Abstract
In this study, a simple one-step blend of isolated soy protein and sucrose was used directly as a wood adhesive for plywood manufacturing. The bonding performance, water resistance, curing performance, and thermal stability of the adhesive were evaluated. The preparation process of the plywood was optimized and the curing mechanism was also investigated. The results demonstrate the following: (1) Sucrose was successfully converted into furan compounds, especially 5-hydroxymethylfurfural (5-HMF), which underwent a sufficient cross-linking reaction with the SPI, and this was the key during the curing of the adhesive. (2) The effect of hot-pressing temperature on the bonding performances was the most significant and played a key role in the success of the test, followed by hot-pressing time, solid content, and adhesive loading. (3) In this study, 200 °C was the critical point at which the adhesive obtained good wet bonding strength and was also the critical temperature at which the effective conversion of sucrose into 5-HMF occurred. (4) The optimum preparation parameters of plywood were a hot-pressing temperature of 216 °C, a hot-pressing time of 1 min/mm, a solid content of 50%, and adhesive consumption of 220 g/m2. Using this process, a bonding strength in warm water of 1.74 MPa, a bonding strength in boiling water of 1.50 MPa, and a wood failure rate of more than 80% were obtained for the plywood.
1. Introduction
Environmental protection is a common theme that has been adopted by the international community in the 21st century, and so the development of environmentally friendly materials is also an inevitable trend. With the increase in people’s awareness regarding environmental protection, the depletion of non-renewable fossil fuel resources around the world, the prominent environmental issues caused by synthetic resins, and their price and other factors, the shift from petrochemical resources to renewable resources has become the most important trend within chemical synthesis today. Therefore, the wood adhesive industry is increasingly looking to use biomass materials to prepare green and environmentally friendly wood adhesives [1,2,3,4,5,6,7]. Proteins are an important type of biological macromolecule, and among them soy protein has the characteristics of widespread sources, high reproducibility, and high reactivity, so the preparation of wood adhesive using soy protein as a raw material has attracted great attention. First produced in the 1920s and 1930s, the soy protein adhesive invented by Davidon and Laucks displayed excellent performance for nearly half a century, and had a profound impact on the production of wood-based panels, especially those consisting of plywood. Since the emergence of the oil crisis, under the double pressure of material shortages and increasingly prominent environmental problems, research on soy protein adhesives gained prominence again. Scholars have carried out extensive and in-depth research on soy protein adhesives, and numerous innovative outcomes have emerged [8,9,10,11,12,13,14,15].
There are lots of studies on the use of synthetic resins as the cross-linkers of soy protein adhesives. By cross-linking with the side group of soy protein, synthetic resins can improve the wet shear strength of soy protein adhesives. Formaldehyde-based adhesives such as urea–formaldehyde resin, melamine–formaldehyde resin, and phenol–formaldehyde resin were initially used as the main cross-linkers, and the prepared soy protein adhesives displayed good bonding strength and water resistance [16,17,18,19,20]. However, the most important feature of soy protein adhesives is their green and environmentally friendly nature, and the introduction of toxic formaldehyde greatly reduces this environmental benefit. This represents the pursuit of superior performance at the expense of the environmental advantages of these adhesives, which is not conducive to the development of the soy protein adhesive industry.
In order to prepare environmentally friendly soy protein adhesives, the environmental impact of the used cross-linkers is very important. Epoxides can be used as the active curing agents of soy protein adhesives that do not release toxic substances. Using epoxides as cross-linkers to modify soy protein adhesives has become the focus of research, mainly focusing on epoxy resin–maleic anhydride, polyamide polyamine-epichlorohydrin, glyceryl ether, and so on. The mechanical properties and water resistance of those modified soy protein adhesives have greatly improved, and they have good biodegradability [21,22,23,24]. In addition, polyvinyl acetate, isocyanate, and melamine glyoxal resins also have similar performances and environmental characteristics to soy protein adhesives modified with epoxy resins, and some of these soy protein adhesives have been industrialized in their production and application [25,26,27,28]. However, these cross-linkers are still based on petrochemicals, and their large-scale introduction obviously violates the original intentions of the development of soy protein adhesives, meaning that the environmental protection benefits are greatly reduced and they cannot be referred to as environmentally friendly soy protein adhesives in the truest sense.
Zhao reported a tannin–sucrose adhesive for the preparation of particle board, and they found that the successful preparation of the adhesive was due to the reaction between tannin and hydroxymethylfurfural derived from sucrose [29,30,31]. The synthesis mechanism of glucose–phenol resin, glucose–melamine resin and fructose–resorcinol resin also involved sugar participating in the polycondensation reaction in the form of 5-hydroxymethylfurfural (5-HMF) [32,33,34,35]. The efficient cross-linking reaction between formaldehyde-based adhesives and soy protein is driven by that fact that formaldehyde can react with active groups in protein molecules such as the hydroxyl group, the carboxyl group, and the amino group. 5-HMF, similarly to formaldehyde, can also react with soy protein [18,19,20,36]. Moreover, compared with formaldehyde, 5-HMF comes from sugar and is safer and more environmentally friendly.
In this study, soy protein and sucrose were used as a fully biomass-based adhesive for preparing plywood. The influence of the preparation methods on the bonding performance was evaluated and a suitable preparation process for plywood was determined.
2. Materials and Methods
2.1. Materials
Soy protein isolate (SPI) with a protein content of 90% was purchased from Shandong Gushen Biotechnology Group Co., Ltd. (Dezhou, China). Sucrose with a purity of 99.0% was purchased from Jinshan Chemical Reagent Co., Ltd. (Chengdu, China). A poplar veneer with a moisture content of 8%–10% and dimensions of 400 mm (length) × 400 mm (width) × 2.0 mm (thickness) was obtained from Shuyang, Jiangsu, China.
