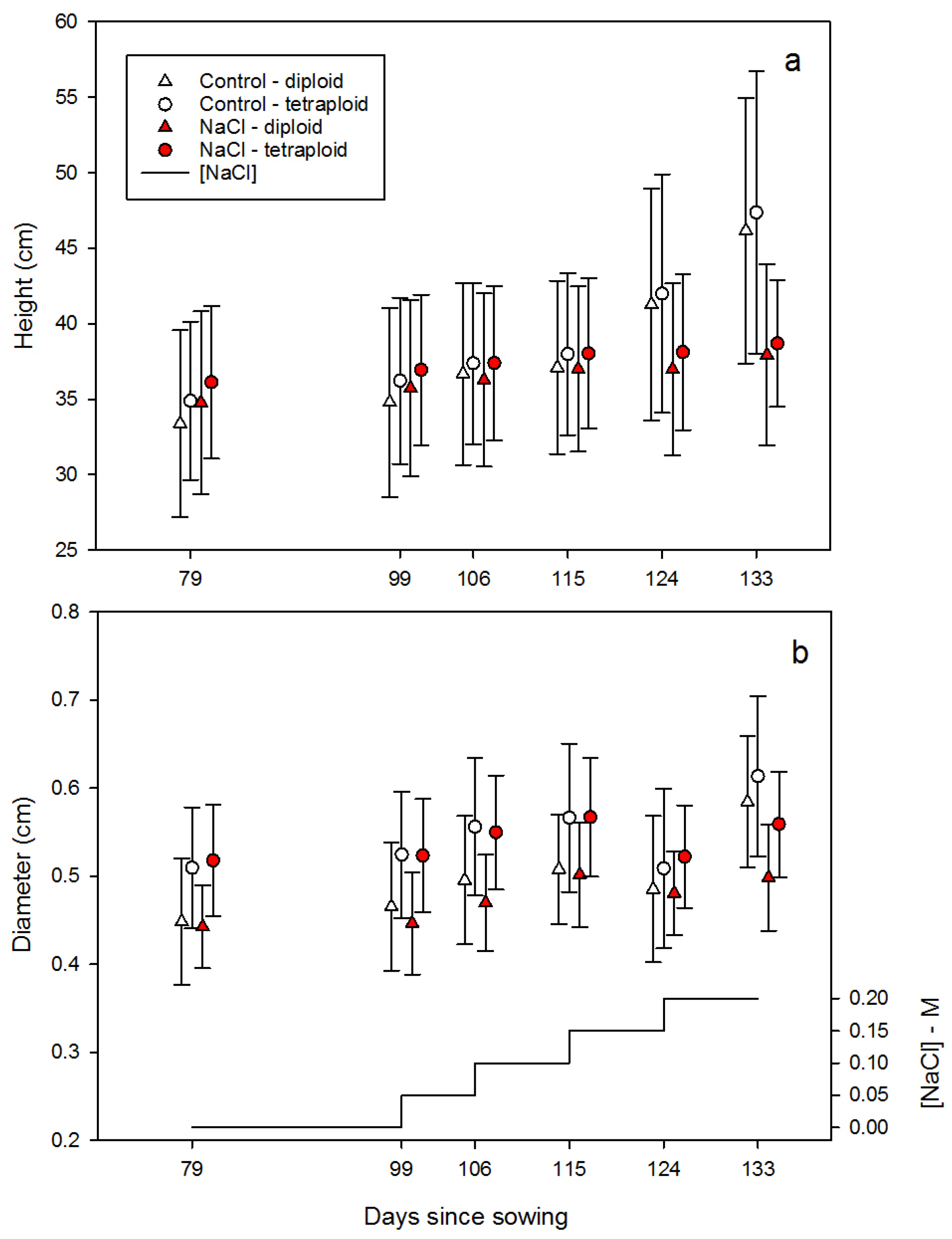
3.2.2. Growth
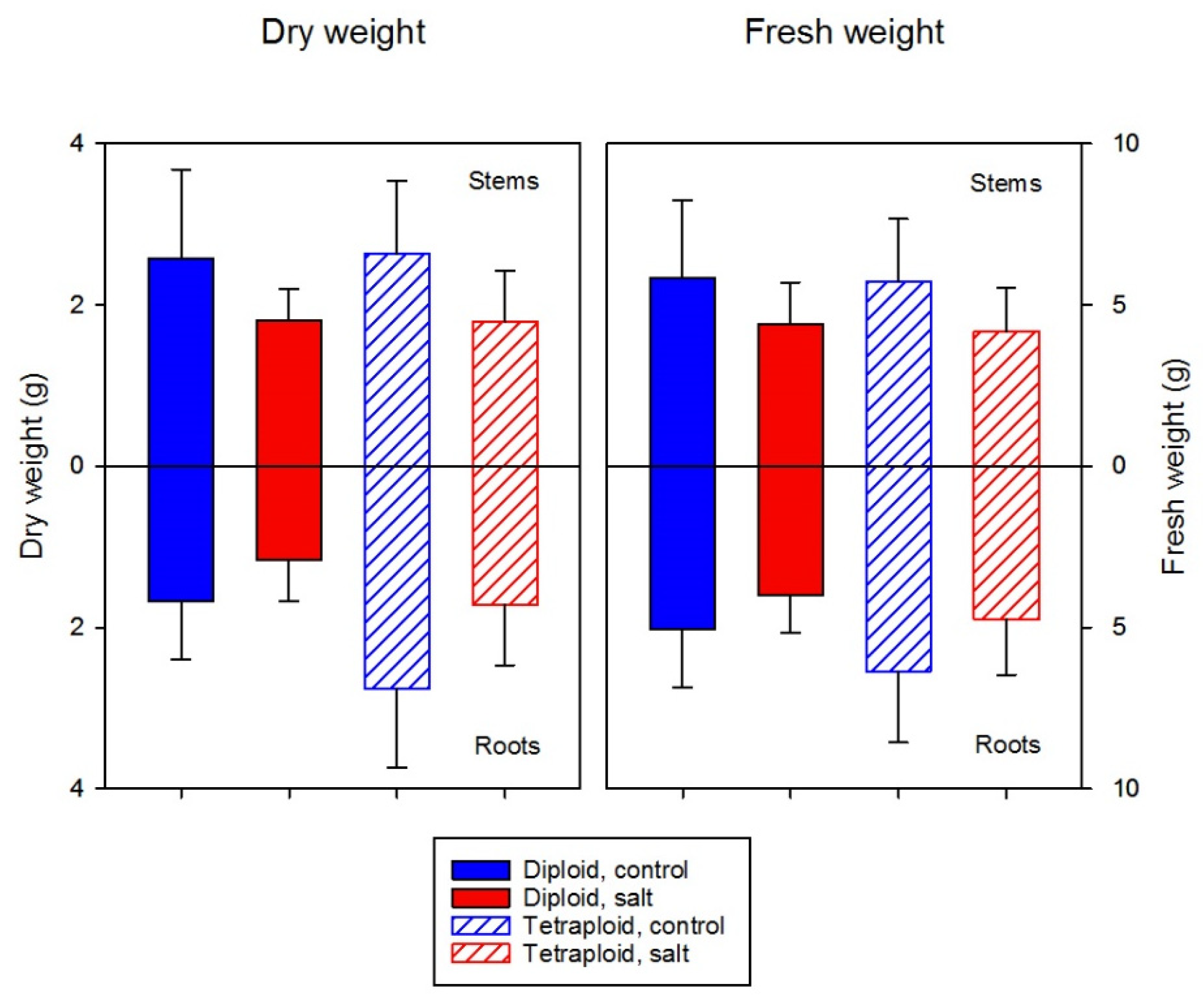
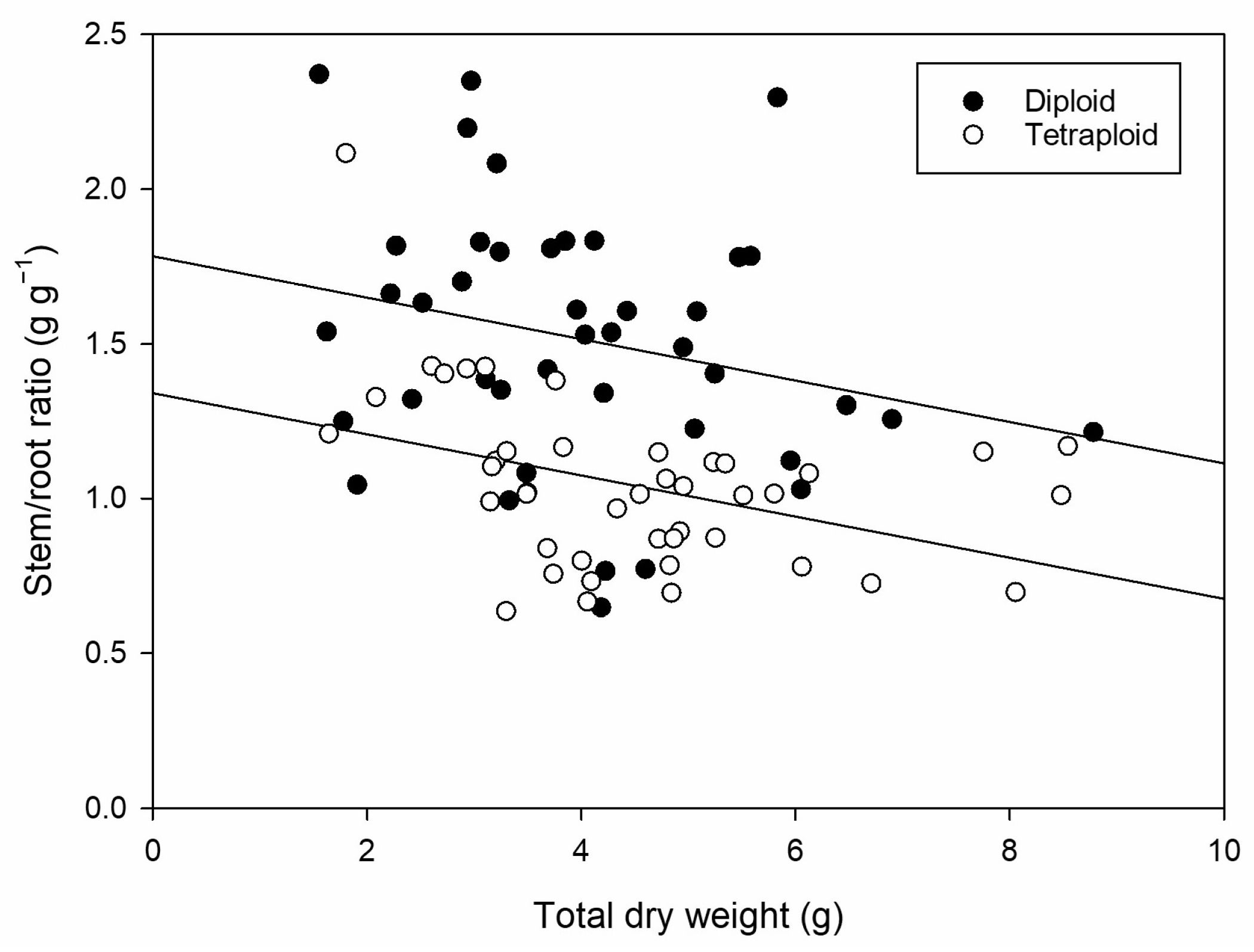
3.2.3. Root-Shoot Biomass
3.2.4. Na+ and Cl− Uptake Under Salt Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Te Beest, M.; Le Roux, J.J.; Richardson, D.M.; Brysting, A.K.; Suda, J.; Kubesová, M.; Pysek, P. The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot. 2012, 109, 19–45. [Google Scholar] [CrossRef]
- Gunn, B.F.; Murphy, D.J.; Walsh, N.G.; Conran, J.G.; Pires, J.C.; Macfarlane, T.D.; Birch, J.L. Evolution of Lomandroideae: Multiple origins of polyploidy and biome occupancy in Australia. Mol. Phylogenet. Evol. 2020, 149, 106836. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Ashman, T.L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An evolutionary and ecological force in stressful times. Plant Cell 2021, 33, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Ræbild, A.; Anamthawat-Jonsson, K.; Egertsdotter, U.; Immanen, J.; Jensen, A.M.; Koutouleas, A.; Martens, H.J.; Nieminen, K.; Olofsson, J.K.; Röper, A.C.; et al. Polyploidy—A tool in adapting trees to future climate changes? A review of polyploidy in trees. For. Ecol. Manag. 2024, 560, 121767. [Google Scholar] [CrossRef]
- Buggs, R.J.; Pannell, J.R. Ecological differentiation and diploid superiority across a moving ploidy contact zone. Evolution 2007, 61, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sumei, C.; Yu, C.; Zhiyong, G.; Dongmei, Y.; Fadi, C. In vitro induced tetraploid of Dendranthemanankingense (Nakai) Tzvel.shows an improved level of abiotic stress tolerance. Sci. Hortic. 2011, 127, 411–419. [Google Scholar] [CrossRef]
