Modeling the Climate-Driven Spread of Pine Wilt Disease for Forest Pest Risk Assessment and Management Using MaxEnt
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Data Collection
2.1.1. Study Area
2.1.2. Data Source
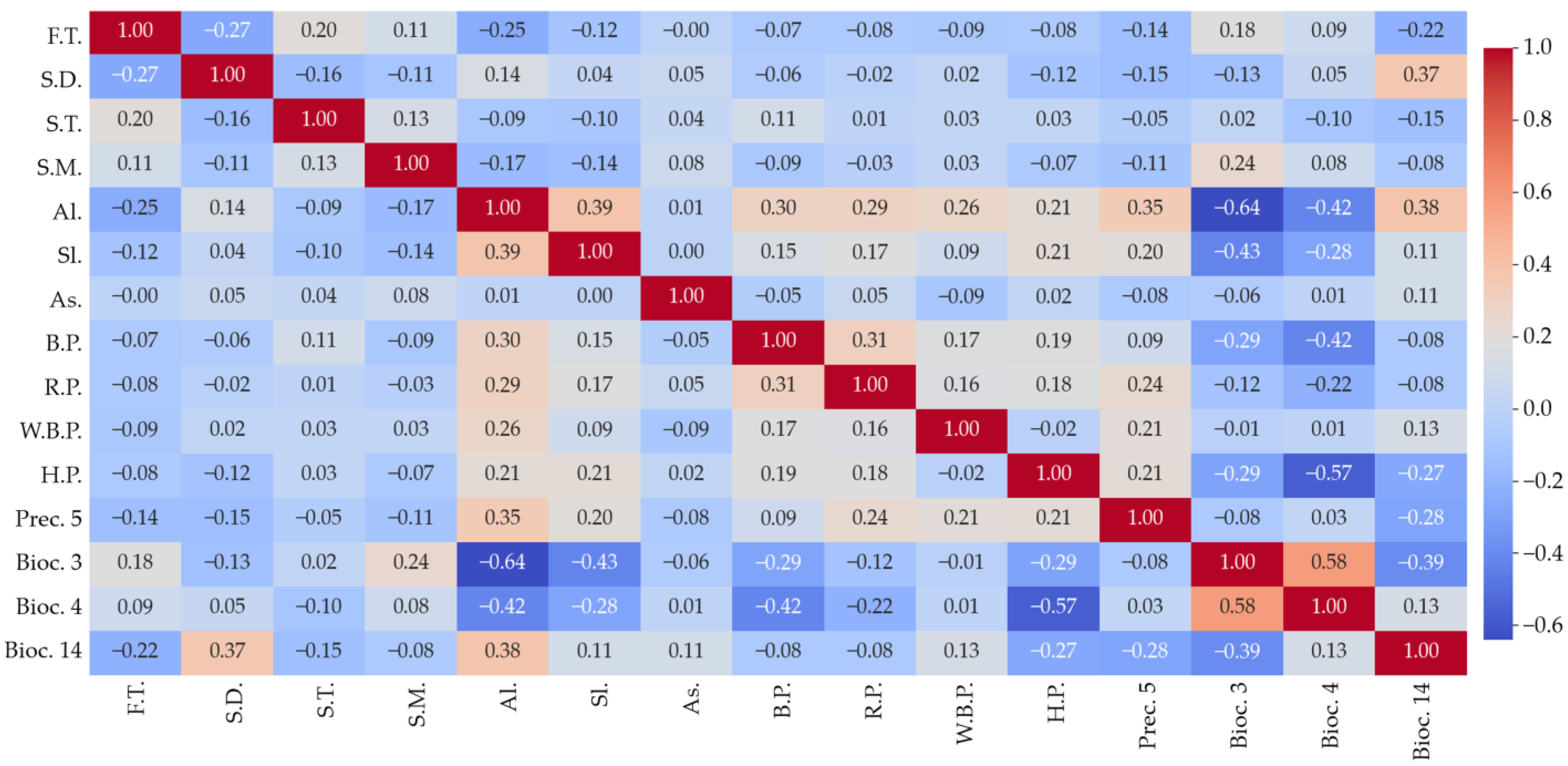
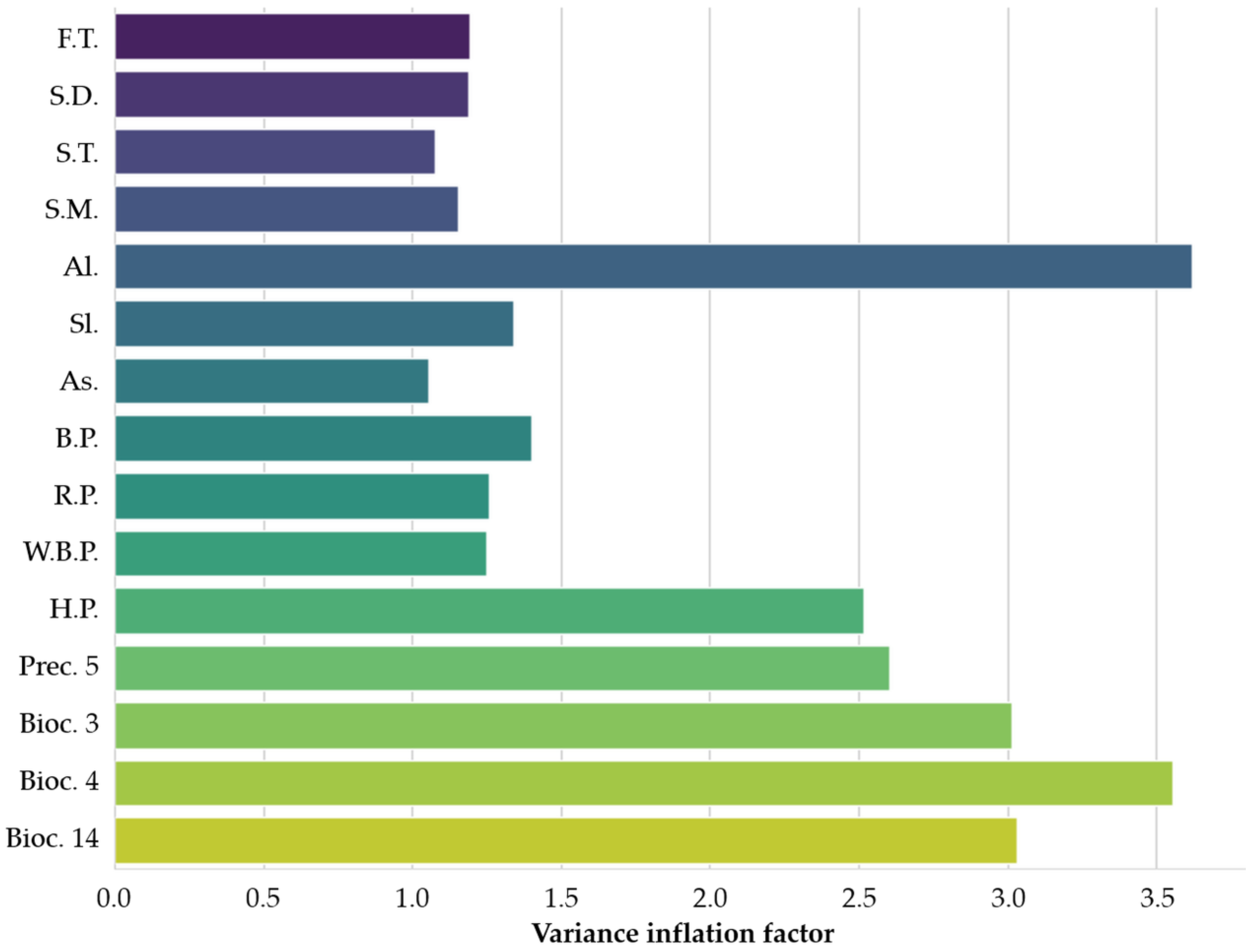
2.1.3. Final Variable Selection
2.2. Model and Analytical Methods
2.2.1. MaxEnt Model Selection and Performance Assessment
2.2.2. Scenario Setting and Analysis
3. Results
3.1. Distribution of Potential PWD Habitats Using MaxEnt
3.1.1. Evaluation of Final Variables
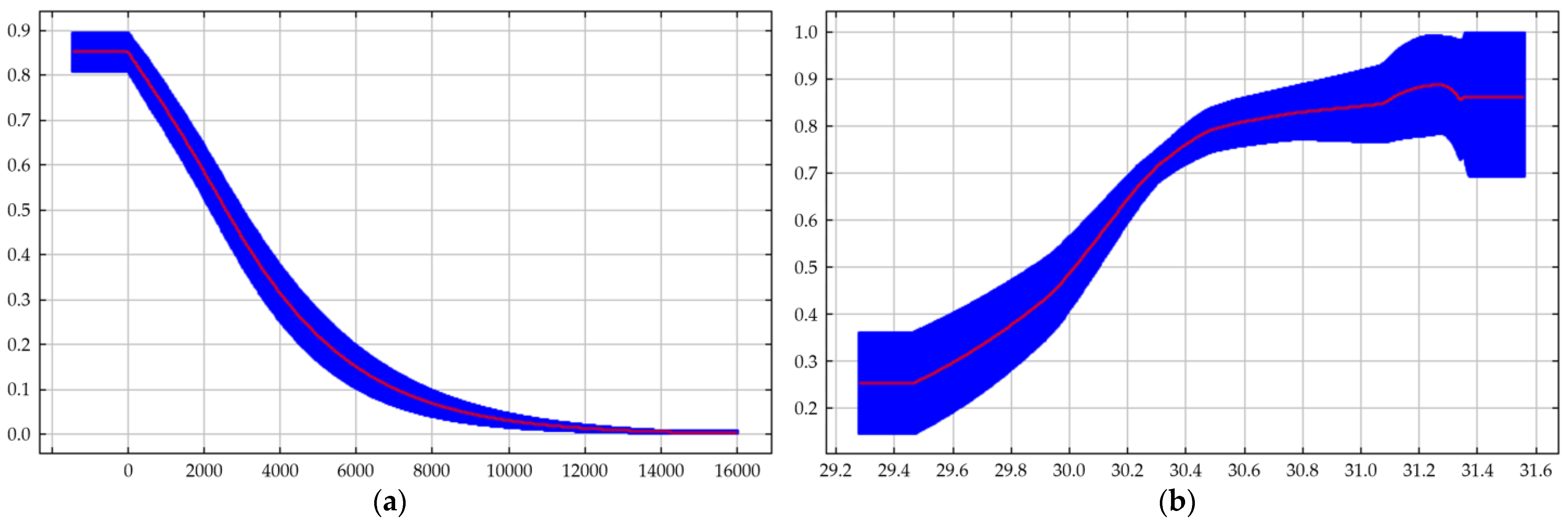
3.1.2. Jackknife Validation
3.1.3. Evaluating Accuracy
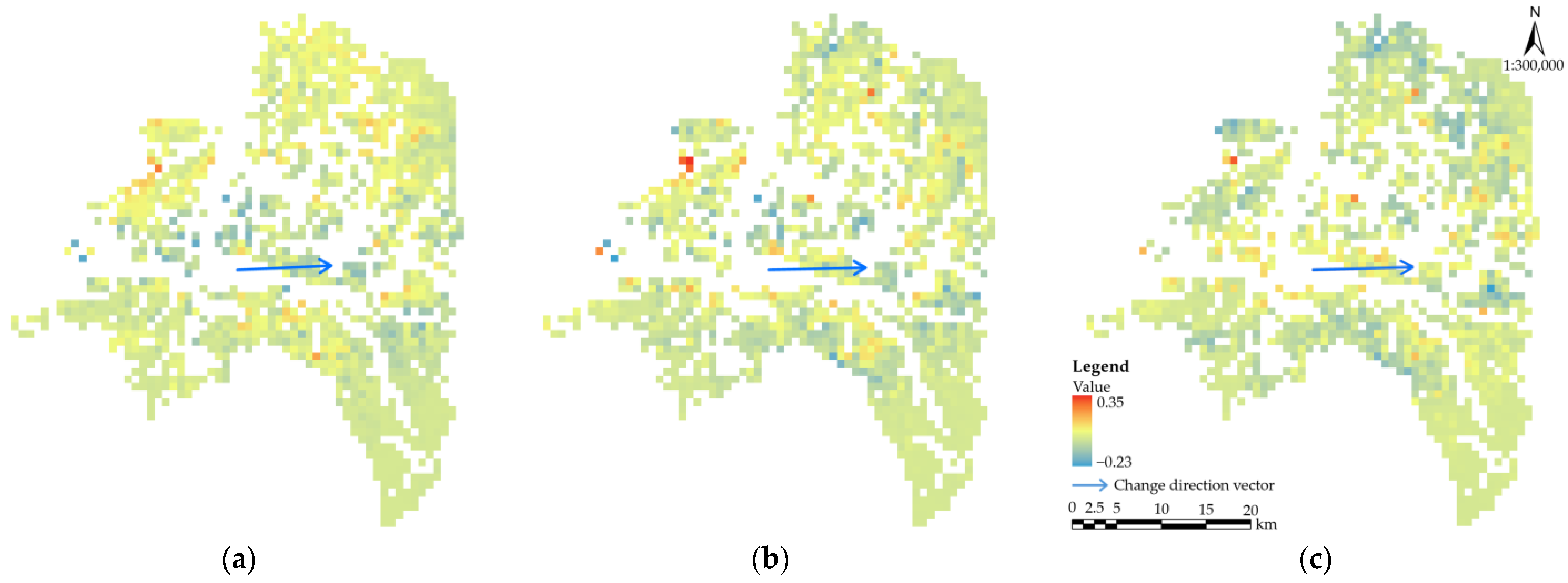
3.2. Changes in the Distribution of Potential PWD Habitats
3.3. Direction of Movement of the Distribution of Potential PWD Habitats
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| Al. | Altitude |
| As. | Aspect |
| B.P. | Building Proximity |
| Bioc. 3 | Bioclimate Variable 3: Isothermality |
| Bioc. 4 | Bioclimate Variable 4: Temperature Seasonality |
| Bioc. 14 | Bioclimate Variable 14: Precipitation of Driest Month |
| CVA | Change Vector Analysis |
| DEM | Digital Elevation Model |
| ESRI | Environmental Systems Research Institute |
| F.T. | Forest Type |
| FCs | Feature Classes |
| H.P. | Historical Proximity |
