1. Introduction
The adaptive evolution of Lauraceae plants is intimately associated with the diversity of their secondary metabolites, notably those implicated in the biosynthesis of terpenoids, flavonoids, and cinnamaldehydes.
Cinnamomum burmanni (Nees & T. Nees) Blume, which belongs to the genus
Cinnamomum in the Lauraceae family, is extensively distributed throughout the tropical and subtropical regions of Southern China. Historically,
C. burmanni has been a valuable medicinal resource in China, renowned for its efficacious therapeutic properties. It comprises over a hundred distinct monoterpene and sesquiterpene compounds, among which are borneol, 1,8-cineol, linalool, and cinnamaldehyde [
1,
2]. These compounds are capable of functioning as antioxidants, osmoprotectants, and signaling molecules, thereby facilitating the plant’s adaptation to unfavorable conditions [
3].
Terpenoids, including camphor and borneol, found within the
Cinnamomum genus, are recognized for their role in conferring stress tolerance [
4]. Research utilizing eco-physiological and metabolomic methodologies has yielded significant insights into the adaptive mechanisms of
C. burmanni in response to various abiotic stresses, encompassing salinity and heavy metal contamination [
5,
6,
7,
8]. It is emphasized that
C. burmanni enhances its resistance to water stress by modulating stomatal conductance and root exudation characteristics [
9]. Likewise, the protective function of the leaf cuticular wax layers in high-temperature environments has been ascertained [
10]. The metabolic profile within the leaves of
C. burmanni has been demonstrated to exhibit a significant responsiveness to alterations in the environment [
7,
10]. The role of terpenoids in facilitating plant–microbe interactions has been elucidated in numerous species, underscoring their significance in nutrient acquisition and stress alleviation [
11,
12,
13]. Furthermore, terpenoids play a dual role in plants, serving not only as mediators of defense mechanisms but also as regulators of allelopathic interactions in the context of interspecific competition [
14]. In conifers, the production of terpenoids is intimately linked with defense mechanisms against herbivory and pathogenic assaults [
15]. The terpenoid pathway is considered a crucial element of its adaptive strategy, wherein particular terpenoids offer defense mechanisms against oxidative stress and herbivory [
16]. The collective evidence suggests that the biosynthesis of terpenoids is a crucial factor in the adaptive evolution of
Cinnamomum species.
Except for terpenoids, cinnamaldehyde is synthesized through the phenylpropanoid pathway, which includes enzymes such as phenylalanine ammonia-lyase (PAL), cinnamate 4-hydroxylase (C4H), and cinnamyl alcohol dehydrogenase (CAD) [
1,
17,
18]. This compound is a distinctive phenylpropanoid found in the leaves of
C. burmanni. Cinnamaldehyde not only provides antimicrobial and antioxidant properties but also enhances thermotolerance by stabilizing cellular membranes during heat stress. In rat models, cinnamaldehyde from
C. burmanni extracts alleviates oxidative liver damage by increasing glutathione peroxidase (GPx) levels and suppressing pro-inflammatory cytokines, such as IL-6 and TNF-α [
17,
19]. Ecological studies have shown that cinnamaldehyde emission fluctuates with environmental changes, reaching its highest levels in populations under high pathogen pressure [
20,
21,
22]. Despite its ecological significance, the regulatory mechanisms of cinnamaldehyde biosynthesis, including the roles of transcription factors (e.g., MYB and bHLH) and epigenetic modifications, are still under-researched in
C. burmanni. Comparative analyses with related species, such as
C. verum, could reveal lineage-specific innovations in phenylpropanoid metabolism [
1,
23,
24,
25]. Flavonoids are crucial in mitigating oxidative stress by scavenging reactive oxygen species (ROS). The adaptive evolution of key flavonoid biosynthesis genes (e.g.,
CHS and
F3H) has been confirmed in related species [
26,
27,
28]. Collectively, these findings underscore that metabolic diversity is a vital strategy for Lauraceae species to flourish in diverse environments.
Despite significant progress in metabolic pathway characterization, comprehensive transcriptomic and proteomic data for
C. burmanni remain limited. Current genomic studies predominantly concentrate on the molecular mechanisms of terpenoid synthesis at the gene expression level, as exemplified in
C. bodinieri [
29],
C. burmanni [
16,
30,
31,
32,
33],
C. longepaniculatum [
34],
C. migao [
35], and
Camphora officinarum (synonymous with
C. camphora [
36,
37,
38,
39,
40,
41,
42,
43,
44]), with relatively little emphasis on protein expression, e.g., C.
officinarum [
45]. The recent available of
C. burmanni reference genome underscores the urgent need for integrated multi-omics approaches to unravel the molecular basis of environmental adaptability [
32,
46]. Existing research has largely relied on phenotypic observations or isolated metabolite analyses leaving systematic investigations of the molecular regulatory networks inadequate. This represents a key bottleneck in understanding its adaptation strategies. The integrated transcriptome-proteome analysis conducted in this study systematically identifies differentially expressed genes (DEGs) and proteome (DEPs) and elucidates their putative collective roles in adaptation. By comparing gene expression profiles, protein translation efficiency, and metabolic pathway dynamics across ecologically divergent populations, we identify critical regulatory nodes in terpenoid and flavonoid biosynthesis linked to environmental adaptation.
2. Results
2.1. Transcriptome Sequencing and Differential Expression Analysis
A total of 15 samples underwent sequencing using Oxford Nanopore Technologies’ third-generation full-length transcriptome sequencing. Following quality control, the clean data for each sample ranged from 3.97 GB to 9.51 GB, with read lengths (N50) spanning 670–1528 base pairs. Full-length sequences, identified by the presence of primer sequences at both ends, yielded 2,624,885 to 7,685,787 full-length reads per sample. After polishing and redundancy removal using minimap, 271,082 non-redundant transcripts were obtained. Based on RNA-Seq analysis, 4,754,313 (BORCB), 8,518,585 (CARCB), 7,063,587 (EUCCB), 4,750,075 (PHYCB), and 5,284,602 (PROCB) reads were generated from the libraries (
Table S1). Expression quantification using CPM (counts per million) revealed distinct transcriptional profiles across samples. Differential expression analysis (|log
2FC| ≥ 1, FDR < 0.01) identified 1479–7361 differentially expressed genes between sample groups.