2.2. Preparation of SPI–Sucrose Adhesive and the Testing of Shear Strength
At room temperature, distilled water was discharged into a round-bottomed triangular flask containing a stirring rod, a thermometer, and a condensing tube. The temperature was increased to 70 °C and different amounts of sucrose and 0.3 g of sodium dodecylbenzene sulfonate were added and stirred for 5 min, and then different amounts of SPI were added gradually and stirred for 45 min. The specific material addition scheme is shown in Table 1. The solid content, viscosity, and pH of adhesives were determined according to GB/T 14074-2017 [37].

Table 1.
The formulation of SPI–sucrose adhesives.
Three-layer poplar plywood was prepared in the lab. The adhesive was coated onto the veneer with a loading of 220 g/m2. After assembling the plywood, it was pressed at room temperature for 10 min and then placed in a drying oven at 60 °C for 1 h. The hot-pressing process involved a temperature of 220 °C, a pressure of 1.2 MPa, and a time of 5 min. The dry and wet bonding strength and wood failure rate were tested according to GB/T17657-2022 [38], and the reported strength was the mean value of 12 samples.
2.3. Orthogonal Experiment
Based on the previous analysis, we fixed the mass ratio of m(SPI)/m(sucrose) at 20:80, and the hot-pressing temperature, hot-pressing time, adhesive loading, and solid content were taken as the four factors in the test, while the water resistance bonding strength was taken as the consideration index. This test was designed according to orthogonal table L16 (44) (Table 2).

Table 2.
Orthogonal experiment design.
2.4. Test of Insoluble Content of Cured Adhesive
A certain amount of each SPI–sucrose adhesive was wrapped in aluminum foil and placed in a drying oven at about 70 °C until the mass was constant. An approximately 2.0 g sample was taken out to dry at 220 °C for 15 min, and then it was removed and ground into powder. A specific weight of powder was wrapped in filter paper and soaked in water at 63 °C for 6 h, and then it was dried at 120 °C until the mass was constant. The insoluble content was calculated according to the change in mass and the reported insoluble content was the mean value of 6 samples.
2.5. Differential Scanning Calorimetry (DSC) Analysis
Before the test, the adhesive samples were frozen and dried. The DSC 204 F1 differential scanning calorimeter produced by NETZSCH in Selb, Germany was used to test the curing performance of the SPI–sucrose adhesives. The adhesives were tested under the following conditions: N2 protection, temperature range 30–300 °C, and a heating rate of 10 °C/min.
2.6. Thermogravimetry (TG) Analysis
The TG 209 F3 thermogravimeter made by NETZSCH in Germany was used to test the thermal stability of the SPI–sucrose adhesives. The cured adhesives were tested under the following conditions: N2 protection; temperature range: 30–700 °C; heating rate: 10 °C/min.
2.7. X-Ray Diffraction (XRD) Analysis
The crystallization properties of the cured adhesive were tested using a TTR XRD (Rigaku Corporation, Tokyo, Japan). The test was carried out under the following conditions: Cu target (λ = 0.154060 nm), 5~90° scanning interval, 0.02° step size, 5 °/min scanning rate, 120 mA tube current, and 40 kV tube voltage.
2.8. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
The structural characteristics of the adhesive were analyzed using a Varian 1000 (Varian, Palo Alto, CA, USA) infrared spectrometer under the following conditions: wave number range: 400~4000 cm−1; resolution: 4 cm−1; number of scans: 32; room temperature: 22~25 °C; relative humidity: ≤60%.
3. Results and Discussion
3.1. Feasibility of SPI–Sucrose as a Wood Adhesive
The solid content of the SPI–sucrose composite adhesive presented in this study is 50%, and the effects of the mass ratio on the bonding properties of the adhesive are shown in Figure 1. Soy protein has a complex tertiary structure, including an α-helix, β-fold, and random curling, and non-covalent bonds in these structures (such as hydrogen, ionic, hydrophobic, and van der Waals forces) are essential for maintaining the stability and shape of the protein [10,11,12,13]. When the concentration of the soy protein solution is high, the distance between protein molecules is shortened, and the non-covalent bond interactions between them become stronger, making it easier for protein molecules to form network structures or polymers, thereby increasing the viscosity [14,39]. In addition, hydrogen bonds or other types of interactions forming between protein molecules and water molecules can restrict the movement of water molecules, thereby increasing the viscosity of the solution. Therefore, as shown in Figure 1a, when the mass ratio of SPI to sucrose is 100:0, 80:20, and 60:40 (with a higher protein concentration), the viscosity is excessively high, nearly exceeding the measurable range of the instrument. Meanwhile, when the mass ratio is 50:50, 40:60, 30:70, and 20:80, the viscosity decreases to 6428, 3405, 1750, and 683 mPa·s, respectively. This indicates that sucrose has an obvious reducing effect on the viscosity of the adhesive. Due to this high viscosity, the solid contents of soy protein adhesives did not exceed 30% in many previous studies [39]. However, due to the viscosity-reducing effect of sucrose, the solid content of the prepared SPI–sucrose adhesives in this work reaches 50% or even higher.
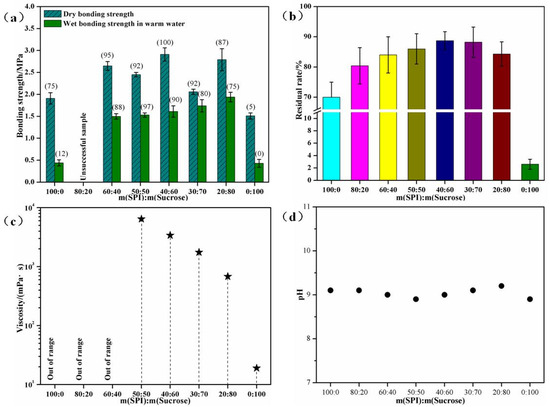
Figure 1.
Bonding performances of SPI–sucrose adhesives. (a) Bonding strength; (b) insoluble contents; (c) viscosity; and (d) pH of cured adhesives. Note: The data in parentheses indicate the wood failure rate.
