A Global Comparative Analysis of Drought Responses of Pines and Oaks
Abstract
1. Introduction
2. Material and Methods
2.1. Data Collection
2.2. Data Analysis
3. Results
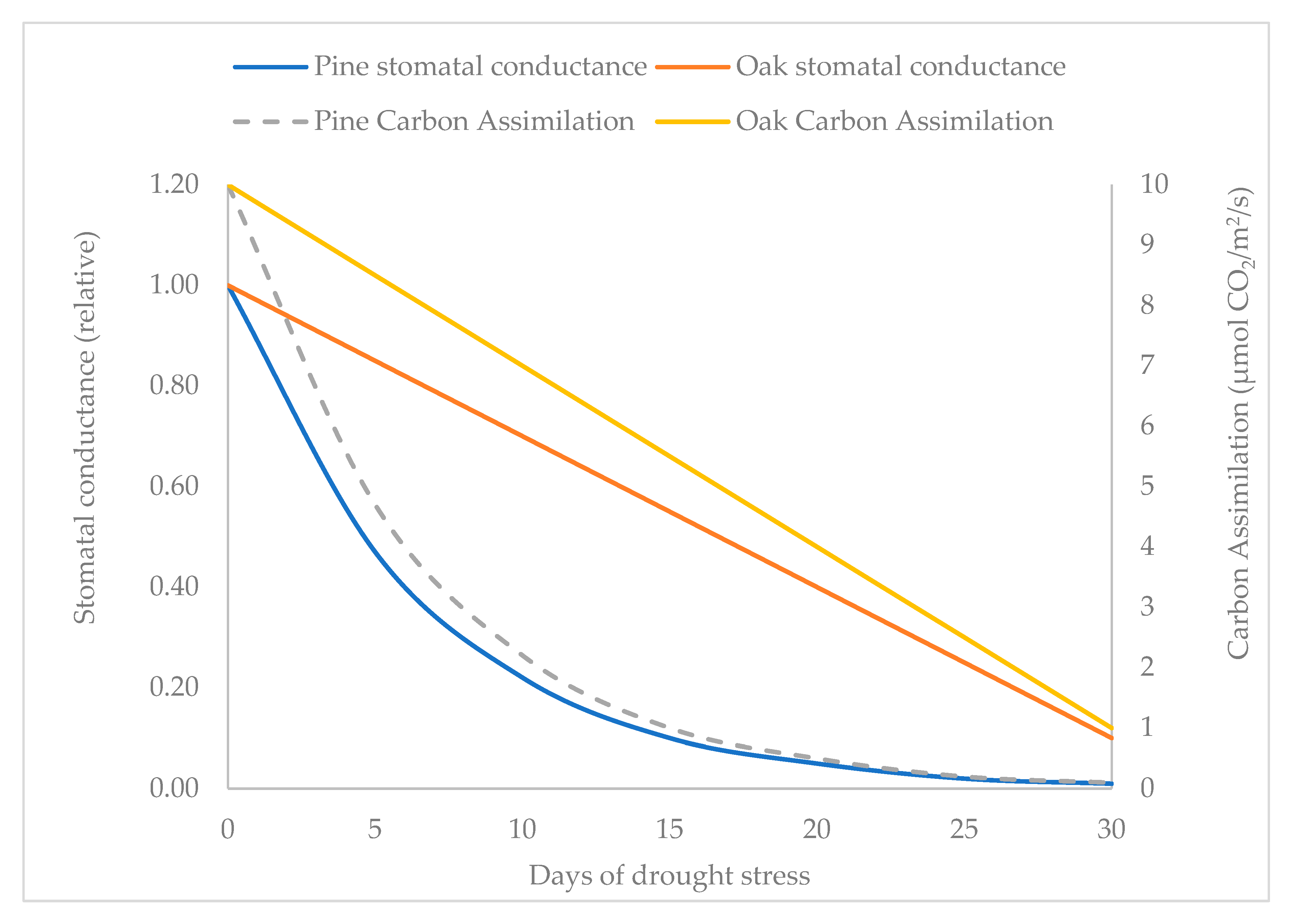
3.1. Water Balance in Response to Drought: Generalizations
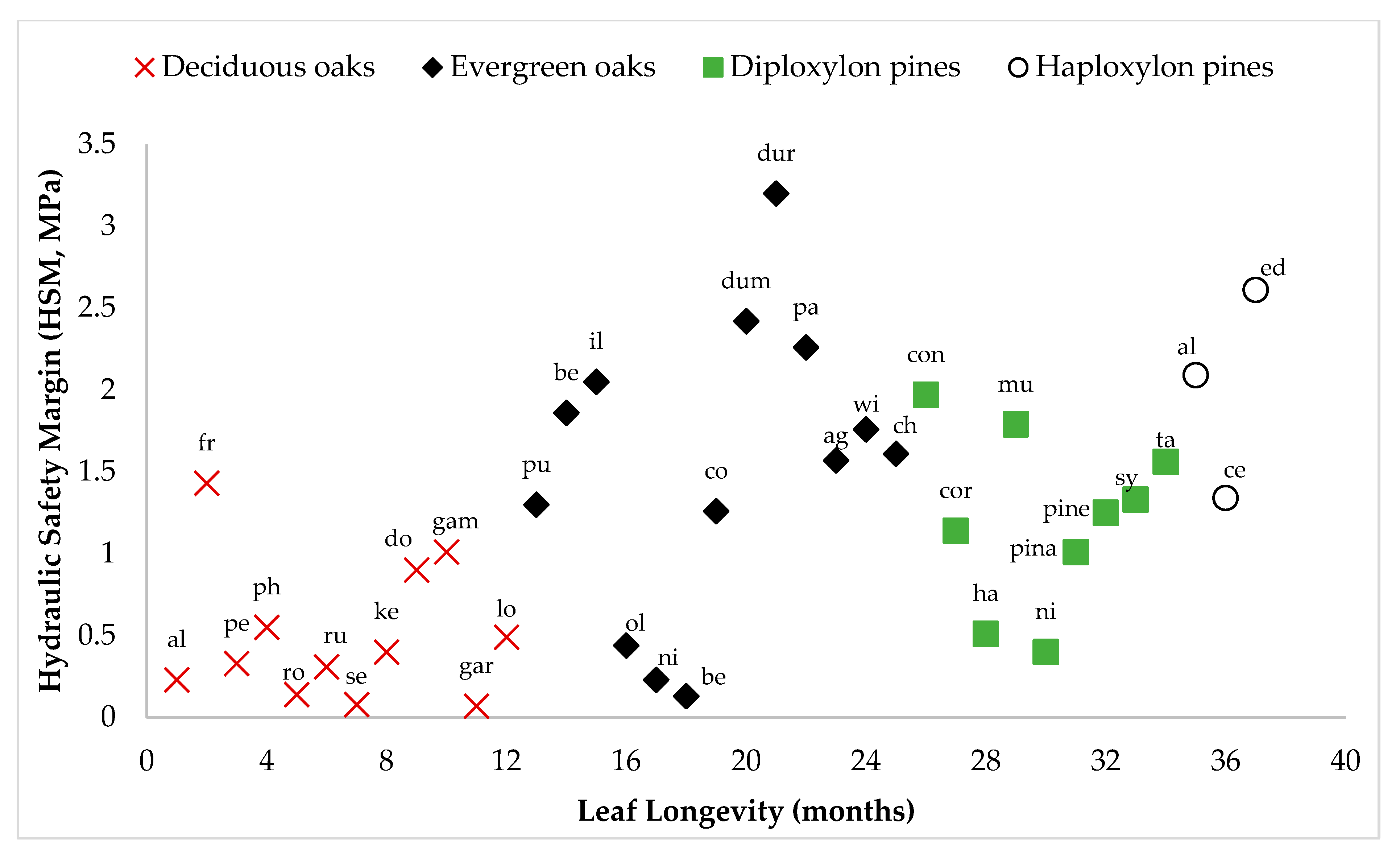
3.2. Hydraulic Safety Margin
3.3. Growth and Carbon Recovery
3.4. Terpene Emissions and Antioxidants
3.5. Climate Change Effect
4. Discussion
4.1. Contrasting Drought Responses and Hydraulic Safety Margin
4.2. Costs Associated with Drought Responses
4.3. Drought Response in Relation to Ecological Succession and Co-Occurrence
4.4. Recent Changes in Oak and Pine Dominance in an Area Appear to Reflect Climate Change Effect
4.5. Extent of Pine–Oak Complementarity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farjon, A.; Filer, D. An Atlas of the World’s Conifers: An Analysis of Their Distribution, Biogeography, Diversity and Conservation Status; Brill: Leiden, The Netherlands, 2013. [Google Scholar]
- Denk, T.; Grimm, G.W.; Manos, P.S.; Deng, M.; Hipp, A.L. An updated infrageneric classification of the oaks: Review of previous taxonomic schemes and synthesis of evolutionary patterns. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Gil-Pelegrín, E., Peguero-Pina, J., Sancho-Knapik, D., Eds.; Tree Physiology; Springer: Cham, Switzerland, 2017; Volume 7, pp. 13–38. [Google Scholar]
- Gernandt, D.S.; López, G.G.; García, S.O.; Liston, A. Phylogeny and classification of Pinus. Taxon 2005, 54, 29–42. [Google Scholar] [CrossRef]
- Kremer, A.; Hipp, A.L. Oaks: An evolutionary success story. New Phytol. 2020, 226, 987–1011. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Zobel, D.B. Pine-Oak Type: A unique resilient global system with high potential for nature-based solutions. Ecol. Indic. 2025, 175, 113536. [Google Scholar] [CrossRef]
- Farjon, A. The Kew review: Conifers of the world. Kew Bull. 2018, 73, 8. [Google Scholar] [CrossRef]
- Thompson, J.D. Plant Evolution in the Mediterranean; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Farjon, A.; Styles, B.T. Pinus (Pinaceae). In Flora Neotropica Monograph 75; New York Botanical Garden: New York, NY, USA, 1997. [Google Scholar]
- Gernandt, D.S.; Pérez-de la Rosa, J.A. Biodiversidad de Pinophyta (coníferas) en México. Rev. Mex. Biodivers. 2014, 85, 126–133. [Google Scholar] [CrossRef]
- Blondel, J.; Aronson, J. Biology and Wildlife of the Mediterranean Region; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Singh, R.D.; Gumber, S.; Joshi, H.; Singh, S.P. Allocation to tree bark in pine and oak species in fire affected mixed forests across the Northern Hemisphere. For. Ecol. Manag. 2022, 509, 120081. [Google Scholar] [CrossRef]
- Singh, S.P.; Gumber, S.; Singh, R.D.; Pandey, R. Differentiation of diploxylon and haploxylon pines in spatial distribution, and adaptational traits. AES 2023, 43, 1–10. [Google Scholar] [CrossRef]
- Forest Survey of India (FSI). Indian State of Forest Report 2021; Forest Survey of India: Dehradun, India, 2021. [Google Scholar]
- Sheffer, E. A review of the development of Mediterranean pine–oak ecosystems after land abandonment and afforestation: Are they novel ecosystems? Ann. For. Sci. 2012, 69, 429–443. [Google Scholar] [CrossRef]
- Barbéro, M.; Quezel, P. Les forêts de Méditerranée Orientale dans une perspective d’Ecologie appliquée à la Sylviculture méditerranéenne. Pascal Fr. Bibliogr. Databases 1981, 2, 227–239. [Google Scholar]
- Barbéro, M.; Loisel, R.; Quézel, P.; Richardson, D.M.; Romane, F. Pines of the Mediterranean Basin. In Ecology and Biogeography of Pinus; Richardson, D.M., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 153–170. [Google Scholar]
- Yu, F.; Wang, D.X.; Shi, X.X.; Yi, X.F.; Huang, Q.P.; Hu, Y.N. Effects of environmental factors on tree seedling regeneration in a pine-oak mixed forest in the Qinling Mountains, China. J. Mt. Sci. 2013, 10, 845–853. [Google Scholar] [CrossRef]
- Chai, Z.; Fan, D.; Wang, D. Environmental factors and underlying mechanisms of tree community assemblages of pine-oak mixed forests in the Qinling Mountains, China. J. Plant Biol. 2016, 59, 347–357. [Google Scholar] [CrossRef]
- Champion, H.G.; Seth, S.K. A Revised Survey of the Forest Types of India; Government of India Press: New Delhi, India, 1968. [Google Scholar]
- Singh, J.S.; Singh, S.P. Forests of the Himalaya: Structure, Functioning and Impact of Man; Gyanodaya Prakashan: Nainital, India, 1992. [Google Scholar]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.G. Tree responses to drought. Tree Physiol. 2011, 31, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Breshears, D.D.; Myers, O.B.; Meyer, C.W.; Barnes, F.J.; Zou, C.B.; Allen, C.D.; McDowell, N.G.; Pockman, W.T. Tree die-off in response to global change-type drought: Mortality insights from a decade of plant water potential measurements. Front. Ecol. Environ. 2009, 7, 185–189. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- McDowell, N.G.; Fisher, R.A.; Xu, C.; Domec, J.C.; Hölttä, T.; Mackay, D.S.; Sperry, J.S.; Boutz, A.; Dickman, L.; Gehres, N.; et al. Evaluating theories of drought-induced vegetation mortality using a multimodel–experiment framework. New Phytol. 2013, 200, 304–321. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Reyna, T.; Martínez-Vilalta, J.; Retana, J. Regeneration patterns in Mexican pine-oak forests. For. Ecosyst. 2019, 6, 50. [Google Scholar] [CrossRef]