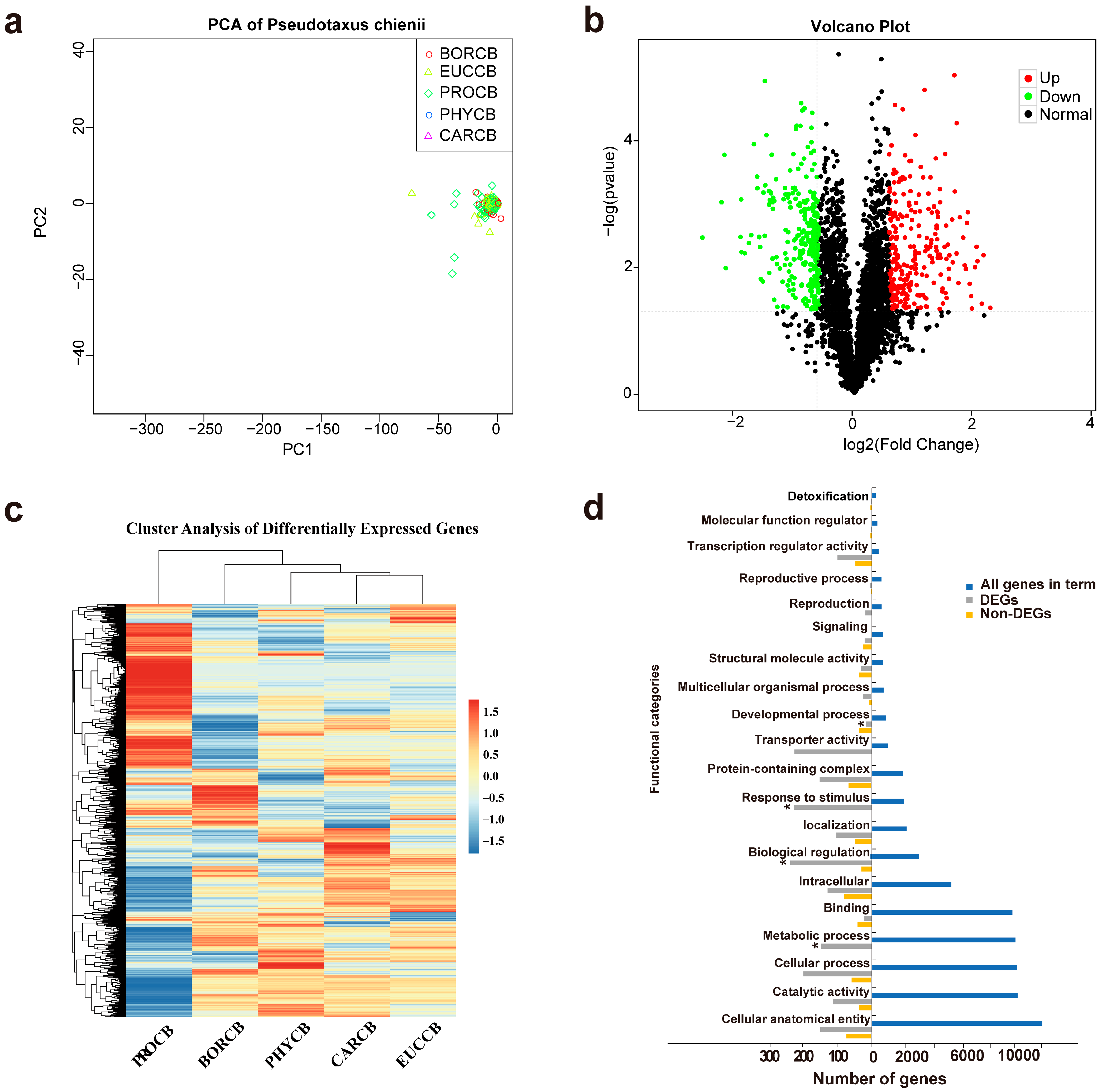
Principal component analysis (PCA) revealed distinct clustering patterns among the 15 samples, with the first two principal components (PC1 and PC2) accounting for 86.5% and 21.8% of the total transcriptional variance, respectively (
Figure 1a). The samples were categorized into five distinct clusters, each corresponding to their respective biological categories (BORCB, CARCB, EUCCB, PHYCB, and PROCB). This categorization highlighted significant inter-group heterogeneity and consistent intra-group reproducibility. Notably, the PROCB samples displayed the greatest divergence along PC1, consistent with their unique transcriptional profile enriched in metabolic pathways. Volcano plots highlighted significant differential expression across pairwise comparisons (|log
2FC| ≥ 1, FDR < 0.01). In the comparison between PROCB and non-PROCB, 7074 genes were found to be differentially expressed, with 3377 genes up-regulated and 3697 genes down-regulated (
Figure 1b). Cluster analysis of differentially expressed genes showed that terpenoid-type
C. burmanni exhibits a significant abundance in gene expression compared to non-terpenoid types (
Figure 1c). Highly up-regulated DEGs included kinases and transporters linked response to stimulus, biological regulation and matabolic process, while down-regulated DEGs were enriched in developmental process pathways (
Figure 1d). A subset of outliers with extreme log
2FC values (>1) corresponded to novel isoforms absent in reference annotations, underscoring the utility of long-read sequencing in capturing unannotated transcriptional diversity.
2.2. TMT-Based Proteomic Analysis and Functional Enrichment of DEPs
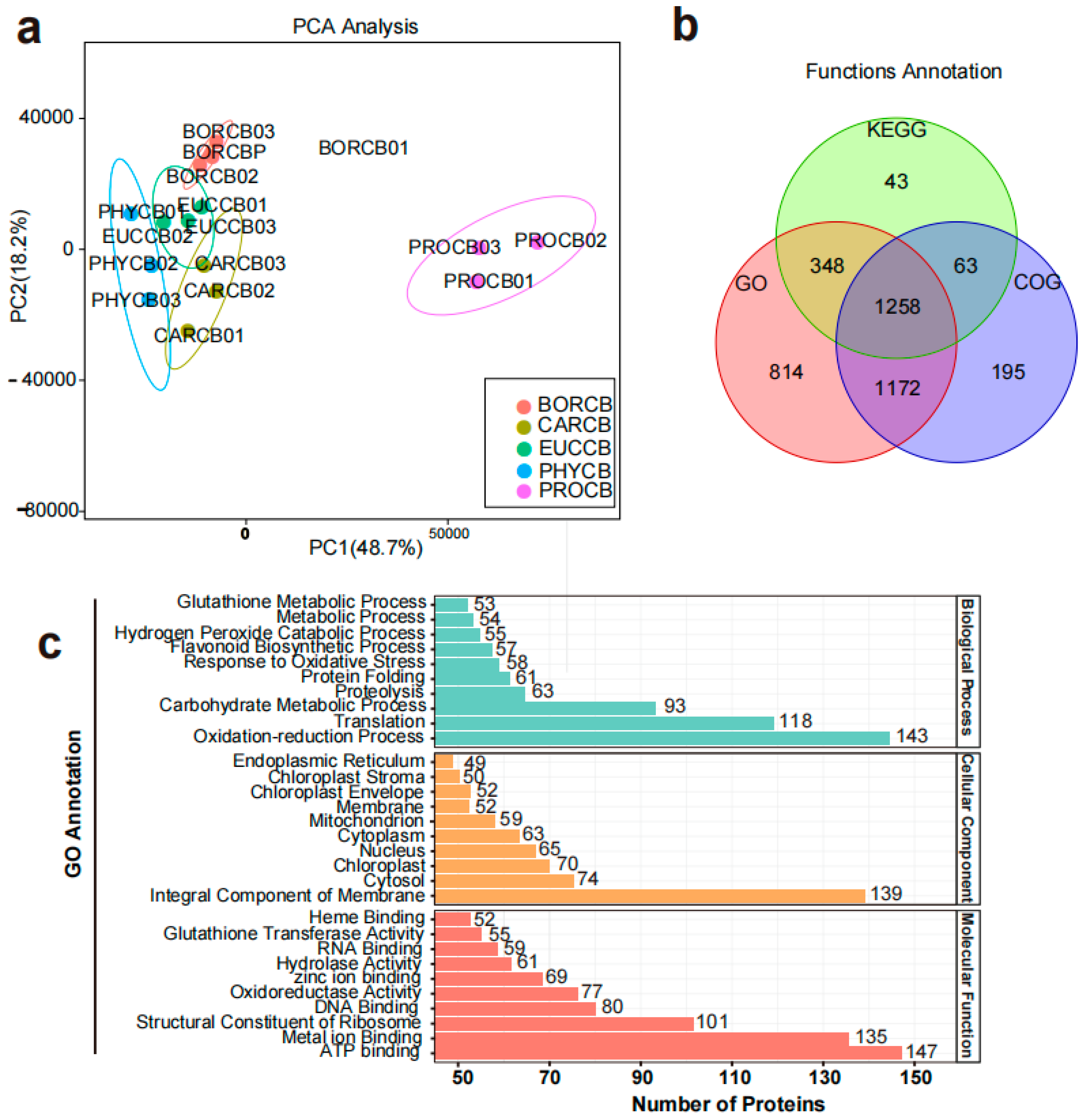
Principal component analysis was performed to characterize global protein profiles and variations across the samples. The PCA results were highly reproducible and robust, with the first two principal components (PC1 and PC2) explaining 48.7% and 18.2% of the total variance, respectively (
Figure 2a). Protein functions were annotated based on Gene Ontology (GO), Clusters of Orthologous Groups (COG), and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases. Among the 4348 proteins identified (
Table S2), 1258 exhibited significant homology to previously uncharacterized proteins in these databases (
Figure 2b). GO enrichment analysis in DEPs revealed that the most represented terms were oxidation–reduction processes (18.94%) in biological process, integral components of membrane (20.65%) in cellular component, and ATP binding (17.58%) in molecular function (
Figure 2c). Notably, flavonoid biosynthetic process and response to oxidative stress accounted for 7.55% and 7.68% of the biological processes, respectively. Carbon metabolism (17.54%), phenylpropanoid biosynthesis (7.92%) and flavonoid biosynthesis (5.77%) within metabolism, as well as ribosome (5.31%) and protein processing in endoplasmic reticulum (7.82%) within genetic information processing was significantly revealed in the KEGG enrichment analysis.