- Meng, H.; Jiang, S.; Hua, S.; Li, Y.; Guo, W.; Jiiang, L. Comparison Between a Tetraploid Turnip and Its Diploid Progenitor (Brassica rapa L.). Agric. Sci. China 2011, 10, 1671–2927. [Google Scholar] [CrossRef]
- Yan, K.; Wu, C.; Zhang, L.; Chen, X. Contrasting photosynthesis and photoinhibition in tetraploid and its autodiploid honeysuckle (Lonicera japonica Thunb.) under salt stress. Front. Plant Sci. 2015, 6, 227. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Liu, L.; Meng, F. Physiological and Proteomic Responses of Diploid and Tetraploid Black Locust (Robinia pseudoacacia L.) subjected to Salt Stress. Int. J. Mol. Sci. 2013, 14, 20299–20325. [Google Scholar] [CrossRef]
- Chao, D.-Y.; Dilkes, B.; Luo, H.; Douglas, A.; Yakubova, E.; Lahner, B.; Salt, D.E. Polyploids exhibit higher potassium uptake and salinity tolerance in Arabidopsis. Science 2013, 341, 658–659. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhao, S.; Lu, X.; He, N.; Gao, L.; Dou, J.; Bie, Z.; Liu, W. Genome duplication improves the resistance of watermelon root to salt stress. Plant Physiol. Biochem. 2018, 133, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Saleh, B.; Allario, T.; Dambier, D.; Ollitrault, P.; Morillon, R. Tetraploid citrus rootstocks are more tolerant to salt stress than diploid. Comptes Rendus Biol. 2008, 331, 703–710. [Google Scholar] [CrossRef]
- Mouhaya, W.; Allario, T.; Brumos, J.; Andrés, F.; Froelicher, Y.; Luro, F.; Talon, M.; Ollitrault, P.; Morillon, R. Sensitivity to high salinity in tetraploid citrus seedlings increases with water availability and correlates with expression of candidate genes. Funct. Plant Biol. 2010, 37, 674–685. [Google Scholar] [CrossRef]