| KFS | Korea Forest Service |
| MaxEnt | Maximum Entropy |
| NGII | National Geographic Information Institute |
| PWD | Pine Wilt Disease |
| Prec. 5 | May Precipitation |
| R.P. | Road Proximity |
| RMs | Regularization Multipliers |
| ROC | Receiver Operating Characteristic |
| S.D. | Stand Density |
| S.M. | Soil Moisture |
| S.T. | Soil Texture |
| SD | Standard Deviation |
| SSP | Shared Socioeconomic Pathways |
| VIF | Variance Inflation Factor |
| W.B.P. | Water Body Proximity |
Appendix A
References
- Ye, J.-r. Epidemic status of pine wilt disease in China and its prevention and control techniques and counter measures. Sci. Silvae Sin. 2019, 55, 1–10. [Google Scholar]
- Faria, J.M.; Sousa, E.; Bonifácio, L.; Carrasquinho, I.; Varela, A.R.; Inácio, M.L. Bursaphelenchus. In Compendium of Phytopathogenic Microbes in Agro-Ecology: Vol. 3, Bacteria, Protozoa, Algae and Nematodes; Springer: Berlin/Heidelberg, Germany, 2025; pp. 353–386. [Google Scholar]
- Futai, K.; Ishiguro, H. Hidden Threats: The Unnoticed Epidemic System of Pine Wilt Disease Driven by Sexually Mature Monochamus Beetles and Asymptomatic Trees. Biology 2025, 14, 485. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Á. Bursaphelenchus xylophilus, the pinewood nematode: Its significance and a historical review. Acta Biol. Szeged. 2011, 55, 213–217. [Google Scholar]
