Response of Soil Organic Carbon and Microbial Metabolic Pathways in Guangxi Karst Regions to Different Vegetation Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Plot Design
2.3. Soil Sample Processing
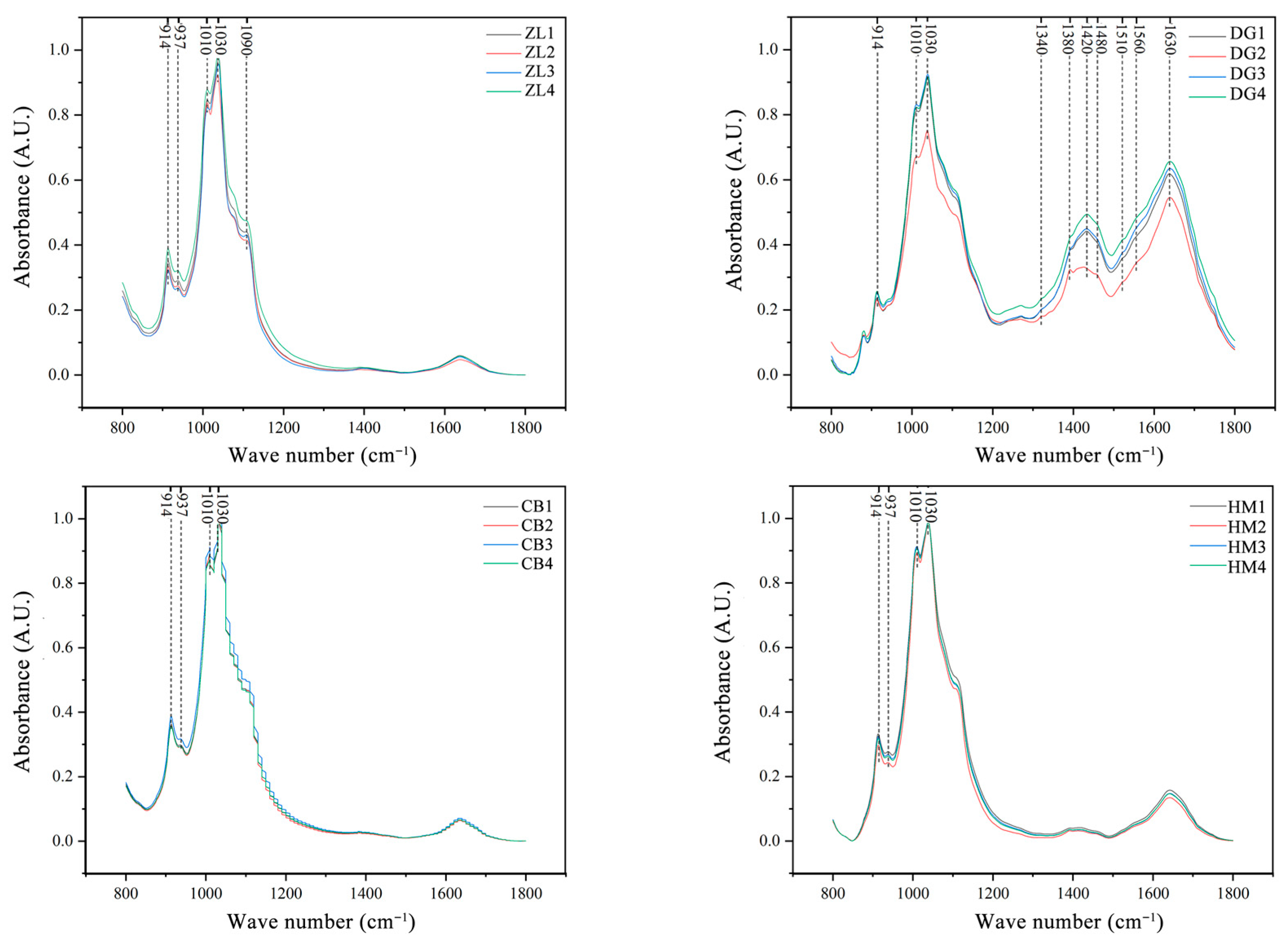
2.4. Determination of Soil Organic Carbon Structure
2.5. DNA Extraction and Analysis
2.6. Data Processing
2.7. Statistical Analysis of Data
3. Results
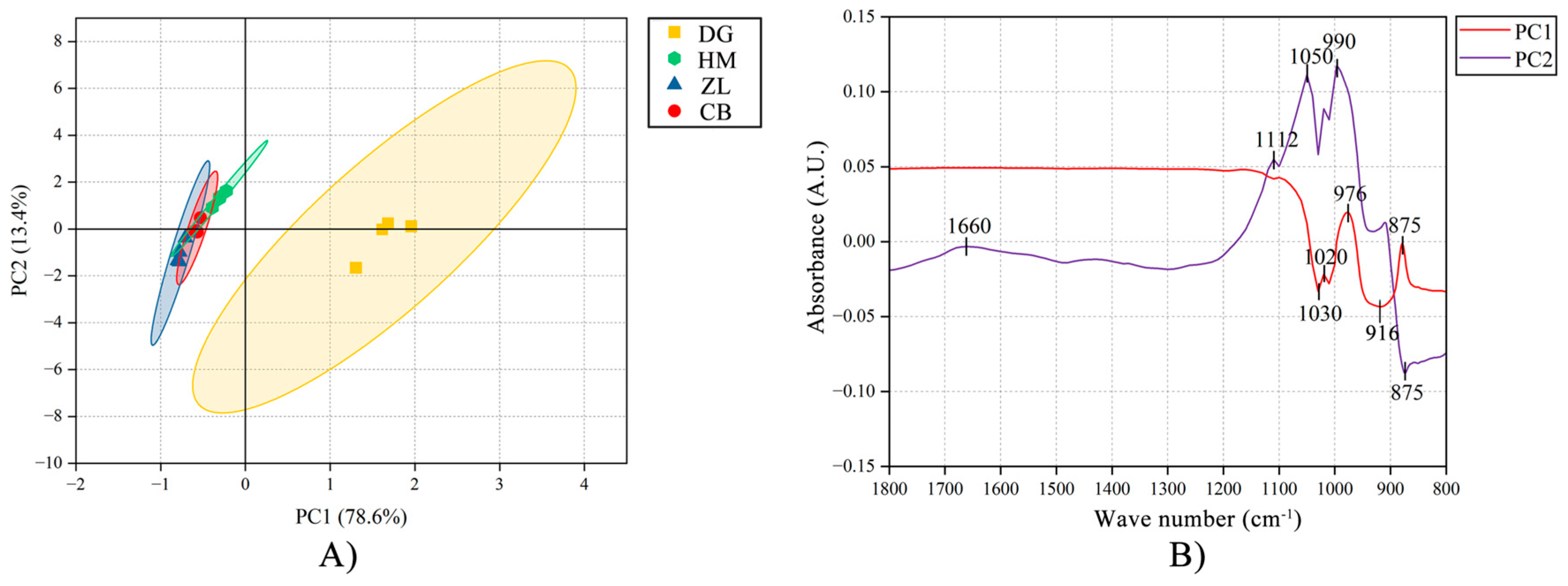
3.1. Molecular Structures of Different Vegetation Types
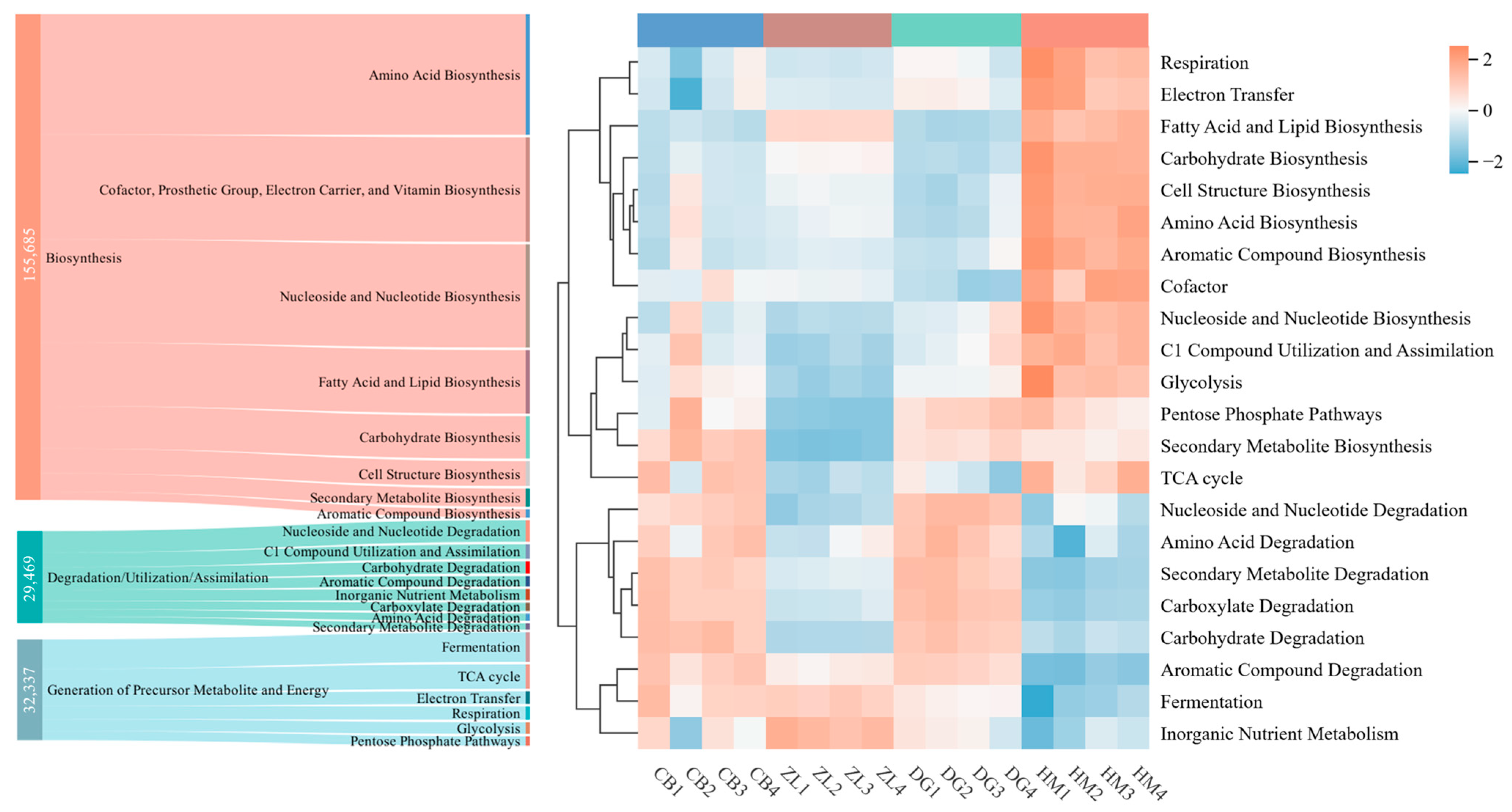
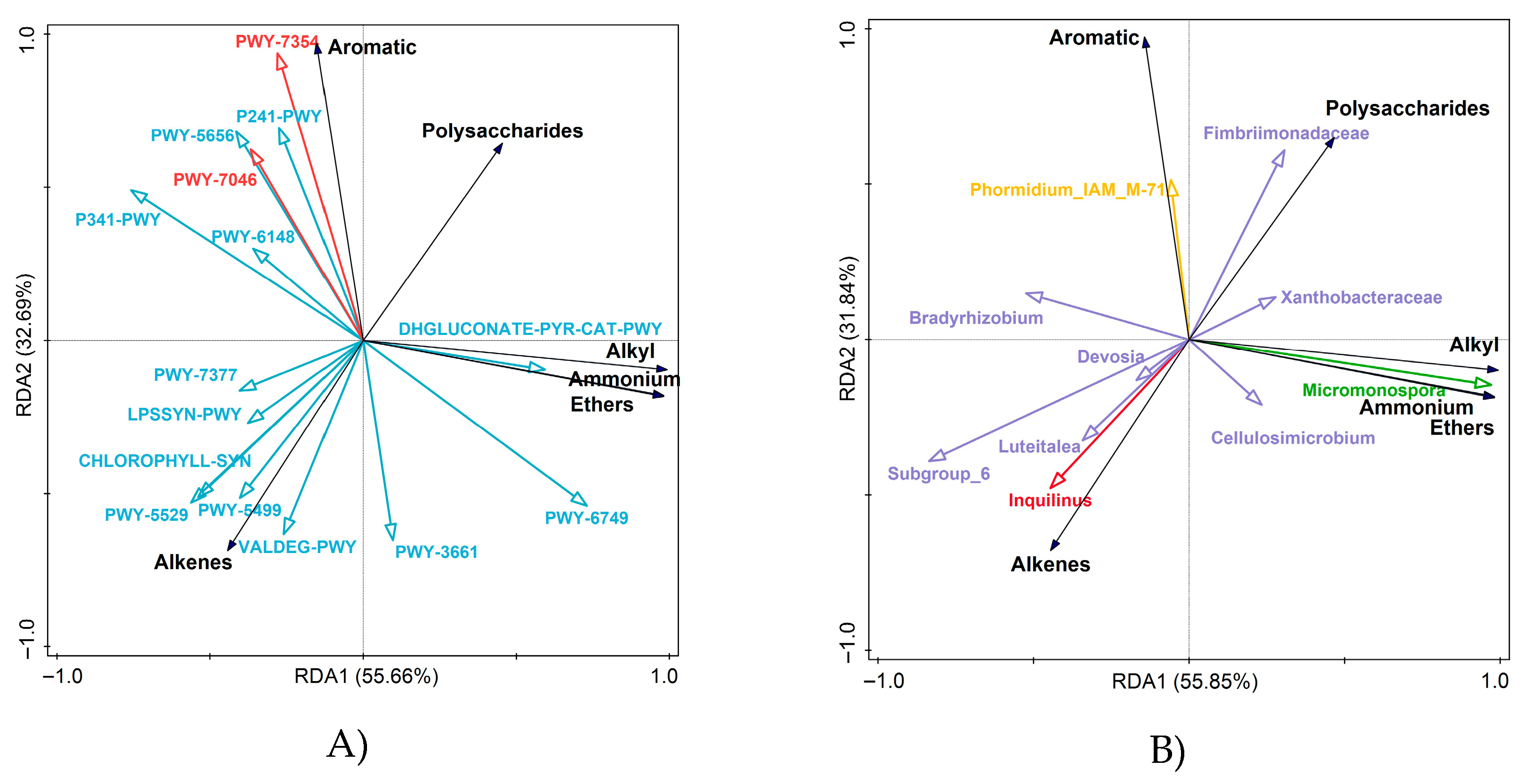
3.2. Responses of Functional Pathways in Soil Microbial Communities of Different Vegetation Types to Molecular Structure
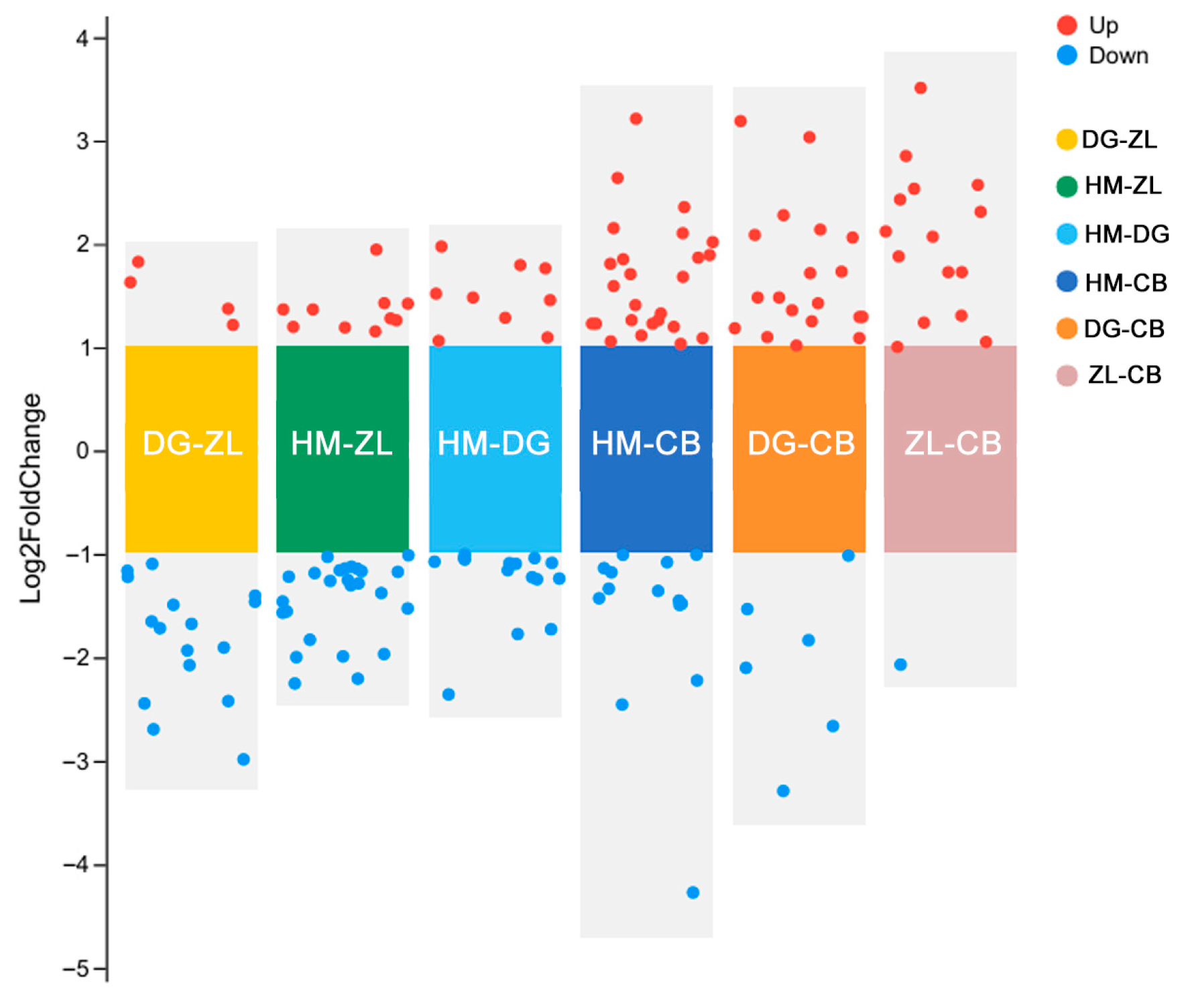
3.3. Differential Functional Pathway Interpretation and Species Composition Analysis
4. Discussion
4.1. Vegetation Effects on Organic Carbon Structure
4.2. Carbon Structure-Driven Selection of Functional Pathways
4.3. Pathway-Mediated Shifts in Microbial Community Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, J.; Liu, X.; Meng, W.; Chen, X. Recent advances in studies of soil organic carbon stability in Karst areas. Front. For. Glob. Change 2024, 7, 1453615. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Sollins, P.; Homann, P.; Caldwell, B.A. Stabilization and destabilization of soil organic matter: Mechanisms and controls. Geoderma 1996, 74, 65–105. [Google Scholar] [CrossRef]
- Chen, J.Q.; Jia, Y.N.; He, Q.F.; Jiang, K.; Chen, C.; Ye, K. Effect of Land Use on the Stability of Soil Organic Carbon in a Karst Region. Huan Jing Ke Xue=Huanjing Kexue 2024, 45, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Deng, Y.; Huang, J.; He, L.; Tang, Q.; Xiao, Y. A quantitative study of the influence of soil organic carbon and pore characteristics on the stability of aggregates of the karst peak-cluster depression area in Southwest China. J. Soils Sediments 2023, 23, 312–330. [Google Scholar] [CrossRef]
