Morphological Anatomy, Developmental Characteristics of the Reproductive System in Arhopalus rusticus (Coleoptera: Cerambycidae) and Their Impacts on the Transmission Potential of Bursaphelenchus xylophilus (Aphelenchida: Parasitaphelenchidae)
Abstract
1. Introduction
2. Materials and Methods
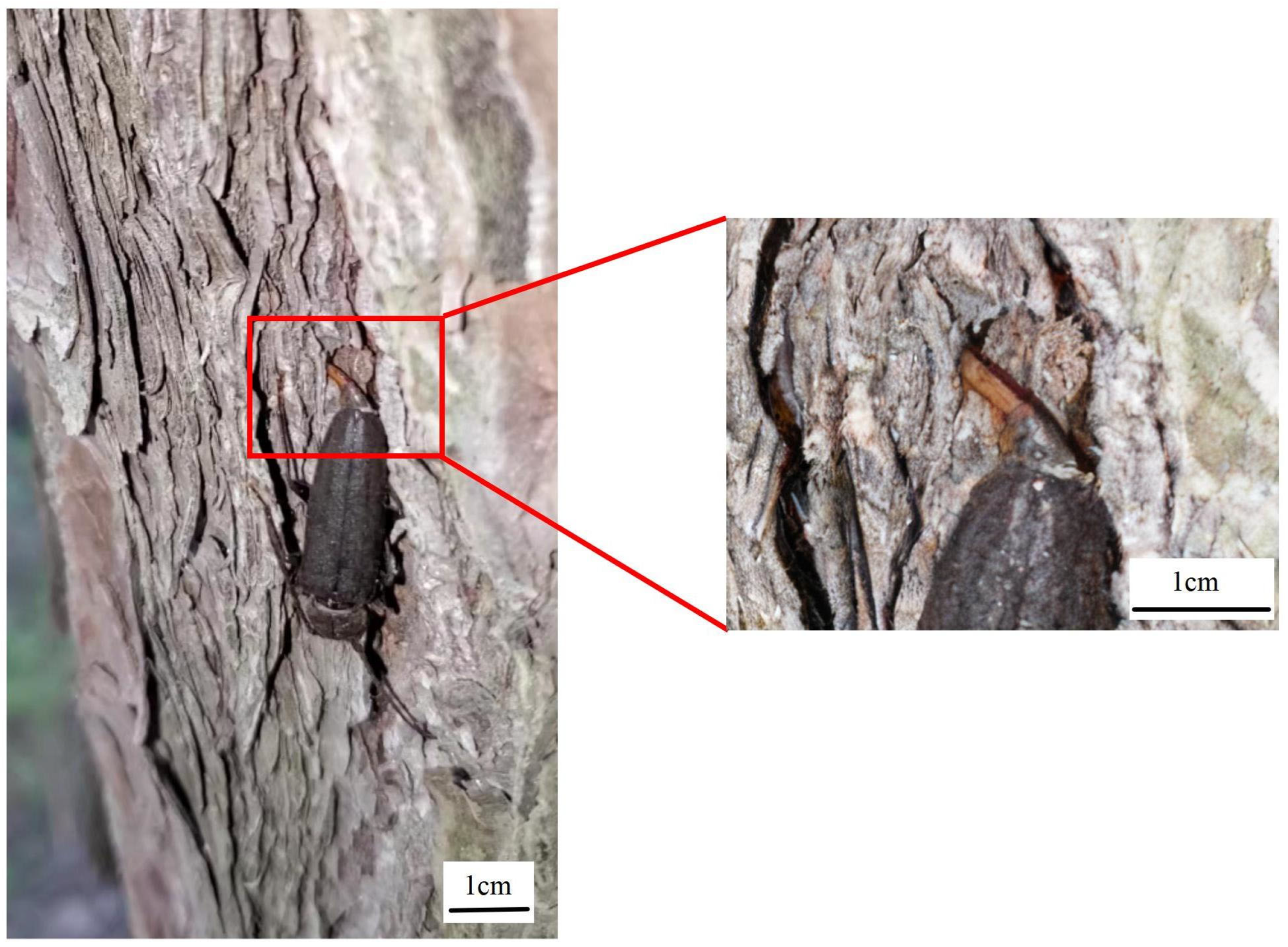
2.1. Experimental Design and Sample Collection
2.2. Anatomy and Image Acquisition Methods
2.3. Determination of Soluble Total Sugar and Protein Content
2.4. Analysis Methods
3. Results
3.1. Morphological Anatomy of the Reproductive System of A. rusticus
3.1.1. Morphological Anatomy of the Female Reproductive System
3.1.2. Anatomy of the Male Reproductive System
3.2. The Differences in the Structure of External Reproductive Organs and the Oviposition Methods of the Females of A. rusticus and M. alternatus
3.2.1. Structural Differences in the Female Genitalia
3.2.2. The Differences in Oviposition Methods Between A. rusticus and M. alternatus
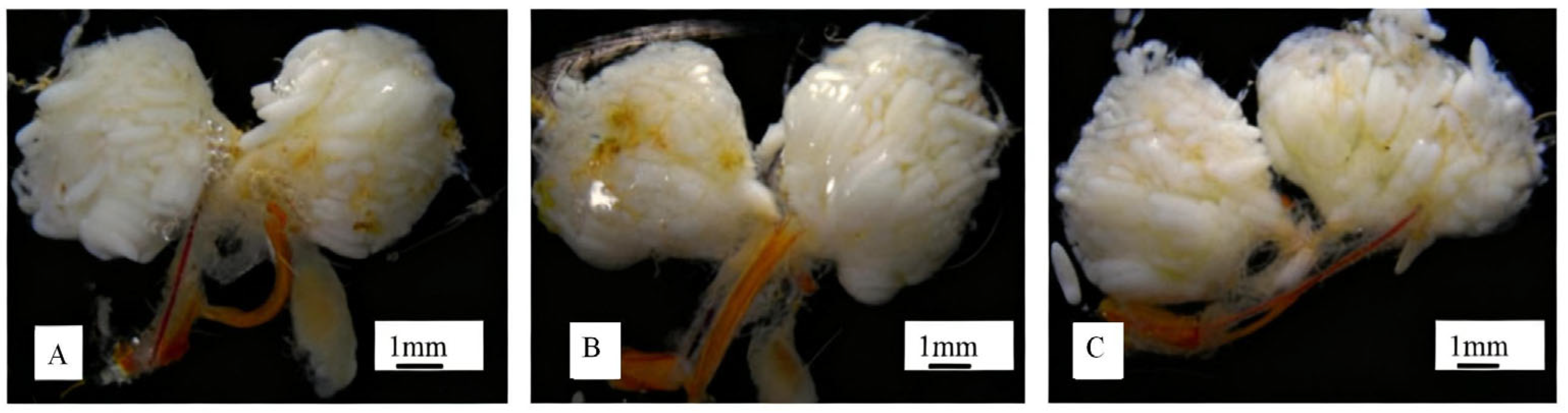
3.3. The Development of the Ovaries of Adult Female A. rusticus at Different Ages
3.4. Changes in Total Soluble Sugar and Protein Content of Ovaries Before and After Gnawing
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, R. Study on Occurrence of Arhopalus rusticus in Coastal Areas of Shandong Province and the Nematodes Carried by Adults. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2018. (In Chinese). [Google Scholar]
- Peng, C.L. Taxonomic and Phylogeny of Chinese Spondylidinae (Coleoptera, Cerambycidae). Master’s Thesis, Southwest University, Chongqing, China, 2019. (In Chinese). [Google Scholar]
- Shi, S.Q. Systematics of Aswiminae and Disteniidae from China. Master’s Thesis, Southwest University, Chongqing, China, 2012. (In Chinese). [Google Scholar]
- Ye, J.R. Epidemic status of pine wilt disease in China and its prevention and control techniques and counter measures. J. For. Res. 2019, 55, 1–10. [Google Scholar]
- Linit, M.J.; Kondo, E.; Smith, M.T. Insects associated with the pinewood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae), in Missouri. Environ. Entomol. 1983, 12, 467–470. [Google Scholar] [CrossRef]
- Jurc, M.; Bojovic, S.; Fernández, M.F.; Jurc, D. The attraction of cerambycids and other xylophagous beetles, potential vectors of Bursaphelenchus xylophilus, to semio-chemicals in Slovenia. Phytoparasitica 2012, 40, 337–349. [Google Scholar] [CrossRef]