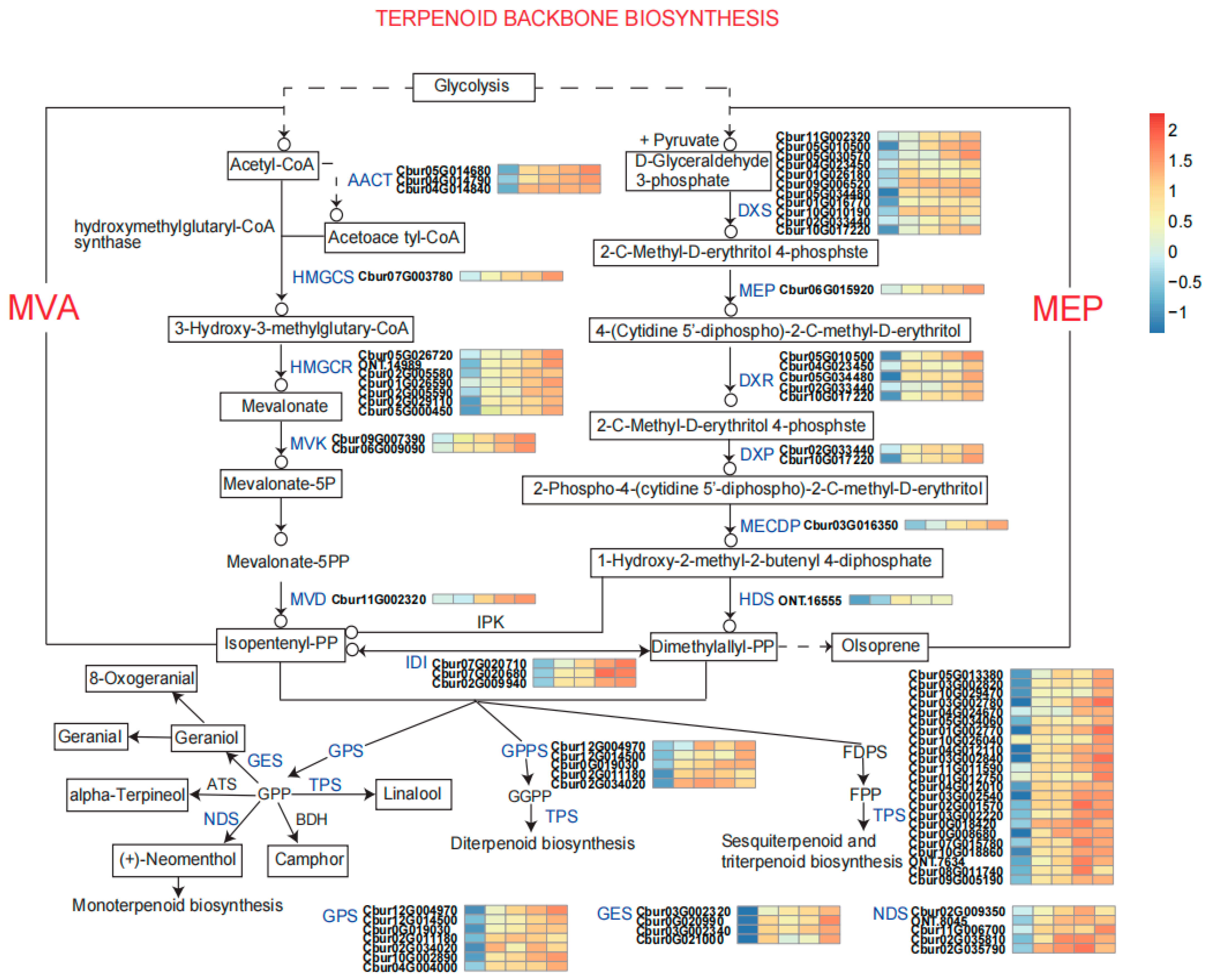
Based on the significant enrichment of DEPs in terpenoid and cinnamaldehyde biosynthetic pathways identified by KEGG analysis, this study conducted a systematic investigation into terpenoid backbone biosynthesis. As illustrated in
Figure 3, total 17 enzymes mainly involved in terpenoid backbone biosynthesis pathway were identified. The precursor supply diverges via glycolysis-derived acetyl-CoA, which serves as a central metabolic node branching into both the mevalonate (MVA) and methylerythritol phosphate (MEP) pathways. In the MVA pathway, acetyl-CoA is sequentially converted to acetoacetyl-CoA by acetoacetyl-CoA thiolase (AACT), followed by the synthesis of 3-Hydroxy-3-methylglutary-CoA via HMG-CoA synthase (HMGCS). Corresponding differential protein analysis revealed that the expression levels of AACT and HMGCR were significantly higher in terpenoid-containing
C. burmanni (BORCB, EUCCB, PHYCB and CARCB) compared to non-terpenoid
C. burmanni (PROCB). Moreover, the expression levels of both AACT and HMGCS exhibited a marked increasing trend from monoterpene-type (BORCB and EUCCB) to diterpenoid-type (PHYCB) and sesquiterpene-type
C. burmanni (CARCB), with peak expression observed in CARCB. HMG-CoA reductase (HMGCR) catalyzes the rate-limiting step, producing mevalonate. This undergoes phosphorylation (by MVK, PMK) and decarboxylation (by MVD) to yield isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP).
Concurrently, the MEP pathway commences with the condensation of pyruvate and glyceraldehyde-3-phosphate, catalyzed by DXS, to yield deoxyxylulose 5-phosphate (DXP). Compared to PROCB, the expression of DXS was significantly upregulated in BORCB and EUCCB. DXP is subsequently reduced to MEP by DXR, followed by a sequence of phosphorylation and cyclization reactions mediated by MCT, CMK, HDS, and HDR, ultimately generating the C5 building blocks isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). The expression level of DXR was generally higher in terpenoid-containing C. burmanni than in PROCB. These universal terpenoid precursors are channeled into downstream biosynthesis: IPP isomerase (IPI) catalyzes their interconversion, while prenyltransferases-including GPPS, FPPS, and GGPPS-extend the carbon chains to form geranyl diphosphate (GPP), farnesyl diphosphate (FPP), and geranylgeranyl diphosphate (GGPP), respectively. The expression of GPPS was significantly upregulated in PHYCB and CARCB compared to PROCB.