- Dwinell, L.D. An Overview of the Pine Wood Nematode Ban in North America. Available online: https://play.google.com/books/reader?id=mfy537QflSgC&pg=GBS.PA4&hl=en (accessed on 1 August 2025).
- Kwon, T.-S.; Lim, J.-H.; Sim, S.-J.; Kwon, Y.-D.; Son, S.-K.; Lee, K.-Y.; Kim, Y.-T.; Park, J.-W.; Shin, C.-H.; Ryu, S.-B. Distribution patterns of Monochamus alternatus and M. saltuarius (Coleoptera: Cerambycidae) in Korea. J. Korean Soc. For. Sci. 2006, 95, 543–550. [Google Scholar]
- Han, H.; Chung, Y.-J.; Shin, S.-C. First report of pine wilt disease on Pinus koraiensis in Korea. Plant Dis. 2008, 92, 1251. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, J.; Yoo, N.S.; Jung, J.-K. Spatiotemporal Dynamics of Trunk-Injected Pesticide Residue for Management of Pine Wilt Disease in Pinus koraiensis. Forests 2024, 15, 1996. [Google Scholar] [CrossRef]
- Korea Forest Service. 2024 Statistical Yearbook of Forestry; Korea Forest Service: Daejeon, Republic of Korea, 2024; pp. 140–141, 178.
- Korea Forest Service. Plan for the Surveillance and Control of Forest Pests and Diseases. Available online: https://www.forest.go.kr/kfsweb/cop/bbs/selectBoardArticle.do?bbsId=BBSMSTR_1069&mn=NKFS_06_09_01&nttId=3203081 (accessed on 1 August 2025).