- Yu, P.; Li, Q.; Jia, H.; Zheng, W.; Wang, M.; Zhou, D. Carbon stocks and storage potential as affected by vegetation in the Songnen grassland of northeast China. Quat. Int. 2013, 306, 114–120. [Google Scholar] [CrossRef]
- Bargali, K.; Manral, V.; Padalia, K.; Bargali, S.S.; Upadhyay, V.P. Effect of vegetation type and season on microbial biomass carbon in Central Himalayan forest soils, India. Catena 2018, 171, 125–135. [Google Scholar] [CrossRef]
- Yu, P.; Li, Y.; Liu, S.; Ding, Z.; Zhang, A.; Tang, X. The quantity and stability of soil organic carbon following vegetation degradation in a salt-affected region of Northeastern China. Catena 2022, 211, 105984. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Liu, X.; Xiao, L. Changes in Soil Aggregate Fractions, Stability, and Associated Organic Carbon and Nitrogen in Different Land Use Types in the Loess Plateau, China. Sustainability 2022, 14, 3963. [Google Scholar] [CrossRef]
- Thangavel, R.; Manjaiah, K.; Arunachalam, A.; Hazarika, S.; Choudhury, B.; Arumugam, B.; Tomar, M.S.; Mishra, V.K. Tree species traits and soil biochemical properties drive carbon stability and temperature sensitivity of soil aggregates in agroforestry systems of subtropical northeast India. Res. Sq. 2025, PPR964757. [Google Scholar] [CrossRef]
- Deng, L.; Liu, G.B.; Shangguan, Z.P. Land-use conversion and changing soil carbon stocks in China’s ‘Grain-for-Green’ Program: A synthesis. Glob. Change Biol. 2014, 20, 3544–3556. [Google Scholar] [CrossRef]
- Golchin, A.; Oades, J.; Skjemstad, J.; Clarke, P. Structural and dynamic properties of soil organic-matter as reflected by 13C natural-abundance, pyrolysis mass-spectrometry and solid-state 13C NMR-spectroscopy in density fractions of an oxisol under forest and pasture. J. Soil Res. 1995, 33, 59–76. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Zhang, Y.; Zhang, J.; Wang, Y.; Zhu, J. Broadleaf Trees Increase Soil Aggregate Stability in Mixed Forest Stands of Southwest China. Forests 2023, 14, 2402. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z.-p. Afforestation Drives Soil Carbon and Nitrogen Changes in China. Land Degrad. Dev. 2017, 28, 151–165. [Google Scholar] [CrossRef]