- Sonneveld, B.G.J.S.; Keyzer, M.A.; Zikhali, P.; Merbis, M.D. National Land Degradation Assessment Senegal and Review of Global Socio-Economic Parameters in the LADA Data Base; Land Degradation Assessment Project, LADA Project Paper (Centre for World Food Projects (SOW-VU) Project Papers); Centre for World Food Studies: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Pandey, S.; Zhang, W.; Assmann, S.M. Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 2007, 581, 2325–2336. [Google Scholar] [CrossRef]
- Sambou, A.; Ndour, B.; Cheng, S.; Senghor, E. Ligneous species tolerance in acid sulphated and saline soils of sine saloum: Case of rural community of Djilass and Loul Secene. J. Sustain. Dev. 2010, 3, 174. [Google Scholar] [CrossRef]
- Fall, D.; Bakhoum, N.; Fall, F.; Diouf, F.; Ly, M.O.; Diouf, M.; Gully, D.; Hocher, V.; Diouf, D. Germination, growth and physiological responses of Senegalia senegal (L.) Britton, Vachellia seyal (Delile) P. Hurter and Prosopis juliflora (Swartz) DC to salinity stress in greenhouse conditions. Afr. J. Biot. 2016, 15, 37. [Google Scholar]
- Sarr, M.S.; Seiler, J.R.; Sullivan, J. Growth and physiology of Senegalia senegal (L.) Britton seedlings as influenced by seed origin and salinity and fertility treatments. Forests 2017, 8, 388. [Google Scholar] [CrossRef]
- Odee, D.W.; Wilson, J.; Omondi, S.; Perry, A.; Cavers, S. Rangewide ploidy variation and evolution in Acacia senegal: A north-south divide? AoB Plants 2015, 7, lv011. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.M.; Nielsen, L.R.; Hansen, J.K.; Ræbild, A.; Kjær, E.D. Study of quantitative genetics of gum arabic production complicated by variability in ploidy level of Acacia senegal (L.) Willd. Tree Genet. Genomes 2015, 11, 80–92. [Google Scholar] [CrossRef]
