Nitrogen–Calcium Stoichiometry Regulates Growth and Physiology in Mongolian Pine (Pinus sylvestris var. mongolica)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Environment
2.2. Experimental Design
2.3. Determination of the Growth and Physiological Characteristics of Mongolian Pine Seedlings
2.3.1. Photosynthetic Parameters
2.3.2. Photosynthetic Pigments
2.3.3. Photosynthetic Products
2.3.4. Antioxidant Enzymes Activities
2.3.5. Plant Heights and Basal Diameters
2.3.6. Biomass
2.4. Statistical Analysis
3. Results
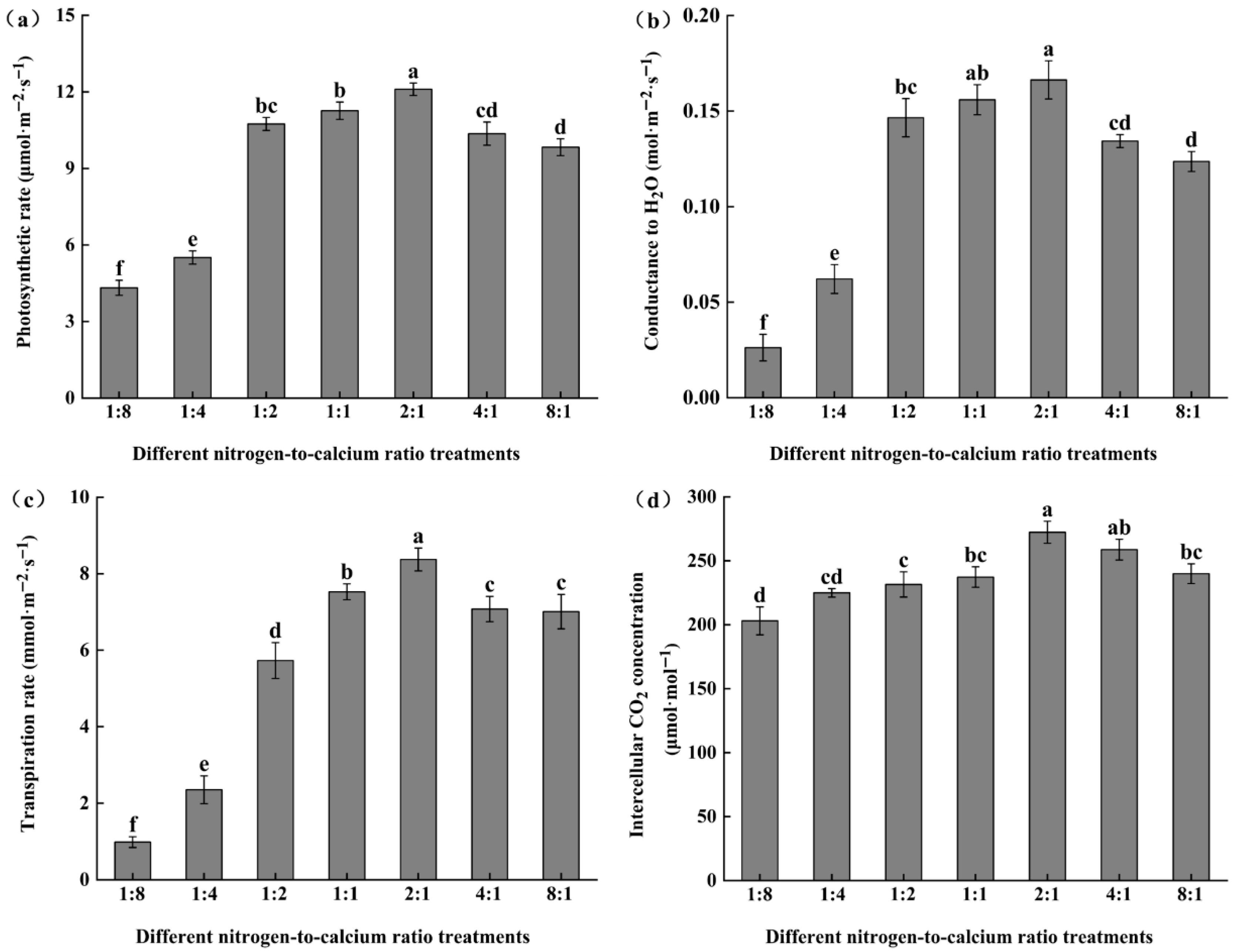
3.1. Photosynthetic Parameters
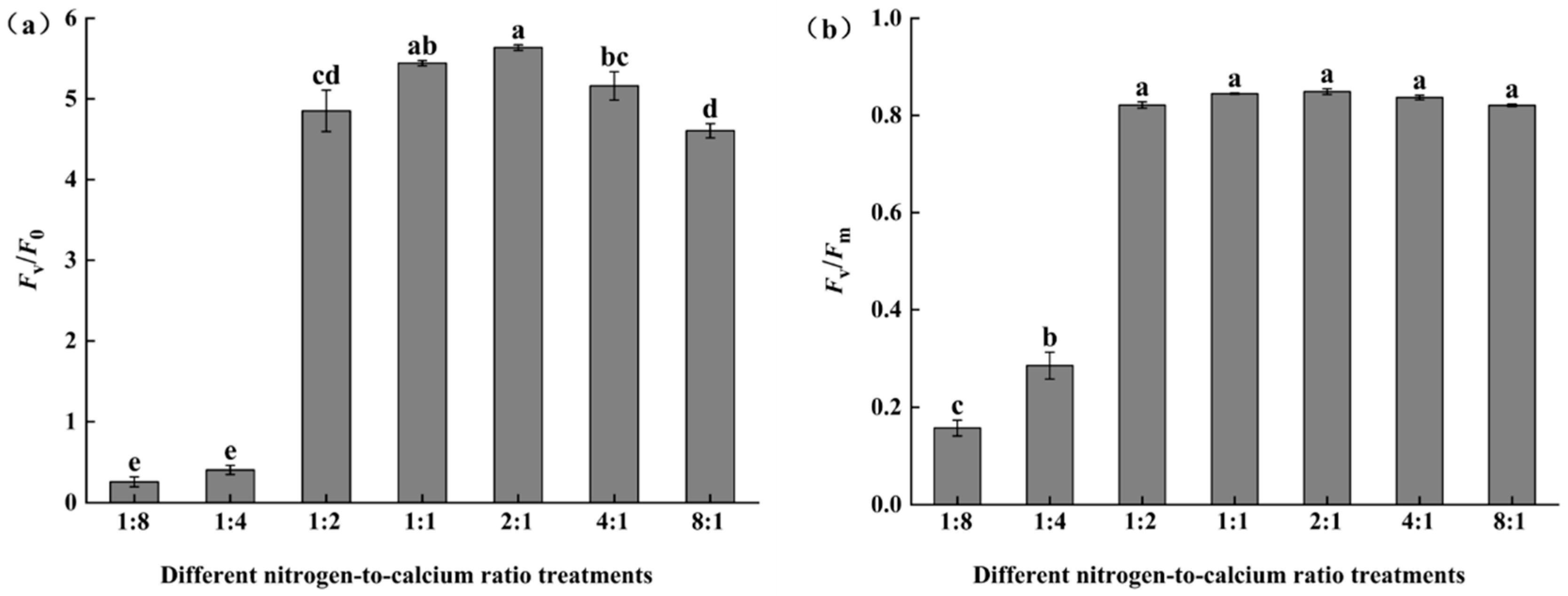
3.2. Chlorophyll Fluorescence Characteristics
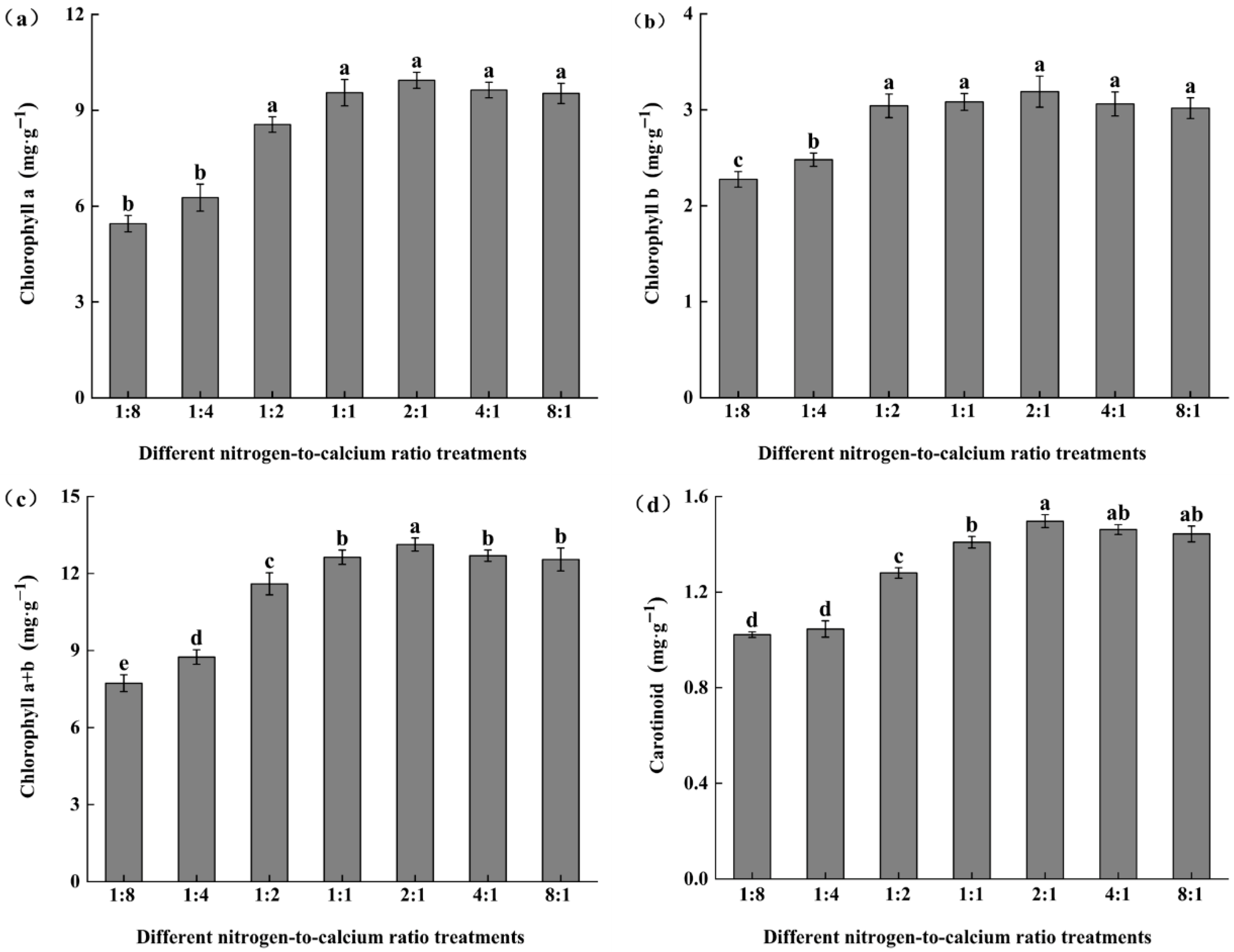
3.3. Photosynthetic Pigments
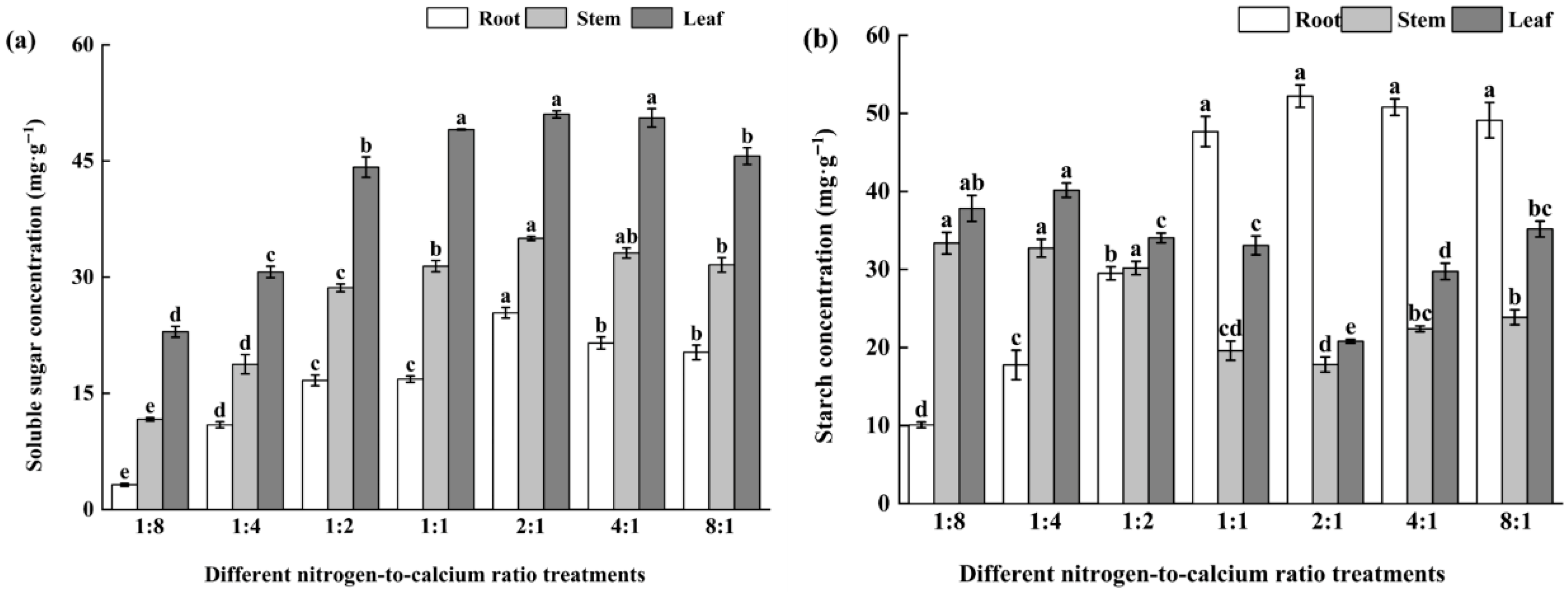
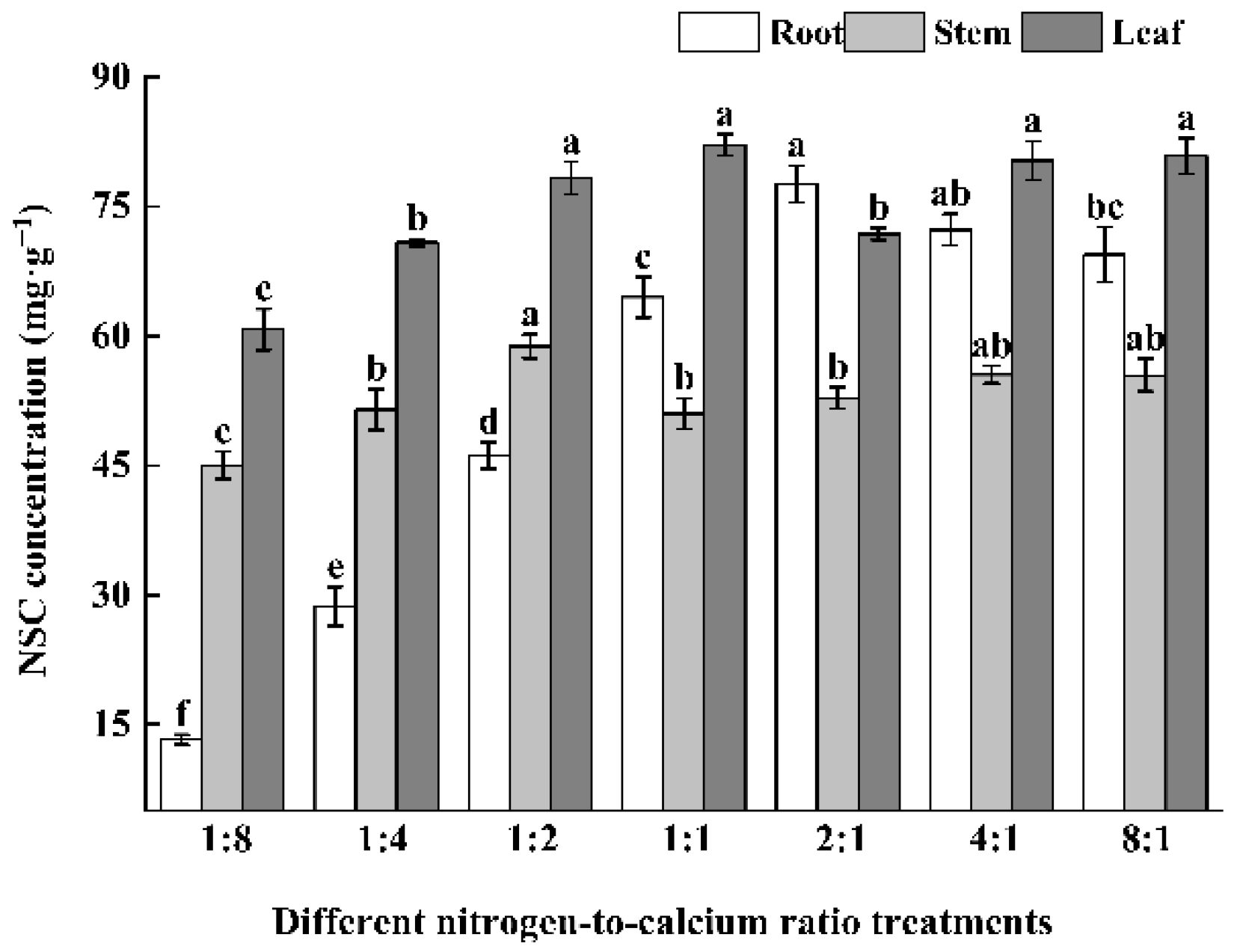
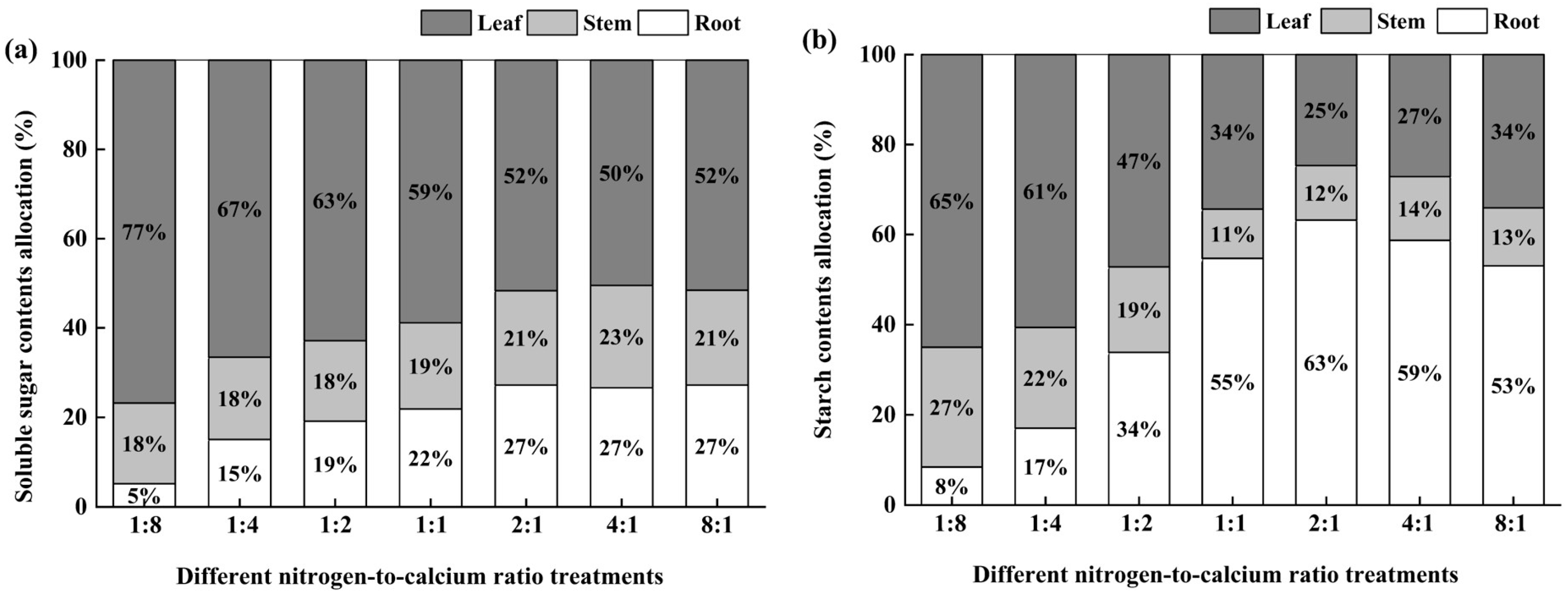
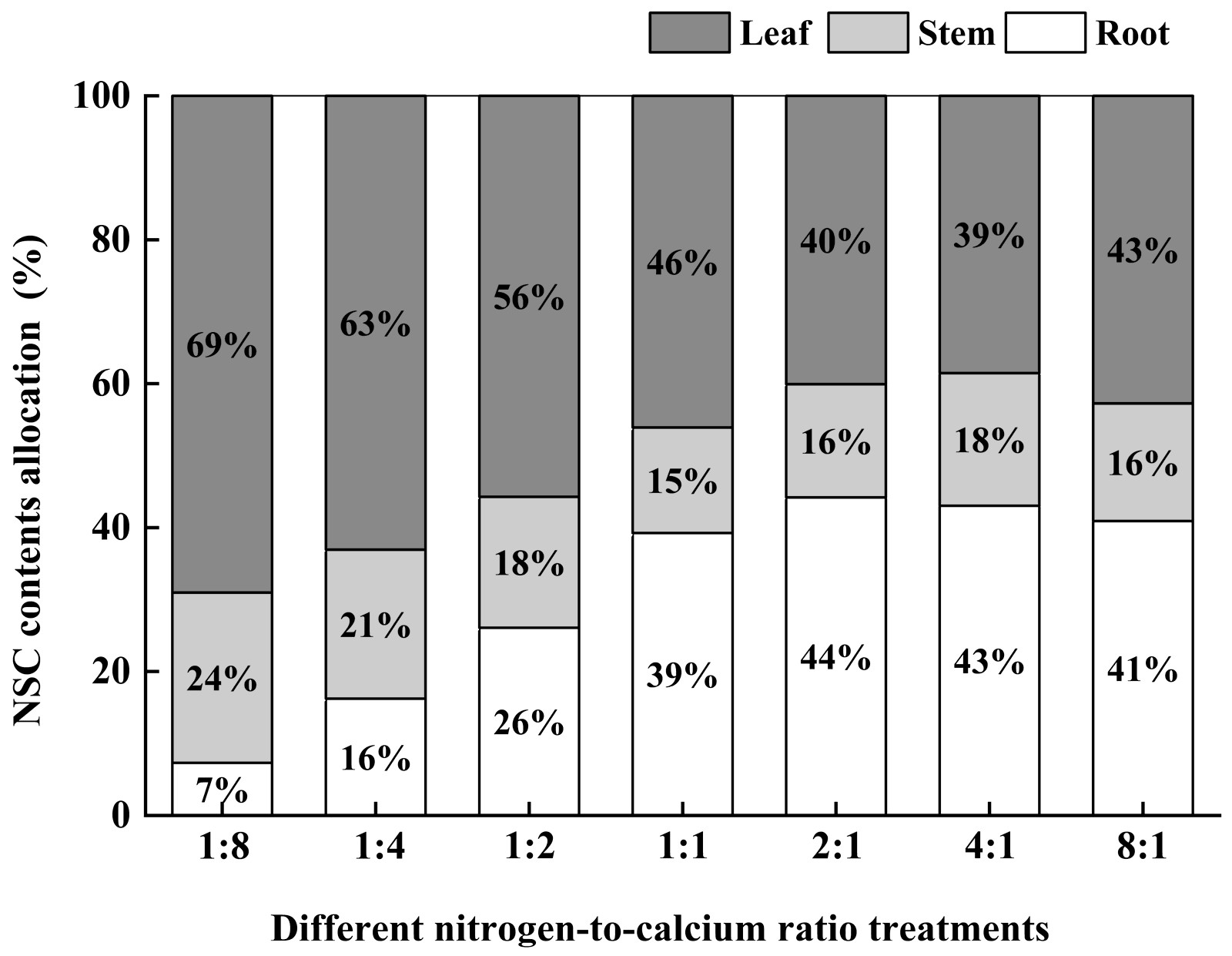
3.4. Photosynthetic Products and Allocation
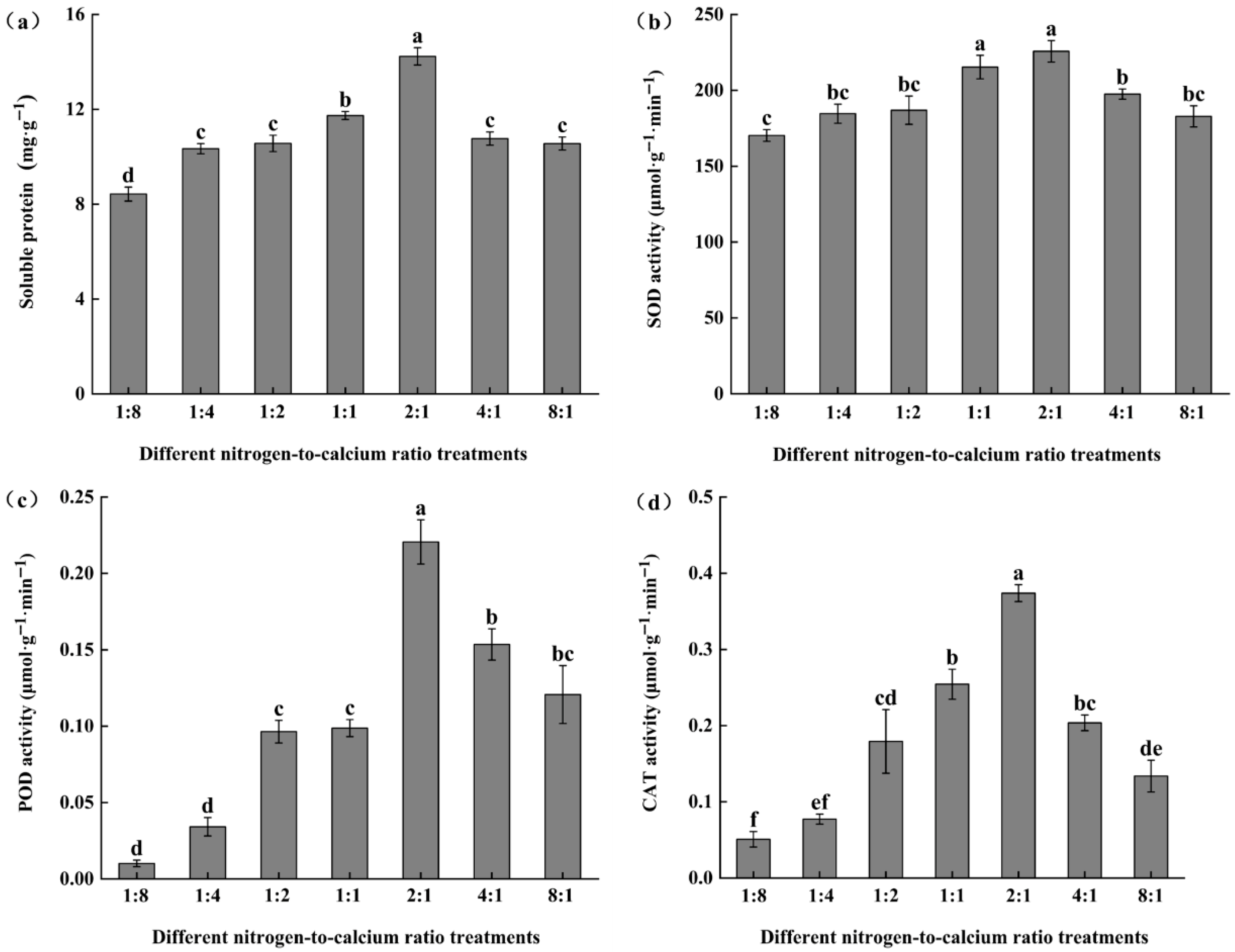
3.5. Antioxidant Enzymes Activities
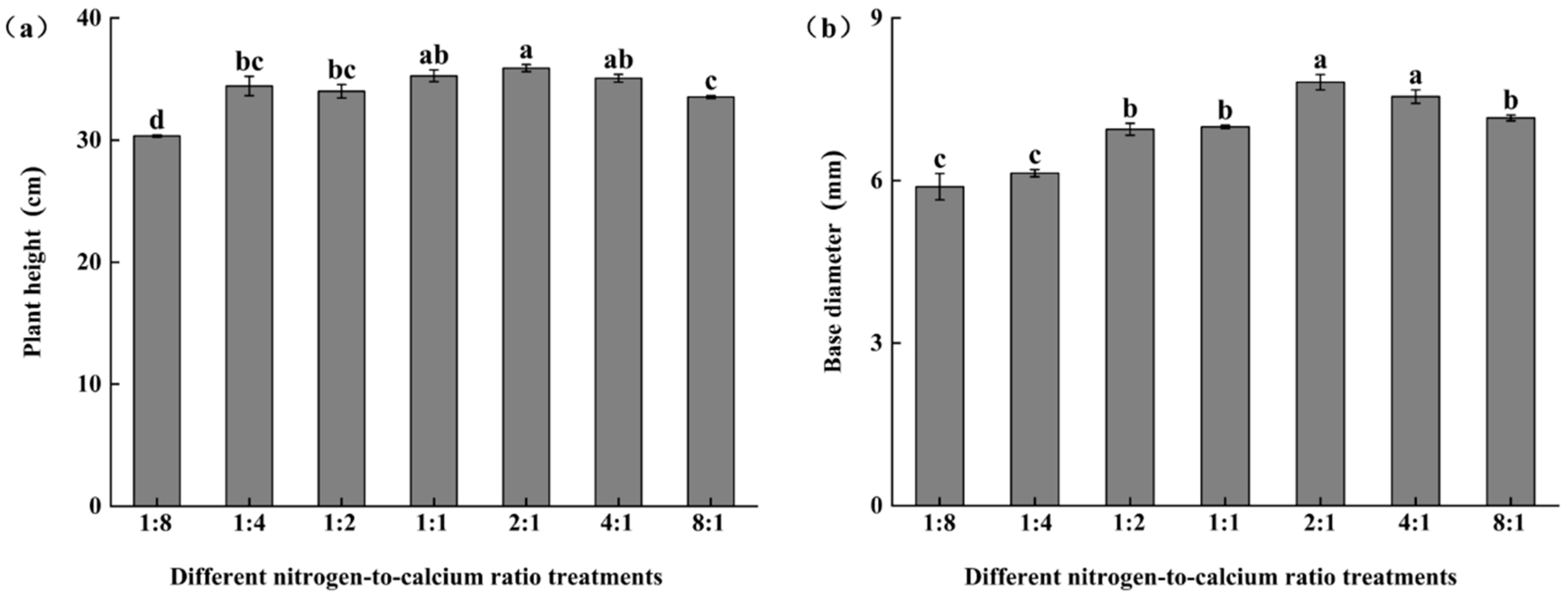
3.6. Plant Height and Basal Diameter
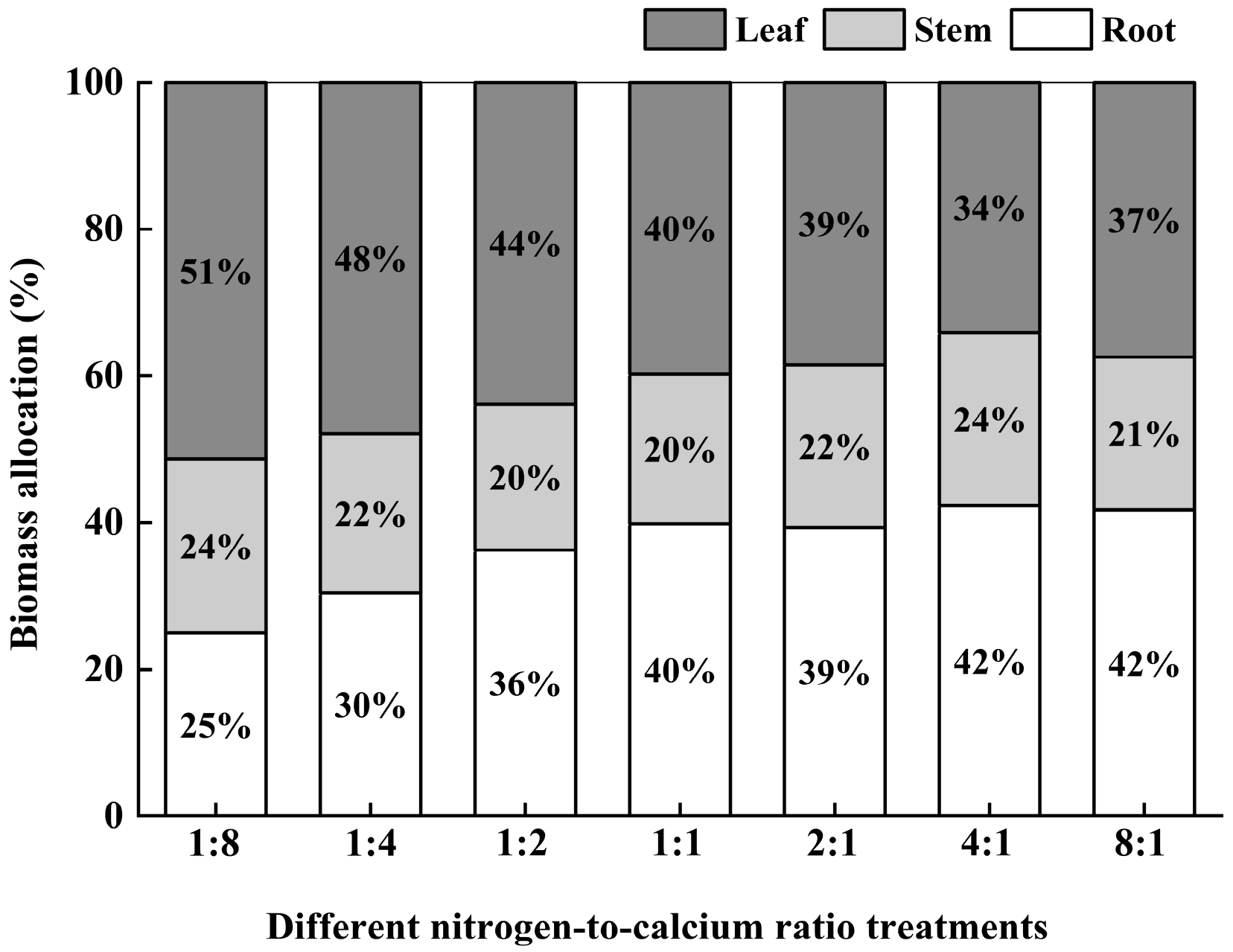
3.7. Biomass and Its Allocation
3.8. Effects of Different N-Ca Ratios on the Average Membership Functions of Mongolian Pine Seedlings
4. Discussion
4.1. An Optimal Nitrogen-to-Calcium Ratio Exists for the Growth of Mongolian Pine Seedlings
4.2. Imbalanced Nitrogen-to-Calcium Ratios Inhibit the Growth and Physiological Activities of Mongolian Pine Seedlings
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ohyama, T. Nitrogen as a Major Essential Element of Plants. Nitrogen Assim. Plants. 2010, 37, 1–17. [Google Scholar]
- Wei, C.Z.; Yu, Q.; Bai, E.; Lü, X.T.; Li, Q.; Xia, J.Y.; Kardol, P.; Liang, W.J.; Wang, Z.W.; Han, X.G. Nitrogen Deposition Weakens Plant–Microbe Interactions in Grassland Ecosystems. Glob. Change Biol. 2013, 19, 3688–3697. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.; Li, J.Y.; He, Y.D.; Shankar, A.; Saxena, A.; Tiwari, A.; Maturi, K.C.; Solanki, M.K.; Singh, V.; Eissa, M.A.; et al. Role of Calcium Nutrition in Plant Physiology: Advances in Research and Insights into Acidic Soil Conditions—A Comprehensive Review. Plant Physiol. Biochem. 2024, 210, 108602. [Google Scholar] [CrossRef]