- Tang, X.; Liu, S.; Liu, J.; Zhou, G. Effects of vegetation restoration and slope positions on soil aggregation and soil carbon accumulation on heavily eroded tropical land of Southern China. J. Soils Sediments 2010, 10, 505–513. [Google Scholar] [CrossRef]
- Dou, Y.; Yang, Y.; An, S.; Zhu, Z. Effects of different vegetation restoration measures on soil aggregate stability and erodibility on the Loess Plateau, China. Catena 2020, 185, 104294. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Hatano, R. Impact of burning on soil organic carbon of maize-upland rice system in Mae Chaem Basin of Northern Thailand. Geoderma 2021, 392, 115002. [Google Scholar] [CrossRef]
- Hu, P.-L.; Liu, S.-J.; Ye, Y.-Y.; Zhang, W.; Wang, K.-L.; Su, Y.-R. Effects of environmental factors on soil organic carbon under natural or managed vegetation restoration. Land Degrad. Dev. 2018, 29, 387–397. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.; Long, J.; Luo, K.; Huang, Z. Responses of soil labile organic carbon stocks and the carbon pool management index to different vegetation restoration types in the Danxia landform region of southwest China. PLoS ONE 2025, 20, e0318195. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Ng, E.L.; Patti, A.F.; Rose, M.T.; Schefe, C.R.; Wilkinson, K.; Smernik, R.J.; Cavagnaro, T.R. Does the chemical nature of soil carbon drive the structure and functioning of soil microbial communities? Soil Biol. Biochem. 2014, 70, 54–61. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Paul, E.A. Dynamics of organic matter in soils. Plant Soil 1984, 76, 275–285. [Google Scholar] [CrossRef]
- Paul, E.A. Chapter 1—Soil Microbiology, Ecology, and Biochemistry: An Exciting Present and Great Future Built on Basic Knowledge and Unifying Concepts. In Soil Microbiology, Ecology and Biochemistry, 4th ed.; Paul, E.A., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 1–14. [Google Scholar]
