Altered Functional Traits in Larix principis-rupprechtii Mayr Seedlings: Responses and Divergence Across Altitudes
Abstract
1. Introduction
2. Materials and Methods
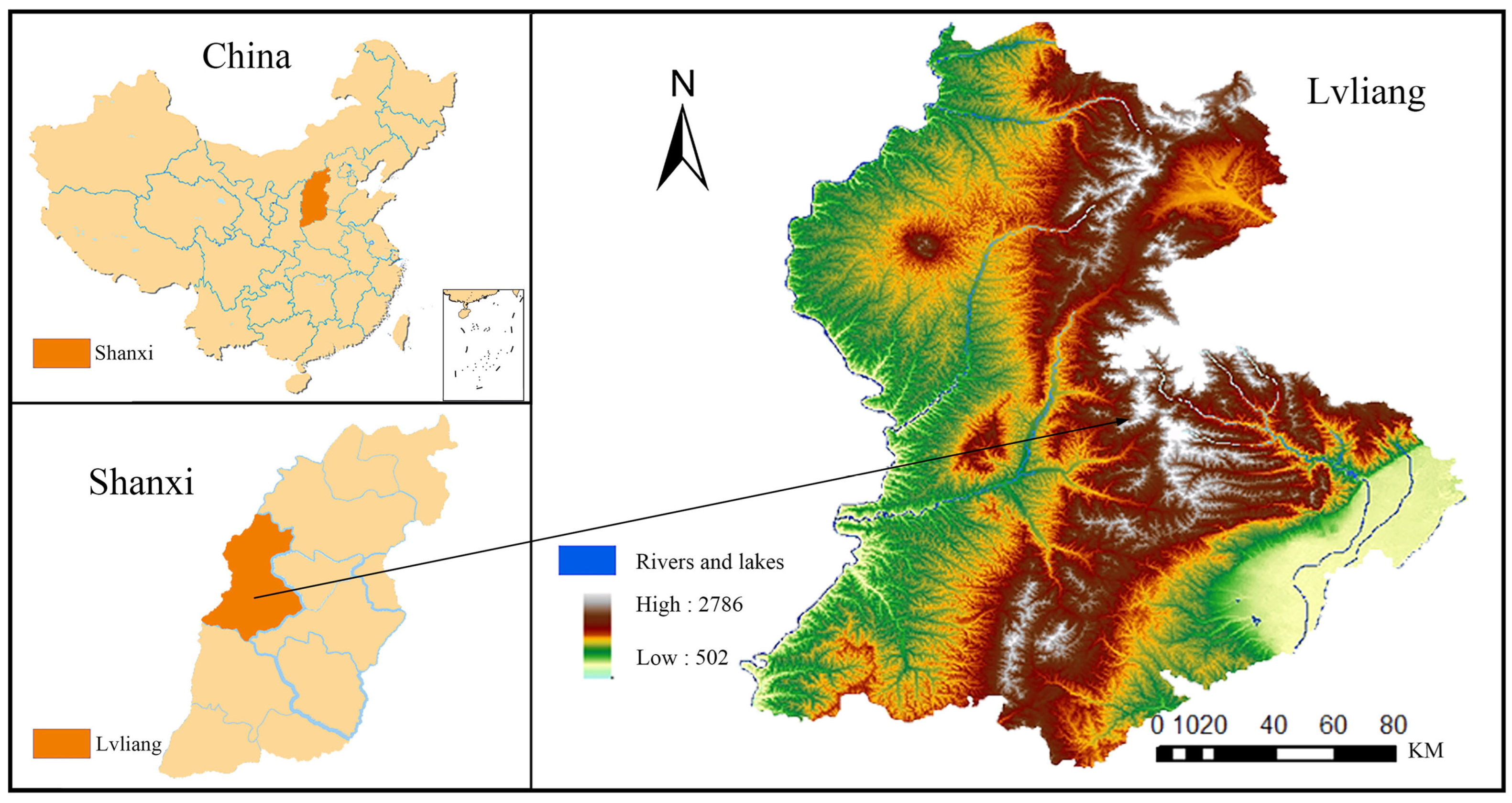
2.1. Study Area
2.2. Plot Selection and Experimental Design
2.3. Sample Collection and Measurements
2.3.1. Measurement of Physiological Functional Traits
2.3.2. Measurement of Photosynthetic Traits
2.3.3. Measurement of Morphological Structural Traits
2.3.4. Measurement of Nutrient Utilisation Capacity
2.4. Data Analysis
3. Results
3.1. Altitudinal Adaptation of Leaf Physiological Traits in Larix principis-rupprechtii Seedlings from Different Provenances
3.1.1. Effects of Provenance Elevation and Transplant Elevation on Physiological Traits in Seedlings of Larix principis-rupprechtii
3.1.2. The Influence of Seed Source Altitude and Transplantation Altitude on Chlorophyll Content and Fluorescence of Larix principis-rupprechtii
3.1.3. The Influence of Seed Source Altitude and Transplanting Altitude on Leaf Morphological Traits of Larix principis-rupprechtii
3.1.4. The Influence of Seed Source Altitude and Transplanting Altitude on the Nutrient Utilisation Ability of Larix principis-rupprechtii
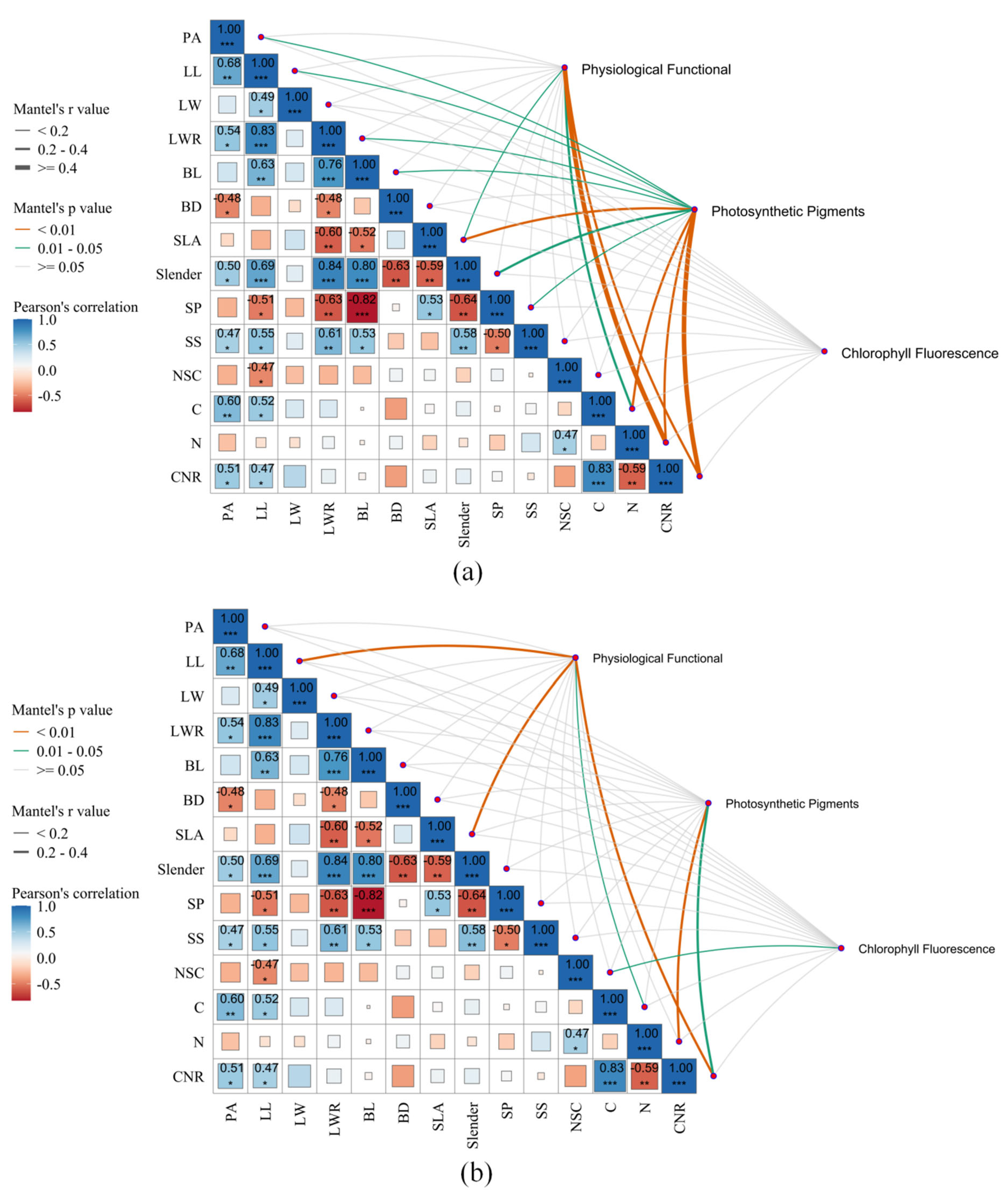
3.2. Correlation Analysis of Leaf Functional Traits of Larix principis-rupprechtii Across Elevational Gradients
3.3. Pathway Differences Among Functional Traits of Larix principis-rupprechtii at Different Elevational Gradients
4. Discussion
4.1. Responses of Leaf Functional Traits in Seedlings of Larix principis-rupprechtii to Transplant Elevation
4.2. Correlations Among Leaf Functional Traits of Larix principis-rupprechtii Seedlings Across Elevational Gradients
4.3. Pathway Differences in Which Leaf Traits Affect Physiological Traits Across Elevational Gradients