- Vayreda, J.; Gracia, M.; Martinez-Vilalta, J.; Retana, J. Patterns and drivers of regeneration of tree species in forests of peninsular Spain. J. Biogeogr. 2013, 40, 1252–1265. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Pons, X.; Ninyerola, M.; Vayreda, J.; Peñuelas, J. Large-scale recruitment limitation in Mediterranean pines: The role of Quercus ilex and forest successional advance as key regional drivers. Glob. Ecol. Biogeogr. 2014, 23, 371–384. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Escudero, A.; Mediavilla, S.; Scoffoni, C.; Zailaa, J.; Cavender-Bares, J.; Álvarez-Arenas, T.G.; Molins, A.; Alonso-Forn, D.; Ferrio, J.P.; et al. Deciduous and evergreen oaks show contrasting adaptive responses in leaf mass per area across environments. New Phytol. 2021, 230, 521–534. [Google Scholar] [CrossRef]
- Keeley, J.E.; Fotheringham, C.J.; Morais, M. Reexamining fire suppression impacts on brushland fire regimes. Science 1999, 284, 1829–1832. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Verdú, M. Plant persistence traits in fire-prone ecosystems of the Mediterranean basin: A phylogenetic approach. Oikos 2005, 109, 196–202. [Google Scholar] [CrossRef]
- Singh, R.D.; Gumber, S.; Sundriyal, R.C.; Ram, J.; Singh, S.P. Chir pine forest and pre-monsoon drought determine spatial, and temporal patterns of forest fires in Uttarakhand Himalaya. Trop. Ecol. 2024, 65, 32–42. [Google Scholar] [CrossRef]
- Martínez-Ferrí, E.; Balaguer, L.; Valladares, F.; Chico, J.M.; Manrique, E. Energy dissipation in drought-avoiding and drought-tolerant tree species at midday during the Mediterranean summer. Tree Physiol. 2000, 20, 131–138. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Valladares, F.; Sánchez-Gómez, D. Ecophysiological traits associated with drought in Mediterranean tree seedlings: Individual responses versus interspecific trends in eleven species. Plant Biol. 2006, 8, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Aranda, I.; Martin-Benito, D.; Sánchez-Gómez, D.; de Simón, B.F.; Gea-Izquierdo, G. Different drought-tolerance strategies of tree species to cope with increased water stress under climate change in a mixed forest. Physiol. Plant. 2024, 176, e14562. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Landsberg, J.; Waring, R. Water relations in tree physiology: Where to from here? Tree Physiol. 2017, 37, 18–32. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A new look at water transport regulation in plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Munson, S.M.; Bradford, J.B.; Hultine, K.R. An integrative ecological drought framework to span plant stress to ecosystem transformation. Ecosystems 2021, 24, 739–754. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. Short-term responses of terpene emission rates to experimental changes of PFD in Pinus halepensis and Quercus ilex in summer field conditions. Environ. Exp. Bot. 1999, 42, 61–68. [Google Scholar] [CrossRef]
- Kopaczyk, J.M.; Warguła, J.; Jelonek, T. The variability of terpenes in conifers under developmental and environmental stimuli. Environ. Exp. Bot. 2020, 180, 104197. [Google Scholar] [CrossRef]
- Turtola, S.; Manninen, A.M.; Rikala, R.; Kainulainen, P. Drought stress alters the concentration of wood terpenoids in Scots pine and Norway spruce seedlings. J. Chem. Ecol. 2003, 29, 1981–1995. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Johnson, D.M.; Lachenbruch, B.; McCulloh, K.A.; Woodruff, D.R. Xylem hydraulic safety margins in woody plants: Coordination of stomatal control of xylem tension with hydraulic capacitance. Funct. Ecol. 2009, 23, 922–930. [Google Scholar] [CrossRef]
- Delzon, S.; Cochard, H. Recent advances in tree hydraulics highlight the ecological significance of the hydraulic safety margin. New Phytol. 2014, 203, 355–358. [Google Scholar] [CrossRef]
- Skelton, R.P.; Anderegg, L.D.; Diaz, J.; Kling, M.M.; Papper, P.; Lamarque, L.J.; Delzon, S.; Dawson, T.E.; Ackerly, D.D. Evolutionary relationships between drought-related traits and climate shape large hydraulic safety margins in western North American oaks. Proc. Natl. Acad. Sci. USA 2021, 118, e2008987118. [Google Scholar] [CrossRef]
- Llusià, J.; Peñuelas, J. Seasonal patterns of terpene content and emission from seven Mediterranean woody species in field conditions. Am. J. Bot. 2000, 87, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sabillón, D.; Cremades, L.V. Diurnal and seasonal variation of monoterpene emission rates for two typical Mediterranean species (Pinus pinea and Quercus ilex) from field measurements—Relationship with temperature and PAR. Atmos. Environ. 2001, 35, 4419–4431. [Google Scholar] [CrossRef]
- Blanch, J.S.; Peñuelas, J.; Llusià, J. Sensitivity of terpene emissions to drought and fertilization in terpene-storing Pinus halepensis and non-storing Quercus ilex. Physiol. Plant. 2007, 131, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Blanch, J.S.; Penuelas, J.; Sardans, J.; Llusia, J. Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol. Plant. 2009, 31, 207–218. [Google Scholar] [CrossRef]