- Bonanomi, G.; De Filippis, F.; Cesarano, G.; La Storia, A.; Zotti, M.; Mazzoleni, S.; Incerti, G. Linking bacterial and eukaryotic microbiota to litter chemistry: Combining next generation sequencing with 13C CPMAS NMR spectroscopy. Soil Biol. Biochem. 2019, 129, 110–121. [Google Scholar] [CrossRef]
- Kell, D.B. Breeding crop plants with deep roots: Their role in sustainable carbon, nutrient and water sequestration. Ann. Bot. 2011, 108, 407–418. [Google Scholar] [CrossRef]
- Longbottom, T.L.; Wahab, L.; Min, K.; Jurusik, A.K.; Moreland, K.C.; Dolui, M.; Thao, T.; Gonzales, M.L.; Rojas, Y.; Alvarez, J.; et al. What’s Soil Got to Do with Climate Change? GSA Today 2022, 32, 4–10. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, W.; Zhou, Y.; Yin, Y. Soil Organic Carbon Chemical Functional Groups under Different Revegetation Types Are Coupled with Changes in the Microbial Community Composition and the Functional Genes. Forests 2019, 10, 240. [Google Scholar] [CrossRef]
- Kara, Ö.; Bolat, İ. The effect of different land uses on soil microbial biomass carbon and nitrogen in Bartin Province. Turk. J. Agric. For. 2008, 32, 281–288. [Google Scholar]
- Huang, Q.; Wang, B.; Shen, J.; Xu, F.; Li, N.; Jia, P.; Jia, Y.; An, S.; Amoah, I.D.; Huang, Y. Shifts in C-degradation genes and microbial metabolic activity with vegetation types affected the surface soil organic carbon pool. Soil Biol. Biochem. 2024, 192, 109371. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, W.; Zhu, K.; Ge, Y.; Li, W.; Liao, N. Similarities and differences in the microbial structure of surface soils of different vegetation types. PeerJ 2023, 11, e16260. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Gerke, H.H. Characterizing organic matter of soil aggregate coatings and biopores by Fourier transform infrared spectroscopy. Eur. J. Soil Sci. 2004, 55, 219–228. [Google Scholar] [CrossRef]
- Claesson, M.J.; O’Sullivan, O.; Wang, Q.; Nikkilä, J.; Marchesi, J.R.; Smidt, H.; de Vos, W.M.; Ross, R.P.; O’Toole, P.W. Comparative Analysis of Pyrosequencing and a Phylogenetic Microarray for Exploring Microbial Community Structures in the Human Distal Intestine. PLoS ONE 2009, 4, e6669. [Google Scholar] [CrossRef]
