Determinants of Needleleaf and Broadleaf Decomposition Rates Under and Outside the Parent Tree Stand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Litterfall Observation
2.3. In Situ Decomposition Experiment
2.4. Decomposition Rate of Tusam and Ebony Leaves Under and Outside Their Stand Origin
2.5. Chemical Analysis of Tusam and Ebony Leaves
2.6. Decomposing Agent
2.7. Soil Chemistry
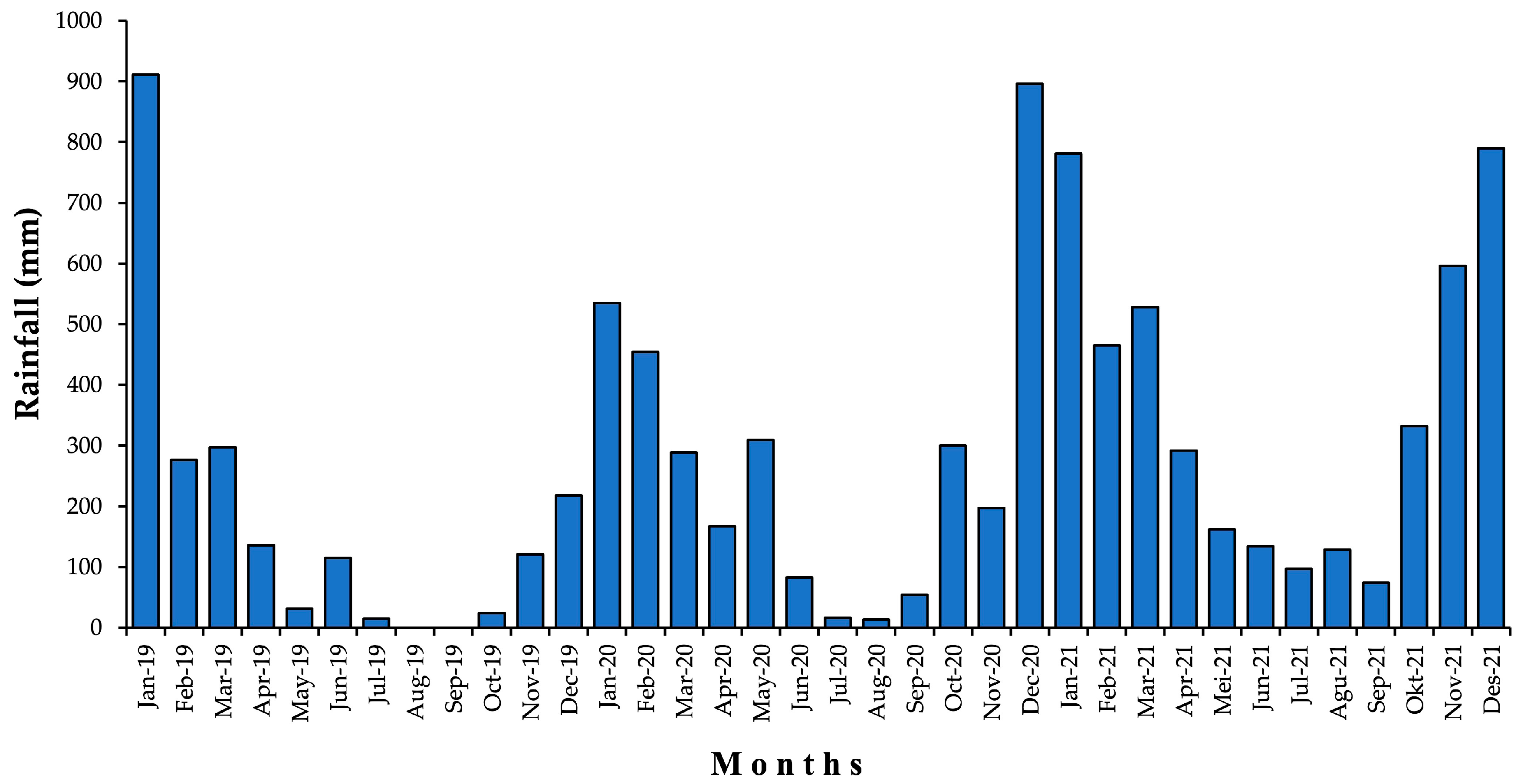
2.8. Climate and Soil Moisture
2.9. Data Analyses
3. Results
3.1. Litterfall and In Situ Decomposition Rate Among the Forest Communities
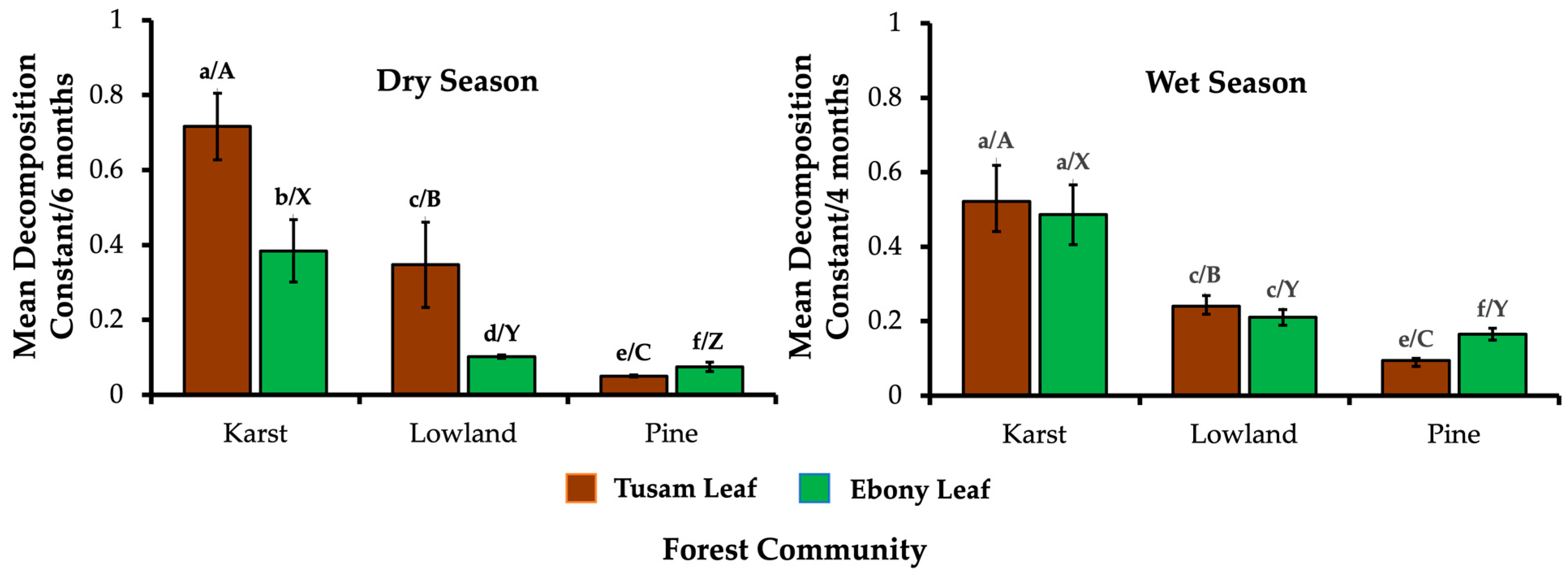
3.2. Decomposition Rate of Tusam and Ebony Leaves Under and Outside Its Stand Origin
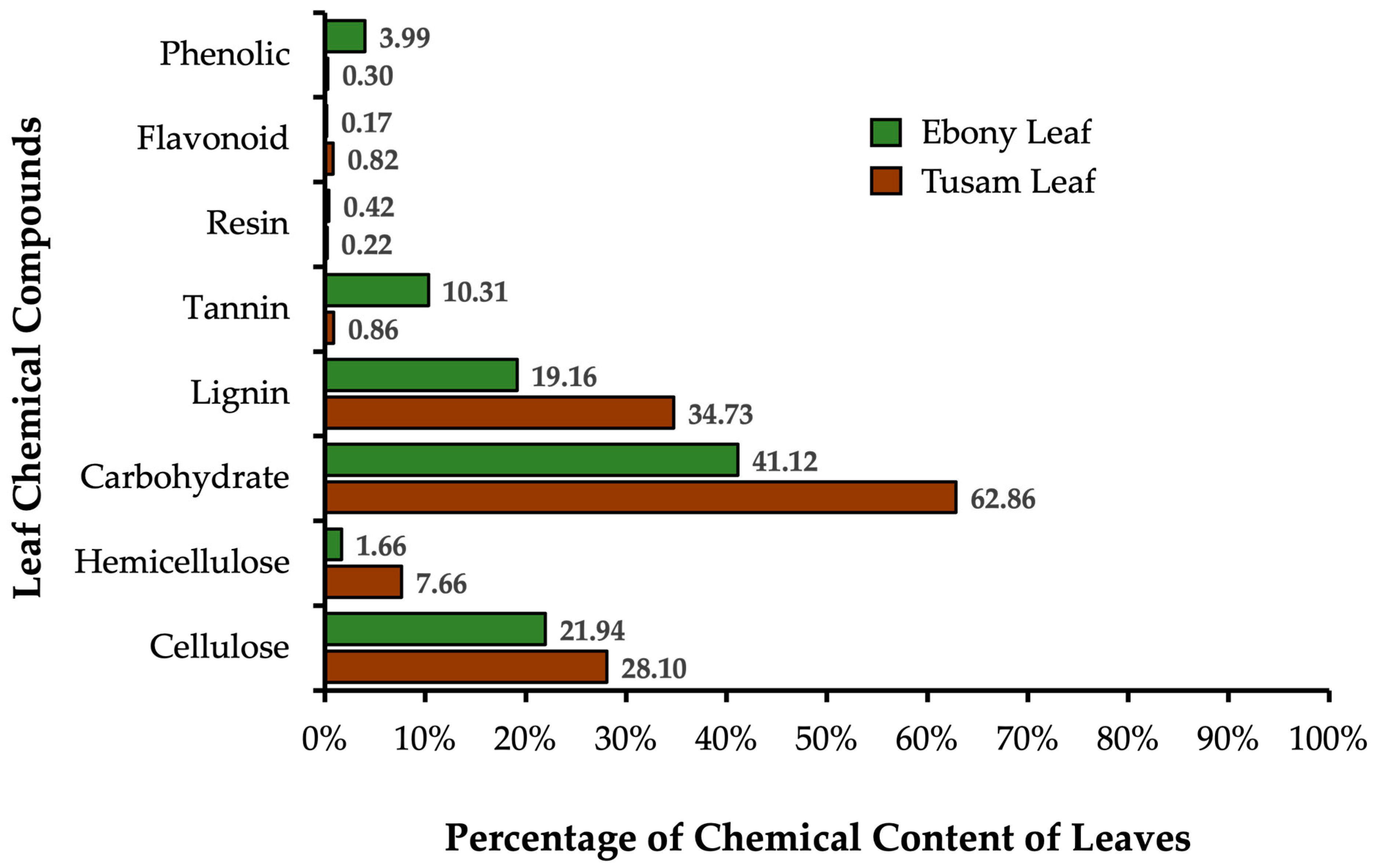
3.3. Chemical Composition of Leaf Litter
3.4. Macroscopic Fungi
3.5. Macrofauna
3.6. Soil Chemical Properties
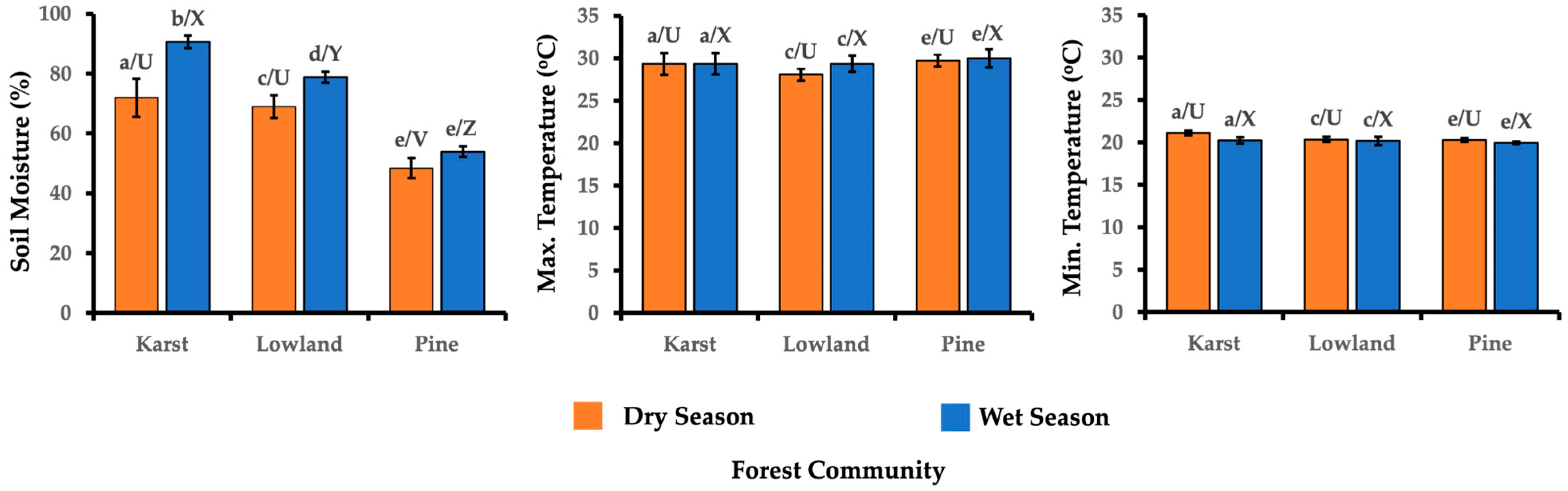
3.7. Soil Moisture and Maximum and Minimum Temperature
3.8. The Correlation Between Biological Factors and the Decomposed Leaf Sample
3.9. The Correlation Between Extrinsic Physical Factors and Decomposed Leaf Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qu, H.; Zhao, X.; Wang, S.; Lian, J.; Tang, X.; Wang, X.; Zhang, R.; Medina-Roldán, E. Abiotic factors affect leaf litter mass loss more strongly than initial litter traits under sand burial conditions. Catena 2021, 196, 104900. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Lebret, M.; Nys, C.; Forgeard, F. Litter production in an Atlantic beech (Fagus sylvatica L.) time sequence. Ann. For. Sci. 2001, 58, 755–768. [Google Scholar] [CrossRef]
- Ge, X.; Zeng, L.; Xiao, W.; Huang, Z.; Geng, X.; Tan, B. Effect of litter substrate quality and soil nutrients on forest litter decomposition: A review. Acta Ecol. Sin. 2013, 33, 102–108. [Google Scholar] [CrossRef]
