The Multifunctional Role of Salix spp.: Linking Phytoremediation, Forest Therapy, and Phytomedicine for Environmental and Human Benefits
Abstract
1. Introduction
Methodology
2. Phytoremediation Potential of Willow Species
2.1. Phytoremediation Mechanisms of Willow
2.2. Salix Ecological Adaptability, Economic Benefits and Microbial Synergies
3. Volatile Emissions and Forest Therapy: Willow-Derived Biogenic Volatile Organic Compounds
Willow Volatile Molecules Enhancing Forest Therapy and Human Well-Being
4. Therapeutic Applications and Pharmacological Properties of Willow and Its Bioactive Compounds
4.1. Historical Therapeutic Use of Willow
4.2. Willow in Modern Medicine
5. Urban Willows: Integrating Green Infrastructure and Public Health
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cozma, P.; Roșca, M.; Minuț, M.; Gavrilescu, M. Phytoremediation: A sustainable and promising bio-based approach to heavy metal pollution management. Sci. Total Environ. 2025, 1001, 180458. [Google Scholar] [CrossRef] [PubMed]
- Rajoo, K.S.; Karam, D.S.; Abdullah, M.Z. The physiological and psychosocial effects of forest therapy: A systematic review. Urban For. Urban Green. 2020, 54, 126744. [Google Scholar] [CrossRef]
- Wuytack, T.; Verheyen, K.; Wuyts, K.; Kardel, F.; Adriaenssens, S.; Samson, R. The potential of biomonitoring of air quality using leaf characteristics of white willow (Salix alba L.). Environ. Monit. Assess. 2010, 171, 197–204. [Google Scholar] [CrossRef]
- Puk, T. Nature-based regenerative healing: Nature and neurons. Eur. J. Ecopsychol. 2024, 9, 111–139. [Google Scholar]
- Vujcic, M.; Tomicevic-Dubljevic, J.; Grbic, M.; Lecic-Tosevski, D.; Vukovic, O.; Toskovic, O. Nature based solution for improving mental health and well-being in urban areas. Environ. Res. 2017, 158, 385–392. [Google Scholar] [CrossRef]
- Stigsdotter, U.K.; Palsdottir, A.M.; Burls, A.; Chermaz, A.; Ferrini, F.; Grahn, P. Nature-based therapeutic interventions. In Forests, Trees and Human Health; Springer: Berlin/Heidelberg, Germany, 2010; pp. 309–342. [Google Scholar]
- Santamour, F.S.; McArdle, A.J. Cultivars of Salix babylonica and other weeping willows. Arboric. Urban For. 1988, 14, 180–184. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Wiczkowski, W.; Szawara-Nowak, D.; Kaszubski, W.; Goraj-Koniarska, J.; Mitrus, J.; Saniewski, M.; Horbowicz, M. Effect of natural light on the development of adventitious roots in stem cuttings of Salix babylonica” Tortuosa”: Histological and metabolic evaluation. J. Elem. 2025, 30, 57–75. [Google Scholar] [CrossRef]
- Papale, D.; Guidolotti, G.; Mattioni, M.; Nicolini, G.; Sabbatini, S.; Sconocchia, P.; Antoniella, G.; Barbati, A.; Cecca, D.; Chiti, T. When a Natural Disaster Becomes an Opportunity for a Holistic Assessment of Ecosystem Restoration Strategies; AGU Fall Meeting Abstracts: New Orleans, LA, USA, 2024; p. B21A-02. [Google Scholar]
- Zhu, Y.; Gu, H.; Li, H.; Lam, S.S.; Verma, M.; Ng, H.S.; Sonne, C.; Liew, R.K.; Peng, W. Phytoremediation of contaminants in urban soils: A review. Environ. Chem. Lett. 2024, 22, 355–371. [Google Scholar] [CrossRef]
- Wang, L.; Lun, X.; Wang, Q.; Wu, J. Biogenic volatile organic compounds emissions, atmospheric chemistry, and environmental implications: A review. Environ. Chem. Lett. 2024, 22, 3033–3058. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Anand, U.; Kumar, R.; Ghorai, M.; Aftab, T.; Jha, N.K.; Rajapaksha, A.U.; Bundschuh, J.; Bontempi, E.; Dey, A. Phytoremediation and sequestration of soil metals using the CRISPR/Cas9 technology to modify plants: A review. Environ. Chem. Lett. 2023, 21, 429–445. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Abdalla, N.; Alshaal, T.; Elhenawy, A.S.; Shams, M.S.; Faizy, S.E.-D.; Belal, E.-S.B.; Shehata, S.A.; Ragab, M.I.; Amer, M.M. Giant reed for selenium phytoremediation under changing climate. Environ. Chem. Lett. 2015, 13, 359–380. [Google Scholar] [CrossRef]