- Naz, M.; Afzal, M.R.; Raza, M.A.; Pandey, S.; Qi, S.S.; Dai, Z.C.; Du, D.L. Calcium (Ca2+) Signaling in Plants: A Plant Stress Perspective. S. Afr. J. Bot. 2024, 169, 464–485. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Rossiello, R.O.P. Mineral Nitrogen in Plant Physiology and Plant Nutrition. Crit. Rev. Plant Sci. 1995, 14, 111–148. [Google Scholar] [CrossRef]
- Weil, S.; Barker, A.V.; Zandvakili, O.R.; Etemadi, F. Plant Growth and Calcium and Potassium Accumulation in Lettuce under Different Nitrogen Regimes of Ammonium and Nitrate Nutrition. J. Plant Nutr. 2020, 44, 270–281. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Lashari, A.A. Role of Nitrogen for Plant Growth and Development: A Review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Pandey, N. Role of Plant Nutrients in Plant Growth and Physiology. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018. [Google Scholar]
- Paradisone, V.; Navarro-León, E.; Ruiz, J.M.; Esposito, S.; Blasco, B. Calcium Silicate Ameliorates Zinc Deficiency and Toxicity Symptoms in Barley Plants through Improvements in Nitrogen Metabolism and Photosynthesis. Acta Physiol Plant. 2021, 43, 154. [Google Scholar] [CrossRef]
- Wang, J.G.; Geng, Y.; Zhang, J.L.; Li, L.; Guo, F.; Yang, S.; Zou, J.; Wan, S.B. Increasing Calcium and Decreasing Nitrogen Fertilizers Improves Peanut Growth and Productivity by Enhancing Photosynthetic Efficiency and Nutrient Accumulation in Acidic Red Soil. Agronomy 2023, 13, 1924. [Google Scholar] [CrossRef]
- Weng, X.H.; Li, H.; Zhou, Y.B.; Ren, C.S.; Zhang, S.Z.; Liu, L.Y. Relative Availability of Nitrogen and Calcium Regulates the Growth of Poplar Seedlings Due to Transcriptome Changes. Forests 2023, 14, 1899. [Google Scholar] [CrossRef]
- Milani, M.; Pradella, E.M.; Heintze, W.; Schafer, G.; Bender, R.J. Nitrogen and Calcium Fertilization on the Growth and Development of Gerbera Cultivated in Pots for Cut Flowers. Ornam. Hortic. 2021, 27, 288–295. [Google Scholar] [CrossRef]
- Ozyhar, T.; Marchi, M.; Facciotto, G.; Bergante, S.; Luster, J. Combined Application of Calcium Carbonate and Npks Fertilizer Improves Early-Stage Growth of Poplar in Acid Soils. For. Ecol. Manag. 2022, 514, 120211. [Google Scholar] [CrossRef]