- Llusia, J.; Roahtyn, S.; Yakir, D.; Rotenberg, E.; Seco, R.; Guenther, A.; Penuelas, J. Photosynthesis, stomatal conductance and terpene emission response to water availability in dry and mesic Mediterranean forests. Trees 2016, 30, 749–759. [Google Scholar] [CrossRef]
- Anderson, S.C.; Elsen, P.R.; Hughes, B.B.; Tonietto, R.K.; Bletz, M.C.; Gill, D.A.; Holgerson, M.A.; Kuebbing, S.E.; McDonough MacKenzie, C.; Meek, M.H.; et al. Trends in ecology and conservation over eight decades. Front. Ecol. Environ. 2021, 19, 274–282. [Google Scholar] [CrossRef]
- Li, T.; Singh, S.P.; Singh, R.D.; Cui, L.; Pandey, R. Responses of Pine and Oak Species to Drought: A Bibliometric Analysis. Int. J. Ecol. Environ. Sci. 2025, 51, 411–423. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Maxwell, J.T.; Pederson, N.; D’Orangeville, L.; Ficklin, D.L.; Phillips, R.P. Drought legacies are dependent on water table depth, wood anatomy and drought timing across the eastern US. Ecol. Lett. 2019, 22, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Primack, R.B.; Zipf, L.; Pardo, S.; Gallinat, A.S.; Panchen, Z.A. Leaf longevity in temperate evergreen species is related to phylogeny and leaf size. Oecologia 2019, 191, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Garkoti, S.C.; Zobel, D.B.; Singh, S.P. Comparison of water relations of seedlings and trees of two Himalayan oaks. Int. J. Ecol. Environ. Sci. 2000, 26, 213–222. [Google Scholar]
- Cavender-Bares, J.; Bazzaz, F.A. Changes in drought response strategies with ontogeny in Quercus rubra: Implications for scaling from seedlings to mature trees. Oecologia 2000, 124, 8–18. [Google Scholar] [CrossRef]
- Mediavilla, S.; Herranz, M.; González-Zurdo, P.; Escudero, A. Ontogenetic transition in leaf traits: A new cost associated with the increase in leaf longevity. J. Plant Ecol. 2014, 7, 567–575. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Colangelo, M.; Matías, L.; Ripullone, F.; Camarero, J.J. Shifts in growth responses to climate and exceeded drought-vulnerability thresholds characterize dieback in two Mediterranean deciduous oaks. Forests 2020, 11, 714. [Google Scholar] [CrossRef]
- Kaproth, M.A.; Fredericksen, B.W.; González-Rodríguez, A.; Hipp, A.L.; Cavender-Bares, J. Drought response strategies are coupled with leaf habit in 35 evergreen and deciduous oak (Quercus) species across a climatic gradient in the Americas. New Phytol. 2023, 239, 888–904. [Google Scholar] [CrossRef]
- Forner, A.; Valladares, F.; Aranda, I. Mediterranean trees coping with severe drought: Avoidance might not be safe. Environ. Exp. Bot. 2018, 155, 529–540. [Google Scholar] [CrossRef]
- Ozcelik, M.S.; Şengönül, K. Transpiration of Anatolian black pine and sessile oak forest stands in a sub-humid region of Turkey. Ann.For. Res. 2021, 64, 111–128. [Google Scholar] [CrossRef]
- Martín-Sánchez, R.; Peguero-Pina, J.J.; Alonso-Forn, D.; Ferrio, J.P.; Sancho-Knapik, D.; Gil-Pelegrín, E. Summer and winter can equally stress holm oak (Quercus ilex L.) in Mediterranean areas: A physiological view. Flora 2022, 290, 152058. [Google Scholar] [CrossRef]
- Tsiouvaras, C.N.; Noitsakis, B.; Papanastasis, V.P. Clipping intensity improves growth rate of kermes oak twigs. For. Ecol. Manag. 1986, 15, 229–237. [Google Scholar] [CrossRef]
- Tsiouvaras, C.N. Long-term effects of clipping on production and vigor of kermes oak (Quercus coccifera). For. Ecol. Manag. 1988, 24, 159–166. [Google Scholar] [CrossRef]
- Espelta, J.M.; Sabaté, S. Resprouting dynamics. In Ecology of Mediterranean Evergreen Oak Forests; Roda, F., Retana, J., Gracia, C.A., Bellot, J., Eds.; Berlin, Springer: Berlin/Heidelberg, Germany, 1999; pp. 61–73. [Google Scholar]
- Pausas, J.G. Bark thickness and fire regime. Funct. Ecol. 2015, 29, 315–327. [Google Scholar] [CrossRef]
- Pausas, J.G.; Su, W.; Luo, C.; Shen, Z. A shrubby resprouting pine with serotinous cones endemic to Southwest China. Ecology 2021, 102, 1–4. [Google Scholar] [CrossRef]
- González-Muñoz, N.; Castro-Díez, P.; Fierro-Brunnenmeister, N. Establishment success of coexisting native and exotic trees under an experimental gradient of irradiance and soil moisture. Environ. Manag. 2011, 48, 764–773. [Google Scholar] [CrossRef]
- Sharma, C.M.; Baduni, N.P.; Gairola, S.; Ghildiyal, S.K.; Suyal, S. Effects of slope aspects on forest compositions, community structures and soil properties in natural temperate forests of Garhwal Himalaya. J. For. Res. 2010, 21, 331–337. [Google Scholar] [CrossRef]
- Tyagi, V.; Singh, S.P.; Singh, R.D.; Gumber, S. Chir pine and banj oak responses to pre-monsoon drought across slope aspects and positions in Central Himalaya. Environ. Monit. Assess. 2023, 195, 258. [Google Scholar] [CrossRef]
- Farjon, A. A Handbook of the World’s Conifers: Revised and Updated Edition; Brill: Leiden, The Netherlands, 2010. [Google Scholar]
- Zweifel, R.; Rigling, A.; Dobbertin, M. Species-specific stomatal response of trees to drought–a link to vegetation dynamics? J. Veg. Sci. 2009, 20, 442–454. [Google Scholar] [CrossRef]
- Roncal-Garcia, S.; Soto-Pinto, L.; Castellanos-Albores, J.; Ramirez-Marcial, N.; de Jong, B. Agroforestry systems and carbon stocks in indigenous communities from Chiapas, Mexico. Interciencia 2008, 33, 200–206. [Google Scholar]