- Zhou, Y.; Clark, M.; Su, J.; Xiao, C. Litter decomposition and soil microbial community composition in three Korean pine (Pinus koraiensis) forests along an altitudinal gradient. Plant Soil 2015, 386, 171–183. [Google Scholar] [CrossRef]
- Luo, D.; Cheng, R.; Shi, Z.; Wang, W. Decomposition of leaves and fine roots in three subtropical plantations in China affected by litter substrate quality and soil microbial community. Forests 2017, 8, 412. [Google Scholar] [CrossRef]
- Cassani, M.T.; Sabatté, M.L.; Rubín, M.A.R.; Sfeir, A.J.; Massobrio, M.J. Litter decomposition by soil fauna: Effect of land use in agroecosystems. Heliyon 2021, 7, e08127. [Google Scholar] [CrossRef]
- Glassman, S.I.; Weihe, C.; Li, J.; Albright, M.B.; Looby, C.I.; Martiny, A.C.; Treseder, K.K.; Allison, S.D.; Martiny, J.B.H. Decomposition responses to climate depend on microbial community composition. Proc. Natl. Acad. Sci. USA 2018, 115, 11994–11999. [Google Scholar] [CrossRef]
- Sembiring, M.; Munawaroh, H.; Mukhlis, M.; Hidayat, B.; Sabrina, T. Soil macrofauna diversity in andisol after eight years of Mount Sinabung eruption in Sumatra, Indonesia. Biodiversitas 2021, 22, 3024–3030. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Zhang, W.; Zhang, Q.; Lu, D.; Zhang, Y.; Zheng, X.; Xu, S.; Wang, G.G. Litter decomposition and nutrient release from monospecific and mixed litters: Comparisons of litter quality, fauna and decomposition site effects. J. Ecol. 2022, 110, 1673–1686. [Google Scholar] [CrossRef]
- Jacob, M.; Viedenz, K.; Polle, A.; Thomas, F.M. Leaf litter decomposition in temperate deciduous forest stands with a decreasing fraction of beech (Fagus sylvatica). Oecologia 2010, 164, 1083–1094. [Google Scholar] [CrossRef]