- Kovačević, B.; Milović, M.; Kesić, L.; Pajnik, L.P.; Pekeč, S.; Stanković, D.; Orlović, S. Interclonal Variation in Heavy Metal Accumulation Among Poplar and Willow Clones: Implications for Phytoremediation of Contaminated Landfill Soils. Plants 2025, 14, 567. [Google Scholar] [CrossRef]
- Liu, J.; Jia, H.; Zhu, K.; Zhao, S.; Lichtfouse, E. Formation of environmentally persistent free radicals and reactive oxygen species during the thermal treatment of soils contaminated by polycyclic aromatic hydrocarbons. Environ. Chem. Lett. 2020, 18, 1329–1336. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Sharma, V.K.; Dionysiou, D.D. The arms race of environmental scientists to purify contaminated water. Environ. Chem. Lett. 2024, 22, 2607–2609. [Google Scholar] [CrossRef]
- Etim, E. Phytoremediation and its mechanisms: A review. Int. J. Environ. Bioenergy 2012, 2, 120–136. [Google Scholar]
- Saier Jr, M.; Trevors, J. Phytoremediation. Water Air Soil Pollut. 2010, 205 (Suppl. S1), 61–63. [Google Scholar] [CrossRef]
- Newman, L.A.; Reynolds, C.M. Phytodegradation of organic compounds. Curr. Opin. Biotechnol. 2004, 15, 225–230. [Google Scholar] [CrossRef]
- Limmer, M.; Burken, J. Phytovolatilization of organic contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Oves, M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 2009, 7, 1–19. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Can, H.; Dogan, I. Phytoremediation using genetically engineered plants to remove metals: A review. Environ. Chem. Lett. 2021, 19, 669–698. [Google Scholar] [CrossRef]
- Mille, T.; Graindorge, P.H.; Morel, C.; Paoli, J.; Lichtfouse, E.; Schroeder, H.; Grova, N. The overlooked toxicity of non-carcinogenic polycyclic aromatic hydrocarbons. Environ. Chem. Lett. 2024, 22, 1563–1567. [Google Scholar] [CrossRef]
- Vervaeke, P.; Luyssaert, S.; Mertens, J.; Meers, E.; Tack, F.M.G.; Lust, N. Phytoremediation prospects of willow stands on contaminated sediment: A field trial. Environ. Pollut. 2003, 126, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Nissim, W.G.; Jerbi, A.; Lafleur, B.; Fluet, R.; Labrecque, M. Willows for the treatment of municipal wastewater: Performance under different irrigation rates. Ecol. Eng. 2015, 81, 395–404. [Google Scholar] [CrossRef]
- Dimitriou, I.; Aronsson, P. Willows for energy and phytoremediation in Sweden. Unasylva 2005, 56, 47. [Google Scholar]
- Landberg, T.; Greger, M. Phytoremediation Using Willow in Industrial Contaminated Soil. Sustainability 2022, 14, 8449. [Google Scholar] [CrossRef]
- Robinson, B.H.; Mills, T.M.; Petit, D.; Fung, L.E.; Green, S.R.; Clothier, B.E. Natural and induced cadmium-accumulation in poplar and willow: Implications for phytoremediation. Plant Soil 2000, 227, 301–306. [Google Scholar] [CrossRef]
- Pulford, I.D.; Riddell-Black, D.; Stewart, C. Heavy Metal Uptake by Willow Clones from Sewage Sludge-Treated Soil: The Potential for Phytoremediation. Int. J. Phytoremediation 2006, 4, 59–72. [Google Scholar] [CrossRef]
- Volk, T.; Abrahamson, L.; Nowak, C.; Smart, L.; Tharakan, P.; White, E. The development of short-rotation willow in the northeastern United States for bioenergy and bioproducts, agroforestry and phytoremediation. Biomass Bioenergy 2006, 30, 715–727. [Google Scholar] [CrossRef]
- Weih, M.; Nordh, N.-E. Characterising willows for biomass and phytoremediation: Growth, nitrogen and water use of 14 willow clones under different irrigation and fertilisation regimes. Biomass Bioenergy 2002, 23, 397–413. [Google Scholar] [CrossRef]