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LSP | Light Saturation Point |
| LCP | Light Compensation Point |
| WUE | Water-Use Efficiency |
| Tr | Transpiration Rate |
| Pn | Net Photosynthetic Rate |
| Amax | Maximum Net Photosynthetic Rate |
| Chla | Chlorophyll a |
| Chlb | Chlorophyll b |
| Tchlab | Total Chlorophyll ab |
| Chla/b | Chlorophyll ab ratio |
| Car | Carotenoids |
| CarChl | The Ratio of Carotenoids to Chlorophyll |
| NPQ | Non-Photochemical Quenching Coefficient |
| ETR | Electron Transfer Rate |
| qP | Photochemical Quenching |
| BL | New Branch Length |
| BD | New Branch Diameter |
| LL | Leaf Length |
| LW | Leaf Width |
| LWR | Leaf Length-to-width Ratio |
| PA | Project Area |
| SLA | Specific Leaf Area |
| SS | Soluble Sugar |
| SP | Soluble Protein |
| NSC | Non-Structural Carbohydrates |
| CNR | Carbon–Nitrogen Ratio |
References
- Veal, A.J. Climate Change 2021—The Physical Science Basis. Chem. Int. 2021, 43, 22–23. [Google Scholar] [CrossRef]
- Perkins-Kirkpatrick, S.E.; Lewis, S.C. Increasing trends in regional heatwaves. Nat. Commun. 2020, 11, 3357. [Google Scholar] [CrossRef]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The projected timing of abrupt ecological disruption from climate change. Nature 2020, 580, 496. [Google Scholar] [CrossRef] [PubMed]
- Ripple, W.J.; Wolf, C.; Newsome, T.M.; Barnard, P.; Moomaw, W.R. World Scientists’ Warning of a Climate Emergency. Bioscience 2020, 70, 8–12. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D.; et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Change 2015, 5, 424–430. [Google Scholar] [CrossRef]
- Christian, K. Alpine Plant Life; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Ma, J.; Kang, F.; Cheng, X.; Han, H. Response of soil organic carbon and nitrogen to nitrogen deposition in a Larix principis-rupprechtii plantation. Sci. Rep. 2018, 8, 8638. [Google Scholar] [CrossRef]