- Kwon, K.C.; Han, S.A.; Lee, D.K.; Jung, I.K.; Seo, Y.J.; Hong, E.P.; Choi, H.S. The site characteristics and stand structure of Pinus densiflora Forests in the Republic of Korea. J. Korean Soc. For. Sci. 2021, 110, 496–503. [Google Scholar]
- Li, M.; Li, H.; Ding, X.; Wang, L.; Wang, X.; Chen, F. The detection of pine wilt disease: A literature review. Int. J. Mol. Sci. 2022, 23, 10797. [Google Scholar] [CrossRef]
- Kim, C.; Park, B.-J. A Study on Improving the Quantitative Analysis Method for the Control Performance of Pine Wilt Disease. J. Korean Soc. For. Sci. 2024, 113, 259–270. [Google Scholar]
- Lee, M.-G.; Cho, H.-B.; Youm, S.-K.; Kim, S.-W. Detection of pine wilt disease using time series UAV imagery and deep learning semantic segmentation. Forests 2023, 14, 1576. [Google Scholar] [CrossRef]
- Sánchez-Mercado, A.; Ferrer-Paris, J.; Franklin, J. Mapping species distributions: Spatial inference and prediction. Oryx 2010, 44, 615. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. A comparison of absolute performance of different correlative and mechanistic species distribution models in an independent area. Ecol. Evol. 2016, 6, 5973–5986. [Google Scholar] [CrossRef] [PubMed]
- Hayat, U.; Shi, J.; Wu, Z.; Rizwan, M.; Haider, M.S. Which SDM Model, CLIMEX vs. MaxEnt, Best Forecasts Aeolesthes sarta Distribution at a Global Scale under Climate Change Scenarios? Insects 