- Enda, N.; Mamiya, Y. Transmission of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) By Monochamus alternatus (Coleoptera: Cerambycidae). Nematology 1972, 18, 159–162. [Google Scholar]
- Ridley, G.; Bain, J.; Dick, M. Exotic nematode found in pine trees in Melbourne, Victoria. N. Z. J. For. 2001, 46, 41–42. [Google Scholar]
- Zhang, J.J.; Zhang, R.Z.; Chen, J.Y. Species and their dispersal ability of Monochamusas vectors to transmit Bursaphelenchus xylophilus. J. Zhejiang Agric. For. Univ. 2007, 24, 350–356. (In Chinese) [Google Scholar]
- Zhao, J.N.; Yu, S.M.; Wang, H.J.; Yao, J.F.; Ding, D.G. Pine borers in Huangshan Scenie Area and the potential for carrying nematodes by them. For. Pest Dis. 2004, 23, 15–18. (In Chinese) [Google Scholar]
- Chen, Q. Spatial Distribution and Control Techniques of Arhopalus rusticus (Innaeus). Master’s Thesis, Beijing Forestry University, Beijing, Chian, 2018. (In Chinese). [Google Scholar]
- Lv, Y.C.; Chen, G.F.; Zhang, X.D.; Chi, Z.Q.; Wang, C.Z.; Xu, J.; Dai, Q.H.; Zhang, Q.F.; Liu, R.M. Occurrence and damage status of Arhopalus rusticus in China and management countermeasures. J. Shandong For. Sci. Technol. 2021, 51, 96–100. (In Chinese) [Google Scholar]
- Wu, H.W.; Luo, Y.Q.; Yu, H.Y.; Tao, J.; Xie, C.C.; Ren, L.L.; Wang, C.Z.; Zhang, X.W.; Zhao, H.H.; Liu, Z.Y.; et al. Ability of Arhopalus rusticus in carrying and transmitting Bursaphelenchus xylophilus. For. Pest Dis. 2022, 41, 29–36. (In Chinese) [Google Scholar]
- Wang, Y. Vector Longicorn Beetles of Bursaphelenchus xylophilus and the Mechanism of Bursaphelenchus xylophilus Detaching from Vector Longicorn Beetles. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2020. (In Chinese). [Google Scholar]
- Hou, R.; Tian, S.G.; Liu, X.; Shao, Z.M.; Liu, Z.Y.; Lu, X.P. Control effect of several pesticides on larvae of Arhopalus rusticus (L.). For. Pest Dis. 2019, 38, 38–41. (In Chinese) [Google Scholar]
- Goble, T.A.; Hajek, A.E.; Jackson, M.A.; Gardescu, S. Microsclerotia of Metarhizium brunneum F52 applied in hydromulch for control of Asian longhorned beetles (Coleoptera: Cerambycidae). Econ. Entomol. 2015, 108, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Yu, N. Researches on Biological Characteristics and Adult Emergence Period Prediction of Arhopalus rusticus. Master’s Thesis, Shandong Agriculture University, Tai′an, China, 2017. (In Chinese). [Google Scholar]