- Liu, T.; Peng, D.; Tan, Z.; Guo, J.; Zhang, Y. Effects of stand density on soil respiration and labile organic carbon in different aged Larix principis-rupprechtii plantations. Ecol. Process. 2021, 10, 44. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Jia, J.; Zhang, Z.; Guo, M. Responses of radial growth of Larix principis-rupprechtii to abrupt warming. Chin. J. Appl. Ecol. 2023, 34, 2629–2636. [Google Scholar]
- Brinkmann, N.; Eugster, W.; Zweifel, R.; Buchmann, N.; Kahmen, A. Temperate tree species show identical response in tree water deficit but different sensitivities in sap flow to summer soil drying. Tree Physiol. 2016, 36, 1508–1519. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, E.B.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Luca, D.N.; Chiara, V.; Renato, B.; Paolo, G.; Alessandro, C.; Valter, D.C.; Luciano, D.M.; Michele, D.M.; Gabriele, G.; Chiara, L.; et al. Contrasting multitaxon responses to climate change in Mediterranean mountains. Sci. Rep. 2021, 11, 4438. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.M.; Zhang, J.; Wang, X.Y.; Ge, Z.X.; Zhang, Z.D. Predicting the growth suitability of Larix principis-rupprechtii based on site index under different climatic scenarios. Front. Plant Sci. 2023, 14, 1097688. [Google Scholar] [CrossRef] [PubMed]
- Tiphaine, P.; Valérie, D.; Ignacio, M.; Monique, P.; Michel, S.; Ana, S.; Laia, A.-H.; Ricardo, V. Tree-ring isotopes from Araucaria araucana as useful proxies for climate reconstructions. Dendrochronologia 2022, 74, 125979. [Google Scholar] [CrossRef]
- Kristensen, T.N.; Ketola, T.; Kronholm, I. Adaptation to environmental stress at different timescales. Ann. N. Y. Acad. Sci. 2020, 1476, 5–22. [Google Scholar] [CrossRef]