2024, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ouyang, X.; Ding, X.; Wang, Y.; Liu, W.; Liu, Y. Predicting Suitable Habitat for Glipa (Coleoptera: Mordellidae: Mordellinae) Under Current and Future Climates Using MaxEnt Modeling. Insects 2025, 16, 642. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Estay, S.A.; Labra, F.A.; Sepulveda, R.D.; Bacigalupe, L.D. Evaluating habitat suitability for the establishment of Monochamus spp. through climate-based niche modeling. PLoS ONE 2014, 9, e102592. [Google Scholar] [CrossRef]
- Ouyang, X.; Chen, A.; Li, Y.; Han, X.; Lin, H. Predicting the potential distribution of pine wilt disease in China under climate change. Insects 2022, 13, 1147. [Google Scholar] [CrossRef]
- Mori, N.; Yamashita, M.; Inoue, M.N. Integration of satellite remote sensing and MaxEnt modeling for improved detection and management of forest pests. Environ. Monit. Assess. 2024, 196, 616. [Google Scholar] [CrossRef]
- Hirata, A.; Nakamura, K.; Nakao, K.; Kominami, Y.; Tanaka, N.; Ohashi, H.; Takano, K.T.; Takeuchi, W.; Matsui, T. Potential distribution of pine wilt disease under future climate change scenarios. PLoS ONE 2017, 12, e0182837. [Google Scholar] [CrossRef]
- Ikegami, M.; Jenkins, T.A. Estimate global risks of a forest disease under current and future climates using species distribution model and simple thermal model–Pine Wilt disease as a model case. For. Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]