The molecular weight of SPI is high, and it has a high cohesive strength, meaning that the plywood made using pure SPI has a low wet bonding strength of 0.44 MPa, but the wood failure rate is only 12% due to excessive viscosity. Sucrose has a low molecular weight and low cohesive strength, but the plywood made using pure sucrose achieves a wet bonding strength of 0.43 MPa, considering that other authors have proven that in such conditions, 5-HMF may react with the -OH groups of wood [29,30,31], and it is assumed that part of the strength of the bond is due to this interaction. However, the wood failure rate is almost zero. Compared with pure SPI, the dry bonding strength, wet bonding strength, and wood failure rate of the composite SPI–sucrose adhesive are significantly increased, especially the wet strength, which improves gradually with the addition of sucrose. This is because the more sucrose is added, the more 5-HMF is produced during the hot-pressing process. As the cross-linking degree between 5-HMF and SPI increases, the cured adhesive exhibits a high cross-linking density and high cohesive strength. Macroscopically, it is characterized by high water resistance and a high wood failure rate.
As shown in Figure 1c, with the adding of sucrose, the insoluble content of the cured composite SPI–sucrose adhesive first gradually increases and then decreases, but it remains significantly higher than that of pure SPI on the whole. This further confirms the cross-linking reaction between SPI and sucrose. In addition, Figure 1d shows that the SPI–sucrose composite adhesive has a pH of around 9.0, meaning that compared with protein adhesives prepared using a strong alkali and a strong acid, it will not cause alkali damage to wood surfaces.
3.2. Analysis of Curing Mechanism
As can be seen in Figure 2a–d, the two peaks at 2θ = 9.1° and 2θ = 19.4° represent the α-helix and β-folding in the structure of the soy protein, respectively. After the reaction between SPI and sucrose, the characteristic peak of the α-helix becomes significantly broader, indicating a change in the secondary structure of the protein molecules. As the curing temperature increases, this peak becomes wider, indicating that the secondary structure of the protein molecules is further disrupted. This helps to expose the active sites within the globulin, so as to provide more reaction sites for the SPI and sucrose [40,41]. The SPI–sucrose adhesive exhibited a new crystallization peak at 18.8°, indicating the cross-linking reaction between SPI and sucrose. With the increase in curing temperature, the cross-linking degree rises further, thus resulting in a higher crystallinity. The greater molecular weight of SPI and the longer molecular chain after degradation were conducive to stabilizing the overall toughness of the SPI–sucrose adhesive system [39] and avoiding the increase in the brittleness of the cured adhesive caused by high hot-pressing temperature. Therefore, as shown in Figure 2a,d, curing temperatures of 180 and 210 °C are suitable. However, when the curing temperature is 230 °C, the cross-linking degree is too high, thus hindering the directional arrangement of molecules and causing a decrease in crystallinity. Additionally, the high degree of cross-linking causes internal stress to accumulate, resulting in the formation of some voids in the layer of cured adhesive, as seen in Figure 2c.
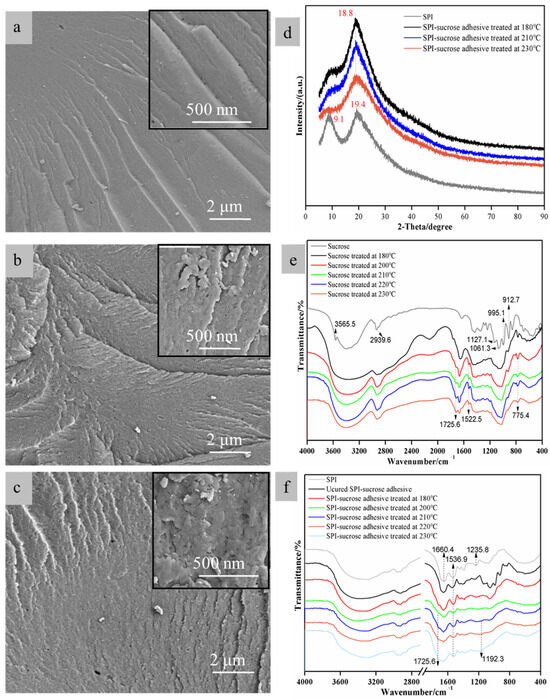
Figure 2.
(a–c) SEM images of the powder of SPI–sucrose adhesives cured at 180, 210 and 230 °C; (d) XRD curves of SPI–sucrose adhesives; (e) FTIR curves of sucrose treated at different temperatures; (f) FTIR curves SPI–sucrose adhesives cured at different temperatures.
As shown in Figure 2e, the peak at 3565.5 cm−1 represents the stretching vibration of free hydroxyl groups in the sucrose molecule. The peak at 2939.6 cm−1 is attributed to the stretching vibration of the methylene group (-CH2-) in the sucrose molecule. The peaks at 1127.1 and 995.1 cm−1 represent the stretching vibrations peak of C-O-C bonds in the sucrose (including glycosidic bonds and glucose ring ether bonds). The peak at 1061.3 cm−1 represents the absorption of hydroxymethyl groups in sucrose, and that at 912.7 cm−1 is related to the stretching vibration of the C-O-C bond in the pyranose ring of sucrose (fructose ring ether bond). After the heat treatment, the absorption peaks at 1127.1 and 995.1 cm−1 nearly disappear, while new absorption peaks appear at 1522.5 and 775.4 cm−1 (C=C and the unsubstituted CH=CH on the furan ring). The results show that sucrose is depolymerized and transformed into furan compounds. At temperatures starting from 200 °C, the peak at 912.7 cm−1 nearly disappears, showing that sucrose degradation is further enhanced. A new peak of the aldehyde C=O group appears at 1725.6 cm−1, and the strength of the peaks at 1725.6 and 1522.5 cm−1 increases with the increase in the heat-treatment temperature, suggesting the formation of furfural or 5-HMF in the product of the cured adhesive. As shown in Scheme 1, sucrose is a disaccharide formed by one molecule of glucose and one molecule of fructose through the 1, 2-glucoside bond, resulting in dehydration, thus producing one molecule of fructose and one molecule of glucose. Glucose is further isomerized to fructose, while fructose is further dehydrated to form 5-HMF [36,42].