- Ogle, K.; Barber, J.J.; Patrick, B.L.; Barron-Gafford, G.A.; Bentley, L.P.; Young, J.M.; Huxman, T.E. Quantifying ecological memory in plant and ecosystem processes. Ecol. Lett. 2015, 18, 221–235. [Google Scholar] [CrossRef]
- Clark, P.W.; D’Amato, A.W.; Evans, K.S.; Schaberg, P.G.; Woodall, C.W. Ecological memory and regional context influence performance of adaptation plantings in northeastern US temperate forests. J. Appl. Ecol. 2021, 59, 314–329. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, X.; Zhang, J.; Zhao, J.; Ge, Z.; Zhang, Z. Predicting the Potential Suitable Distribution of Larix principis-rupprechtii under Climate Change Scenarios. Forests 2022, 13, 1428. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Field, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Liu, X. Review on the Elevation Adaptive Mechanisms of Alpine Plants Based on Different Research Methods. J. Trop. Subtrop. Bot. 2025, 33, 352–362. (In Chinese) [Google Scholar]
- Zhang, W.; Jiang, Y.; Wang, M.; Zhang, L.; Dong, M. Responses of radial growth in Larix principis-rupprechtii to climate change along an elevation gradient on the southern slope of Luya Mountain. Acta Ecol. Sin. 2015, 35, 6481–6488. (In Chinese) [Google Scholar]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tian, S.; Li, Y.; Zhang, Y.; Guo, J. A Study on the Relationship Between the Local Climatic—Geographic Gradient and the Bio-climatic Indices in Guandi Mountains. J. Shanxi Agric. Univ. 1998, 1, 7–11+91–92. [Google Scholar] [CrossRef]
- Han, X.; Huang, C.; Zhang, Y.; Guo, J. Soil Bacterial Community Composition and Biodiversity of Different Degraded Grassland Community Types in the Guandi Mountain Forest Region, Northern China. Acta Laser Biol. Sin. 2020, 29, 446–452+460. (In Chinese) [Google Scholar]