- Berg, B.; Lönn, M.; Ni, X.; Sun, T.; Dong, L.; Gainieks, T.; Santo, A.V.D.; Johansson, M.-J. Decomposition rates in late stages of Scots pine and Norway spruce needle litter: Influence of nutrients and substrate properties over a climate gradient. For. Ecol. Manag. 2022, 522, 120450. [Google Scholar] [CrossRef]
- Sohng, J.; Han, A.R.; Jeong, M.-A.; Park, Y.; Park, B.B.; Park, P.S. Seasonal pattern of decomposition and N, P, and C dynamics in leaf litter in a Mongolian Oak Forest and a Korean Pine Plantation. Forests 2014, 5, 2561–2580. [Google Scholar] [CrossRef]
- Klimek, B.; Niklinska, M. Changes in temperature sensitivity of forest litter during decomposition along an altitudinal gradient in temperate mountains—A reciprocal litter transplantation study. CATENA 2024, 240, 107977. [Google Scholar] [CrossRef]
- Phung, V.L.H.; Oka, K.; Honda, Y.; Hijioka, Y.; Ueda, K.; Seposo, X.T.; Sahani, M.; Mahiyuddin, W.R.W.; Kim, Y. Daily temperature effects on under-five mortality in a tropical climate country and the role of local characteristics. Environ. Res. 2023, 218, 114988. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Porporato, A.; Rodriguez-Iturbe, I. Changes in rainfall seasonality in the tropics. Nat. Clim. Change 2013, 3, 811–815. [Google Scholar] [CrossRef]
- Allison, S.T.; Lu, Y.; Weihe, C.; Goulden, M.C.; Martiny, A.C.; Treseder, K.K.; Martiny, J.B.H. Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 2013, 94, 714–725. [Google Scholar] [CrossRef]
- Putra, P.S.; Achmad, A.; Yamada, T.; Ngakan, P.O. Seasonal Decomposition Rates of Broadleaf and Conifer Wood Litter in Far Eastern Tropical Forest Communities. Int. J. For. Res. 2023, 2023, 9677809. [Google Scholar] [CrossRef]