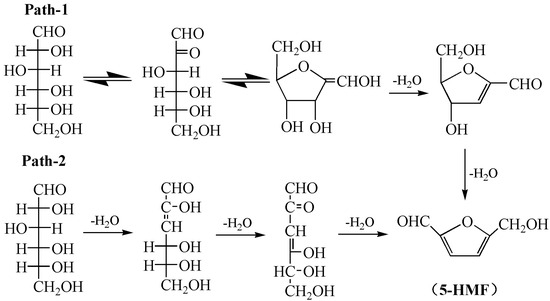
Scheme 1.
Conversion mechanism of sucrose to 5-hydroxymethylfurfural.
As shown in Figure 2f, the C=O stretching vibration of the amide bonds from the soy protein is represented by the peak at 1660.4 cm−1. The peak at 1536.9 cm−1 is a coupling peak representing the N-H bending vibration and C-N stretching vibration of the amide bonds from the soy protein, and the C-N stretching vibration from the amide bonds is presented by the peak at 1235.8 cm−1. The cured product of SPI–sucrose shows a significant decrease in the strength of its peaks at 1725.6 and 1536.9 cm−1, and the peak at 1725.6 cm−1 almost disappears with the increase in curing temperature, indicating that there is a cross-linking reaction between the furan aldehyde group and the amino group of soy protein. In addition, the peak at 775.4 cm−1 disappears, and a new absorption peak appears at 1192.3 cm−1 when the curing temperature is 210~230 °C. This peak is considered to represent a dimethylene ether bond, which may be related to the polycondensation of 5-HMF. 5-HMF and formaldehyde or formaldehyde-based polymers are similar in their chemical structure and can also react with active functional groups in the protein molecules, such as the hydroxyl, carboxyl, and amino groups. However, 5-HMF derived from biomass sugars is more environmentally friendly, and compared to furfural or furfuryl alcohol, 5-HMF can easily form branched structures during co-condensation reactions with soy protein, meaning that it is more conducive to curing into an insoluble and infusible three-dimensional network structure. In addition, DA addition reactions can also occur between 5-HMF furan rings or between double bonds in proteins and furan rings to form overlapping ring structures [39], thus increasing the number of polycondensation reaction sites and further enhancing the three-dimensional network structure of the cured adhesive (Scheme 2).
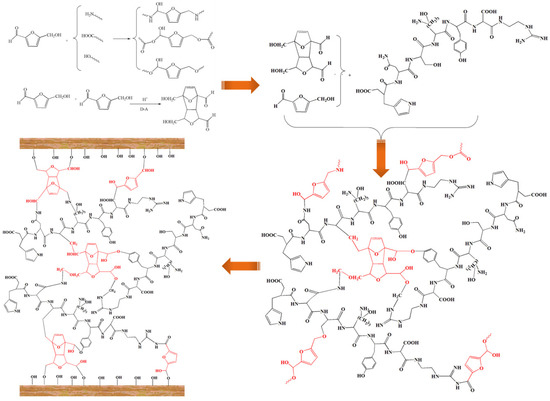
Scheme 2.
Diagram of soy protein adhesive cross-linked with 5-hydroxymethylfurfural.
3.3. Effect of Preparation Parameters on the Bonding Performance of SPI–Sucrose Adhesive
Figure 3a illustrates the relationship between solid content and viscosity for an SPI–sucrose adhesive with a mass ratio of m(SPI)/m(sucrose) of 20:80SPI–sucrose adhesive. The viscosity increases gradually with the increase in solid content. When the solid content ranges from 40% to 70%, the viscosity increases from 2922 to 32,180 mPa·s, and the viscosity increases by more than 10 times. Figure 3b shows that when the solid content is 40% and 50% based on a mass ratio of m(SPI)/m(sucrose) of 20:80, the wet shear strength is 1.61 and 1.74 MPa, and the wood failure rate is 73% and 90%, respectively. However, when the solid content increases to 60% and 70%, the wet bonding strength decreases to 1.27 and 0.90 MPa, and the wood failure rate decreases to 32% and 10%, respectively. This is because the higher the solid content is, the greater the viscosity and the poorer the fluidity and wettability. This not only affects the use of the adhesive, but also causes the uneven distribution of the adhesive during hot-pressing, which further affects the bonding performance. However, the solid content cannot be too low, otherwise there may be a lack of glue on the bonding surface, thus resulting in poor bonding stability. The solid content is generally above 35%. During the curing process, more adhesive bonds are formed on the bonding surface of plywood, and the shear strength and water resistance are also enhanced, so as to obtain stable bonding properties [43]. Therefore, a solid content of 40%~50% is suitable for preparing the SPI–sucrose adhesive presented in this work.
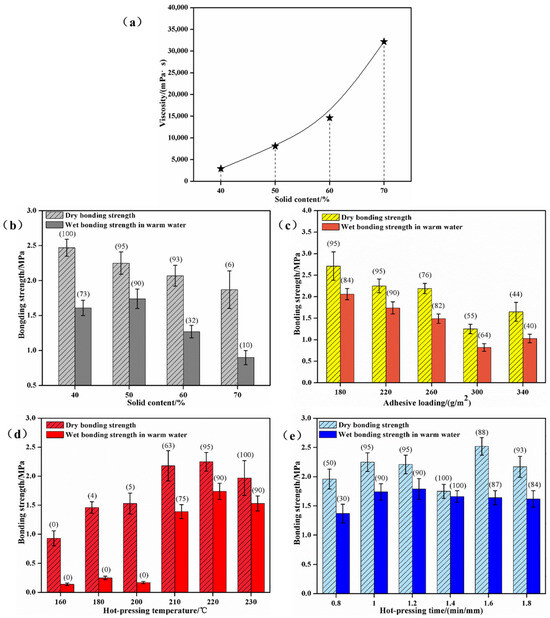
Figure 3.