- Guidi, C.; Frey, B.; Brunner, I.; Meusburger, K.; Vogel, M.E.; Chen, X.; Stucky, T.; Gwiazdowicz, D.J.; Skubała, P.; Bose, A.K.; et al. Soil fauna drives vertical redistribution of soil organic carbon in a long-term irrigated dry pine forest. Glob. Change Biol. 2022, 28, 3145–3160. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, J.; Zhang, Y. Interactive Effects of Climate Warming and Forest Succession on Root Respiration of Natural Secondary Forest in Warm Temperate Zone. J. Soil Water Conserv. 2022, 36, 122–129. (In Chinese) [Google Scholar] [CrossRef]
- Mehta, N.; Chawla, A. Eco-physiological trait variation in widely occurring species of Western Himalaya along elevational gradients reveals their high adaptive potential in stressful conditions. Photosynth. Res. 2024, 159, 29–59. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.; Li, C.; Guo, J.; Zhang, Y. Altitudinal Variation and Acclimation of Physiological Characteristics in Meyer Spruce Seedlings. Natuural Science Edition; J. Shanxi Agric. Univ. 2014, 34, 541–547. (In Chinese) [Google Scholar] [CrossRef]
- Saathoff, A.J.; Jon, W. Gas exchange measurements in the unsteady state. Plant Cell Environ. 2021, 44, 3509–3523. [Google Scholar] [CrossRef]
- Hans, L.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 2008. [Google Scholar]
- Farquhar, G.; Richards, R. Isotopic Composition of Plant Carbon Correlates with Water-Use Efficiency of Wheat Genotypes. Funct. Plant Biol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Maxwell, K. Perspectives in Experimental Botany. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 10. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H.t.; Morgan, H.D.; Heijden, M.G.A.v.d.