- Sun, J.; Gao, P.; Xu, H.; Li, C.; Niu, X. Decomposition dynamics and ecological stoichiometry of Quercus acutissima and Pinus densiflora litter in the Grain to Green Program Area of Northern China. J. For. Res. 2020, 31, 1613–1623. [Google Scholar] [CrossRef]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; University of California Press: Berkeley, CA, USA, 1979. [Google Scholar]
- Sheffer, E.; Canham, C.D.; Kigel, J.; Perevolotsky, A. Countervailing effects on pine and oak leaf litter decomposition in human-altered Mediterranean ecosystems. Oecologia 2015, 177, 1039–1051. [Google Scholar] [CrossRef]
- Talbot, J.M.; Treseder, K.K. Interactions among lignin, cellulose, and nitrogen drive litter chemistry–Decay relationships. Ecology 2012, 93, 345–354. [Google Scholar] [CrossRef]
- Rahman, M.M.; Tsukamoto, J.; Rahman, M.M.; Yoneyama, A.; Mostafa, K.M. Lignin and its effects on litter decomposition in forest ecosystem. Chem. Ecol. 2013, 29, 540–553. [Google Scholar] [CrossRef]
- Rodríguez, B.P.C.; Rodríguez, H.G.; Silva, I.C.; Pando Moreno, M.; Marmolejo Monsiváis, J.G.; Gómez Meza, M.V.; Lazcano Cortez, J. Decomposition models of litter from oak and pine forests in Nuevo León State. Rev. Mex. Cienc. For. 2019, 10, 39–55. [Google Scholar] [CrossRef]
- Santonja, M.; Bousqued-Mélou, A.; Greff, S.; Ormeño, E.; Fernandez, C. Alleptahic effect of volatile organic compounds released from Pinus halepensisi needles and roots. Ecol. Evol. 2019, 9, 8201–8213. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yin, P.; Zeng, S.; Tong, C. Responses of soil macrofaunal community and diversity under different types of vegetation on soil nutrient pools in winter in Emei Mountain, China. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 22125. [Google Scholar] [CrossRef]