Effects of preparation parameters on bonding performances. (a) The relationship between the viscosity and solid content of the adhesive; (b) the relationship between the bonding strength and solid content of the adhesive; (c) the relationship between the bonding strength and adhesive loading; the effects of hot-pressing temperature (d) and hot-pressing time (e) on the bonding strength. Note: The data in parentheses represent the wood failure rate.
When the solid content of SPI–sucrose composite adhesive is 50% and a mass ratio of m(SPI)/m(sucrose) is 20:80, the effects of adhesive loading on the bonding strength are shown in Figure 3c. As seen from Figure 3c, when the adhesive loading increases from 180 to 340 g/m2, the dry bonding strength, wet bonding strength, and wood failure show a decreasing trend in general. When the adhesive loading ranges from 260~340 g/m2, the declining trend in bonding performance is slower than that when the adhesive loading is 180~220 g/m2. The main reason for this is that using more adhesive increases the layer thickness of the cured adhesive, which enhances the inner stress of the cured adhesive and causes it to produce a weak interface layer on the bonding interface.
Based on the results and analysis presented in Figure 3c, with a fixed hot-pressing time of 5 min, adhesive loading of 220 g/m2, and a pressure of 1.2 MPa, the effects of hot-pressing temperature on the bonding strength are shown in Figure 3d. When the hot-pressing temperature is 160 °C, 180 °C and 200 °C, the wet bonding strength is low and no effective wood failure can be observed, while the strength is 0.15, 0.25, and 0.17 MPa, respectively, which does not meet the requirements of Type II plywood in GB/T17657-2022. When the hot-pressing temperature is 210 °C, 220 °C, and 230 °C, the wet bonding strength is significantly improved to 1.39, 1.74, and 1.53 MPa, respectively, while the wood failure rate is also increased to 75%, 90%, and 90%, respectively. It is obvious that the hot-pressing temperature plays a decisive role in the success of this test. A hot-pressing temperature of 200 °C is the critical point at which the adhesive obtains good wet bonding strength, which also indirectly indicates that 200 °C is the critical temperature at which effective conversion of sucrose into 5-HMF occurs under the conditions used in this experiment. The adhesive performance is best at a hot-pressing temperature of 220 °C, while it begins to decline when the hot-pressing temperature is 230 °C. Although increasing the hot-pressing temperature could further promote the formation of 5-HMF, there is also a competitive relationship between the improvement in the bonding performance via cross-linking reactions between 5-HMF and SPI and the decline in the bonding performance through wood degradation. The higher the temperature is, the more the wood degrades, and then the bonding strength decreases rapidly. Therefore, the SPI–sucrose adhesive prepared with a hot-pressing temperature of 200~220 °C is the most suitable.
Based on the results and analysis presented in Figure 3d, with a fixed hot-pressing temperature of 220 °C, adhesive loading of 220 g/m2, and a pressure of 1.2 MPa, the effects of hot-pressing times on the bonding strength are shown in Figure 3e. It can be seen from Figure 3e that the wet shear bonding strength of the plywood reaches its maximum when the pressing time is 5 min. The wet bonding strength decreases when the pressing time continues to be prolonged, thus showing that the curing reaction is completed within 1 min/mm. Combined with Figure 3e,d, the hot-pressing time is found to have little effect on the bonding performance under an effective hot-pressing temperature.
3.4. Results Analysis of the Orthogonal Test
The experiment was designed according to orthogonal table L16(44) with the bonding strength in warm water and bonding strength in boiling water as the assessment indexes, and the results of the orthogonal test are presented in Table 3 and Table 4.

Table 3.
Range analysis of orthogonal experiment results.

Table 4.
Analysis of variance of orthogonal test results.
As seen from Table 2 and Table 3 and Figure 4, the influence of each factor in the orthogonal experiment on the bonding performance is as follows: pressing temperature > pressing time > solid content > adhesive loading. The influence of hot-pressing temperature is the most significant, followed by hot-pressing time, solid content, and adhesive loading, which has no significant influence. The suitable preparing parameters for plywood are a pressing temperature of 216 °C, a pressing time of 5 min, a solid content of 50%, and adhesive loading of 220 g/m2. Under this condition, the plywood has a bonding strength in warm water of 1.74 MPa, a bonding strength in boiling water of 1.50 MPa, and a wood failure rate of more than 80%.
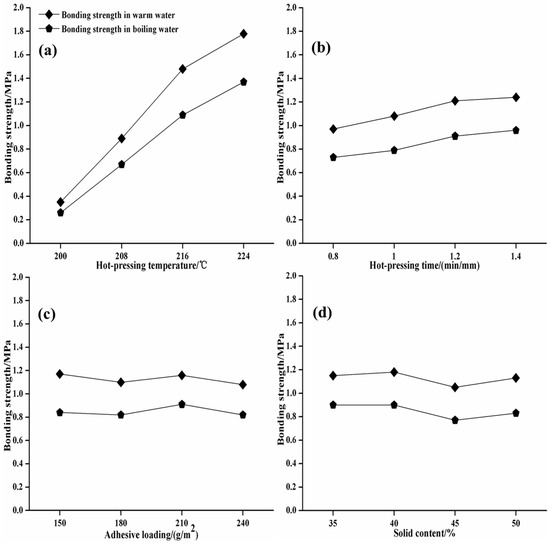
Figure 4.
Effects of hot-pressing temperature (a), hot-pressing time (b), adhesive loading (c) and solid content on the shear strength of the SPI–sucrose adhesive based on orthogonal tests (d).