- Cao, H.Y.; Du, Y.J.; Gao, G.L.; Rao, L.Y.; Ding, G.D.; Zhang, Y. Afforestation with Pinus sylvestris Var. Mongolica Remodelled Soil Bacterial Community and Potential Metabolic Function in the Horqin Desert. Glob. Ecol. Conserv. 2021, 29, e01716. [Google Scholar]
- Li, H.; Huo, Y.; Weng, X.H.; Zhou, Y.B.; Sun, Y.; Zhang, G.Q.; Zhang, S.Z.; Liu, L.Y.; Pei, J.B. Regulation of the Growth of Mongolian Pine (Pinus sylvestris Var. Mongolica) by Calcium-Water Coupling in a Semiarid Region. Ecol. Indic. 2022, 137, 20220441675. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, J.T.; Yu, W.J.; Li, Q.; Feng, Z.D.; Zhang, H. Resilience and Community Dynamics of Understorey Vegetation in Mongolian Pine Plantations at the Southeastern Edge of the Mu Us Sandy Land, China. For. Ecol. Manag. 2024, 555, 121723. [Google Scholar] [CrossRef]
- Zhu, J.J.; Li, E.Q.; Xu, M.L.; Kang, H.Z.; Wu, X.Y. The Role of Ectomycorrhizal Fungi in Alleviating Pine Decline in Semiarid Sandy Soil of Northern China: An Experimental Approach. Ann. For. Sci. 2008, 65, 12. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, P.; Xiao, T.; Cao, W.; Gong, G.L. The Sand Fixation Effects of Three-North Shelter Forest Program in Recent 35 Years. Sci. Geogr. Sin. 2018, 38, 600–609. [Google Scholar]
- Zhu, J.J.; Kang, H.Z.; Tan, H.; Xu, M.L. Effects of Drought Stresses Induced by Polyethylene Glycol on Germination of Pinus sylvestris Var. Mongolica Seeds from Natural and Plantation Forests on Sandy Land. J. For. Res. 2017, 11, 319–328. [Google Scholar]
- Li, J.T.; Xie, Y.Y.; Wulan, T.Y.; Liu, H.Y.; Wang, X.J.; Zheng, Y.; Qi, Q.G.; Gao, Z.X.; Gao, S.Y.; Shen, Z.H. Drought Resilience of Mongolian Scotch Pine (Pinus sylvestris Var. Mongolica) at the Southernmost Edge of Its Natural Distribution: A Comparison of Natural Forests and Plantations. For. Ecol. Manag. 2023, 542, 121104. [Google Scholar] [CrossRef]
- Song, L.N.; Zhu, J.J.; Zheng, X.; Wang, K.; Zhang, J.X.; Hao, G.Y.; Liu, J.H. Comparison of canopy transpiration between Pinus sylvestris var. mongolica and Pinus tabuliformis plantations in a semiarid sandy region of Northeast China. Agric. For. Meteorol. 2022, 314, 108784. [Google Scholar] [CrossRef]
- Khan, A.; Zhang, X.; Zhang, K.B.; Iqbal, A.; Ahmad, A.; Saeed, S.; Hayat, M.; Tang, X.H. Tree distribution pattern, growing stock characteristics and biomass carbon density of mongolian scots pine (Pinus sylvestris var. Mongolica) plantation of Horqin Sandy Land, China. Pak. J. Bot. 2020, 52, 995–1002. [Google Scholar]