- White, T.J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Elsevier: Amsterdam, The Netherlands, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Li, K.; Lu, Y.; Wang, Q.-W.; Ni, R.; Han, R.; Li, C.; Zhang, C.; Shen, W.; Yao, Q.; Gao, Y.; et al. Leaf litter mixtures alter decomposition rate, nutrient retention, and bacterial community composition in a temperate forest. For. Res. 2023, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Chin, J.; Byrd, E.; Clerici, C.; Forster, A.; Oudina, M.; Sung, L.; Rice, K. Chemical and Physical Characterization of Poly(p-phenylene-2,6-benzobisoxazole) Fibers Used in Body Armor: Temperature and Humidity. Aging 2006. [Google Scholar] [CrossRef]
- Pandey, A.; Benjamin, S.; Soccol, C.R.; Nigam, P.; Krieger, N.; Soccol, V.T. The realm of microbial lipases in biotechnology. Biotechnol. Appl. Biochem. 1999, 29, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Du, C.; Ma, F.; Shen, Y.; Wu, K.; Liang, D.; Zhou, J. Detection of soil organic matter from laser-induced breakdown spectroscopy (LIBS) and mid-infrared spectroscopy (FTIR-ATR) coupled with multivariate techniques. Geoderma 2019, 355, 113905. [Google Scholar] [CrossRef]
- Doan, T.K.Q.; Chiang, K.Y. Characteristics and kinetics study of spherical cellulose nanocrystal extracted from cotton cloth waste by acid hydrolysis. Sustain. Environ. Res. 2022, 32, 26. [Google Scholar] [CrossRef]
- Xu, L.; Han, Y.; Yi, M.; Yi, H.; Guo, E.; Zhang, A. Shift of millet rhizosphere bacterial community during the maturation of parent soil revealed by 16S rDNA high-throughput sequencing. Appl. Soil Ecol. 2019, 135, 157–165. [Google Scholar] [CrossRef]
- Peltre, C.; Bruun, S.; Du, C.; Thomsen, I.K.; Jensen, L.S. Assessing soil constituents and labile soil organic carbon by mid-infrared photoacoustic spectroscopy. Soil Biol. Biochem. 2014, 77, 41–50. [Google Scholar] [CrossRef]
- Swetha, N.; Venkata Lakshmi, V.; Mylarappa, M.; Chandruvasan, S.; Harisha, K.S. Development of SiO2/rGO from Rice Husk for Photocatalysis, Antioxidant, Electrochemical and Green Sensor Detection Studies. Silicon 2024, 16, 4037–4059. [Google Scholar] [CrossRef]