- Imanuddin, R.; Hidayat, A.; Rachmat, H.H.; Turjaman, M.; Pratiwi; Nurfatriani, F.; Indrajaya, Y.; Susilowati, A. Reforestation and sustainable management of Pinus merkusii forest plantation in Indonesia: A review. Forests 2020, 11, 1235. [Google Scholar] [CrossRef]
- Lorenz, K.; Preston, C.M.; Krumrei, S.; Feger, K.-H. Decomposition of needle/leaf litter from Scots pine, black cherry, common oak and European beech at a conurbation forest site. Eur. J. For. Res. 2004, 123, 177–188. [Google Scholar] [CrossRef]
- Tuomi, M.; Thum, T.; Järvinen, H.; Fronzek, S.; Berg, B.; Harmon, M.; Trofymow, J.A.; Sevanto, S.; Liski, J. Leaf litter decomposition-estimates of global variability based on Yasso07 model. Ecol. Model. 2009, 220, 3362–3371. [Google Scholar] [CrossRef]
- Gunadi, B.; Verhoef, H.A.; Bedaux, J.J.M. Seasonal dynamics of decomposition of coniferous leaf litter in a forest plantation (Pinus merkusii) in Central Java, Indonesia. Soil Biol. Biochem. 1998, 30, 845–852. [Google Scholar] [CrossRef]
- Rahajoe, J.S.; Susanti, R.; Simbolon, H.; Mansur, M.; Hidayat, A.; Shiodera, S.; Suzuki, E.; Kohyama, T.S. Decomposition rate of some dominant tree species in low montane forest of Gunung Halimun Salak National Park, West Java-Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 762, 12010. [Google Scholar] [CrossRef]
- Junaedi, A.; Mindawati, N.; Pribadi, A.; Hardiwinoto, S. Leaf litter decomposition and nutrient release of three native tree species in a drained tropical peatland in Riau, Indonesia. HAYATI J. Biosci. 2022, 29, 182–191. [Google Scholar] [CrossRef]
- Schmidt, F.H.; Ferguson, J.H. Rainfall Types Based on Wet and Dry Period Ratios for Indonesia with Western New Guinea. Verh. Djawatan Meteorologi dan Geofisika. Djakarta. 1951. Available online: https://www.biblio.com/book/rainfall-types-based-wet-dry-period/d/1303199206 (accessed on 11 March 2020).