- Wani, K.A.; Sofi, Z.M.; Malik, J.A.; Wani, J.A. Phytoremediation of Heavy Metals Using Salix (Willows). In Bioremediation and Biotechnology; Springer: Cham, Switzerland, 2020; Volume 2, pp. 161–174. [Google Scholar]
- Lewandowski, I.; Schmidt, U.; Londo, M.; Faaij, A. The economic value of the phytoremediation function—Assessed by the example of cadmium remediation by willow (Salix ssp.). Agric. Syst. 2006, 89, 68–89. [Google Scholar] [CrossRef]
- Gervais-Bergeron, B.; Chagnon, P.-L.; Labrecque, M. Willow Aboveground and Belowground Traits Can Predict Phytoremediation Services. Plants 2021, 10, 1824. [Google Scholar] [CrossRef]
- Fortin Faubert, M.; Desjardins, D.; Hijri, M.; Labrecque, M. Willows Used for Phytoremediation Increased Organic Contaminant Concentrations in Soil Surface. Appl. Sci. 2021, 11, 2979. [Google Scholar] [CrossRef]
- Yıldırım, K.; Kasım, G.Ç. Phytoremediation potential of poplar and willow species in small scale constructed wetland for boron removal. Chemosphere 2018, 194, 722–736. [Google Scholar] [CrossRef]
- Janssen, J.; Weyens, N.; Croes, S.; Beckers, B.; Meiresonne, L.; Van Peteghem, P.; Carleer, R.; Vangronsveld, J. Phytoremediation of Metal Contaminated Soil Using Willow: Exploiting Plant-Associated Bacteria to Improve Biomass Production and Metal Uptake. Int. J. Phytoremediation 2015, 17, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, M.; Hu, Y.; Vincent, G.; Shang, K. The use of willow microcuttings for phytoremediation in a copper, zinc and lead contaminated field trial in Shanghai, China. Int. J. Phytoremediation 2020, 22, 1331–1337. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Du, W.; Ai, F.; Yin, Y.; Ji, R.; Guo, H. Microbial communities in the rhizosphere of different willow genotypes affect phytoremediation potential in Cd contaminated soil. Sci. Total Environ. 2021, 769, 145224. [Google Scholar] [CrossRef]
- Lin, Z.; Qiao, Y.; Ge, J.; Lu, L.; Xie, R.; Tian, S. Novel plant growth-promoting endophytic bacteria, Stenotrophomonas maltophilia SaRB5, facilitate phytoremediation by plant growth and cadmium absorption in Salix suchowensis. Ecotoxicol. Environ. Saf. 2025, 303, 118967. [Google Scholar] [CrossRef]
- Lin, Z.; Qiao, Y.; Xu, K.; Lu, L.; Shu, Q.-Y.; Tian, S. The endophytic fungus Serendipita indica reshapes rhizosphere soil microbiota to improve Salix suchowensis growth and phytoremediation. J. Hazard. Mater. 2025, 495, 138620. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Kwak, K.-H.; Ryu, Y.-H.; Lee, S.-H.; Baik, J.-J. Impacts of biogenic isoprene emission on ozone air quality in the Seoul metropolitan area. Atmos. Environ. 2014, 96, 209–219. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Q.; Xu, C.; Lun, X.; Wang, L.; Gao, Y.; Huang, L.; Zhang, Q.; Li, L.; Liu, B. Biogenic volatile organic compounds in forest therapy base: A source of air pollutants or a healthcare function? Sci. Total Environ. 2024, 931, 172944. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Yang, Y.; Li, C.; Peng, W. Molecular characteristics of volatile components from willow bark. J. King Saud Univ.-Sci. 2020, 32, 1932–1936. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar] [CrossRef]
- Mozaffar, M.A. Biogenic volatile organic compound emissions from Willow trees. In Student Thesis Series INES; Lund University: Lund, Sweden, 2013. [Google Scholar]
- Tun, K.M.; Minor, M.; Jones, T.; McCormick, A.C. Volatile Profiling of Fifteen Willow Species and Hybrids and Their Responses to Giant Willow Aphid Infestation. Agronomy 2020, 10, 1404. [Google Scholar] [CrossRef]
- Shaoning, L.; Tingting, L.; Xueying, T.; Na, Z.; Xiaotian, X.; Shaowei, L. Comparative Study on the Release of Beneficial Volatile Organic Compounds from Four Deciduous Tree Species. Ecol. Environ. 2023, 32, 123. [Google Scholar]