3.5. Analysis of Curing Performance
As seen in Figure 5a, the cross-linking peak temperature of the SPI–sucrose adhesive presented in this study is 196.4 °C. Figure 5b shows that when the curing temperature ranges from 180 °C to 200 °C, the insoluble content increases by 47.1%, from 22.9% to 70.0%. When the curing temperature is 210 °C, 220 °C, and 230 °C, the insoluble content increases to 77.1%, 84.3%, and 80.8%, respectively. These results further confirm that the critical point at which the adhesive obtains good wet bonding strength and the critical temperature at which the effective conversion of sucrose to 5-HMF occurs is about 200 °C. As shown in Figure 5c, when the curing temperature is maintained at 220 °C and the curing time ranges from 1 to 5 min, the insoluble content is only 18.0%~47.1%. When the curing time is increased to 7–15 min, the insoluble content increases to 80.4%~86.0%. This indicates that the effective cross-linking of 5-HMF and SPI is the result of the comprehensive effect of the hot-pressing temperature and hot-pressing time. While still maintaining the hot-pressing temperature above the critical threshold, using a lower pressing temperature and extending the pressing time can also obtain good bonding performance.
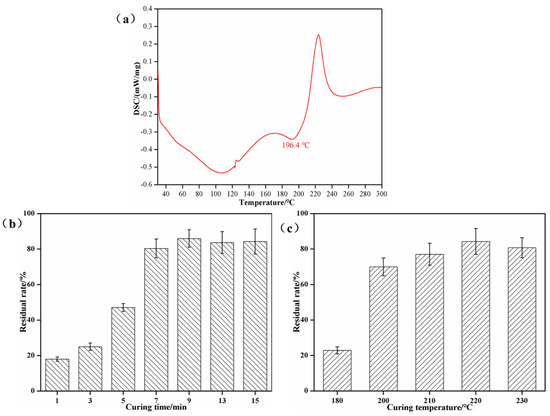
Figure 5.
Curing performance of SPI–sucrose adhesives. (a) DSC curve of SPI–sucrose adhesives; (b) effects of curing temperature on the insoluble contents of cured adhesives; (c) effects of curing time on the insoluble contents of cured adhesives.
To sum up, the SPI–sucrose adhesive presented in this study requires a higher curing temperature to obtain a satisfactory bonding strength. Therefore, reducing the reaction temperature to achieve high conversion of sucrose to 5-HMF is a potential point of focus for subsequent studies. However, sucrose molecules have many oxygen-containing groups and strong electronegativity and can easily adsorb H+ in aqueous solutions [44], thus affecting the effective conversion of sucrose. The following three comments deserve further consideration [45,46,47]. (1) As a new solvent, ionic liquid can promote the isomerization and dehydration of sugar to some extent and can play the role of catalyst and solvent at the same time. (2) The process of the preparation of 5-HMF catalyzed by sucrose can be regarded as consisting of protonation, dehydration, deprotonation, and other elementary reactions. The common acidic catalysts inevitably continue to react with 5-HMF, resulting in insufficient conversion reduction. Bronsted acid is beneficial to the protonation reaction and has a better catalytic effect on fructose. (3) Lewis acid is more favorable for dehydration and deprotonation, and has a good effect on the isomerization of glucose. Moreover, Lewis acid has a stable effect on 5-HMF, and can inhibit the hydration decarboxylation of 5-HMF to a certain extent, thus improving the selectivity and yield of 5-HMF.
3.6. Analysis of Thermal Stability
The results of TG-DTG of SPI–sucrose adhesives are shown in Figure 6. The mass loss of the adhesive is less than 10% at 30~200 °C, which is due to the evaporation of moisture from the adhesive. Starting from 200 °C, the adhesive experiences great weight loss. When the curing temperature is 180 °C, 210 °C, and 230 °C, the temperature at which the maximum weight loss of the cured product of the adhesive occurs is 315.3 °C, 318.4 °C, and 322.7 °C, respectively, showing that increasing the curing temperature improves the thermal stability of the cured adhesive. When the temperature is 700 °C, the residual weight is 34.6%, 36.9% and 40.7%, respectively. Combined with the analysis results of the curing mechanism, it is further demonstrated that the curing temperature has a crucial influence on the degree of cross-linking and the thermal stability of the cured adhesive.
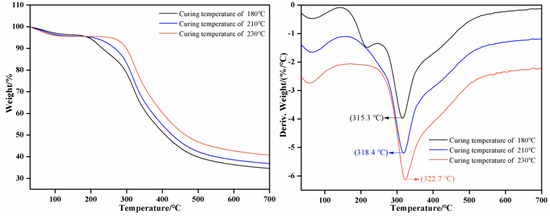
Figure 6.
TG-DTG results of the SPI–sucrose adhesives.
4. Conclusions
In this study, a simple blend of SPI and sucrose was used directly as a wood adhesive to explore its feasibility as a wood adhesive. The results show the following:
- (1)
- The hot-pressing temperature plays a decisive role in the success for the experiment. A temperature of 200 °C was the critical point at which the adhesive obtained good wet bonding strength and was also the critical temperature at which the effective conversion of sucrose into 5-HMF occurred in this experiment.
- (2)
- The optimum preparation parameters of plywood were a hot-pressing temperature of 216 °C, a hot-pressing time of 5 min, a solid content of 50%, and adhesive loading of 220 g/m2. Under these conditions, the plywood had a bonding strength in warm water of 1.74 MPa, a bonding strength in boiling water of 1.50 MPa, and a wood failure rate of more than 80%.
- (3)
- In future studies, ionic solutions, Bronsted acid, and Lewis acid can be used to further reduce the curing temperature of SPI–sucrose adhesives.