- Tang, X.; Yuan, Y.; Li, X.; Zhang, J. Maximum entropy modeling to predict the impact of climate change on pine wilt disease in China. Front. Plant Sci. 2021, 12, 652500. [Google Scholar] [CrossRef]
- Wang, J.; Deng, J.; Yan, W.; Zheng, Y. Habitat suitability of pine wilt disease in Northeast China under climate change scenario. Forests 2023, 14, 1687. [Google Scholar] [CrossRef]
- Yu, L.; Zhan, Z.; Ren, L.; Li, H.; Huang, H.; Luo, Y. Impact of stand-and landscape-level variables on pine wilt disease-caused tree mortality in pine forests. Pest Manag. Sci. 2023, 79, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kiyohara, T. Influence of water stress on development of pine wilting disease caused by Bursaphelenchus lignicolus. Eur. J. For. Pathol. 1978, 8, 97–107. [Google Scholar] [CrossRef]
- Miki, N.; Sakamoto, K.; Nishimoto, T.; Yoshikawa, K.; Hada, Y. Relationship between the incidence of pine wilt disease and the drainage area. J. For. Res. 2001, 6, 181–186. [Google Scholar] [CrossRef]
- Basic Statistics of Gyeongsangbuk-do: Occurrence and Control Status of Forest Pests and Diseases. Available online: https://stat.kosis.kr/statHtml_host/statHtml.do?orgId=216&tblId=DT_21603_E001048&conn_path=I2&dbUser=NSI_IN_216 (accessed on 1 August 2025).