- Diallo, A.M.; Nielsen, L.R.; Kjær, E.D.; Petersen, K.K.; Ræbild, A. Polyploid can confer superiority to West African Acacia senegal (L.) Willd. trees. Front. Plant Sci. 2016, 7, 821. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.M.; Kjær, E.D.; Ræbild, A.; Nielsen, L.R. Coexistence of diploid and polyploid Acacia senegal (L.) Willd. and its implication for interploidy pollination. New For. 2022, 54, 67–82. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Hardikar, S.A.; Pandey, A.N. Growth, water status and nutrient accumulation of seedlings of Acacia senegal in response to soil salinity. Ann. Biol. 2008, 30, 17–28. [Google Scholar]
- Ruiz, M.; Pina, J.A.; Alcayde, E.; Morillon, R.; Navarro, L.; Millo, E.P. Behavior of diploid and tetraploid genotypes of ‘carrizo’ citrange under abiotic stress. Int. Soc. Hort. Sci. 2016, 1065, 1283–1292. [Google Scholar] [CrossRef]
- Lilles, E.B.; Purdy, B.G.; Macdonald, S.E.; Chang, S.X. Growth of aspen and white spruce on naturally saline sites in northern Alberta: Implications for development of boreal forest vegetation on reclaimed saline soils. Can. J. Soil Sci. 2012, 92, 213–227. [Google Scholar] [CrossRef]
- Zohar, Y.; Di Stefano, J.; Bartle, J. Strategy for screening eucalypts for saline lands. Agroforest. Syst. 2010, 78, 127–137. [Google Scholar] [CrossRef]
- Aroca, R. Plant Response to Drought Stress: From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1, pp. 1–33. [Google Scholar]
- Gning, F.; Jourdan, C.; Marone, D.; Ngom, D.; Ræbild, A. Root growth and biomass partitioning of nine juvenile Sahelian agroforestry tree species under drought and irrigation treatments. Plant Soil 2025. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plants responses and adaptation to soil salinity. Innovation 2020, 24, 100017. [Google Scholar] [CrossRef] [PubMed]