- Mandal, S.; Banik, G.C. Forest Degradation and Its Impact on Soil Carbon. In Forest Degradation and Management; Shukla, G., Manohar, K., Raj Kizha, A., Panwar, A., Chakravarty, P., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar]
- Xie, X.M. Soil and Plant Nutrition Experiments; Zhejiang University Press: Hangzhou, China, 2014. [Google Scholar]
- Li, X.J.; Zhang, G.Q.; Li, H.; Sun, Y.; Huo, Y.; Huang, S.L.; Zhang, S.Z.; Liu, L.Y.; Zhou, Y.B. Effects of Exogenous Calcium on the Growth and Physiological Characteristics of Pinus sylvestris Var. Mongolica Seedlings in Sandy Land. Chin. J. Soil Sci. 2021, 52, 1095–1103. [Google Scholar]
- Li, H.; Huang, S.L.; Ren, C.S.; Weng, X.H.; Zhang, S.Z.; Liu, L.Y.; Pei, J.B. Optimal Exogenous Calcium Alleviates the Damage of Snow-Melting Agent to Salix matsudana Seedlings. Front. Plant Sci. 2022, 13, 928092. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplastes. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Weng, X.H.; Li, H.; Ren, C.S.; Zhou, Y.B.; Zhu, W.X.; Zhang, S.Z.; Liu, L.Y. Calcium Regulates Growth and Nutrient Absorption in Poplar Seedlings. Front. Plant Sci. 2022, 13, 887098. [Google Scholar] [CrossRef]
- Perveen, S.; Saeed, M.; Parveen, A.; Javed, M.T.; Zafar, S.; Iqbal, N. Modulation of Growth and Key Physiobiochemical Attributes after Foliar Application of Zinc Sulphate (ZnSO4) on Wheat (Triticum aestivum L.) under Cadmium (Cd) Stress. Physiol. Mol. Biol. Plants. 2020, 26, 1787–1797. [Google Scholar] [CrossRef]
- Ezzat, A.; Szabó, S.; Szabó, Z.; Hegedűs, A.; Berényi, D.; Holb, I.J. Temporal Patterns and Inter-Correlations among Physical and Antioxidant Attributes and Enzyme Activities of Apricot Fruit Inoculated with Monilinia laxa under Salicylic Acid and Methyl Jasmonate Treatments under Shelf-Life Conditions. J. Fungi. 2021, 7, 341. [Google Scholar] [CrossRef]
- Liang, X.X.; Liang, F.; Tan, Z.Q.; Huang, X.Y.; Liang, S.Y. Effects of Fertilization with Different Nitrogen-to Calcium Ratios on Growth and Physiological Traits of Endangered Plant Excentrodendron tonkinense Seedlings. Guihaia 2025, 45, 121–132. [Google Scholar]
- Eckstein, R.L.; Karlsson, P.S. Above-Ground Growth and Nutrient Use by Plants in a Subarctic Environment: Effects of Habitat, Life-Form and Species. Oikos 1997, 79, 311–324. [Google Scholar] [CrossRef]