2.3. Comparative Analysis of Phenylpropanoids Biosynthesis Among Genotypes
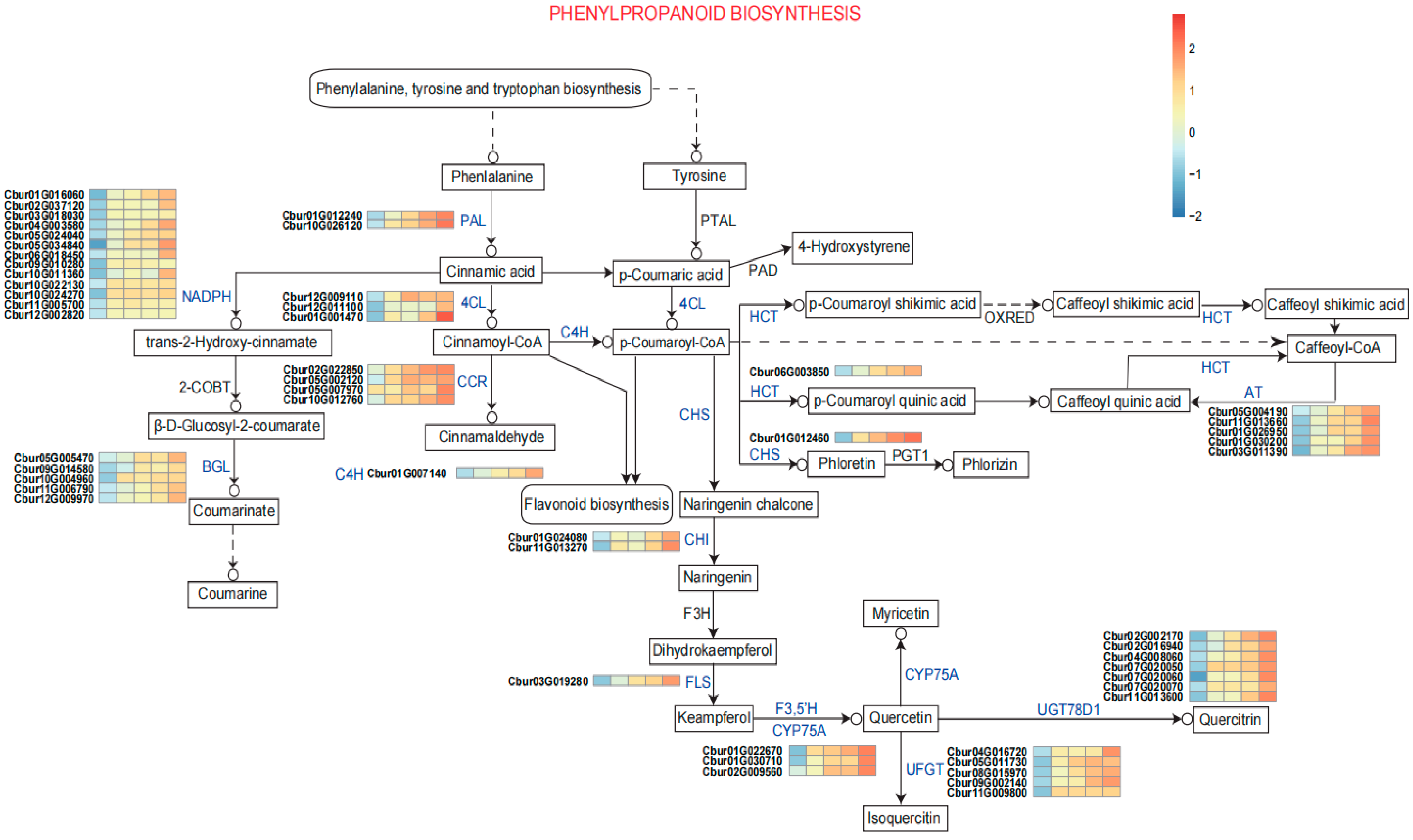
The biosynthesis of phenylpropanoids and flavonoids, as illustrated in
Figure 4, represents a highly coordinated metabolic network in plants, integrating enzymatic catalysis, gene regulation, and substrate channeling to produce structurally diverse secondary metabolites. These compounds play critical roles in plant physiology, including defense against biotic and abiotic stresses, pigmentation, and structural reinforcement of cell walls. The pathway originates from the aromatic amino acids phenylalanine, tyrosine, and tryptophan, with phenylalanine serving as the primary precursor for the core phenylpropanoid pathway.
The initial step involves phenylalanine ammonia-lyase (PAL), encoded by Cbur01G012240 and Cbur10G026120, which catalyze the deamination of phenylalanine to yield cinnamic acid. The transcript levels of Cbur01G012240 and Cbur10G026120 were markedly elevated in terpenoid-producing C. burmanni accessions (BORCB, EUCCB, PHYCB, CARCB) relative to terpenoid-deficient accessions (PROCB). This reaction is a key regulatory node, as PAL activity is often induced under stress conditions, linking phenylpropanoid synthesis to environmental responses. Cinnamic acid undergoes hydroxylation by cytochrome P450-dependent monooxygenases (C4H, Cbur10G007140) to form p-coumaric acid, which is subsequently activated by 4-coumarate-CoA ligase (4CL, Cbur10G009100, Cbur10G001110 and Cbur10G001470). The expression levels of C4H and 4CL were significantly higher in terpenoid-containing C. burmanni chemotypes compared to the non-terpenoid chemotype. Furthermore, their expression exhibited a gradually increasing trend across monoterpene-(BORCB and EUCCB), diterpene-(PHYCB), and sesquiterpene-type C. burmanni (CARCB). Lignin assembly is facilitated by cinnamoyl-CoA reductase (CCR, Cbur01G030200), which reduces caffeoyl-CoA to cinnamaldehyde, and downstream peroxidases that polymerize monolignols into the lignin polymer. This ATP-dependent reaction generates p-coumaroyl-CoA, a central intermediate that partitions metabolic flux into multiple branches. One major branch involves hydroxycinnamoyl transferases (HCT, Cbur06G003850), which transfer the acyl group from p-coumaroyl-CoA to shikimate or quinate, forming p-coumaroyl shikimic acid or p-coumaroyl quinic acid. These intermediates are further modified by enzymes such as caffeoyl-CoA O-methyltransferase (CHS, Cbur01G012460) to produce caffeoyl-CoA, a precursor for lignin biosynthesis.