Author Contributions
Validation, investigation, resources, L.W., W.G., D.L., H.Y., Q.Y., H.L., C.C., T.M., X.Y., X.H. and Y.Y.; conceptualization, methodology, writing—original draft preparation, L.W., W.G. and D.L.; formal analysis, writing—review and editing, supervision, Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (32160348 and 32160346), the Outstanding Youth Science and Technology Talent Project of Guizhou Province, China (YQK [2023]003), the Agriculture Joint Research Program of Yunnan Province (2017FG001(-079)), and the International Joint Research Center for Biomass Materials Open Fund (2023-GH03 and 2023-GH05).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Z.; Zhao, S.; Pang, H.; Zhang, W.; Zhang, S.; Li, J. Developing eco-friendly high-strength soy adhesives with improved ductility through multiphase core–shell hyperbranched polysiloxane. ACS Sustain. Chem. Eng. 2019, 7, 7784–7794. [Google Scholar] [CrossRef]
- Kumar, C.; Leggate, W. An overview of bio-adhesives for engineered wood products. Int. J. Adhes. Adhes. 2022, 118, 103187. [Google Scholar] [CrossRef]
- Todorovic, T.; Norstrom, E.; Khabbaz, F.; Brucher, J.; Malmstrom, E.; Fogelstrom, L. A fully bio-based wood adhesive valorising hemicellulose-rich sidestreams from the pulp industry. Green Chem. 2021, 23, 3322–3333. [Google Scholar] [CrossRef]
- Arias, A.; Gonzalez-Rodriguez, S.; Barros, M.; Salvador, R.; Francisco, A.; Moro Piekarski, C.; Moreira, M. Recent developments in bio-based adhesives from renewable natural resources. J. Clean. Prod. 2021, 314, 127892. [Google Scholar] [CrossRef]
- Ndiwe, B.; Pizzi, A.; Raïdandi, D.; Béda, T.; Konaï, N.; Amirou, S. Particleboard bonded with bio-hardeners of tannin adhesives. Eur. J. Wood Wood Prod. 2019, 77, 1221–1223. [Google Scholar] [CrossRef]
- Lin, H.; Chen, X.; Lei, H.; Zhou, X.; Du, G.; Essawy, H.; Xi, X.; Hou, D.; Song, J.; Cao, M. Synthesis and characterization of a bio-aldehyde-based lignin adhesive with desirable water resistance. Int. J. Biol. Macromol. 2024, 264, 130020. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, C.; Wang, Q.; Tan, H.; Zhang, Y. Preparation of glycidyl methacrylate grafted starch adhesive to apply in high-performance and environment-friendly plywood. Int. J. Biol. Macromol. 2022, 194, 954–961. [Google Scholar] [CrossRef]
- Kallakas, H.; Plaza, N.; Crooks, C.; Turner, D.; Gargulak, M.; Arvanitis, M.A.; Frihart, C.R.; Hunt, C.G. Effect of Protein Surface Hydrophobicity and Surface Amines on Soy Adhesive Strength. Polymers 2024, 16, 202. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, W.; Xu, P.; Cai, X.; Dong, W.; Chen, M.; Du, M.; Liu, T.; Lemstra, P.; Ma, P. The bonding strength, water resistance and flame retardancy of soy protein-based adhesive by incorporating tailor-made core–shell nanohybrid compounds. Chem. Eng. J. 2022, 428, 132390. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Wang, Z.; Chen, H.; Fan, D. Renewable bio-based adhesive fabricated from a novel biopolymer and soy protein. RSC Adv. 2021, 11, 11724–11731. [Google Scholar] [CrossRef]
- Mousavi, S.; Huang, J.; Li, K. A cold-set wood adhesive based on soy protein. Int. J. Adhes. Adhes. 2021, 106, 102801. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Zhou, Y.; Zhang, F.; Zeng, G.; Li, J.; Shi, S.Q.; Li, J. Bioinspired dual-crosslinking strategy for fabricating soy protein-based adhesives with excellent mechanical strength and antibacterial activity. Compos. Part B Eng. 2022, 240, 109987. [Google Scholar] [CrossRef]
- Wei, Y.; Jiang, S.; Li, J.; Aladejana, J.; Zhang, T.; Li, X.; Dong, Y.; Li, J. A soy protein-based adhesive with improved mechanical and electromagnetic shielding properties by employment of core@double-shell BT@PDA@PANI fillers. Chem. Eng. J. 2023, 458, 141512. [Google Scholar] [CrossRef]
- Hunt, C.G.; Lorenz, L.F.; Houtman, C.J.; Valle, E.; Coolidge, T.; Mock, C.; Frihart, C.R. Jet cooking dramatically improves the wet strength of soy adhesives. J. Am. Oil Chem. Soc. 2023, 100, 69–79. [Google Scholar] [CrossRef]
- Pang, H.; Wang, Y.; Chang, Z.; Xia, C.; Han, C.; Liu, H.; Li, J.; Zhang, S.; Huang, Z. Soy meal adhesive with high strength and water resistance via carboxymethylated wood fiber-induced crosslinking. Cellulose 2021, 28, 3569–3584. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Chen, M.; Gao, Q.; Li, J. A high-performance and low-cost soy flour adhesive with a hydroxymethyl melamine prepolymer. Polymers 2018, 10, 909. [Google Scholar] [CrossRef]
- Román, J.K.; Wilker, J.J. Cooking chemistry transforms proteins into high-strength adhesives. J. Am. Chem. Soc. 2019, 141, 1359–1365. [Google Scholar] [CrossRef]
- Gao, Q.; Shi, S.Q.; Zhang, S.; Li, J.; Wang, X.; Ding, W.; Liang, K.; Wang, J. Soybean meal-based adhesive enhanced by MUF resin. J. Appl. Polym. Sci. 2012, 125, 3676–3681. [Google Scholar] [CrossRef]
- Jin, S.; Li, K.; Zhang, X.; Gao, Q.; Zeng, L.; Shi, S.Q.; Li, J. Phytic acid-assisted fabrication for soybean meal/nanofiber composite adhesive via bioinspired chelation reinforcement strategy. J. Hazard. Mater. 2020, 399, 123064. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, F.; Zhang, C.; Tang, J.; Zeng, X.; Wan, X. Versatile value-added application of hyperbranched lignin derivatives: Water-resistance adhesive, UV protection coating, self-healing and skin-adhesive sensing. Chem. Eng. J. 2021, 404, 126358. [Google Scholar] [CrossRef]