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Feng, X.; Lv, S.; Zhang, Y. Adaptive Differences of Larix principis-rupprechtii Regenerated Seedlings from Different Elevations to Elevations of Transplant Sites. Guangxi For. Sci. 2025, 54, 44–51. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, X.; Zhang, J.; Zhang, Y. Response of leaf functional traits and C, N, and P stoichiometry of Fraxinus mandshurica Rupr. to altitude. Acta Bot. Boreal.-Occident. Sin 2024, 44, 792–801. (In Chinese) [Google Scholar]
- Fan, B.L.; Ma, Z.Q.; Gao, P.F.; Lu, J.; Ding, N.N.; Sun, K. Functional Traits of Male and Female Leaves of Hippophae tibetana on the Eastern Edge of the Tibetan Plateau and Their Altitudinal Variability. Plants 2022, 11, 2484. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, X.L. Changes in leaf functional traits of common woody plants across altitudinal gradients in the Lhasa River Basin. J. Sichuan For. Sci. Technol. 2025, 46, 22–31. (In Chinese) [Google Scholar]
- Gabriele, M.; Pieter, D.F.; Norbert, H.; Camilla, W. Global patterns of intraspecific leaf trait responses to elevation. Glob. Change Biol. 2019, 25, 2485–2498. [Google Scholar]
- Liang, J.; Niu, Y.; Xie, J.; Zhang, J. Antioxidase activities and photosynthetic pigment contents in Larix principis-rupprechtii leaves along an altitudinal gradient. Chin. J. Appl. Ecol. 2007, 18, 1414–1419. (In Chinese) [Google Scholar] [CrossRef]
- Xing, H.; Feng, Q.; Shi, Z.M.; Liu, S.; Xu, G.; Chen, J.; Gong, S. Response of leaf traits to altitude in Quercus aquifolioids and Sorbud rehderiana on the eastern edge of the Qinghai–Tibet Plateau, China. Chin. J. Appl. Ecol. 2024, 35, 606–614. (In Chinese) [Google Scholar] [CrossRef]