- Obeng, H.A.; Arhinful, R.; Tessema, D.H.; Nuhu, J.A. The mediating role of organisational stress in the relationship between gender diversity and employee performance in Ghanaian public hospitals. Future Bus. J. 2025, 11, 38. [Google Scholar] [CrossRef]
- Futai, K. Pine wilt in Japan: From first incidence to the present. In Pine Wilt Disease; Springer: Berlin/Heidelberg, Germany, 2008; pp. 5–12. [Google Scholar]
- Ohsawa, M.; Akiba, M. Possible altitude and temperature limits on pine wilt disease: The reproduction of vector sawyer beetles (Monochamus alternatus), survival of causal nematode (Bursaphelenchus xylophilus), and occurrence of damage caused by the disease. Eur. J. For. Res. 2014, 133, 225–233. [Google Scholar] [CrossRef]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The consequence of tree pests and diseases for ecosystem services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef]
- Sim, S.T.; Lee, S.-H.; Lee, C.Y.; Nam, Y. Analysis of occurrence characteristics of pine wilt disease in Korea based on monitoring data from 2016 to 2018. J. Korean Soc. For. Sci. 2021, 110, 280–288. [Google Scholar]
- Lee, S.; Jeong, K.; Park, Y.-J.; Park, J. Prediction of risk area distribution of pine wilt disease using maximum entropy model in Gyeongju-si. J. Korean Cartogr. Assoc. 2021, 21, 43–57. [Google Scholar] [CrossRef]
- Ha, U.R.; Son, E.H.; Seong, H.J.; Kang, H.D.; Lee, D.W.; Kim, H.H. Predicting potential distribution of the pine wilt disease using MaxEnt model in Jinju-si. J. Agric. Life Sci. 2023, 57, 93–104. [Google Scholar] [CrossRef]
- Hao, Z.; Fang, G.; Huang, W.; Ye, H.; Zhang, B.; Li, X. Risk Prediction and Variable Analysis of Pine Wilt Disease by a Maximum Entropy Model. Forests 2022, 13, 342. [Google Scholar] [CrossRef]
- Low, B.W.; Zeng, Y.; Tan, H.H.; Yeo, D.C. Predictor complexity and feature selection affect Maxent model transferability: Evidence from global freshwater invasive species. Divers. Distrib. 2021, 27, 497–511. [Google Scholar] [CrossRef]
- Carvalho Júnior, O.A.; Guimarães, R.F.; Gillespie, A.R.; Silva, N.C.; Gomes, R.A. A new approach to change vector analysis using distance and similarity measures. Remote Sens. 2011, 3, 2473–2493. [Google Scholar] [CrossRef]
- ZhiYong, L.; Wang, F.; Xie, L.; Sun, W.; Falco, N.; Benediktsson, J.A.; You, Z. Diagnostic analysis on change vector analysis methods for LCCD using remote sensing images. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sen. 2021, 14, 10199–10212. [Google Scholar] [CrossRef]
- Chen, L.; Lu, W.; Lamont, B.B.; Liu, Y.; Wei, P.; Xue, W.; Xiong, Z.; Tang, L.; Wang, Y.; Wang, P. Modeling the distribution of pine wilt disease in China using the ensemble models MaxEnt and CLIMEX. Ecol. Evol. 2024, 14, e70277. [Google Scholar] [CrossRef]
- Kim, I.; Nam, Y.; Park, S.; Cho, W.; Choi, K.; Ko, D.W. Enhancing pest control interventions by linking species distribution model prediction and population density assessment of pine wilt disease vectors in South Korea. Front. Ecol. Evol. 2024, 11, 1305573. [Google Scholar] [CrossRef]
- Wang, W.; Peng, W.; Liu, X.; He, G.; Cai, Y. Spatiotemporal dynamics and factors driving the distributions of pine wilt disease-damaged forests in China. Forests 2022, 13, 261. [Google Scholar] [CrossRef]
- Jung, B.-J.; Lee, M.-G.; Kim, S.-W. Prediction of Potential Habitat of Monochamus alternatus Based on Shared Socioeconomic Pathway Scenarios. Forests 2024, 15, 1563. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, Q.; Xie, N.; Yuan, G.; Liao, M.; Gui, Q.; Ding, G. Predicting the global potential distribution of Bursaphelenchus xylophilus using an ecological niche model: Expansion trend and the main driving factors. BMC Ecol. Evol. 2024, 24, 48. [Google Scholar] [CrossRef]
- Forests. Special Issue: Management of Forest Pests and Diseases—3rd Edition. Available online: https://www.mdpi.com/journal/forests/special_issues/7Z97KI0Y2U (accessed on 16 September 2025).