- Calderón, F.J.; Reeves, J.B.; Collins, H.P.; Paul, E.A. Chemical Differences in Soil Organic Matter Fractions Determined by Diffuse-Reflectance Mid-Infrared Spectroscopy. Soil Sci. Soc. Am. J. 2011, 75, 568–579. [Google Scholar] [CrossRef]
- Chen, C.R.; Xu, Z.H.; Mathers, N.J. Soil Carbon Pools in Adjacent Natural and Plantation Forests of Subtropical Australia. Soil Sci. Soc. Am. J. 2004, 68, 282–291. [Google Scholar] [CrossRef]
- Guo, X.; Meng, M.; Zhang, J.; Chen, H.Y.H. Vegetation change impacts on soil organic carbon chemical composition in subtropical forests. Sci. Rep. 2016, 6, 29607. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Zhang, Y.; Duan, W.; Xia, J.; Wu, J.; Deng, T. Vegetation Types Can Affect Soil Organic Carbon and δ13C by Influencing Plant Inputs in Topsoil and Microbial Residue Carbon Composition in Subsoil. Sustainability 2024, 16, 4538. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Lu, B.; Zhang, Y.; Liu, G.; Xue, S. Variation in soil organic carbon stability and driving factors after vegetation restoration in different vegetation zones on the Loess Plateau, China. Soil Tillage Res. 2020, 204, 104727. [Google Scholar] [CrossRef]
- Gnoevoy, V.I.; Hnoievyi, I.; Trishin, A.; Karpiuk, U.; Kyslychenko, V.J.V.S. Content of polysaccharides in vegetative mass of maize in connection with its selection characteristic. Vet. Sci. Technol. Anim. Husb. Nat. Manag. 2019, 3, 29–36. [Google Scholar] [CrossRef]
- Lee, Y.; Lemmetty, J.M.; Nihtilä, H.; Koivula, H.; Samandoulougou, S.; Sawadogo-Lingani, H.; Katina, K.; Maina, N.H. Efficacy of Aflatoxin B1 and Fumonisin B1 Adsorption by Maize, Wheat, and Oat Bran. Toxins 2024, 16, 288. [Google Scholar] [CrossRef]
- Karpiuk, U.; Kyslychenko, V.; Abudayeh, Z. The study of carbohydrates of corn raw materials. ScienceRise Pharm. Sci. 2023, 1, 32–40. [Google Scholar] [CrossRef]