- Putra, P.S.; Achmad, A.; Yamada, T.; Ngakan, P.O. Seasonal litter production patterns in three tropical forests in Sulawesi, Indonesia: Implications for managing secondary forests. Biodiversitas J. Biol. Divers. 2023, 24, 852–860. [Google Scholar] [CrossRef]
- Singh, J.S.; Gupta, S.R. Plant decomposition and soil respiration in terrestrial ecosystems. Bot. Rev. 1977, 43, 449–528. [Google Scholar] [CrossRef]
- Bradford, M.A.; Tordoff, G.M.; Eggers, T.; Jones, T.H.; Newington, J.E. Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 2002, 99, 317–323. [Google Scholar] [CrossRef]
- Riutta, T.; Slade, E.M.; Bebber, D.P.; Taylor, M.E.; Malhi, Y.; Riordan, P.; Macdonald, D.W.; Morecroft, M.D. Experimental evidence for the interacting effects of forest edge, moisture and soil macrofauna on leaf litter decomposition. Soil Biol. Biochem. 2012, 49, 124–131. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 19 February 2020).
- Prescott, C.E.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of broadleaf and needle litter in forests of British Columbia: Influences of litter type, forest type, and litter mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Begum, F.; Zuhra, B.; Khan, A.; Durrani, S.A.; Ali, S.; Ali, K.; Ishaq, S. Study of soil macrofauna in relation with some of selected soil physio-chemical properties at sumayar-nagar in district Hunza-Nagar Gilgit-Baltistan, Pakistan. J. Biodivers. Environ. Sci. 2014, 5, 124–132. [Google Scholar]
- Qi, D.; Wieneke, X.; Tao, J.; Zhou, X.; Desilva, U. Soil pH is the primary factor correlating with soil microbiome in karst rocky desertification regions in the Wushan County, Chongqing, China. Front. Microbiol. 2018, 9, 1027. [Google Scholar] [CrossRef]
- Prommer, J.; Walker, T.W.N.; Wanek, W.; Braun, J.; Zezula, D.; Hu, Y.; Hofhansl, F.; Richter, A. Increased microbial growth, biomass, and turnover drive soil organic carbon accumulation at higher plant diversity. Glob. Change Biol. 2020, 26, 669–681. [Google Scholar] [CrossRef]
- Barros, N.; Gomez-Orellana, I.; Feijóo, S.; Balsa, R. The effect of soil moisture on soil microbial activity studied by microcalorimetry. Thermochim. Acta 1995, 249, 161–168. [Google Scholar] [CrossRef]
- Heil, M.; Baumann, B.; Andary, C.; Linsenmair, K.E.; Mckey, D. Extraction and quantification of “condensed tannins” as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften 2002, 89, 519–524. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Kuiters, A.T. Role of phenolic substances from decomposing forest litter in plant-soil interactions. Acta Bot. Neerl. 1990, 39, 329–348. [Google Scholar] [CrossRef]
- Fenner, N.; Freeman, C. Drought-induced carbon loss in peatlands. Nat. Geosci. 2011, 4, 895–900. [Google Scholar] [CrossRef]
- Cameron, G.N.; LaPoint, T.W. Effects of Tannins on the decomposition of Chinese tallow leaves by terrestrial and aquatic invertebrates. Oecologia 1978, 32, 349–366. [Google Scholar] [CrossRef]
- González-Paleo, L.; Ravetta, D.; Van Tassel, D. From leaf traits to agroecosystem functioning: Effects of changing resource use strategy during silphium domestication on litter quality and decomposition rate. Plant Soil 2022, 471, 655–667. [Google Scholar] [CrossRef]
- Park, B.B.; Ko, Y.; Hernandez, J.O.; Byambadorj, S.-O.; Han, S.H. Growth of deciduous and evergreen species in two contrasting temperate forest stands in Korea: An intersite experiment. Plants 2022, 11, 841. [Google Scholar] [CrossRef]