- Karlsson, T.; Klemedtsson, L.; Rinnan, R.; Holst, T. Leaf-Scale Study of Biogenic Volatile Organic Compound Emissions from Willow (Salix spp.) Short Rotation Coppices Covering Two Growing Seasons. Atmosphere 2021, 12, 1427. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef]
- Engelberth, J. Green Leaf volatiles: A New Player in the Protection against Abiotic stresses? Int. J. Mol. Sci. 2024, 25, 9471. [Google Scholar] [CrossRef]
- Engelberth, J. Green leaf volatiles: Airborne signals that protect against biotic and abiotic stresses. Biol. Life Sci. Forum 2020, 4, 101. [Google Scholar] [CrossRef]
- Karlsson, T.; Rinnan, R.; Holst, T. Variability of BVOC Emissions from Commercially Used Willow (Salix spp.) Varieties. Atmosphere 2020, 11, 356. [Google Scholar] [CrossRef]
- Swanson, L.; Li, T.; Rinnan, R. Contrasting responses of major and minor volatile compounds to warming and gall-infestation in the Arctic willow Salix myrsinites. Sci. Total Environ. 2021, 793, 148516. [Google Scholar] [CrossRef]
- Mezzomo, P.; Leong, J.V.; Vodrážka, P.; Moos, M.; Jorge, L.R.; Volfová, T.; Michálek, J.; de L. Ferreira, P.; Kozel, P.; Sedio, B.E.; et al. Variation in induced responses in volatile and non-volatile metabolites among six willow species: Do willow species share responses to herbivory? Phytochemistry 2024, 226, 114222. [Google Scholar] [CrossRef]
- Toome, M.; Randjärv, P.; Copolovici, L.; Niinemets, Ü.; Heinsoo, K.; Luik, A.; Noe, S.M. Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta 2010, 232, 235–243. [Google Scholar] [CrossRef]
- Hakola, H.; Rinne, J.; Laurila, T. The hydrocarbon emission rates of tea-leafed willow (Salix phylicifolia), silver birch (Betula pendula) and European aspen (Populus tremula). Atmos. Environ. 1998, 32, 1825–1833. [Google Scholar] [CrossRef]
- Füssel, U. Floral Scent in Salix L. and the Role of Olfactory and Visual Cues for Pollinator Attraction of Salix caprea L. Ph.D. Thesis, University of Bayreuth, Bayreuth, Germany, 2007. [Google Scholar]
- Ling, J.; Li, X.; Yang, G.; Yin, T. Volatile metabolites of willows determining host discrimination by adult Plagiodera versicolora. J. For. Res. 2021, 33, 679–687. [Google Scholar] [CrossRef]
- Braccini, C.L.; Vega, A.S.; Coll Aráoz, M.V.; Teal, P.E.; Cerrillo, T.; Zavala, J.A.; Fernandez, P.C. Both Volatiles and Cuticular Plant Compounds Determine Oviposition of the Willow Sawfly Nematus oligospilus on Leaves of Salix spp. (Salicaceae). J. Chem. Ecol. 2015, 41, 985–996. [Google Scholar] [CrossRef]
- Galotta, M.P.; Omacini, M.; Fernández, P.C. Symbiosis with Mycorrhizal Fungi Alters Sesquiterpene but not Monoterpene Profile in the South American Willow Salix humboldtiana. J. Chem. Ecol. 2025, 51, 70. [Google Scholar] [CrossRef]
- Morrison, E.C.; Drewer, J.; Heal, M.R. A comparison of isoprene and monoterpene emission rates from the perennial bioenergy crops short-rotation coppice willow and Miscanthus and the annual arable crops wheat and oilseed rape. GCB Bioenergy 2015, 8, 211–225. [Google Scholar] [CrossRef]
- Fakhrzad, F.; Jowkar, A. Water stress and increased ploidy level enhance antioxidant enzymes, phytohormones, phytochemicals and polyphenol accumulation of tetraploid induced wallflower. Ind. Crops Prod. 2023, 206, 117612. [Google Scholar] [CrossRef]
- Gilhen-Baker, M.; Roviello, V.; Beresford-Kroeger, D.; Roviello, G.N. Old growth forests and large old trees as critical organisms connecting ecosystems and human health. A review. Environ. Chem. Lett. 2022, 20, 1529–1538. [Google Scholar] [CrossRef]
- Roviello, V.; Gilhen-Baker, M.; Roviello, G.N.; Lichtfouse, E. River therapy. Environ. Chem. Lett. 2022, 20, 2729–2734. [Google Scholar] [CrossRef]