- Ni, H.; Jing, X.; Xiao, X.; Zhang, N.; Wang, X.; Sui, Y.; Sun, B.; Liang, Y. Microbial metabolism and necromass mediated fertilization effect on soil organic carbon after long-term community incubation in different climates. ISME J. 2021, 15, 2561–2573. [Google Scholar] [CrossRef]
- Ogawara, H. Comparison of Antibiotic Resistance Mechanisms in Antibiotic-Producing and Pathogenic Bacteria. Molecules 2019, 24, 3430. [Google Scholar] [CrossRef]
- Liu, F.; Hewezi, T.; Lebeis, S.L.; Pantalone, V.; Grewal, P.S.; Staton, M.E. Soil indigenous microbiome and plant genotypes cooperatively modify soybean rhizosphere microbiome assembly. BMC Microbiol. 2019, 19, 201. [Google Scholar] [CrossRef]
- Peregrín-Alvarez, J.M.; Sanford, C.; Parkinson, J. The conservation and evolutionary modularity of metabolism. Genome Biol. 2009, 10, R63. [Google Scholar] [CrossRef] [PubMed]
- Schada von Borzyskowski, L.; Bernhardsgrütter, I.; Erb, T.J. Biochemical unity revisited: Microbial central carbon metabolism holds new discoveries, multi-tasking pathways, and redundancies with a reason. Biol. Chem. 2020, 401, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yuan, C.; Chen, P.; Rong, Z.; Peng, T.; Farooq, T.H.; Wang, G.; Yan, W.; Wang, J. Soil Microbial Community Composition and Diversity Analysis under Different Land Use Patterns in Taojia River Basin. Forests 2023, 14, 1004. [Google Scholar] [CrossRef]
- Watzinger, A.; Prommer, J.; Spiridon, A.; Kisielinska, W.; Hood-Nowotny, R.; Leitner, S.; Wanek, W.; Resch, C.; Heiling, M.; Murer, E.; et al. Functional redundant soil fauna and microbial groups and processes were fairly resistant to drought in an agroecosystem. Biol. Fertil. Soils 2023, 59, 629–641. [Google Scholar] [CrossRef]
- Zhong, C.; Hu, C.; Xu, C.; Zhang, Z.; Hu, G. Metabolomics reveals changes in soil metabolic profiles during vegetation succession in karst area. Front. Microbiol. 2024, 15, 1337672. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Deng, J.; Zhang, J.; Fu, Q.; Wang, T.; Osono, T.; Peng, H.; Robson, T.M.; Kurokawa, H.; Wang, Q.-W. Sunlight promotes aboveground carbon loss by producing polysaccharides from litter decomposition in a temperate forest. J. For. Res. 2025, 36, 22. [Google Scholar] [CrossRef]