- Chen, J.; Li, H.; Hu, T.Y.; Yang, H.L.; Wei, J.H.; Jin, L.; Hao, D.J. Morphology and development of the internal reproductive system Monochamus alternatus (Coleoptera: Cerambycidae) adults. Acta Entomol. Sin. 2023, 66, 1210–1220. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yan, X.Y. Morphology and Function Study of Mouthpartsand Genitalia Structure on Cerambycidae (Coleoptera). Master’s Thesis, Southwest Universitu, Chongqing, China, 2018. (In Chinese). [Google Scholar]
- Zhang, Y.H.; Hao, D.J.; Wang, Y.; Dai, H.C. The mating and ovipositing behavor of Monochamus alernatus. Chin. Bull. Entomol. 2006, 43, 47–49. (In Chinese) [Google Scholar]
- Lin, C.C.; Lu, G.; Zhou, C.M.; Zhao, J.N. Effect of Supplemental Nutritional Materials on the Adult Longevity of Monochamus alternatus. For. Res. 2003, 16, 69–74. (In Chinese) [Google Scholar]
- Lin, C.C.; Lai, M.H.; Lu, G.; Cai, D.Y.; Zhou, C.M.; Zhao, J.N. Effect of Supplemental Nutritional Materials on the Fecundity of Female Adults of Monochamus alternatus. For. Res. 2003, 16, 398–403. (In Chinese) [Google Scholar]
- Li, Z.Q.; Zhou, P.Y.; Li, L.J.; Xie, F.; Lang, W.; Han, H.J. The supplement nutrient and ovipositing behavior of Monochamus alternatus adult on Pinus kesiya var. langbianensis. Plant Quar. 2009, 23, 21–24. (In Chinese) [Google Scholar]
- Hanks, L.M. Influence of the larval host plant on reproductive strategies of cerambycid beetles. Annu. Rev. Entomol. 1999, 44, 483–505. [Google Scholar] [CrossRef]
- Wang, L.C.; Chen, F.M.; Dong, X.Y.; Tian, C.L.; Wang, Y. A study on feeding and oviposition characteristics of Monochamus alternatus. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2023, 47, 219–224. (In Chinese) [Google Scholar]
- Zhao, S.G.; Chen, G.F.; Qu, H.C.; Xu, S.L.; Lu, Y.H.; Wang, J. Advances in studies on biological and ecological characteristics of Monochamus saltuarius. For. Pest Dis. 2021, 40, 37–43. [Google Scholar]
- Heisuke, S.; Takeshi, S.; Mitsunori, K. Transmission of Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle (Nematoda, Aphelenchoididae) by Monochamus salturarius (Gehler) (Coleoptera, Cerambycidae). J. Jpn. For. Soc. 1987, 69, 492–496. [Google Scholar]
- Gao, N.; Yao, H.X.; Jiang, X.F.; Zhang, X.; Li, Y.; Lu, X.P. Niche of Monochamus alternatus and Arhopalus rusticus in Pinus thunbergii infected with Bursaphelenchus xylophilus. For. Pest Dis. 2013, 32, 4–7. [Google Scholar]
- Man, H.Y. Study on the Spatial-Temporal Niche Characteristics and It’s Influencing Factors Between Monochamus alternatus and Arhopabs rusticus. Master’s Thesis, Shandong Agriculture University, Tai′an, China, 2022. [Google Scholar]