- Frihart, C.; Satori, H. Soy flour dispersibility and performance as wood adhesive. J. Adhes. Sci. Technol. 2013, 27, 2043–2052. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Gao, Q.; Zhang, S.; Li, J. Properties of soybean-flour-based adhesives enhanced by attapulgite and glycerol polyglycidyl ether. Ind. Crop. Prod. 2014, 59, 35–40. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Yi, Z.; Gao, Q.; Li, J. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crop. Prod. 2015, 74, 613–618. [Google Scholar] [CrossRef]
- Xu, F.; Dong, Y.; Zhang, W.; Zhang, S.; Li, L.; Li, J. Preparation of cross-linked soy protein isolate-based environmentally-friendly films enhanced by PTGE and PAM. Ind. Crop. Prod. 2015, 67, 373–380. [Google Scholar] [CrossRef]
- Hemmil, V.; Adamopoulos, S.; Karlssonb, O.; Kumar, A. Development of sustainable bio-adhesives for engineered wood panels—A Review. RSC Adv. 2017, 7, 38604–38630. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, S.; Pang, J.; Gao, Z. Plywood with soy protein-acrylate hybrid adhesive. Adv. Mater. Res. 2014, 884, 108–111. [Google Scholar] [CrossRef]
- Zeng, N.; Xie, J.; Ding, C. Properties of the soy protein isolate/PVAc latex blend adhesives. Adv. Mater. Res. 2012, 550, 1103–1107. [Google Scholar] [CrossRef]
- Chen, N.; Zeng, Q.; Lin, Q.; Rao, J. Development of defatted soy flour based bio-adhesives using Viscozyme L. Ind. Crop. Prod. 2015, 76, 198–203. [Google Scholar] [CrossRef]
- Zhao, Z.; Umemura, K. Investigation of a new natural particleboard adhesive composed of tannin and sucrose. J. Wood Sci. 2014, 60, 269–277. [Google Scholar] [CrossRef]
- Zhao, Z.; Umemura, K. Investigation of a new natural particleboard adhesive composed of tannin and sucrose. 2. Effect of pressing temperature and time on board properties, and characterization of adhesive. BioResources 2015, 10, 2444–2460. [Google Scholar] [CrossRef]
- Zhao, Z.; Umemura, K.; Kanayama, K. Effects of the addition of citric acid on tannin-sucrose adhesive and physical properties of the particleboard. BioResources 2015, 11, 1319–1333. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Z.; Umemura, K. Further exploration of sucrose-citric acid adhesive: Synthesis and application on plywood. Polymers 2019, 11, 1875. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, K.; Kang, H.; Chen, S. Synthesis and response surface optimization of the phenol- glucose resin in non-aqueous phase. Thermosetting Resin 2015, 30, 29–32. [Google Scholar]
- Long, Y.; Yang, K.; Zhang, Y. Synthesis of melamine-glucose resin adhesive. Mater. Res. Appl. 2008, 2, 409–412. [Google Scholar]
- Chen, Z.; Liu, S.; Guo, J.; Chen, S. Optimization for Synthesis of Fructose-Resorcinol Resin Adhesive by Response Surface Methodology. Polym. Mater. Sci. Eng. 2012, 28, 8–11. [Google Scholar]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, l597–1600. [Google Scholar] [CrossRef] [PubMed]
- GB/T 14074-2017; Test Method for Adhesives and Their Resins Used in Wood Industry. Standards Press: Beijing, China, 2017.
- GB/T 17657-2022; Test Method for Physical and Chemical Properties of Wood-Based Panels and Veneer Panels. Standards Press: Beijing, China, 2022.
- Xiao, G.; Liang, J.; Wu, Z.; Lei, H.; Gong, F.; Gu, W.; Tu, Y.; Li, D. A composite whole-biomass tannin–sucrose–soy protein wood adhesive with high performance. Forests 2023, 14, 1250. [Google Scholar] [CrossRef]
- Liu, D.; Chen, H.; Chang, P.R.; Wu, Q.; Li, K.; Guan, L. Biomimetic soy protein nanocomposites with calcium carbonate crystalline arrays for use as wood adhesive. Bioresour. Technol. 2010, 101, 6235–6241. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Lei, H.; Zhang, B.; Chen, X.; Du, G. Environmentally friendly chitosan adhesives for plywood bonding. Int. J. Adhes. Adhes. 2022, 112, 103027. [Google Scholar] [CrossRef]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Ding, X.; Tang, M.; Gong, F.; Yuan, S.; Duan, J. Study on the preparation process optimization of plywood based on a full biomass tannin-sucrose wood adhesive. J. Renew. Mater. 2023, 11, 3245–3259. [Google Scholar] [CrossRef]
- Li, X.; Jiang, S.; Li, J.; Li, K.; Li, J. Highly dispersed manganese dioxide nanoparticles anchored on diatomite surface by sol-gel method and its performance on soybean meal-based adhesive. J. Appl. Polym. Sci. 2022, 139, 51719. [Google Scholar] [CrossRef]
- Liu, Y.; Kerton, F.M. Mechanistic studies on the formation of 5-hydroxymethylfurfural from the sugars fructose and glucose. Pure Appl. Chem. 2021, 93, 463–478. [Google Scholar] [CrossRef]
- Pyo, S.; Sayed, M.; Hatti-Kaul, R. Batch and Continuous Flow Production of 5-Hydroxymethylfurfural from a High Concentration of Fructose Using an Acidic Ion Exchange Catalyst. Org. Process Res. Dev. 2019, 23, 952–960. [Google Scholar] [CrossRef]
- Munshi, M.; Lomate, S.; Deshpande, R.; Rane, V.; Kelkar, A. Synthesis of acrolein by gas-phase dehydration of glycerol over silica supported Bronsted acidic ionic liquid catalysts. J. Chem. Technol. Biot. 2010, 85, 1319–1324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).