- Roviello, V.; Roviello, G.N. Less COVID-19 deaths in southern and insular Italy explained by forest bathing, Mediterranean environment, and antiviral plant volatile organic compounds. Environ. Chem. Lett. 2022, 20, 7–17. [Google Scholar] [CrossRef]
- Li, Q. Effect of forest bathing trips on human immune function. Environ. Health Prev. Med. 2010, 15, 9–17. [Google Scholar] [CrossRef]
- dos Santos, É.R.; Maia, J.G.S.; Fontes-Júnior, E.A.; do Socorro Ferraz Maia, C. Linalool as a therapeutic and medicinal tool in depression treatment: A review. Curr. Neuropharmacol. 2022, 20, 1073–1092. [Google Scholar] [CrossRef]
- Alkanat, M.; Alkanat, H.Ö. D-Limonene reduces depression-like behaviour and enhances learning and memory through an anti-neuroinflammatory mechanism in male rats subjected to chronic restraint stress. Eur. J. Neurosci. 2024, 60, 4491–4502. [Google Scholar] [CrossRef]
- Bandiera, B.; Natale, F.; Rinaudo, M.; Sollazzo, R.; Spinelli, M.; Fusco, S.; Grassi, C. Olfactory stimulation with multiple odorants prevents stress-induced cognitive and psychological alterations. Brain Commun. 2024, 6, fcae390. [Google Scholar] [CrossRef]
- Linck, V.d.M.; da Silva, A.L.; Figueiró, M.; Caramao, E.B.; Moreno, P.R.H.; Elisabetsky, E. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef]
- d’Alessio, P.A.; Bisson, J.-F.; Béné, M.C. Anti-stress effects of d-limonene and its metabolite perillyl alcohol. Rejuvenation Res. 2014, 17, 145–149. [Google Scholar] [CrossRef]
- Amenduni, A.; Massari, F.; Palmisani, J.; de Gennaro, G.; Brattoli, M.; Tutino, M. Chemical characterization of odor active volatile organic compounds emitted from perfumes by GC/MS-O. Environ. Eng. Manag. J. (EEMJ) 2016, 15, 1963–1969. [Google Scholar]
- Pino, J.A.; Trujillo, R. Characterization of odour-active compounds of sour guava (Psidium acidum [DC.] Landrum) fruit by gas chromatography-olfactometry and odour activity value. Flavour. Fragr. J. 2021, 36, 207–212. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Ahamed, M.K.; Majid, A.A. In vivo anti-inflammatory activity of β-caryophyllene, evaluated by molecular imaging. Mol. Med. Chem. 2015, 1, 6. [Google Scholar]
- Rahimi, K.; Zalaghi, M.; Shehnizad, E.G.; Salari, G.; Baghdezfoli, F.; Ebrahimifar, A. The effects of alpha-pinene on inflammatory responses and oxidative stress in the formalin test. Brain Res. Bull. 2023, 203, 110774. [Google Scholar] [CrossRef]
- Ozah, E.O.; Ben-Azu, B.; Chimezie, J.; Friday, F.B.; Esuku, D.T.; Chijioke, B.S.; Iwhiwhu, P.; Moses, A.S.; Nekabari, M.K.; Oyovwi, O.M.; et al. Sabinene confers protection against cerebral ischemia in rats: Potential roles of antioxidants, anti-inflammatory effects, and astrocyte-neurotrophic support. Neurol. Res. 2025, 1–21. [Google Scholar] [CrossRef]
- Bilbrey, J.A.; Ortiz, Y.T.; Felix, J.S.; McMahon, L.R.; Wilkerson, J.L. Evaluation of the terpenes β-caryophyllene, α-terpineol, and γ-terpinene in the mouse chronic constriction injury model of neuropathic pain: Possible cannabinoid receptor involvement. Psychopharmacology 2022, 239, 1475–1486. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Park, B.-I.; Kim, B.-S.; Kim, K.-J.; You, Y.-O. Sabinene suppresses growth, biofilm formation, and adhesion of Streptococcus mutans by inhibiting cariogenic virulence factors. J. Oral Microbiol. 2019, 11, 1632101. [Google Scholar] [CrossRef]
- Gardiner, A. Douglas Fir (Pseudotsuga menziesii): The New “King of the Conifer Oils”? Int. J. Prof. Holist. Aromather. 2025, 14, 23. [Google Scholar]
- Ambroziak, T. The Tipsiness of Black Spruce. Aromather. J. 2020. Available online: https://naha.org/assets/product-downloads/NAHAJournal-Winter2020-FinalFix-HQOptEml.pdf#page=23 (accessed on 3 November 2025).