- Kim, S.G.; Lee, J.S.; Bae, H.H.; Kim, J.T.; Son, B.Y.; Kim, S.L.; Baek, S.B.; Shin, S.; Jeon, W.T. Physiological and Proteomic Analyses of Korean F1 Maize (Zea mays L.) Hybrids under Water-Deficit Stress during Flowering. Appl. Biol. Chem. 2019, 62, 32. [Google Scholar] [CrossRef]
- Farooq, T.H.; Shakoor, A.; Rashid, M.H.U.; Zhang, S.Y.; Wu, P.F.; Yan, W.D. Annual Growth Progression, Nutrient Transformation, and Carbon Storage in Tissues of Cunninghamia lanceolata Monoculture in Relation to Soil Quality Indicators Influenced by Intraspecific Competition Intensity. J. Soil Sci. Plant Nutr. 2021, 21, 3146–3158. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Vogt, K.A.; Vogt, D.J.; Wargo, P.M.; Tilley, J.P.; Siccama, T.G.; Sigurdardottir, R.; Ludwig, D. Nitrogen and Calcium Additions Increase Forest Growth in Northeastern USA Spruce–Fir Forests. Can. J. For. Res. 2007, 37, 1574–1585. [Google Scholar] [CrossRef]
- Yadav, M.R.; Kumar, R.; Parihar, C.M.; Yadav, R.K.; Jat, S.L.; Ram, H.; Meena, R.K.; Singh, M.; Birbal; Verma, A.P.; et al. Strategies for Improving Nitrogen Use Efficiency: A Review. Agric. Rev. 2017, 38, 29–40. [Google Scholar] [CrossRef]
- Zhang, T.Z.; Li, Y.N.; Li, H.; Zhang, S.Z.; Zhou, Y.B. Optimal Soil Calcium for the Growth of Mulberry Seedlings Is Altered by Nitrogen Addition. Forests 2023, 14, 399. [Google Scholar] [CrossRef]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. Fertilizers and Fertilization Strategies Mitigating Soil Factors Constraining Efficiency of Nitrogen in Plant Production. Plants 2022, 11, 1855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, Y.Y.; Sheng, D.C.; Zhang, S.Y.; Gu, S.C.; Yan, Y.; Zhao, F.C.; Wang, P.; Huang, S.B. Optimizing Root System Architecture to Improve Root Anchorage Strength and Nitrogen Absorption Capacity under High Plant Density in Maize. Field Crops Res. 2023, 303, 109109. [Google Scholar] [CrossRef]
- Voitsekhovskaja, O.V.; Tyutereva, E.V. Chlorophyll B in Angiosperms: Functions in Photosynthesis, Signaling and Ontogenetic Regulation. J. Plant Physiol. 2015, 189, 51–64. [Google Scholar] [CrossRef]
- Ullah, A.; Sun, H.; Yang, X.Y.; Zhang, X.L. Drought Coping Strategies in Cotton: Increased Crop Per Drop. Plant Biotechnol. J. 2017, 15, 271–284. [Google Scholar] [CrossRef]