- Tumajer, J.; Altman, J.; Lehejcek, J. Linkage between growth phenology and climate-growth responses along landscape gradients in boreal forests. Sci. Total Environ. 2023, 905, 167153. [Google Scholar] [CrossRef]
- Liu, X.; Gan, L.; Liu, C.; Sun, Y.; Wei, X.; Xu, M.; Han, C.; Tian, B.; Jia, X.; Zha, T. Variation and Adaptation Strategies in Leaf Traits of Main Woody Plants in the Larix principis-rupprechtii Community in Baihua Mountain, Beijing. Sci. Silvae Sin. 2023, 59, 12–23. (In Chinese) [Google Scholar]
- Richter, D.D.; Markewitz, D.; Trumbore, S.E.; Wells, C.G. Rapid accumulation and turnover of soil carbon in a re-establishing forest. Nature 1999, 400, 56–58. [Google Scholar] [CrossRef]
- Grether, F.G. Environmental change, phenotypic plasticity, and genetic compensation. Am. Nat. 2005, 166, E115–E123. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B. Leaf structure (specific leaf area) modulates photosynthesis-nitrogen relations: Evidence from within and across species and functional groups. Funct. Ecol. 1998, 12, 948–958. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- Bibi, Z.; Maqsood, M.J.; Idrees, A.; Rafique, H.; Butt, A.A.; Ali, R.; Arif, Z.; Nabi, M.U. Exploring the Role of Phenotypic Plasticity in Plant Adaptation to Changing Climate: A Review. Asian J. Res. Crop Sci. 2024, 9, 1–9. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Albert, C.H.; Francesco, d.B.; Isabelle, B.; Gilles, P.; Sandra, L.; Wilfried, T. On the importance of intraspecific variability for the quantification of functional diversity. Oikos 2012, 121, 116–126. [Google Scholar] [CrossRef]
- Hepworth, C.; Caine, R.S.; Harrison, E.L.; Sloant, J.; Gray, J.E. Stomatal development: Focusing on the grasses. Curr. Opin. Plant Biol. 2018, 41, 1–7. [Google Scholar] [CrossRef]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917. [Google Scholar] [CrossRef]