- Tang, M.; Hou, W.; Gong, J.; Jin, J.; Malik, K.; Wang, C.; Kong, X.; Chen, X.; Wang, L.; Chen, L.; et al. Changes in bacterial community structure and root metabolites in Themeda japonica habitat under slight and strong karst rocky desertification. Appl. Soil Ecol. 2024, 195, 105227. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.-J.; Wang, L. Irradiation and nitrogen regulate growth and physiology in Horsfieldia hainanensis seedlings. Biol. Plant. 2023, 67, 19–27. [Google Scholar] [CrossRef]
| ArC | PSC | AlkC | AmC | EtC | OleC | |
|---|---|---|---|---|---|---|
| CB | 6.10 ± 0.13 b | 132.04 ± 2.36 a | - | - | - | 16.48 ± 0.37 d |
| ZL | 6.24 ± 0.31 b | 92.18 ± 12.29 b | - | - | - | 14.61 ± 0.58 c |
| DG | 105.79 ± 20.52 a | 152.48 ± 14.26 a | 38.82 ± 4.45 b | 3.76 ± 3.76 b | 3.52 ± 3.53 b | 8.82 ± 0.83 b |
| HM | 4.83 ± 0.15 b | 147.58 ± 3.61 a | 416.87 ± 2.30 a | 503.94 ± 1.73 a | 578.18 ± 1.62 a | 11.12 ± 0.35 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, K.; Xu, S.; Wang, L.; Wu, S.; Zhu, W.; Liao, N.; Li, W. Response of Soil Organic Carbon and Microbial Metabolic Pathways in Guangxi Karst Regions to Different Vegetation Types. Forests 2025, 16, 1664. https://doi.org/10.3390/f16111664
Zhu K, Xu S, Wang L, Wu S, Zhu W, Liao N, Li W. Response of Soil Organic Carbon and Microbial Metabolic Pathways in Guangxi Karst Regions to Different Vegetation Types. Forests. 2025; 16(11):1664. https://doi.org/10.3390/f16111664
Chicago/Turabian StyleZhu, Keye, Sheng Xu, Lei Wang, Siqi Wu, Wenxu Zhu, Nanyan Liao, and Wuzheng Li. 2025. "Response of Soil Organic Carbon and Microbial Metabolic Pathways in Guangxi Karst Regions to Different Vegetation Types" Forests 16, no. 11: 1664. https://doi.org/10.3390/f16111664
APA StyleZhu, K., Xu, S., Wang, L., Wu, S., Zhu, W., Liao, N., & Li, W. (2025). Response of Soil Organic Carbon and Microbial Metabolic Pathways in Guangxi Karst Regions to Different Vegetation Types. Forests, 16(11), 1664. https://doi.org/10.3390/f16111664