- van Zonneveld, M.; Jarvis, A.; Dvorak, W.; Lema, G.; Leibing, C. Climate change impact predictions on Pinus patula and Pinus tecunumanii populations in Mexico and Central America. For. Ecol. Manag. 2009, 257, 1566–1576. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Viguera, B.; Cabrera, M.; Cañellas, I. Drought induced decline could portend widespread pine mortality at the xeric ecotone in managed mediterranean pine-oak woodlands. For. Ecol. Manag. 2014, 320, 70–82. [Google Scholar] [CrossRef]
- Novick, K.; Jo, I.; D’Orangeville, L.; Benson, M.; Au, T.F.; Barnes, M.; Denham, S.; Fei, S.; Heilman, K.; Hwang, T.; et al. The drought response of eastern US oaks in the context of their declining abundance. BioScience 2022, 72, 333–346. [Google Scholar] [CrossRef]
- Bose, A.K.; Doležal, J.; Scherrer, D.; Altman, J.; Ziche, D.; Martínez-Sancho, E.; Bigler, C.; Bolte, A.; Colangelo, M.; Dorado-Liñán, I.; et al. Revealing legacy effects of extreme droughts on tree growth of oaks across the Northern Hemisphere. Sci. Total Environ. 2024, 926, 172049. [Google Scholar] [CrossRef]
- Kuster, T.M.; Arend, M.; Günthardt-Goerg, M.S.; Schulin, R. Root growth of different oak provenances in two soils under drought stress and air warming conditions. Plant Soil 2013, 369, 61–71. [Google Scholar] [CrossRef]
- Pathak, G.C.; Joshi, H.; Singh, R.D.; Tewari, A.; Pandey, R.; Singh, S.P. Vertical root distribution in Himalayan trees: About half of roots occur below 30 cm, the generally sampled depth. Trop. Ecol. 2021, 62, 479–491. [Google Scholar] [CrossRef]
- Rodriguez-Robles, U.; Arredondo, T.; Smart, D. Wood anatomical and physiological differences between semiarid pine and oak, stand up for their tree ring sensibility to precipitation variability. Agric. For. Meteorol. 2023, 338, 109530. [Google Scholar] [CrossRef]
- Canadell, J.; Jackson, R.B.; Ehleringer, J.B.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. Maximum rooting depth of vegetation types at the global scale. Oecologia 1996, 108, 583–595. [Google Scholar] [CrossRef]
- Enderle, L.; Gribbe, S.; Coners, H.; Leuschner, C.; Hertel, D. Impact of a 2 °C Warmer Climate on the Fine Root System of European Beech, Sessile Oak, Scots Pine, and Douglas Fir in Central European Lowland Forests: L. Enderle and others. Ecosystems 2025, 28, 49. [Google Scholar] [CrossRef]
- Aldea, J.; Bravo, F.; Vazquez-Pique, J.; Ruiz-Peinado, R.; del Rio, M. Differences in stem radial variation between Pinus pinaster Ait. and Quercus pyrenaica Willd. may release inter-specific competition. For. Ecol. Manag. 2021, 481, 118779. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Wei, Z.; Jiang, J.; Zhang, Q.; Lv, X. Water use by trees is linked to precipitation: A case study of a mixed forest in a hilly area in southern China. Ecol. Indic. 2022, 143, 109343. [Google Scholar] [CrossRef]
- Gentilesca, T.; Battipaglia, G.; Borghetti, M.; Colangelo, M.; Altieri, S.; Ferrara, A.M.; Lapolla, A.; Rita, A.; Ripullone, F. Evaluating growth and intrinsic water-use efficiency in hardwood and conifer mixed plantations. Trees 2021, 35, 1329–1340. [Google Scholar] [CrossRef]
- Pardos, M.; Del Río, M.; Pretzsch, H.; Jactel, H.; Bielak, K.; Bravo, F.; Brazaitis, G.; Defossez, E.; Engel, M.; Godvod, K.; et al. The greater resilience of mixed forests to drought mainly depends on their composition: Analysis along a climate gradient across Europe. For. Ecol. Manag. 2021, 481, 118687. [Google Scholar] [CrossRef]
- Chergui, B.; Fahd, S.; Santos, X. Quercus suber forest and Pinus plantations show different post-fire resilience in Mediterranean north-western Africa. Ann. For. Sci. 2018, 75, 64. [Google Scholar] [CrossRef]
- Amsten, K.; Cromsigt, J.P.; Kuijper, D.P.; Loberg, J.M.; Churski, M.; Niklasson, M. Fire-and herbivory-driven consumer control in a savanna-like temperate wood-pasture: An experimental approach. J. Ecol. 2021, 109, 4103–4114. [Google Scholar] [CrossRef]
- Coop, J.D. Postfire futures in southwestern forests: Climate and landscape influences on trajectories of recovery and conversion. Ecol. Appl. 2023, 33, e2725. [Google Scholar] [CrossRef] [PubMed]
- Vigren, C.; Vospernik, S.; Morin, X.; Toïgo, M.; Bielak, K.; Bravo, F.; Heym, M.; Löf, M.; Pach, M.; Ponette, Q.; et al. Divergent regional volume growth responses of Scots Pine and Oak stands to climate change in Europe. Sci. Total Environ. 2025, 969, 178858. [Google Scholar] [CrossRef] [PubMed]
- Picon, C.; Guehl, J.M.; Ferhi, A. Leaf gas exchange and carbon isotope composition responses to drought in a drought-avoiding (Pinus pinaster) and a drought-tolerant (Quercus petraea) species under present and elevated atmospheric CO2 concentrations. Plant Cell Environ. 1996, 19, 182–190. [Google Scholar] [CrossRef]
- Kolb, T.E.; Stone, J.E. Differences in leaf gas exchange and water relations among species and tree sizes in an Arizona pine–oak forest. Tree Physiol. 2000, 20, 1–12. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Florit, A.; Vega, A.; Serrano, L.; Voltas, J. Δ13C and tree-ring width reflect different drought responses in Quercus ilex and Pinus halepensis. Oecologia. 2003, 137, 512–518. [Google Scholar] [CrossRef]
- Asbjornsen, H.; Vogt, K.A.; Ashton, M.S. Synergistic responses of oak, pine and shrub seedlings to edge environments and drought in a fragmented tropical highland oak forest, Oaxaca, Mexico. For. Ecol. Manag. 2004, 192, 313–334. [Google Scholar] [CrossRef]