| Final Variable | Description | Variable Type |
|---|---|---|
| F.T. | Forest type (tree species) | Nominal |
| S.D. | Stand density | Ordinal |
| S.M. | Soil moisture | Ordinal |
| S.T. | Soil texture | Nominal |
| W.B.P. | Water body proximity: Euclidean distance analysis | Continuous |
| Al. | Altitude | Continuous |
| As. | Aspect | Continuous |
| Sl. | Slope | Continuous |
| R.P. | Road proximity: Euclidean distance analysis | Continuous |
| B.P. | Building proximity: Euclidean distance analysis | Continuous |
| H.P. | Historical proximity: Euclidean distance analysis from 2022 PWD occurrence points based on 2023 | Continuous |
| Prec. 5 | May precipitation | Continuous |
| Bioc. 3 | 100) | Continuous |
| Bioc. 4 | 100) | Continuous |
| Bioc. 14 | Bioclimate 14: precipitation of driest month | Continuous |
| Final Variable | Contribution (%) | Importance (%) |
|---|---|---|
| H.P. | 30.6 | 30.0 |
| Bioc. 3 | 12.4 | 16.5 |
| Bioc. 14 | 9.9 | 14.3 |
| S.T. | 8.6 | 3.2 |
| S.M. | 7.1 | 1.7 |
| S.D. | 4.7 | 0.6 |
| W.B.P. | 3.7 | 3.8 |
| As. | 3.5 | 3.0 |
| B.P. | 3.3 | 3.7 |
| R.P. | 3.3 | 3.7 |
| Prec. 5 | 3.2 | 5.4 |
| F.T. | 2.8 | 1.0 |
| Al. | 2.7 | 3.3 |
| Sl. | 2.3 | 4.1 |
| Bioc. 4 | 2.1 | 5.8 |
| Time Period | Near Future (2021–2040) | Far Future (2041–2060) | Post-Near Future |
|---|---|---|---|
| Increase (a) | 689 (54.0) 1 | 618 (48.4) | 582 (45.6) |
| Decrease (b) | 587 (46.0) | 658 (51.6) | 694 (54.4) |
| Difference (a − b) | 102 (8.0) | −40 (−3.1) | −112 (−8.8) |
| Total | 1276 (100.0) | 1276 (100.0) | 1276 (100.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, M.; Lee, C.; Kim, H. Modeling the Climate-Driven Spread of Pine Wilt Disease for Forest Pest Risk Assessment and Management Using MaxEnt. Forests 2025, 16, 1677. https://doi.org/10.3390/f16111677
Ha M, Lee C, Kim H. Modeling the Climate-Driven Spread of Pine Wilt Disease for Forest Pest Risk Assessment and Management Using MaxEnt. Forests. 2025; 16(11):1677. https://doi.org/10.3390/f16111677
Chicago/Turabian StyleHa, Manleung, Chongkyu Lee, and Hyun Kim. 2025. "Modeling the Climate-Driven Spread of Pine Wilt Disease for Forest Pest Risk Assessment and Management Using MaxEnt" Forests 16, no. 11: 1677. https://doi.org/10.3390/f16111677
APA StyleHa, M., Lee, C., & Kim, H. (2025). Modeling the Climate-Driven Spread of Pine Wilt Disease for Forest Pest Risk Assessment and Management Using MaxEnt. Forests, 16(11), 1677. https://doi.org/10.3390/f16111677