- Kaur, H.; Kohli, S.K.; Khanna, K.; Bhardwaj, R. Scrutinizing the Impact of Water Deficit in Plants: Transcriptional Regulation, Signaling, Photosynthetic Efficacy, and Management. Physiol. Plant. 2021, 172, 935–962. [Google Scholar] [CrossRef]
- Liu, H.; Song, S.B.; Zhang, H.; Li, Y.H.; Niu, L.J.; Zhang, J.H.; Wang, W. Signaling Transduction of Aba, Ros, and Ca2+ in Plant Stomatal Closure in Response to Drought. Int. J. Mol. Sci. 2022, 23, 14824. [Google Scholar] [CrossRef]
- Zou, W.T.; Chen, S.; Teng, J.C. Effects of Nitrogen and Calcium on Anti-Oxidative Enzyme System and Active Oxygen of Flue-Cured Tobacco Leaf during Fast Growing Period. J. Northwest AF Univ. 2016, 44, 97–103. [Google Scholar]
- Zhang, G.W.; Liu, Z.L.; Zhou, J.G.; Zhu, Y.L. Effects of Ca(NO3)2 Stress on Oxidative Damage, Antioxidant Enzymes Activities and Polyamine Contents in Roots of Grafted and Non-Grafted Tomato Plants. Plant Growth Regul. 2008, 56, 7–19. [Google Scholar] [CrossRef]
- Hammond, J.P.; White, P.J. Sucrose Transport in the Phloem: Integrating Root Responses to Phosphorus Starvation. J. Exp. Bot. 2007, 59, 93–109. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of Osmoprotectants in Improving Salinity and Drought Tolerance in Plants: A Review. Rev. Environ. Sci. Bio Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Al-Qurainy, F.; Foolad, M.R. Chapter Five—Drought Tolerance: Roles of Organic Osmolytes, Growth Regulators, and Mineral Nutrients. Adv. Agron. 2011, 111, 249–296. [Google Scholar]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell. 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Teraoka, K. Accumulation of Sugars in Cucumber Leaves during Calcium Starvation. Plant Cell Physiol. 1980, 21, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Karthika, K.S.; Rashmi, I.; Parvathi, M.S. Biological Functions, Uptake and Transport of Essential Nutrients in Relation to Plant Growth. In Plant Nutrients and Abiotic Stress Tolerance; Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, K., Eds.; Springer: Singapore, 2018; pp. 1–49. [Google Scholar]
- Wolf, S.; Hématy, K.; Höfte, H. Growth Control and Cell Wall Signaling in Plants. Annu. Rev. Plant Biol. 2012, 63, 381–407. [Google Scholar] [CrossRef]