- Baquedano, F.J.; Castillo, F.J. Comparative ecophysiological effects of drought on seedlings of the Mediterranean water-saver Pinus halepensis and water-spenders Quercus coccifera and Quercus ilex. Trees. 2006, 20, 689–700. [Google Scholar] [CrossRef]
- Singh, S.P.; Zobel, D.B.; Garkoti, S.C.; Tewari, A.; Negi, C.M.S. Patterns in water relations of central Himalayan trees. Trop. Ecol. 2006, 47, 159–182. [Google Scholar]
- Zweifel, R.; Steppe, K.; Sterck, F.J. Stomatal regulation by microclimate and tree water relations: Interpreting ecophysiological field data with a hydraulic plant model. J. Exp. Bot. 2007, 58, 2113–2131. [Google Scholar] [CrossRef]
- Baquedano, F.J.; Valladares, F.; Castillo, F.J. Phenotypic plasticity blurs ecotypic divergence in the response of Quercus coccifera and Pinus halepensis to water stress. Eur. J. For. Res. 2008, 127, 495–506. [Google Scholar] [CrossRef]
- Chirino, E.; Bellot, J.; Sánchez, J.R. Daily sap flow rate as an indicator of drought avoidance mechanisms in five Mediterranean perennial species in semi-arid southeastern Spain. Trees. 2011, 25, 593–606. [Google Scholar] [CrossRef]
- Himmelsbach, W.; Trevino-Garza, E.J.; González-Rodríguez, H.; González-Tagle, M.A.; Gómez Meza, M.V.; Aguirre Calderón, O.A.; Eduardo Estrada Castillón, A.; Mitlöhner, R. Acclimatation of three co-occurring tree species to water stress and their role as site indicators in mixed pine-oak forests in the Sierra Madre Oriental, Mexico. Eur. J. For. Res. 2012, 131, 355–367. [Google Scholar] [CrossRef]
- Klein, T.; Shpringer, I.; Fikler, B.; Elbaz, G.; Cohen, S.; Yakir, D. Relationships between stomatal regulation, water-use, and water-use efficiency of two coexisting key Mediterranean tree species. For. Ecol. Manag. 2013, 302, 34–42. [Google Scholar] [CrossRef]
- Aguadé, D.; Poyatos, R.; Rosas, T.; Martínez-Vilalta, J. Comparative drought responses of Quercus ilex L. and Pinus sylvestris L. in a montane forest undergoing a vegetation shift. Forests. 2015, 6, 2505–2529. [Google Scholar] [CrossRef]
- Mayoral, C.; Calama, R.; Sánchez-González, M.; Pardos, M. Modelling the influence of light, water and temperature on photosynthesis in young trees of mixed Mediterranean forests. New For. 2015, 46, 485–506. [Google Scholar] [CrossRef]
- Renninger, H.J.; Carlo, N.J.; Clark, K.L.; Schäfer, K.V. Resource use and efficiency, and stomatal responses to environmental drivers of oak and pine species in an Atlantic Coastal Plain forest. Front. Plant Sci. 2015, 6, 297. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Liu, Z.; Chen, L.; Yu, X. Distinguish water utilization strategies of trees growing on earth-rocky mountainous area with transpiration and water isotopes. Ecol. Evol. 2017, 7, 10640–10651. [Google Scholar] [CrossRef]
- Martínez-Sancho, E.; Vásconez Navas, L.K.; Seidel, H.; Dorado-Liñán, I.; Menzel, A. Responses of contrasting tree functional types to air warming and drought. Forests 2017, 8, 450. [Google Scholar] [CrossRef]
- Forner, A.; Valladares, F.; Bonal, D.; Granier, A.; Grossiord, C.; Aranda, I. Extreme droughts affecting Mediterranean tree species’ growth and water-use efficiency: The importance of timing. Tree Physiol. 2018, 38, 1127–1137. [Google Scholar] [CrossRef]
- Nolan, R.H.; Hedo, J.; Arteaga, C.; Sugai, T.; de Dios, V.R. Physiological drought responses improve predictions of live fuel moisture dynamics in a Mediterranean forest. Agric. For. Meteorol. 2018, 263, 417–427. [Google Scholar] [CrossRef]
- Ouyang, L.; He, W.; Huang, K.; Zhou, C.; Gu, D.; Huang, Y.; Zhao, P. Seasonal water use strategy of canopy tree species and possible implication for their coexistence in a subtropical secondary forest. Ecohydrology. 2019, 12, e2129. [Google Scholar] [CrossRef]
- Yi, K.; Smith, J.W.; Jablonski, A.D.; Tatham, E.A.; Scanlon, T.M.; Lerdau, M.T.; Novick, K.A.; Yang, X. High heterogeneity in canopy temperature among co-occurring tree species in a temperate forest. J. Geophys. Res.-Biogeosci. 2020, 125, e2020JG005892. [Google Scholar] [CrossRef]
- Asbjornsen, H.; McIntire, C.D.; Vadeboncoeur, M.A.; Jennings, K.A.; Coble, A.P.; Berry, Z.C. Sensitivity and threshold dynamics of Pinus strobus and Quercus spp. in response to experimental and naturally occurring severe droughts. Tree Physiol. 2021, 41, 1819–1835. [Google Scholar] [CrossRef]
- Bayar, E.; Deligöz, A. Ecophysiological behavior of Mediterranean woody species under summer drought. Bosque. 2021, 42, 311–321. [Google Scholar]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. STOTEN. 2021, 779, 146466. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Aranda, I.; Cañellas, I.; Dorado-Liñán, I.; Olano, J.M.; Martin-Benito, D. Contrasting species decline but high sensitivity to increasing water stress on a mixed pine–oak ecotone. J. Ecol. 2021, 109, 109–124. [Google Scholar] [CrossRef]
- Moreno, M.; Simioni, G.; Cailleret, M.; Ruffault, J.; Badel, E.; Carrière, S.; Davi, H.; Gavinet, J.; Huc, R.; Limousin, J.M.; et al. Consistently lower sap velocity and growth over nine years of rainfall exclusion in a Mediterranean mixed pine-oak forest. Agric. For. Meteorol. 2021, 308, 108472. [Google Scholar] [CrossRef]
- Pardos, M.; Calama, R. Adaptive strategies of seedlings of four mediterranean co-occurring tree species in response to light and moderate drought: A nursery approach. Forests 2022, 13, 154. [Google Scholar] [CrossRef]