- North, M.; Oakley, B.; Fiegener, R.; Gray, A.; Barbour, M. Influence of light and soil moisture on Sierran mixed-conifer understory communities. Plant Ecol. 2005, 177, 13–24. [Google Scholar] [CrossRef]
- Yusef, H.M.; Allam, M.E. The effect of light on growth and sporulation of certain fungi. Mycopathol. Mycol. Appl. 1967, 33, 81–89. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Soil moisture as a factor affecting the microbiological and biochemical activity of soil. Plant Soil Environ. 2016, 62, 250–255. [Google Scholar] [CrossRef]


| Species | Existence | Degraded Compound | ||
|---|---|---|---|---|
| Karst | Lowland | Pine | ||
| Bacteria | ||||
| Bacillus cereus Frankland and Frankland 1889. | √ | Cellulose, hemicellulose, lignin | ||
| Burkholderia sp. | √ | Cellulose, hemicellulose, lignin | ||
| Burkholderia ubonensis Yabuuchi et al., 2000 | √ | Phosphor | ||
| Burkholderia cepacia (Palleroni and Holmes 1981) Yabuuchi et al., 1993 | √ | √ | Cellulose, lignin | |
| Bacillus thuringiensis Berliner 1915 | √ | Cellulose, hemicellulose | ||
| Burkholderia cenocepacia Vandamme et al., 2003 | √ | √ | Phosphor, potassium | |
| Number of bacterial species | 4 | 2 | 2 | |
| Fungi | ||||
| Trichoderma virens (J.H. Miller, Giddens and A.A. Foster) Arx, 1987 | √ | Cellulose, hemicellulose | ||
| Aspergillus aculeatus Iizuka 1953 | √ | Cellulose, hemicellulose | ||
| Aspergillus terreus Thom 1918 | √ | √ | Cellulose, hemicellulose, lignin | |
| Aspergillus japonicus Saito 1906 | √ | √ | Cellulose | |
| Penicillium pinophilum Hedgc. 1907 | √ | Cellulose, hemicellulose, lignin | ||
| Trichoderma sp. | √ | Cellulose, hemicellulose, lignin | ||
| Aspergillus sp. | √ | Cellulose, lignin | ||
| Penicillium citrinum Thom 1910 | √ | Cellulose, lignin | ||
| Cladosporium tenuissimum Cooke 1878 | √ | Cellulose, hemicellulose, lignin | ||
| Talaromyces pinophilus (Hedgc.) Samson, Yilmaz, Frisvad and Seifert 2011 | √ | √ | √ | Cellulose, phosphor |
| Number of fungal species | 5 | 6 | 3 | |
| Total Bacterial and Fungal | 9 | 8 | 5 | |
| Parameter | Forest Communities | ||
|---|---|---|---|
| Karst | Lowland | Pine | |
| Caught Using Pitfall Traps in the Dry Season (per 567.163 cm2) | |||
| Mean number of species | 4.8 (±0.34) a | 4.1 (±0.34) b | 3.3 (±0.34) c |
| Mean density of macrofauna | 36.4 (±4.02) d | 34.2 (±4.02) d | 17.2 (±4.02) e |
| Caught Using Pitfall Traps in the Wet Season (per 567.163 cm2) | |||
| Mean number of species | 5.6 (±0.43) a | 5.6 (±0.43) a | 4.0 (±0.43) b |
| Mean density of macrofauna | 73.6 (±5.45) c | 60.1 (±5.45) c | 38.2 (±5.45) d |
| Caught Using Square Ring Samples in the Dry Season (per 4000 cm2) | |||
| Mean number of species | 5.8 (±0.27) a | 6.5 (±0.27) a | 4.3 (±0.27) b |
| Mean density of macrofauna | 69.6 (±12.57) c | 24.0 (±12.57) d | 35.2 (±12.57) cd |
| Caught Using Square Ring Samples in the Wet Season (per 4000 cm2) | |||
| Mean number of species | 10.0 (±0.44) a | 9.1 (±0.44) a | 6.4 (±0.44) b |
| Mean density of macrofauna | 39.1 (±2.85) c | 41.3 (±2.85) c | 20.6 (±2.85) d |
| Parameter | Forest Communities | ||
|---|---|---|---|
| Karst | Lowland | Pine | |
| Chemical Properties | |||
| pH | 6.28 (±0.05) a | 6.39 (±0.05) a | 5.93 (±0.05) b |
| C (%) | 2.30 (±0.11) c | 2.33 (±0.11) c | 1.61 (±0.11) d |
| N (%) | 0.22 (±0.01) e | 0.22 (±0.01) e | 0.17 (±0.01) e |