- López-Lefebre, L.R.; Rivero, R.M.; García, P.C.; Sanchez, E.; Ruiz, J.M.; Romero, L. Effect of Calcium on Mineral Nutrient Uptake and Growth of Tobacco. J. Sci. Food Agric. 2001, 81, 1334–1338. [Google Scholar] [CrossRef]
- Mu, X.H.; Chen, Y.L. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Kong, L.G.; Xie, Y.Y.; Hu, L.; Si, J.S.; Wang, Z.S. Excessive Nitrogen Application Dampens Antioxidant Capacity and Grain Filling in Wheat as Revealed by Metabolic and Physiological Analyses. Sci. Rep. 2017, 7, 43363. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A. Role of Nitrogen (N) in Plant Growth, Photosynthesis Pigments, and N Use Efficiency: A Review. Agrisost 2022, 28, 1–8. [Google Scholar]
- Fujita, M.; Hasanuzzaman, M. Approaches to Enhancing Antioxidant Defense in Plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Vicidomini, C.; Palumbo, R.; Moccia, M.; Roviello, G.N. Oxidative Processes and Xenobiotic Metabolism in Plants: Mechanisms of Defense and Potential Therapeutic Implications. J. Xenobiot. 2024, 14, 1541–1569. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Tariq, A.; Zeng, F.J.; Graciano, C.; Zhang, B. Nitrogen Application Mitigates Drought-Induced Metabolic Changes in Alhagi Sparsifolia Seedlings by Regulating Nutrient and Biomass Allocation Patterns. Plant Physiol. Biochem. 2020, 155, 828–841. [Google Scholar] [CrossRef] [PubMed]
| N-Ca | Leaf Biomass (g) | Stem Biomass (g) | Root Biomass (g) | Total Biomass (g) |
|---|---|---|---|---|
| 1:8 | 9.14 ± 0.328 e | 4.23 ± 0.632 d | 4.44 ± 0.344 f | 16.52 ± 0.329 f |
| 1:4 | 10.98 ± 0.409 cd | 4.96 ± 0.675 cd | 6.97 ± 0.817 e | 22.92 ± 0.437 e |
| 1:2 | 11.55 ± 0.379 bc | 5.23 ± 0.322 bcd | 9.55 ± 0.347 d | 25.74 ± 0.909 d |
| 1:1 | 12.10 ± 0.134 b | 6.19 ± 0.900 b | 13.12 ± 0.312 b | 30.29 ± 0.535 c |
| 2:1 | 14.27 ± 0.826 a | 8.19 ± 0.484 a | 14.56 ± 0.726 a | 36.78 ± 1.283 a |
| 4:1 | 10.65 ± 0.456 d | 7.38 ± 1.067 a | 13.21 ± 0.169 b | 33.55 ± 0.448 b |
| 8:1 | 10.54 ± 1.039 d | 5.87 ± 0.512 bc | 11.74 ± 0.131 c | 30.21 ± 0.512 c |
| Parameter | 1:8 | 1:4 | 1:2 | 1:1 | 2:1 | 4:1 | 8:1 |
|---|---|---|---|---|---|---|---|
| Plant height | 0.022 | 0.683 | 0.613 | 0.817 | 0.919 | 0.785 | 0.538 |
| Base diameter | 0.046 | 0.154 | 0.505 | 0.525 | 0.882 | 0.766 | 0.596 |
| Fv/Fm | 0.045 | 0.221 | 0.960 | 0.993 | 0.999 | 0.982 | 0.960 |