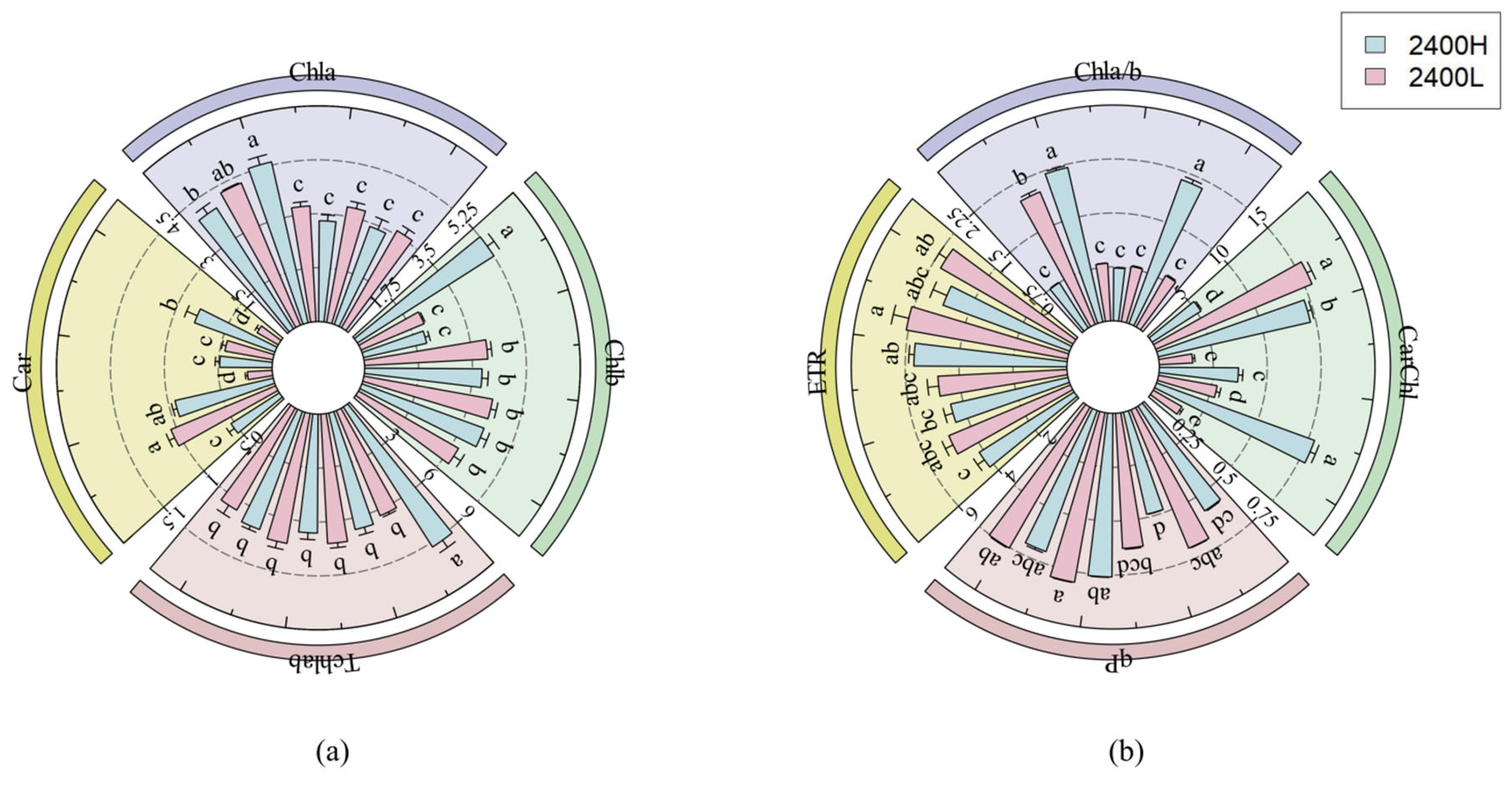
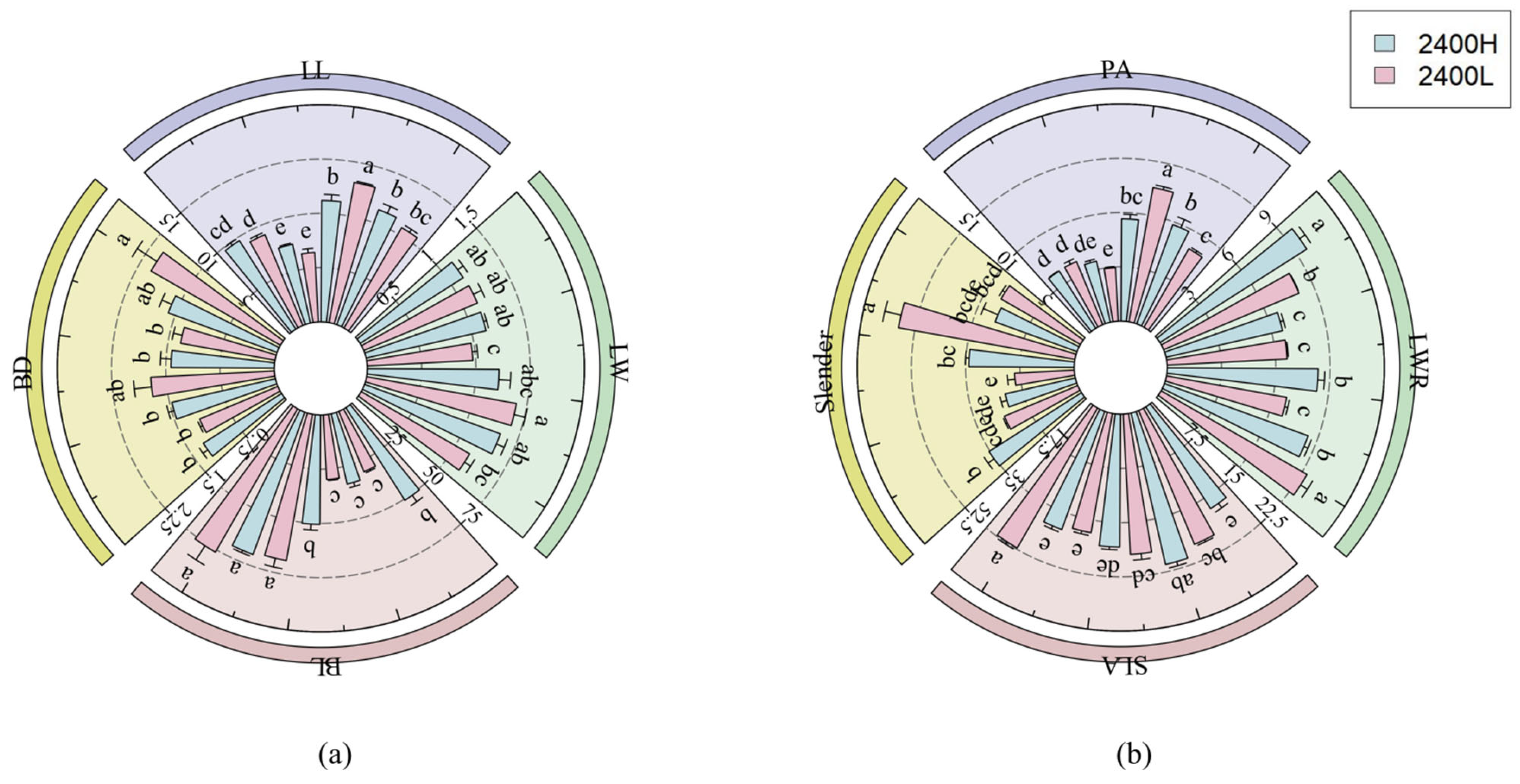
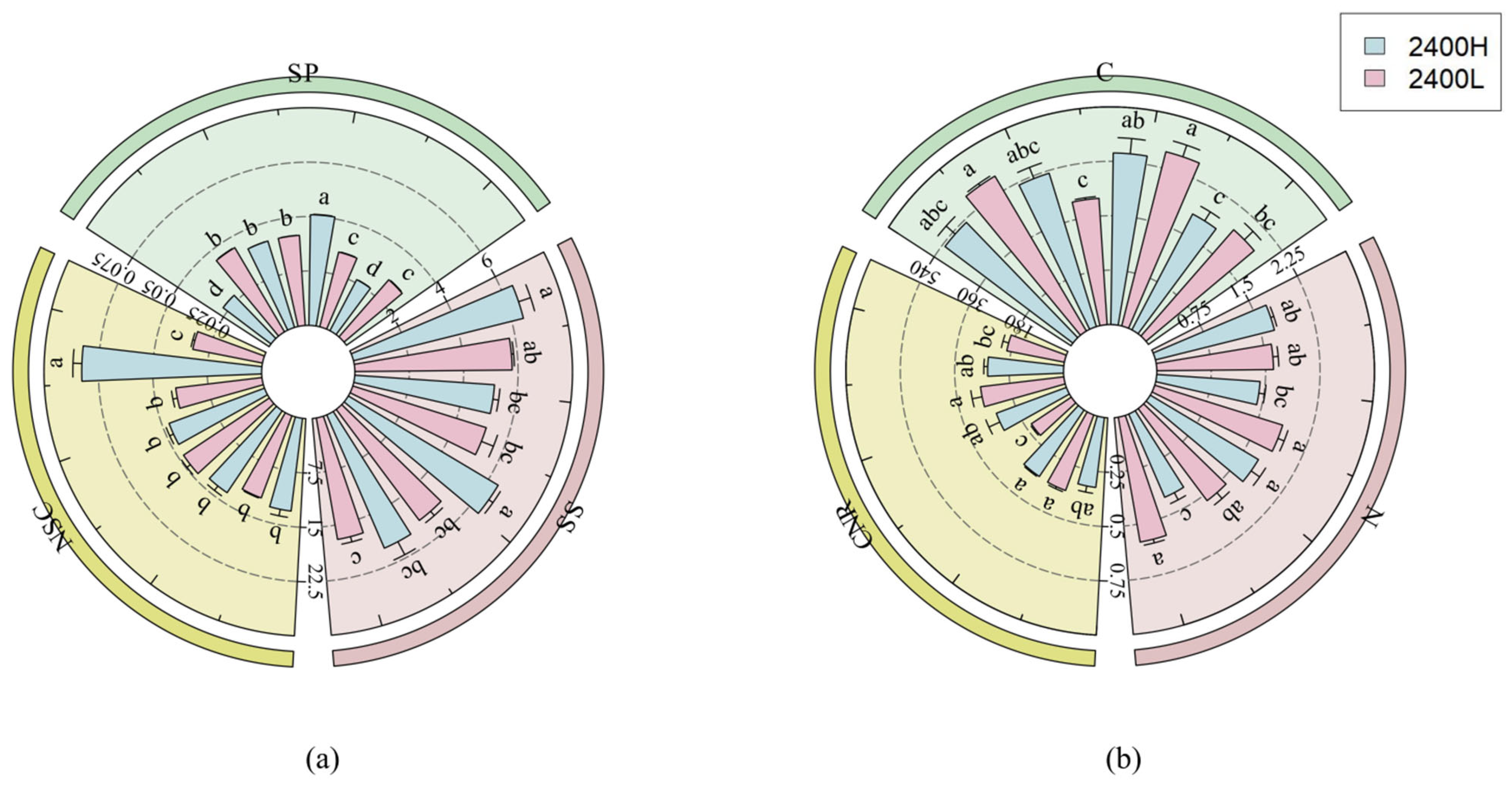


| High Elevation | Interpretation Amount (%) | F | p |
|---|---|---|---|
| Model | 48.110 | 3.520 | <0.001 |
| Chla | 7.320 | 3.240 | 0.037 |
| Tchlab | 3.510 | 3.330 | 0.032 |
| PA | 12.210 | 10.690 | <0.001 |
| LW | 7.270 | 2.190 | 0.109 |
| SLA | 7.830 | 2.870 | 0.040 |
| SS | 5.690 | 0.950 | 0.417 |
| NSC | 4.280 | 1.340 | 0.262 |
| Low Elevation | Interpretation Amount (%) | F | p |
| Model | 58.650 | 4.850 | <0.001 |
| Chla | 2.110 | 3.710 | 0.029 |
| Chlb | 1.940 | 3.740 | 0.025 |
| LL | 1.200 | 2.810 | 0.048 |
| LW | 1.360 | 2.480 | 0.067 |
| LWR | 7.950 | 3.070 | 0.031 |
| SLA | 8.670 | 3.320 | 0.032 |
| SP | 35.420 | 14.820 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Xie, J.; Liu, T.; Guo, J.; Zhang, Y.; Yang, M. Altered Functional Traits in Larix principis-rupprechtii Mayr Seedlings: Responses and Divergence Across Altitudes. Forests 2025, 16, 1665. https://doi.org/10.3390/f16111665
Deng J, Xie J, Liu T, Guo J, Zhang Y, Yang M. Altered Functional Traits in Larix principis-rupprechtii Mayr Seedlings: Responses and Divergence Across Altitudes. Forests. 2025; 16(11):1665. https://doi.org/10.3390/f16111665
Chicago/Turabian StyleDeng, Jiayi, Jiangkai Xie, Tairui Liu, Jinping Guo, Yunxiang Zhang, and Meng Yang. 2025. "Altered Functional Traits in Larix principis-rupprechtii Mayr Seedlings: Responses and Divergence Across Altitudes" Forests 16, no. 11: 1665. https://doi.org/10.3390/f16111665
APA StyleDeng, J., Xie, J., Liu, T., Guo, J., Zhang, Y., & Yang, M. (2025). Altered Functional Traits in Larix principis-rupprechtii Mayr Seedlings: Responses and Divergence Across Altitudes. Forests, 16(11), 1665. https://doi.org/10.3390/f16111665