- Huang, P. Three important forest pest species of the genus Monochamus. Plant Quar. 2008, 234–235. [Google Scholar] [CrossRef]
- Whitehouse, H.J. Forest fires and insects: Palaeoentomological research from a subfossil burntforest. Palaeogeogr. Palaeoecol. 2000, 164, 231–246. [Google Scholar] [CrossRef]






| Tissues and Organs | Total Carbohydrates | Total Proteins |
|---|---|---|
| Ovary before gnawing | 0.9415 ± 0.0892 | 24.0092 ± 18.1527 |
| Ovary after gnawing | 0.8526 ± 0.0949 | 19.7379 ± 7.1393 |
| Male reproductive system before gnawing | 0.2390 ± 0.1297 | 4.8818 ± 1.5119 |
| Male reproductive system after gnawing | 0.2420 ± 0.2623 | 6.4472 ± 1.7081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Ren, G.; Wang, J.; Zhong, K.; Chang, Z.; Li, D.; Ma, A.; Qu, Y.; Shi, L.; Duan, B.; et al. Morphological Anatomy, Developmental Characteristics of the Reproductive System in Arhopalus rusticus (Coleoptera: Cerambycidae) and Their Impacts on the Transmission Potential of Bursaphelenchus xylophilus (Aphelenchida: Parasitaphelenchidae). Forests 2025, 16, 1754. https://doi.org/10.3390/f16121754
Wang M, Ren G, Wang J, Zhong K, Chang Z, Li D, Ma A, Qu Y, Shi L, Duan B, et al. Morphological Anatomy, Developmental Characteristics of the Reproductive System in Arhopalus rusticus (Coleoptera: Cerambycidae) and Their Impacts on the Transmission Potential of Bursaphelenchus xylophilus (Aphelenchida: Parasitaphelenchidae). Forests. 2025; 16(12):1754. https://doi.org/10.3390/f16121754
Chicago/Turabian StyleWang, Mengxiao, Guangjuan Ren, Jing Wang, Kai Zhong, Zongtao Chang, Dongqin Li, Anbao Ma, Yongyun Qu, Lei Shi, Beining Duan, and et al. 2025. "Morphological Anatomy, Developmental Characteristics of the Reproductive System in Arhopalus rusticus (Coleoptera: Cerambycidae) and Their Impacts on the Transmission Potential of Bursaphelenchus xylophilus (Aphelenchida: Parasitaphelenchidae)" Forests 16, no. 12: 1754. https://doi.org/10.3390/f16121754
APA StyleWang, M., Ren, G., Wang, J., Zhong, K., Chang, Z., Li, D., Ma, A., Qu, Y., Shi, L., Duan, B., Wu, H., & Zhang, X. (2025). Morphological Anatomy, Developmental Characteristics of the Reproductive System in Arhopalus rusticus (Coleoptera: Cerambycidae) and Their Impacts on the Transmission Potential of Bursaphelenchus xylophilus (Aphelenchida: Parasitaphelenchidae). Forests, 16(12), 1754. https://doi.org/10.3390/f16121754