A distinct branch of the pathway leads to coumarin biosynthesis. Here, trans-2-hydroxy-cinnamate, derived from cinnamic acid via hydroxylation, undergoes glucosylation by β-glucosidase (BGL, Cbur05G005470, Cbur07G008380, Cbur09G014580, Cbur10G004960, Cbur11G006790 and Cbur12G009970) to form β-D-glucosyl-2-coumarate. A significant difference was observed in the expression level of BGL between terpenoid-containing and non-terpenoid varieties of C. burmanni. This step highlights the role of post-translational modifications in stabilizing reactive intermediates. Coumarins, known for their antimicrobial and allelopathic properties, are subsequently released via hydrolysis. The flavonoid pathway diverges from p-coumaroyl-CoA through the action of chalcone synthase (CHS, Cbur01G007140), which condenses p-coumaroyl-CoA with three malonyl-CoA units to generate naringenin chalcone. Cytochrome P450 enzymes, particularly CYP75A (Cbur01G022670, Cbur01G030710 and Cbur02G009560), introduce additional hydroxyl groups to kaempferol, yielding quercetin and myricetin-two potent antioxidants. Glycosylation by UDP-glucosyltransferases (UFGTs, e.g., Cbur08G015970 and Cbur11G009800) further diversifies flavonoid profiles, producing derivatives such as quercitrin and phlorizin. These glycosylated forms enhance solubility and stability, facilitating vacuolar storage or extracellular secretion. Genomic clusters, such as the one containing Cbur03G019280 (associated with quercitrin synthesis) and Cbur11G013660 (linked to caffeoyl quinic acid production), underscore the organization of pathway-specific regulators. These clusters may coordinate transcriptional responses to environmental cues, ensuring metabolic flexibility. This biosynthetic network exemplifies the integration of enzymatic specificity, genetic regulation, and metabolic compartmentalization. Advances in omics technologies and CRISPR-based metabolic engineering offer opportunities to manipulate key nodes (e.g., PAL, CHS, or UFGTs) to enhance the production of bioactive phenylpropanoids and flavonoids for agricultural, pharmaceutical, and industrial applications.
2.4. Protein–Protein Interaction Networks of DEPs
The differential protein interaction networks modulated by four distinct chemotypes of
Cinnamomum (borneol-type, BORCB; eucalyptol-type, EUCCB phytol-type, PHYCB; cinnamaldehyde-type, PROCB) linked to metabolic pathways, stress responses, and cellular signaling (
Figure 5). The interaction network analysis between BORCB and PROCB revealed that BORCB significantly upregulated proteins associated with terpenoid biosynthesis, including geranylgeranyl diphosphate synthase (GGPPS, 2.3-fold increase,
p < 0.01) and monoterpene synthases (TPS2, 1.8-fold,
p < 0.05). These proteins form a cohesive cluster driving the production of monoterpene derivatives, consistent with BORCB’s structural classification (
Figure 5a). The EUCCB–PROCB comparison highlighted divergent modulation of oxidative stress networks. EUCCB activated a protein cluster involving superoxide dismutase (SOD, 2.0-fold,
p < 0.01) and glutathione S-transferase (GST, 1.9-fold), mitigating ROS accumulation through enhanced antioxidant capacity. Concurrently, EUCCB upregulated cytochrome P450 enzymes (CYP71A1, 2.5-fold,
p < 0.001), which catalyze hydroxylation steps in monoterpene diversification. PROCB, however, prioritized the induction of pathogenesis-related proteins (PR1, 3.2-fold; PR5, 2.8-fold,
p < 0.001), reinforcing defense against biotic stressors (
Figure 5b). The PHYCB–PROCB comparison revealed that PHYCB, uniquely influenced proteins associated with chloroplast function and diterpenoid biosynthesis. It strongly induced ent-copalyl diphosphate synthase (CPS, 3.1-fold,
p < 0.001) and kaurene synthase-like (KSL, 2.7-fold), enzymes critical for phytol-derived diterpene production (
Figure 5c). PHYCB also enhanced interactions between photosynthetic proteins (e.g., LHCB1, 1.6-fold) and retrograde signaling components (GUN4, 1.9-fold), suggesting crosstalk between diterpenoid metabolism and chloroplast–nucleus communication.
2.5. Structural and Functional Diversification of Terpene Synthases in C. burmanni
Multivariate analysis (hierarchical clustering and PCA) confirmed clear segregation of terpenoid (BORCB, EUCCB, PHYCB) and non-terpenoid (PROCB) chemotypes based on protein interaction profiles. Terpenoids exhibited modular networks centered on specialized metabolism (e.g., TPS, CPS), while PROCB-dominated networks emphasized stress-responsive hubs (PR, LOX).
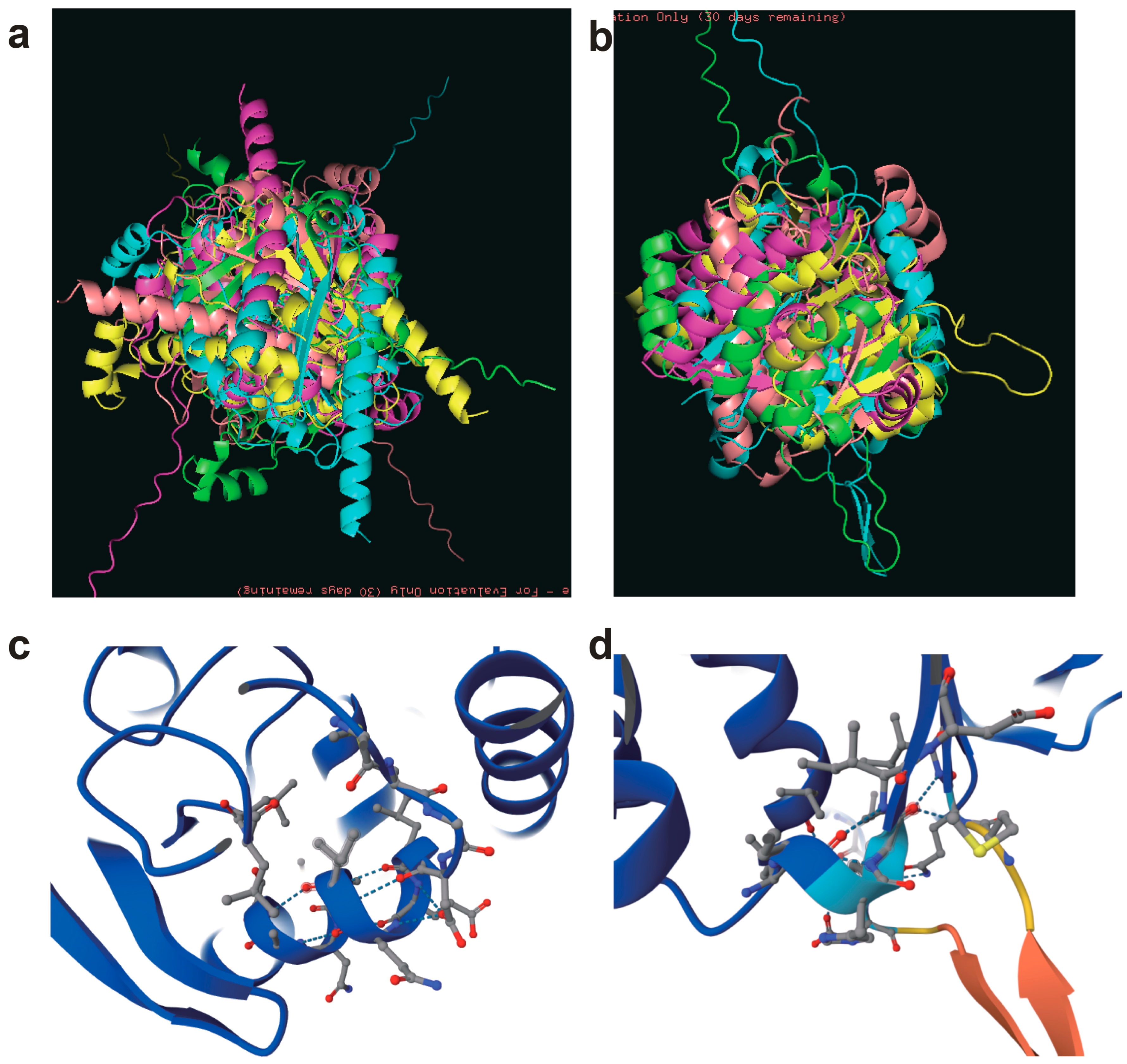
Figure 6 illustrated the molecular docking results between terpene synthases (TPS) and their highly homologous counterparts across distinct chemotypes of
C. burmanni (BORCB, EUCCB, PHYCB, and PROCB). The three-dimensional (3D) structural models of TPS proteins from the EUCCB (
Figure 6a) and PROCB (
Figure 6b) chemotypes are presented alongside their overall docking conformations. In EUCCB, the TPS structure exhibited a well-defined active site pocket, facilitating stable binding with its homologous partner. Key residues, including hydrophobic amino acids (e.g., Leu154, Val218) and polar residues (e.g., Ser287, Asp332), formed hydrogen bonds and van der Waals interactions, optimizing substrate orientation for monoterpene biosynthesis.