- Kanezaki, M.; Terada, K.; Ebihara, S. Effect of olfactory stimulation by L-menthol on laboratory-induced dyspnea in COPD. Chest 2020, 157, 1455–1465. [Google Scholar] [CrossRef]
- Eccles, R. Menthol: Effects on nasal sensation of airflow and the drive to breathe. Curr. Allergy Asthma Rep. 2003, 3, 210–214. [Google Scholar] [CrossRef]
- Karimi, I.; Modaresi, M.; Cheshmekaboodi, F.; Miraghaee, S.S. The Effects of Aromatic Water of Salix aegyptiaca L. and its Major Component, 1, 4-Dimethoxybenzene, on Lipid and Lipoprotein Profiles and Ethology of Normolipidemic Rabbits. Int. J. Clin. Toxicol. 2015, 2, 55–63. [Google Scholar] [CrossRef]
- Dacho, V.; Szolcsányi, P. Synthesis and olfactory properties of seco-analogues of lilac aldehydes. Molecules 2021, 26, 7086. [Google Scholar] [CrossRef]
- Kim, M.; Sowndhararajan, K.; Choi, H.J.; Park, S.J.; Kim, S. Olfactory stimulation effect of aldehydes, nonanal, and decanal on the human electroencephalographic activity, according to nostril variation. Biomedicines 2019, 7, 57. [Google Scholar] [CrossRef]
- Suresh, A.S.; Sood, A.; Vellapandian, C. The Role of Ocimene in Decreasing α-Synuclein Aggregation using Rotenone-induced Rat Model. Cent. Nerv. Syst. Agents Med. Chem.-Cent. Nerv. Syst. Agents 2024, 24, 304–316. [Google Scholar] [CrossRef]
- Schmid, B.; Kötter, I.; Heide, L. Pharmacokinetics of salicin after oral administration of a standardised willow bark extract. Eur. J. Clin. Pharmacol. 2001, 57, 387–391. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Schmidt, M.; Jäggi, R.; Metz, J.; Khayyal, M.T. Willow bark extract: The contribution of polyphenols to the overall effect. Wien. Med. Wochenschr. 2007, 157, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, J.G. Medicinal potential of willow: A chemical perspective of aspirin discovery. J. Saudi Chem. Soc. 2010, 14, 317–322. [Google Scholar] [CrossRef]
- Oketch-Rabah, H.A.; Marles, R.J.; Jordan, S.A.; Low Dog, T. United States Pharmacopeia Safety Review of Willow Bark. Planta Medica 2019, 85, 1192–1202. [Google Scholar] [CrossRef]
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, Pharmacology and Medicinal Uses of Plants of the Genus Salix: An Updated Review. Front. Pharmacol. 2021, 12, 593856. [Google Scholar] [CrossRef] [PubMed]
- Maistro, E.L.; Terrazzas, P.M.; Perazzo, F.F.; Gaivão, I.O.N.D.M.; Sawaya, A.C.H.F.; Rosa, P.C.P. Salix alba (white willow) medicinal plant presents genotoxic effects in human cultured leukocytes. J. Toxicol. Environ. Health Part A 2020, 82, 1223–1234. [Google Scholar] [CrossRef]
- Warmiński, K.; Stolarski, M.J.; Gil, Ł.; Krzyżaniak, M. Willow bark and wood as a source of bioactive compounds and bioenergy feedstock. Ind. Crops Prod. 2021, 171, 113976. [Google Scholar] [CrossRef]
- Romesberg, F.; El-Shemy, H.A.; Aboul-Enein, A.M.; Aboul-Enein, K.M.; Fujita, K. Willow Leaves’ Extracts Contain Anti-Tumor Agents Effective against Three Cell Types. PLoS ONE 2007, 2, e178. [Google Scholar]
- Gaffin, S.R.; Rosenzweig, C.; Kong, A.Y. Adapting to climate change through urban green infrastructure. Nat. Clim. Change 2012, 2, 704. [Google Scholar] [CrossRef]