| C/N (%) | 10.75 (±0.32) f | 10.63 (±0.32) f | 9.63 (±0.32) f |
| P (ppm) | 12.11 (±0.48) gh | 10.40 (±0.48) g | 12.73 (±0.48) h |
| Ca (kg−1) | 6.42 (±0.71) i | 6.10 (±0.71) i | 5.40 (±0.71) i |
| Mg (kg−1) | 0.90 (±0.44) j | 1.95 (±0.44) j | 2.01 (±0.44) j |
| K (kg−1) | 0.48 (±0.05) k | 0.36 (±0.05) k | 0.43 (±0.05) k |
| Na (kg−1) | 0.47 (±0.05) l | 0.41 (±0.05) l | 0.36 (±0.05) l |
| CEC (kg−1) | 19.79 (±0.67) m | 20.33 (±0.67) m | 18.15 (±0.67) m |
| BS (%) | 41.63 (±4.29) n | 44.88 (±4.29) n | 45.63 (±4.29) n |
| Leaf Sample | Forest Type | Season | Biological Factors | |
|---|---|---|---|---|
| Macroscopic Fungi Colony Cover | Macrofauna Abundance | |||
| Tusam | Karst | Dry | r = 0.433 p = 0.211 | r = 0.681 * p = 0.030 |
| Wet | r = 0.830 ** p = 0.003 | r = 0.861 ** p = 0.001 | ||
| Lowland | Dry | r = 0.340 p = 0.336 | r = 0.975 ** p < 0.0001 | |
| Wet | r = 0.871 ** p = 0.001 | r = 0.817 ** p = 0.004 | ||
| Pine | Dry | r = −0.207 p = 0.567 | r = −0.361 p = 0.305 | |
| Wet | r = 0.037 p = 0.918 | r = 0.653 * p = 0.041 | ||
| Ebony | Karst | Dry | r = 0.629 p = 0.051 | r = −0.222 p = 0.538 |
| Wet | r = 0.096 p = 0.792 | r = 0.309 p = 0.386 | ||
| Lowland | Dry | r = 0.171 p = 0.637 | r = −0.118 p = 0.745 | |
| Wet | r = 0.632 * p = 0.050 | r = 0.700 * p = 0.024 | ||
| Pine | Dry | r = −0.197 p = 0.586 | r = −0.361 p = 0.305 | |
| Wet | r = 0.057 p = 0.876 | r = −0.507 p = 0.140 | ||
| Leaf Sample | Forest Type | Season | Physical Factors | |
|---|---|---|---|---|
| Soil pH | Soil Moisture | |||
| Tusam | Karst | Dry | r = −0.141 p = 0.698 | r = −0.158 p = 0.663 |
| Wet | r = 0.677 * p = 0.032 | r = 0.797 ** p = 0.006 | ||
| Lowland | Dry | r = 0.437 p = 0.206 | r = 0.088 p = 0.81 | |
| Wet | r = 0.666 * p = 0.036 | r = 0.543 p = 0.105 | ||
| Pine | Dry | r = 0.479 p = 0.161 | r = −0.553 p = 0.098 | |
| Wet | r = 0.008 p = 0.983 | r = −0.275 p = 0.442 | ||
| Ebony | Karst | Dry | r = −0.357 p = 0.311 | r = 0.613 p = 0.059 |
| Wet | r = 0.774 ** p = 0.009 | r = 0.364 p = 0.301 | ||
| Lowland | Dry | r = 0.033 p = 0.928 | r = 0.012 p = 0.973 | |
| Wet | r = 0.744 ** p = 0.014 | r = 0.44 p = 0.203 | ||
| Pine | Dry | r = −0.007 p = 0.984 | r = −0.273 p = 0.445 | |
| Wet | r = −0.173 p = 0.634 | r = −0.278 p = 0.436 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra, P.S.; Mas’ud, W.; Hamzah, A.S.; Nasri, N.; Achmad, A.; Yamada, T.; Ngakan, P.O. Determinants of Needleleaf and Broadleaf Decomposition Rates Under and Outside the Parent Tree Stand. Forests 2025, 16, 1678. https://doi.org/10.3390/f16111678
Putra PS, Mas’ud W, Hamzah AS, Nasri N, Achmad A, Yamada T, Ngakan PO. Determinants of Needleleaf and Broadleaf Decomposition Rates Under and Outside the Parent Tree Stand. Forests. 2025; 16(11):1678. https://doi.org/10.3390/f16111678
Chicago/Turabian StylePutra, Putu Supadma, Wardiman Mas’ud, Andi Siady Hamzah, Nasri Nasri, Amran Achmad, Toshihiro Yamada, and Putu Oka Ngakan. 2025. "Determinants of Needleleaf and Broadleaf Decomposition Rates Under and Outside the Parent Tree Stand" Forests 16, no. 11: 1678. https://doi.org/10.3390/f16111678
APA StylePutra, P. S., Mas’ud, W., Hamzah, A. S., Nasri, N., Achmad, A., Yamada, T., & Ngakan, P. O. (2025). Determinants of Needleleaf and Broadleaf Decomposition Rates Under and Outside the Parent Tree Stand. Forests, 16(11), 1678. https://doi.org/10.3390/f16111678