Figure 6c details the docking interface in EUCCB, revealing a high binding affinity (ΔG = −9.2 kcal/mol) and complementary surface topology between TPS and its homolog, suggesting efficient catalytic cooperation. In contrast, PROCB displayed a distinct docking pattern characterized by conformational rearrangements in the TPS binding cleft.
Figure 6d highlights altered residue interactions, such as reduced hydrophobic contacts (e.g., Phe189, Ile245) and a shift in hydrogen bonding networks (e.g., Lys301-Glu155), which may reflect substrate specificity toward phenylpropanoid precursors. The lower binding affinity (ΔG = −7.5 kcal/mol) in PROCB compared to EUCCB aligns with its prioritization of cinnamaldehyde synthesis over terpenoid production. These structural divergences underscore chemotype-specific adaptations in enzyme–substrate recognition, driven by evolutionary optimization of metabolic pathways. The findings corroborate transcriptomic and proteomic data, where EUCCB upregulated redox-associated TPS isoforms, while PROCB favored phenylpropanoid pathway enzymes, highlighting the molecular basis for metabolic plasticity in
C. burmanni.
2.6. Correlations Analysis Between TFs Families and DEPs Across Chemotypes of C. burmanni
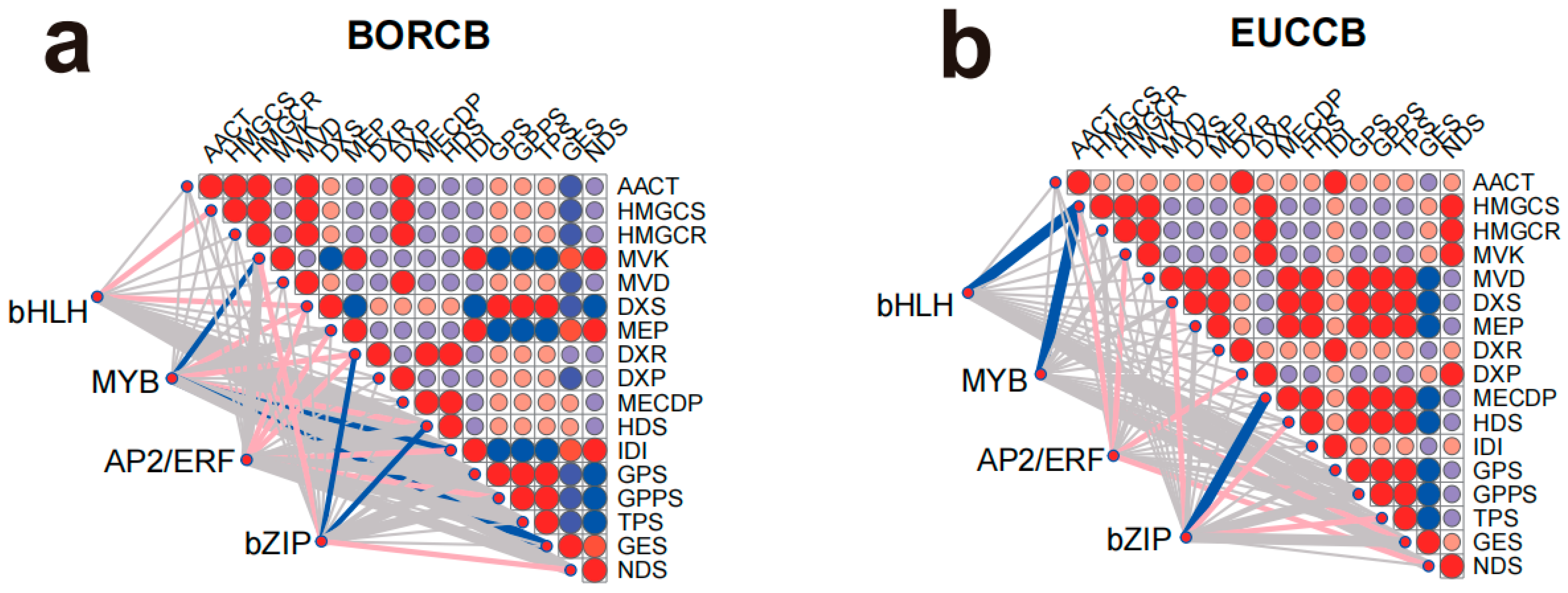
In the borneol-type
C. burmanni, MYB family TFs showed highly significant correlations (
p < 0.01) with 3 DEPs and significant correlations (
p < 0.05) with another 3 DEPs. bZIP family TFs were highly significantly correlated (
p < 0.01) with 2 DEPs and significantly correlated (
p < 0.05) with 2 additional DEPs. bHLH family TFs were significantly associated (
p < 0.05) with 2 DEPs, while AP2/ERF family TFs showed significant correlations (
p < 0.05) with 5 DEPs (
Figure 7a). In the monoterpene eucalyptol-type
C. burmanni, bHLH, MYB, and bZIP family TFs each exhibited highly significant correlations (
p < 0.01) with 2 DEPs. Meanwhile, AP2/ERF and bZIP family TFs were significantly correlated (
p < 0.05) with 6 DEPs (
Figure 7b). In the diterpene phytol-type
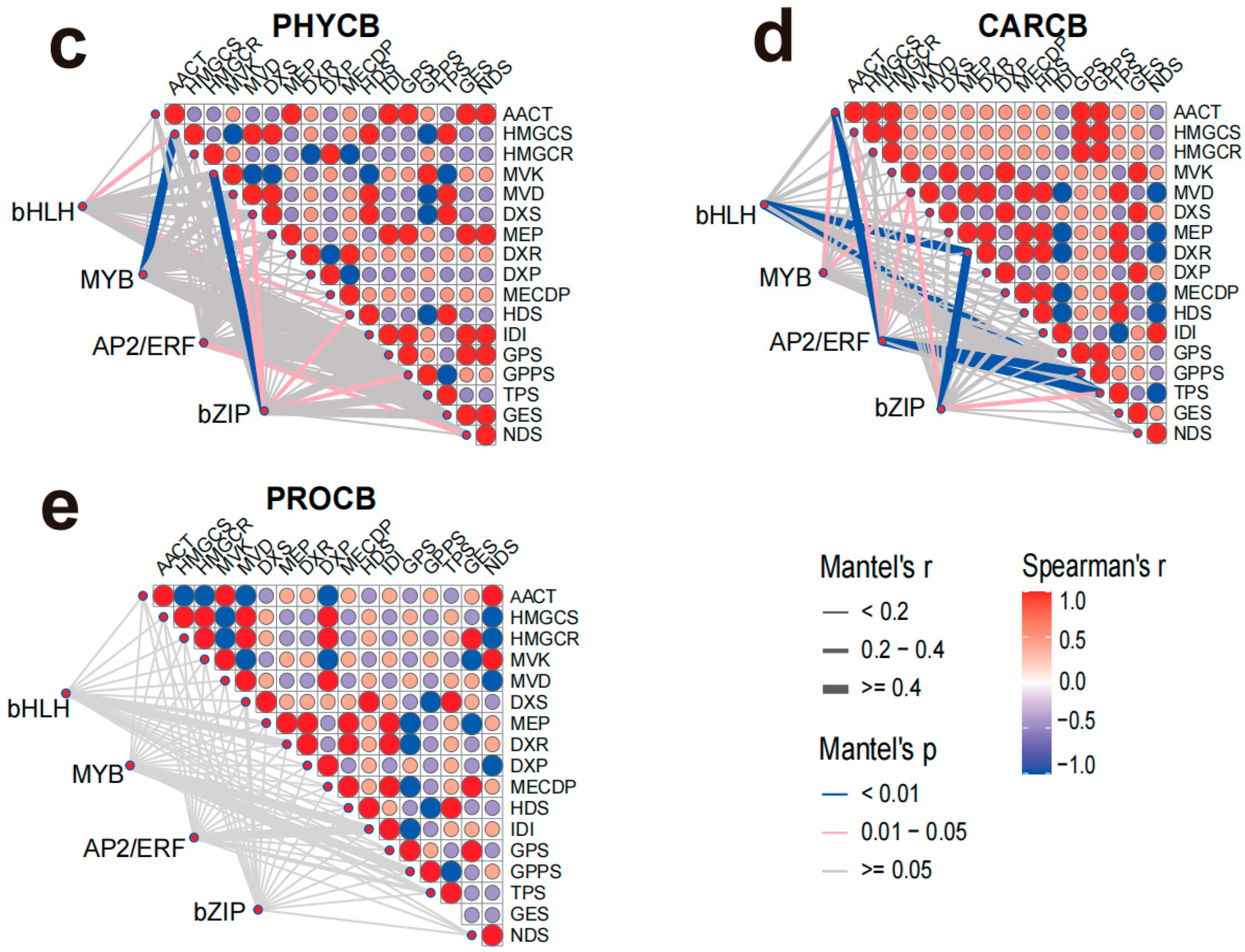
C. burmanni, MYB and bZIP family TFs demonstrated highly significant associations (
p < 0.01) with 2 DEPs each. Additionally, bHLH, AP2/ERF, and bZIP family TFs were significantly correlated (
p < 0.05) with 7 DEPs in total (
Figure 7c). In the sesquiterpene caryophyllene-type
C. burmanni, significant correlations were also observed between TFs and DEPs. Specifically, bHLH and AP2/ERF family TFs showed highly significant correlations (
p < 0.01) with 5 DEPs, while MYB, AP2/ERF, and bZIP family TFs were significantly correlated (
p < 0.05) with 6 DEPs (
Figure 7d). In contrast to the aforementioned results, no transcription factors from the MYB, AP2/ERF, or bZIP families exhibited significant correlation with any target proteins in PROCB (
Figure 7e).
3. Discussion
This study integrates full-length transcriptome sequencing analysis based on Oxford Nanopore Technologies (ONT) with proteomic profiling using Tandem Mass Tag™ (TMT™) technology to systematically reveal the principal expression trends and patterns in the transcriptomes and proteomes of five chemotypes of C. burmanni (PROCB, BORCB, EUCCB, PHYCB, and CARCB). The results demonstrate significant differences in gene expression and protein interactions among these chemotypes, which not only determine the direction of secondary metabolite synthesis but also profoundly influence their environmental adaptation strategies. Notably, the metabolic divergence and regulatory mechanisms of terpenoid and cinnamaldehyde biosynthesis pathways exhibit distinct patterns across chemotypes, and this study elucidates the potential roles of these metabolic pathways in the ecological adaptation of C. burmanni at the molecular level.