- Hanna, E.; Comín, F.A. Urban green infrastructure and sustainable development: A review. Sustainability 2021, 13, 11498. [Google Scholar] [CrossRef]
- Di Stasio, L.; Gentile, A.; Tangredi, D.N.; Piccolo, P.; Oliva, G.; Vigliotta, G.; Cicatelli, A.; Guarino, F.; Guidi Nissim, W.; Labra, M.; et al. Urban Phytoremediation: A Nature-Based Solution for Environmental Reclamation and Sustainability. Plants 2025, 14, 2057. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Wang, W.J.; Xie, Z. Therapeutic plant landscape design of urban forest parks based on the Five Senses Theory: A case study of Stanley Park in Canada. Int. J. Geoheritage Parks 2022, 10, 97–112. [Google Scholar] [CrossRef]
- Chen, B.; Qi, X. Protest response and contingent valuation of an urban forest park in Fuzhou City, China. Urban For. Urban Green. 2018, 29, 68–76. [Google Scholar] [CrossRef]
- Volenec, Z.M.; Abraham, J.O.; Becker, A.D.; Dobson, A.P. Public parks and the pandemic: How park usage has been affected by COVID-19 policies. PLoS ONE 2021, 16, e0251799. [Google Scholar] [CrossRef]
- Morales-Rodríguez, C.; Vannini, A.; Scanu, B.; González-Moreno, P.; Turco, S.; Drais, M.I.; Brandano, A.; Varo Martínez, M.Á.; Mazzaglia, A.; Deidda, A. Challenges to Mediterranean Fagaceae ecosystems affected by Phytophthora cinnamomi and climate change: Integrated pest management perspectives. Curr. For. Rep. 2025, 11, 9. [Google Scholar] [CrossRef]
- Kröel-Dulay, G.; Ransijn, J.; Schmidt, I.K.; Beier, C.; De Angelis, P.; De Dato, G.; Dukes, J.S.; Emmett, B.; Estiarte, M.; Garadnai, J. Increased sensitivity to climate change in disturbed ecosystems. Nat. Commun. 2015, 6, 6682. [Google Scholar] [CrossRef] [PubMed]
- Bressler, A.; Vidon, P.; Hirsch, P.; Volk, T. Valuation of ecosystem services of commercial shrub willow (Salix spp.) woody biomass crops. Environ. Monit. Assess. 2017, 189, 137. [Google Scholar] [CrossRef] [PubMed]
- Rousset, J.; Menoli, S.; François, A.; Gaucherand, S.; Evette, A. Developing Nature-based Solutions in the Alps: An Ex-situ Experiment to Select Willows for Subalpine Soil and Water Bioengineering Structures. Environ. Manag. 2025, 75, 1950–1962. [Google Scholar] [CrossRef] [PubMed]
- Weih, M. Genetic and environmental variation in spring and autumn phenology of biomass willows (Salix spp.): Effects on shoot growth and nitrogen economy. Tree Physiol. 2009, 29, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Read, P.; Garton, S.; Tormala, T. Willows (Salix spp.). In Trees II; Springer: Berlin/Heidelberg, Germany, 1989; pp. 370–386. [Google Scholar]

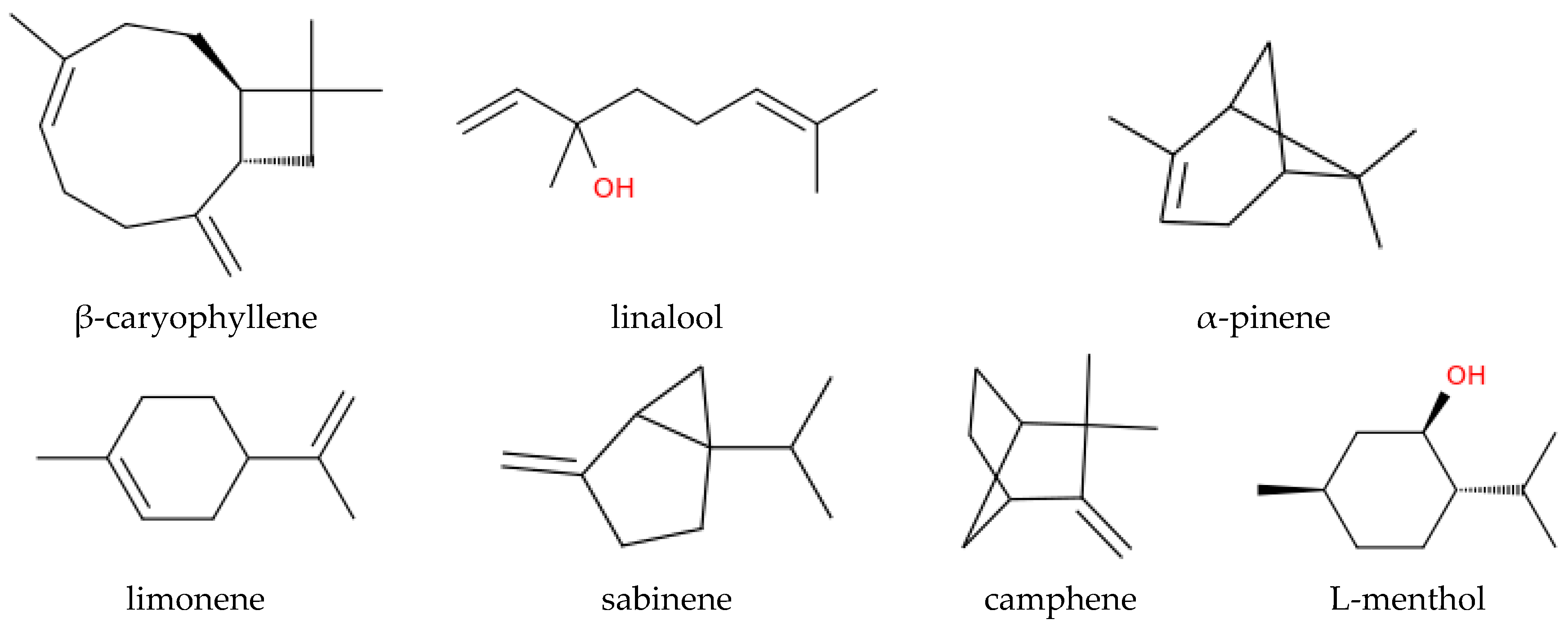
| Compound | Salix Species | Reference | Beneficial Effect | Reference |
|---|---|---|---|---|
| Isoprene | Salix viminalis, Salix myrsinites | Karlsson et al., 2021, Swanson et al., 2021 | — | — |
| β-Caryophyllene | Salix viminalis, Salix nigra | Karlsson et al., 2021, Braccini et al., 2015 | Anti-inflammatory, anxiolytic, immune-modulating | Dahham et al., 2015, Bilbrey et al., 2022 |
| Ocimene (cis- and trans-) | Salix viminalis, Salix nigra | Karlsson et al., 2021, Braccini et al., 2015 | Pleasant scent, neuroprotective | Suresh et al., 2024 |