- Blackman, C.J.; Billon, L.M.; Cartailler, J.; Torres-Ruiz, J.M.; Cochard, H. Key hydraulic traits control the dynamics of plant dehydration in four contrasting tree species during drought. Tree Physiol. 2023, 43, 1772–1783. [Google Scholar] [CrossRef]
- Davi, H.; Dufrêne, E.; Francois, C.; Le Maire, G.; Loustau, D.; Bosc, A.; Rambal, S.; Granier, A.; Moors, E. Sensitivity of water and carbon fluxes to climate changes from 1960 to 2100 in European forest ecosystems. Agric. For. Meteorol. 2006, 141, 35–56. [Google Scholar] [CrossRef]
- Sanz-Pérez, V.; Castro-Díez, P.; Joffre, R. Seasonal carbon storage and growth in Mediterranean tree seedlings under different water conditions. Tree Physiol. 2009, 29, 1105–1116. [Google Scholar] [CrossRef]
- Pita, G.; Gielen, B.; Zona, D.; Rodrigues, A.; Rambal, S.; Janssens, I.A.; Ceulemans, R. Carbon and water vapor fluxes over four forests in two contrasting climatic zones. Agric. For. Meteorol. 2013, 180, 211–224. [Google Scholar] [CrossRef]
- Martín-Gómez, P.; Aguilera, M.; Pemán, J.; Gil-Pelegrín, E.; Ferrio, J.P. Contrasting ecophysiological strategies related to drought: The case of a mixed stand of Scots pine (Pinus sylvestris) and a submediterranean oak (Quercus subpyrenaica). Tree Physiol. 2017, 37, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.E.; Muir, J.P.; Morgan, C.L.; Moore, G.W. Tortoise or hare: Will resprouting oaks or reseeding pines dominate following severe wildfire? For. Ecol. Manag. 2018, 408, 54–66. [Google Scholar] [CrossRef]
- Zavala, M.A.; Espelta, J.M.; Retana, J. Constraints and trade-offs in Mediterranean plant communities: The case of holm oak-Aleppo pine forests. Bot. Rev. 2000, 66, 119–149. [Google Scholar] [CrossRef]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Fonti, P.; Rigling, A. Drought-induced adaptation of the xylem in Scots pine and pubescent oak. Tree Physiol. 2009, 29, 1011–1020. [Google Scholar] [CrossRef]
- Forner, A.; Aranda, I.; Granier, A.; Valladares, F. Differential impact of the most extreme drought event over the last half century on growth and sap flow in two coexisting Mediterranean trees. Plant Ecol. 2014, 215, 703–719. [Google Scholar] [CrossRef]
- Esperón-Rodríguez, M.; Barradas, V.L. Ecophysiological vulnerability to climate change: Water stress responses in four tree species from the central mountain region of Veracruz, Mexico. Reg. Environ. Change. 2015, 15, 93–108. [Google Scholar] [CrossRef]
- Sperlich, D.; Chang, C.T.; Peñuelas, J.; Gracia, C.; Sabaté, S. Seasonal variability of foliar photosynthetic and morphological traits and drought impacts in a Mediterranean mixed forest. Tree Physiol. 2015, 35, 501–520. [Google Scholar] [CrossRef]
- Fernández-de-Uña, L.; Rossi, S.; Aranda, I.; Fonti, P.; González-González, B.D.; Cañellas, I.; Gea-Izquierdo, G. Xylem and leaf functional adjustments to drought in Pinus sylvestris and Quercus pyrenaica at their elevational boundary. Front. Plant Sci. 2017, 8, 1200. [Google Scholar] [CrossRef] [PubMed]
- Bello, J.; Vallet, P.; Perot, T.; Balandier, P.; Seigner, V.; Perret, S.; Couteau, C.; Korboulewsky, N. How do mixing tree species and stand density affect seasonal radial growth during drought events? For. Ecol. Manag. 2019, 432, 436–445. [Google Scholar] [CrossRef]
- Martín-Gómez, P.; Rodríguez-Robles, U.; Ogée, J.; Wingate, L.; Sancho-Knapik, D.; Peguero-Pina, J.; dos Santos Silva, J.V.; Gil-Pelegrín, E.; Pemán, J.; Ferrio, J.P. Contrasting stem water uptake and storage dynamics of water-saver and water-spender species during drought and recovery. Tree Physiol. 2023, 43, 1290–1306. [Google Scholar] [CrossRef]
- Pompa-García, M.; Camarero, J.J.; Vivar-Vivar, E.D. Contrasting climate drivers of seasonal growth in western vs. eastern Mexican mountain conifer forests. For. Ecosyst. 2023, 10, 100091. [Google Scholar] [CrossRef]
- Steger, D.N.; Peters, R.L.; Blume, T.; Hurley, A.G.; Balanzategui, D.; Balting, D.F.; Heinrich, I. Site matters-Canopy conductance regulation in mature temperate trees diverges at two sites with contrasting soil water availability. Agric. For. Meteorol. 2024, 345, 109850. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I. Deuterium labelling of roots provides evidence of deep water access and hydraulic lift by Pinus nigra in a Mediterranean forest of NE Spain. Environ. Exp. Bot. 2003, 49, 201–208. [Google Scholar] [CrossRef]
- Domínguez Núñez, J.A.; Planelles González, R.; Rodríguez Barreal, J.A.; Saiz de Omeñaca González, J.A. The effect of Tuber melanosporum Vitt. mycorrhization on growth, nutrition, and water relations of Quercus petraea Liebl., Quercus faginea Lamk., and Pinus halepensis Mill. seedlings. New For. 2008, 35, 159–171. [Google Scholar] [CrossRef]
- Rincón, A.; Valladares, F.; Gimeno, T.E.; Pueyo, J.J. Water stress responses of two Mediterranean tree species influenced by native soil microorganisms and inoculation with a plant growth promoting rhizobacterium. Tree Physiol. 2008, 28, 1693–1701. [Google Scholar] [CrossRef]
- Rodríguez-Robles, U.; Arredondo, J.T.; Huber-Sannwald, E.; Vargas, R. Geoecohydrological mechanisms couple soil and leaf water dynamics and facilitate species coexistence in shallow soils of a tropical semiarid mixed forest. New Phytol. 2015, 207, 59–69. [Google Scholar] [CrossRef]