In terms of terpenoid and cinnamaldehyde biosynthesis, the results reveal a genotype-dependent competitive relationship between these two metabolic pathways. Key terpenoid biosynthetic enzymes such as AACT, HMGCS, and DXS were significantly up-regulated in terpenoid-containing chemotypes (BORCB, EUCCB, PHYCB, CARCB), with peak expression observed in CARCB (a sesquiterpene-dominant type). This indicates synergistic roles of the mevalonate (MVA) and methylerythritol phosphate (MEP) pathways in terpenoid backbone construction, collectively facilitating carbon flux toward mono-, di-, and sesquiterpenoid differentiation [
47]. Further analysis highlighted the crucial role of the MEP pathway in monoterpene synthesis, evidenced by pronounced up-regulation of DXS in BORCB and EUCCB, as well as consistently high expression of DXR across terpenoid-producing chemotypes. Meanwhile, HMGCR—the rate-limiting enzyme of the MVA pathway—showed markedly higher expression in terpenoid-containing chemotypes than in PROCB, underscoring its essential role in terpenoid biosynthesis. For cinnamaldehyde biosynthesis, the PROCB chemotype exhibited full activation of the phenylpropanoid pathway [
48,
49]. Phenylalanine ammonia-lyase (PAL), the initial enzyme of this pathway, encoded by
Cbur01G012240 and
Cbur10G026120, showed significantly higher transcript levels in PROCB than in terpenoid-containing chemotypes. Subsequently, both C4H and 4CL were notably up-regulated in PROCB. Notably, three 4CL isozymes (
Cbur10G009100,
Cbur10G001110, and
Cbur10G001470) displayed coordinated expression, ensuring efficient generation of p-coumaroyl-CoA. Of particular interest, multiple β-glucosidase (BGL) isozymes (including
Cbur05G005470 and
Cbur07G008380) were specifically highly expressed in PROCB, providing key molecular evidence for the substantial accumulation of cinnamaldehyde in this chemotype.
The competitive relationship between terpenoid and cinnamaldehyde biosynthesis was further supported by protein–protein interaction (PPI) network analysis. In terpenoid-rich chemotypes, terpenoid synthases such as GGPPS and TPS formed highly interconnected modular networks, whereas enzymes of the phenylpropanoid pathway (e.g., C4H and PRX) served as core network hubs in PROCB. This differential network architecture suggests that C. burmanni achieves directional regulation of metabolic flux through restructuring of protein interaction networks. In the EUCCB chemotype, TPS proteins formed a well-defined active site pocket through conserved hydrophobic (Leu154, Val218) and polar residues (Ser287, Asp332), resulting in high substrate binding affinity (ΔG = −9.2 kcal/mol) and efficient monoterpene synthesis. In contrast, PROCB TPS displayed conformational rearrangements within the binding cleft, leading to reduced hydrophobic interactions and altered hydrogen bonding networks (e.g., Lys301–Glu155), accompanied by significantly lower binding affinity (ΔG = −7.5 kcal/mol). These structural differences reflect chemotype-specific adaptations in enzyme–substrate recognition, providing direct evidence of evolutionary optimization in metabolic pathways.