| α-Farnesene | Salix spp. | Karlsson et al., 2021 | — | — |
| Hexanal | Salix viminalis | Toome et al., 2010 | Calming scent, stress reduction | Pino and Trujillo, 2021 |
| Nonanal | Salix babylonica | Shaoning et al., 2023 | — | — |
| Linalool | Salix viminalis | Karlsson et al., 2021 | Sedative, anxiolytic, mood-enhancing | dos Santos et al., 2022, Linck et al., 2010 |
| (E)-4,8-Dimethyl-1,3,7-nonatriene | Salix myrsinites | Swanson et al., 2021 | — | — |
| α-Pinene | Salix cinerea, Salix spp. | Mezzomo et al., 2024, Morrison et al., 2015 | Anti-inflammatory, bronchodilatory, cognitive support | Rahimi et al., 2023, Allenspach and Steuer, 2021, Gardiner, 2025 |
| Delta-3-carene | Salix spp. | Morrison et al., 2015 | — | — |
| β-Pinene | Salix spp. | Morrison et al., 2015 | — | — |
| Limonene | Salix phylicifolia, Salix spp. | Hakola et al., 1998, Morrison et al., 2015 | Antidepressant, stress reduction | Alkanat and Alkanat, 2024, d’Alessio et al., 2014 |
| Sabinene | Salix phylicifolia | Hakola et al., 1998 | Antioxidant, anti-inflammatory | Ozah et al., 2025, Park et al., 2019 |
| Camphene | Salix phylicifolia | Hakola et al., 1998 | Respiratory stimulant, antimicrobial | Ambroziak, 2020 |
| 1,4-Dimethoxybenzene | Salix caprea, Salix atrocinerea | Füssel, 2007 | Floral scent, mood-enhancing | Karimi et al., 2015 |
| Lilac aldehyde | Salix caprea, Salix atrocinerea | Füssel, 2007 | Floral aroma, calming effect | Dacho and Szolcsányi, 2021 |
| Decanal | Salix babylonica, Salix nigra | Shaoning et al., 2023, Braccini et al., 2015 | Soothing scent, insect-repellent | Kim et al., 2019 |
| Undecane | Salix nigra | Braccini et al., 2015 | — | — |
| Cis-3-hexenyl acetate | Salix suchowensis | Ling et al., 2021 | Calming, masking other scents | Pino and Trujillo, 2021 |
| Cis-3-hexen-1-ol | Salix babylonica | Shaoning et al., 2023 | Fresh green aroma, stress reduction | Bandiera et al., 2024 |
| L-menthol | Salix babylonica | Shaoning et al., 2023 | Cooling, analgesic, respiratory relief | Kanezaki et al., 2020, Eccles, 2003 |
| Azulene (chamomile blue) | Salix babylonica | Shaoning et al., 2023 | Anti-inflammatory, calming | Ozah et al., 2025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roviello, G.N. The Multifunctional Role of Salix spp.: Linking Phytoremediation, Forest Therapy, and Phytomedicine for Environmental and Human Benefits. Forests 2025, 16, 1808. https://doi.org/10.3390/f16121808
Roviello GN. The Multifunctional Role of Salix spp.: Linking Phytoremediation, Forest Therapy, and Phytomedicine for Environmental and Human Benefits. Forests. 2025; 16(12):1808. https://doi.org/10.3390/f16121808
Chicago/Turabian StyleRoviello, Giovanni N. 2025. "The Multifunctional Role of Salix spp.: Linking Phytoremediation, Forest Therapy, and Phytomedicine for Environmental and Human Benefits" Forests 16, no. 12: 1808. https://doi.org/10.3390/f16121808
APA StyleRoviello, G. N. (2025). The Multifunctional Role of Salix spp.: Linking Phytoremediation, Forest Therapy, and Phytomedicine for Environmental and Human Benefits. Forests, 16(12), 1808. https://doi.org/10.3390/f16121808