| Fv/F0 | 0.021 | 0.047 | 0.847 | 0.954 | 0.988 | 0.903 | 0.803 |
| Chlorophyll a | 0.118 | 0.249 | 0.671 | 0.777 | 0.840 | 0.791 | 0.768 |
| Chlorophyll b | 0.059 | 0.235 | 0.718 | 0.753 | 0.845 | 0.734 | 0.697 |
| Chlorophyll a+b | 0.009 | 0.193 | 0.705 | 0.892 | 0.980 | 0.902 | 0.876 |
| Carotinoid | 0.037 | 0.082 | 0.531 | 0.776 | 0.945 | 0.878 | 0.843 |
| Photosynthetic rate | 0.068 | 0.206 | 0.815 | 0.875 | 0.973 | 0.771 | 0.709 |
| Conductance to H2O | 0.050 | 0.275 | 0.804 | 0.862 | 0.928 | 0.727 | 0.660 |
| Transpiration rate | 0.035 | 0.211 | 0.646 | 0.877 | 0.986 | 0.819 | 0.811 |
| Intercellular CO2 concentration | 0.020 | 0.275 | 0.351 | 0.418 | 0.825 | 0.667 | 0.449 |
| Soluble sugar | 0.01 | 0.21 | 0.48 | 0.65 | 0.97 | 0.70 | 0.56 |
| Starch | 0.04 | 0.30 | 0.44 | 0.85 | 0.92 | 0.86 | 0.77 |
| NSC | 0.02 | 0.25 | 0.46 | 0.73 | 0.95 | 0.76 | 0.64 |
| Soluble protein | 0.056 | 0.333 | 0.364 | 0.535 | 0.895 | 0.394 | 0.364 |
| POD activity | 0.017 | 0.120 | 0.387 | 0.397 | 0.919 | 0.631 | 0.490 |
| CAT activity | 0.043 | 0.117 | 0.402 | 0.612 | 0.946 | 0.470 | 0.275 |
| SOD activity | 0.059 | 0.267 | 0.300 | 0.711 | 0.861 | 0.453 | 0.241 |
| Average membership function value | 0.045 | 0.237 | 0.570 | 0.722 | 0.921 | 0.720 | 0.611 |
| Comprehensive sorting | 7 | 6 | 5 | 2 | 1 | 3 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Li, H.; Huo, Y.; Weng, X.; Wang, H. Nitrogen–Calcium Stoichiometry Regulates Growth and Physiology in Mongolian Pine (Pinus sylvestris var. mongolica). Forests 2025, 16, 1809. https://doi.org/10.3390/f16121809
Huang S, Li H, Huo Y, Weng X, Wang H. Nitrogen–Calcium Stoichiometry Regulates Growth and Physiology in Mongolian Pine (Pinus sylvestris var. mongolica). Forests. 2025; 16(12):1809. https://doi.org/10.3390/f16121809
Chicago/Turabian StyleHuang, Shenglan, Hui Li, Yan Huo, Xiaohang Weng, and Hongbo Wang. 2025. "Nitrogen–Calcium Stoichiometry Regulates Growth and Physiology in Mongolian Pine (Pinus sylvestris var. mongolica)" Forests 16, no. 12: 1809. https://doi.org/10.3390/f16121809
APA StyleHuang, S., Li, H., Huo, Y., Weng, X., & Wang, H. (2025). Nitrogen–Calcium Stoichiometry Regulates Growth and Physiology in Mongolian Pine (Pinus sylvestris var. mongolica). Forests, 16(12), 1809. https://doi.org/10.3390/f16121809