- Läuchli, A.; Epstein, E. Plant responses to saline and sodic conditions. In Agricultural Salinity Assessment and Management; Tanji, K.K., Ed.; ASCE Manuals and Reports on Engineering Practice; ASCE: New York, NY, USA, 2010; pp. 113–137. [Google Scholar]
- Levin, D.A. The Role of Chromosomal Change in Plant Evolution; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Ramsey, J.; Schemske, D.W. Neopolyploidy in flowering plants. Annu. Rev. Ecol. Syst. 2002, 33, 589–639. [Google Scholar] [CrossRef]
- Paterson, A.H.; Chapman, B.A.; Kissinger, J.C.; Bowers, J.E.; Feltus, F.A.; Estill, J.C. Many gene and domain families have convergent fates following independent whole-genome duplication events in Arabidopsis, Oryza, Saccharomyces and Tetraodon. Trends. Genet. 2006, 22, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Alix, K.; Gérard, P.R.; Schwarzacher, T.; Heslop-Harrison, J.S. Polyploidy and interspecific hybridization: Partners for adaptation, speciation and evolution in plants. Ann. Bot. 2017, 120, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Hojsgaard, D.; Hörandl, E. The rise of apomixis in natural plant populations. Front. Plant Sci. 2019, 10, 358. [Google Scholar] [CrossRef] [PubMed]

| Sites | N | Freq. Diploid (%) | Freq. Polyploid (%) | Latitude | Longitude | Annual Rainfall (mm) | Electric Conductivity (µS/cm) | pH Water | pH KCl |
|---|---|---|---|---|---|---|---|---|---|
| Kamb | 30 | 67 | 33 | 15°31′32″ N | 15°26′06″ W | 400 | 36.67 | 6.4 | 5.7 |
| Diery | 30 | 77 | 23 | 15°23′47″ N | 15°22′54″ W | 408 | 14.75 | 6.1 | 4.9 |
| Gniby | 28 | 100 | 0 | 14°26′11″ N | 15°38′07″ W | 555 | 25.65 | 6.9 | 5.8 |
| Velor | 30 | 43 | 57 | 14°03′26″ N | 16°15′25″ W | 636 | 802 | 6.3 | 5.9 |