- Belovitch, M.; Brantley, S.; Aubrey, D.P. Interspecific variation in the timing and magnitude of hydraulic redistribution in a forest with distinct water sources. Plant Soil. 2022, 472, 451–464. [Google Scholar] [CrossRef]
- Poulos, H.M. Tree mortality from a short-duration freezing event and global-change-type drought in a Southwestern piñon-juniper woodland, USA. PeerJ. 2014, 2, e404. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Shestakova, T.A.; del Castillo, J.; Voltas, J. Oak competition dominates interspecific interactions in growth and water-use efficiency in a mixed pine–oak Mediterranean forest. Forests. 2021, 12, 1093. [Google Scholar] [CrossRef]
- Leuzinger, S.; Körner, C. Tree species diversity affects canopy leaf temperatures in a mature temperate forest. Agric. For. Meteorol. 2007, 146, 29–37. [Google Scholar] [CrossRef]
- Martínez-Sancho, E.; Dorado-Liñán, I.; Hacke, U.G.; Seidel, H.; Menzel, A. Contrasting hydraulic architectures of scots pine and sessile oak at their southernmost distribution limits. Front. Plant Sci. 2017, 8, 598. [Google Scholar] [CrossRef]
- Zalloni, E.; Battipaglia, G.; Cherubini, P.; Saurer, M.; De Micco, V. Contrasting physiological responses to Mediterranean climate variability are revealed by intra-annual density fluctuations in tree rings of Quercus ilex L. and Pinus pinea L. Tree Physiol. 2018, 38, 1213–1224. [Google Scholar] [CrossRef]
- Bello, J.; Hasselquist, N.J.; Vallet, P.; Kahmen, A.; Perot, T.; Korboulewsky, N. Complementary water uptake depth of Quercus petraea and Pinus sylvestris in mixed stands during an extreme drought. Plant Soil. 2019, 437, 93–115. [Google Scholar] [CrossRef]
- Champagne, E.; Turgeon, R.; Munson, A.D.; Raymond, P. Seedling response to simulated browsing and reduced water availability: Insights for assisted migration plantations. Forests 2021, 12, 1396. [Google Scholar] [CrossRef]

| Drought Response Mechanism | Parameters (Units) |
|---|---|
| Plant Water Relations and Hydraulic Responses | Predawn water potential (ΨPD, MPa); midday water potential (ΨMD, MPa); leaf water potential (Ψleaf, MPa); daily change in tree water potential (Ψ∆, MPa); stem water potential (Ψstem, MPa); sap flow rate (%); stomatal conductance (gs, mol m−2s−1); transpiration rate; xylem embolism; percentage loss of conductivity (PLC); and hydraulic safety margin (Ψ50 and Ψ88, MPa) |
| Carbon Dynamics and Metabolism | Non-structural carbon (NSC) concentration (%); carbon reserve depletion; respiration; net photosynthetic rate; water-use efficiency (WUE/iWUE, µmol CO2 mol−1 H2O), gross primary productivity (GPP) variation |
| Drought and Growth Impact | Radial growth (mm); xylem formation; sap flow recovery; leaf area increment (LAI) change (%); leaf senescence timing (days); post-drought physiological recovery |
| Root Traits and Belowground Adaptations | Root depth and distribution; hydraulic lift; mycorrhizal symbiosis; microbial enhancement of water uptake; soil–water access |
| Climate and Environmental Drivers | Long-term drought impact; rainfall exclusion; temperature and CO2 effects; species range shift; mortality/recruitment |
| Terpene Emission and Secondary Metabolites | Monoterpene/sesquiterpene concentration (µg G DM−1h−1); stress-induced emission under drought/temperature stress |
| External Factors | Mycorrhization; rhizobacteria; irradiance, canopy position; browsing pressure; site aspect |
| Pine | Oak | |
|---|---|---|
| Plant response to drought in terms of Ψplant when stomatal closure occurs (n = 15) - Daily change in Ψplant (n = 7) - Stomatal conductance (n = 12) - Decrease in sap flow density | Higher (less negative, average ΨMD being −1.96 MPa) Ψplant; early during the drought (in all studies) Narrow (average 0.50 MPa) Generally lower; stomata sunken and located in pits Higher | Lower (more negative, average ΨMD being −2.61 MPa) Ψplant; late during the drought (in all studies) Wide (average 0.79 MPa) Generally higher; stomata flush with or slightly below the epidermis Lower |
| Water-Use Efficiency (WUE) | Higher | Lower |
| Transpiration pattern | Peaked early in the day, but fell rapidly as VPD increased; seasonal rapid decline with drought (in all studies) | More uniform in the day, sustained under moderate stress; maintained longer transpiration during the years (in all studies) |
| Root-related responses - Seasonal change in roots in response to soil drying - Deep soil carbon sequestration - Access of roots to deep water in rock crevices and fissures (scarce data) - Aerenchyma tissues in roots | Decrease in root soil depth Lower Less common; shallow root domination, strong tap roots only at seedling stage; high fine root and mycorrhizal density in top 20 cm of soil Less developed | Root growth extended to deeper soil Higher More common because oaks have three-layered epidermises with Ca-oxalate crystals; deeper root domination, deep penetration of mycorrhizal hyphae, high fine root density even below 20 cm of soil depth More developed in some species (e.g., Q. robur, Q. macrocarpa) |
| Drought effect on carbon reserve (n = 7) | More depletion; lower C reserve) | Less depletion; higher C reserve |
| Leaf-specific transpiration rate | Lower | Higher |