| Ngane | 25 | 30 | 70 | 14°12′06″ N | 16°12′09″ W | 608 | 1909 | 6.2 | 5.8 |
| Pete | 30 | 100 | 0 | 16°03′02″ N | 14°01′04″ W | 298 | 24.3 | 6.6 | 5.6 |
| Ourouss | 30 | 100 | 0 | 15°34′56″ N | 13°21′54″ W | 400 | 19.75 | 6.7 | 5.8 |
| Semme | 30 | 93 | 7 | 15°11′00″ N | 12°56′50″ W | 486 | 20.99 | 6.4 | 5.3 |
| Bakel | 30 | 33 | 67 | 14°52′16″ N | 12°29′20″ W | 535 | 19.96 | 6.0 | 4.9 |
| Seoudji | 30 | 100 | 0 | 14°19′46″ N | 12°26′43″ W | 655 | 27.69 | 6.2 | 5.2 |
| Traits | Slope | SE | t-Value | Pr > t |
|---|---|---|---|---|
| Longitude | 0.62 | 0.66 | 0.95 | 0.37 |
| Latitude | 0.19 | 0.9 | 0.21 | 0.84 |
| pH water | −71 | 32 | −2.2 | 0.06 |
| pH KCl | −3 | 30 | −0.1 | 0.93 |
| log(EC) | 32 | 10 | 3 | 0.02 |
| Rainfall | 0.13 | 0.08 | 1.6 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diallo, A.M.; Kjær, E.D.; Nielsen, L.R.; Ræbild, A. Polyploid Advantage? Comparing Salt Stress Responses of Di- and Tetraploid Acacia senegal (L.) Willd. Seedlings. Forests 2025, 16, 412. https://doi.org/10.3390/f16030412
Diallo AM, Kjær ED, Nielsen LR, Ræbild A. Polyploid Advantage? Comparing Salt Stress Responses of Di- and Tetraploid Acacia senegal (L.) Willd. Seedlings. Forests. 2025; 16(3):412. https://doi.org/10.3390/f16030412
Chicago/Turabian StyleDiallo, Adja Madjiguene, Erik Dahl Kjær, Lene Rostgaard Nielsen, and Anders Ræbild. 2025. "Polyploid Advantage? Comparing Salt Stress Responses of Di- and Tetraploid Acacia senegal (L.) Willd. Seedlings" Forests 16, no. 3: 412. https://doi.org/10.3390/f16030412
APA StyleDiallo, A. M., Kjær, E. D., Nielsen, L. R., & Ræbild, A. (2025). Polyploid Advantage? Comparing Salt Stress Responses of Di- and Tetraploid Acacia senegal (L.) Willd. Seedlings. Forests, 16(3), 412. https://doi.org/10.3390/f16030412