| Growth-related parameters - Shoot growth during spring - Leaf loss - Cessation of xylem formation - Change in leaf mass per unit area (LMA) - Stem circumference increment - Effect of mixed pine–oak stand on stem circumference increment | More decrease More and early during the drought Early in the drought Increased More decrease Decreased from pure stand to mixed stand | Less decrease Less and late during the drought Late in the drought Increased Less decrease Increased from pure stand to mixed stand |
| Decrease in conduit area and radial lumen diameter | More | Less |
| Effect of mycorrhization on Ψplant | Increases | Increases |
| High irradiance effect on drought plants | Unaffected | Adversely affected |
| Guttation to manage high xylem pressure (scanty information) | Rare, needles lack hydathodes for guttation and rely on resin ducts and cuticular transpiration to manage high xylem pressure | Guttation through leaf margins or hydathodes; few species, particularly in seedlings, guttation involves an active root pressure mechanism facilitated by deeper roots |
| Climate change effect - Predicted effect on GPP from 1960–2100 - Regeneration - Post-drought recovery (intensified drought due to climate change) - Climate change effect on competition - Hot drought effect | Decrease More negative or less positive effects Less Unaffected More unfavorable | Increase Less negative or more positive effects More, through resprouting Modulated Less unfavorable or favorable |
| Pine Species | Oak Species | |
|---|---|---|
| Isohydry/anisohydry | Isohydry, increases with needle longevity, so more in Haploxylon pines than in Diploxylon pines | Anisohydry, more in deciduous (4–6 months leaf longevity) than in evergreen species (1–3 years leaf longevity). |
| Hydraulic safety margin | Increases with needle longevity, so more in Haploxylon pines than in Diploxylon pines | Less in deciduous (0.53 MPa) than in evergreen species (1.77 MPa) [46]. |
| Leaf mass per area (LMA, gm−2) | Increases with leaf longevity from ~170 to 314 gm−2 | Higher in evergreen species (average 130 ± 6) than in deciduous species (average 92 ± 2) [29]. |
| Seral status (support comes from Mediterranean regions, China, Mexico, USA, and the Himalayas, but not universal) | Generally early-seral | Generally late-seral; however, some oaks (e.g., Q. faginea) may dominate through resprouting immediately after disturbance (early-seral role). |
| Genera | Species Group | Ψ50 Safety Margin (MPa) | Ψ88 Safety Margin (MPa) | Drop in Ψ from Safety Margin Ψ50 to Ψ88 (MPa) | Ψmin Seasonal (MPa) |
|---|---|---|---|---|---|
| Quercus | Deciduous | 0.55 ± 0.19 | 2.03 ± 0.69 | 1.88 ± 0.51 | −2.86 ± 0.50 |
| (n = 8) | (n = 8) | (n = 9) | (n = 9) | ||
| Evergreen | 1.14 ± 0.47 | 1.66 ± 1.12 | 2.80 ± 1.36 | −3.37 ± 0.41 | |
| (n = 4) | (n = 4) | (n = 4) | (n = 6) | ||
| All oaks | 0.75 ± 0.20 (n = 12) | 1.91 ± 0.56 (n = 12) | 2.16 ± 0.53 (n = 13) | −2.19 ± 0.17 (n = 15) | |
| Pinus | Diploxylon | 1.22 ± 0.18 | 2.73 ± 0.28 | 1.51 ± 0.25 | −2.26 ± 0.21 |
| (n = 9) | (n = 9) | (n = 9) | (n = 10) | ||
| Haploxylon | 2.01 ± 0.37 | 3.09 ± 0.721 | 1.08 ± 1.73 | −1.92 ± 1.73 | |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | ||
| All pines | 1.42 ± 0.18 (n = 12) | 2.82 ± 0.26 (n = 12) | 1.40 ± 0.21 (n = 12) | −3.07 ± 0.34 (n = 13) |
| Pine and Oak Species/Pair | Site/Country (Reference) | Findings |
|---|---|---|
| P. halepensis; Q. ilex | Catalonia, NE Spain [41] | Both pine and oak emitted large and similar amounts of monoterpenes (~20 µg G DM−1h−1). |
| P. halepensis; Q. coccifera; Q. ilex | Barcelona, Spain [47] | Terpene emission was 5.64% and 1.65% of C fixation in summer in Q. coccifera and Q. ilex, respectively, and 5.39% in pine. |
| P. pinea; Q. ilex | Mediterranean Sea Shore, Spain [48] | The oak (21.1 ± 19.8 µg (g d.w.)−1 h−1) emitted about 3 times more terpene than the pine (6.5 ± 5.4 µg (g d.w.)−1 h−1). |
| P. halepensis; Q. ilex | Bellaterra, Barcelona, Spain [49] | Both oak and pine produced a large and similar amounts of monoterpenes (31.45 and 31.71 mu g g(−1) DM h(−1), respectively). However, the responses of oak were faster and stronger than that of pine. |
| P. halepensis; Q. ilex | Barcelona, Spain [50] | Terpene concentration is much more in the unstressed pine than in the oak. The drought treatment (reduction to 1/3 of full watering) significantly increased the total terpene concentrations in both pine and oak species but more in oak (119%) than in pine (54%). |
| P. halepensis; Q. calliprinos; Q. ithaburensis | Israel [51] | At a mesic site, terpenes increased 2–3 fold in Q. calliprinos and Q. ithaburensis, and 5.8-fold in the pine when stressed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.P.; Gumber, S.; Singh, R.D.; Li, T.; Pandey, R. A Global Comparative Analysis of Drought Responses of Pines and Oaks. Forests 2025, 16, 1660. https://doi.org/10.3390/f16111660
Singh SP, Gumber S, Singh RD, Li T, Pandey R. A Global Comparative Analysis of Drought Responses of Pines and Oaks. Forests. 2025; 16(11):1660. https://doi.org/10.3390/f16111660
Chicago/Turabian StyleSingh, Surendra P., Surabhi Gumber, Ripu Daman Singh, Tong Li, and Rajiv Pandey. 2025. "A Global Comparative Analysis of Drought Responses of Pines and Oaks" Forests 16, no. 11: 1660. https://doi.org/10.3390/f16111660
APA StyleSingh, S. P., Gumber, S., Singh, R. D., Li, T., & Pandey, R. (2025). A Global Comparative Analysis of Drought Responses of Pines and Oaks. Forests, 16(11), 1660. https://doi.org/10.3390/f16111660