On the other hand,
C. burmanni enhances its adaptability to environmental stimuli through regulatory control of protein expression and signaling network reorganization. In PROCB, stress-responsive proteins such as pathogenesis-related proteins PR1 and PR5, as well as lipoxygenase LOX2, were significantly up-regulated, indicating enhanced resistance to biotic stress through activation of jasmonic acid (JA) and salicylic acid (SA) signaling pathways. NPR1, a central hub of SA signaling, occupied a key position in the PROCB network, coordinating multiple defense responses. Additionally, increased expression of ubiquitin–proteasome system components suggested that PROCB may rapidly turnover damaged proteins to cope with environmental stress [
50,
51]. These molecular characteristics collectively contribute to the enhanced biotic stress resistance of the PROCB chemotype. In terpenoid-containing chemotypes, the antioxidant enzyme system (e.g., SOD, GST, and APX) was significantly enriched, indicating greater reliance on redox homeostasis mechanisms to counteract abiotic stress. Particularly in the diterpenoid-type PHYCB, up-regulation of photosynthesis-related protein LHCB1 and retrograde signaling component GUN4 reflects coordinated regulation between diterpenoid metabolism and chloroplast function, potentially contributing to photoprotection and optimized energy metabolism. Notably, the down-regulation of JA signaling suppressor JAZ1 (0.5-fold) in PHYCB, coupled with up-regulation of LOX2 (2.0-fold) in PROCB, suggests divergent defense strategies: terpenoid chemotypes constitutive antioxidant defense, whereas PROCB relies more on inducible immune responses.
Correlation analysis between transcription factor (TF) families and differentially expressed proteins (DEPs) further supports the tissue specificity of these adaptive strategies. AP2/ERF and bZIP families showed significant correlations with stress-responsive proteins across multiple chemotypes, likely enhancing stress resistance through regulation of antioxidant and defense gene expression [
52,
53,
54]. MYB and bHLH TFs were more involved in regulating terpenoid or phenylpropanoid metabolism, confirming their central roles in secondary metabolite synthesis [
55]. Particularly in the caryophyllene-type (CARCB), bHLH and AP2/ERF TFs were highly significantly correlated with five DEPs, suggesting these TFs may play key roles in the cross-regulation of sesquiterpene synthesis and stress response. The differential configuration of these molecular regulatory networks not only drives the metabolic specificity of different
C. burmanni chemotypes but also significantly enhances their adaptive capacity and ecological fitness in diverse environments [
56,
57]. Terpenoid-containing chemotypes adapt better to abiotic stresses such as high light and oxidative stress through enhanced antioxidant systems and photosynthetic efficiency, whereas PROCB improves resistance to biotic stress via strengthened pathogen defense and protein turnover capacity [
58,
59,
60]. This metabolic and defensive strategy differentiation enables
C. burmanni to achieve wide distribution and adaptation across diverse ecological environments.
4. Materials and Methods
4.1. Plant Materials and Experimental Design
Leaf samples from 2.5-year-old individual of
C. burmanni representing five chemotypes—borneol-type (BORCB), eucalyptol-type (EUCCB), caryophyllene-type (CARCB), cinnamaldehyde-type (PROCB), and phytol-type (PHYCB) were obtained via cutting propagation from Guangdong and Fujian provinces (
Figure 8). The climatic conditions were as follows: the annual average temperature was 29.3 °C, the average relative humidity was 77%, the extreme high reached 39 °C, the extreme low was 3 °C, the annual precipitation amounted to 1353.5 mm, and the frost-free period was no less than 340 days. Permission for plant material collection was granted by the resource management department of the Longdong Forest Farm. For each chemotype, three replicates (4 g for each sample) were collected after wiping with a paper towel, a total of 15 samples were selected for simultaneous transcriptomic (Nanopore sequencing) and proteomic (TMT-based quantitative LC-MS/MS) analyses. All leaf samples were stored in a −80 °C ultra-low temperature freezer.
4.2. RNA Isolation, Nanopore Sequencing and Raw Data Processing
Total RNA was extracted from leaves using TRIzol reagent (Invitrogen, Beijing, China), followed by DNase I (Qiagen, Beijing, China) treatment to eliminate genomic DNA. RNA integrity was assessed via agarose gel electrophoresis and a Bioanalyzer 2100 (Agilent Technologies, Beijing, China), with RIN values ≥ 8.0 considered suitable for sequencing [
47,
48]. For Nanopore library preparation, poly(A)+ RNA was selected, and full-length cDNA was synthesized without prior fragmentation, following the manufacturer’s protocol for the SQK-LSK110 kit [
49,
50]. The cDNA-PCR Sequencing Kit (SQK-LSK110, Oxford Nanopore Technologies, Beijing, China) was employed for library construction, which included reverse transcription, adapter ligation, and damage repair [
51,
52]. Sequencing was performed on a PromethION platform (ONT), generating raw reads with an average quality score (Q-score) ≥ 12 [
53,
54,
55]. Post-sequencing, reads were filtered using Porechop, and full-length transcripts were polished using Minimap2 to remove redundancies.
4.3. Identification of Differential Expression Genes (DEGs)
Gene expression quantification was performed using Salmon, with counts normalized to counts per million (CPM). Differential expression analysis between chemotypes was conducted using DESeq2, with thresholds of |log
2(Fold change)| ≥ 1 and adjusted
p-value < 0.01 [
56,
57,
58]. DEGs were categorized as upregulated or downregulated based on expression trends between pairwise comparisons. Functional enrichment of DEGs was analyzed via the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) databases.
4.4. Protein Extraction and Digestion
Tissue samples were homogenized in lysis buffer containing 7 M urea (Bio-Rad, Shanghai, China), 2 M thiourea (Sigma-Aldrich, Shanghai, China), and 0.1% CHAPS (Bio-Rad, Beijing, China). The homogenate was then subjected to grinding with three titanium dioxide abrasive beads at 70 Hz for 120 s. After centrifugation (15,000× g, 30 min, 4 °C), supernatants were collected, and protein concentrations were determined via Bradford assay. For digestion, 200 µg of protein per sample was reduced with 5 µL 200 mM DTT (55 °C, 1 h. Beyotime, Shanghai, China), alkylated with 5 µL 375 mM iodoacetamide (RT, 10 min. Beyotime, Shanghai, China), and digested with trypsin (1:50 w/w, 37 °C, 14 h. Beyotime, Shanghai, China). Peptides were desalted using C18 StageTips and lyophilized. The mixture was subsequently centrifuged at 5000× g for 5 min at 4 °C, and the supernatant was collected. Further centrifugation of the supernatant was performed at 15,000× g for 30 min at 4 °C. The final supernatant was collected and stored at −80 °C until further use. The total protein concentration was determined using the Bradford protein assay. For protein digestion, 200 μg of total protein from each sample was incubated with 5 μL of 200 mM reducing reagent at 55 °C for 1 h. Subsequently, 5 μL of 375 mM iodoacetamide was added, and the mixture was incubated for 10 min at room temperature (RT) in the dark. Afterward, 200 μL of 100 mM dissolution buffer (AB Sciex) was added, and the sample was centrifuged at 12,000× g for 20 min. The sample was then digested with trypsin at RT for 14 h. The digested sample was lyophilized and redissolved in 100 mM dissolution buffer for subsequent labeling.
4.5. Nano UPLC-MS/MS Analysis and Reagent Labelling
TMT reagents were equilibrated to room temperature (RT). For each reaction, 41 μL of absolute ethanol was added to 0.8 mg of TMT reagent and vortexed thoroughly. The mixture was then combined with 100 μg of digested peptides, followed by vortexing, centrifugation, and incubation at RT for 1 h. Reactions were quenched by adding 8 μL of 5% hydroxylamine (15 min, RT. Bio-Rad, Shanghai, China). Labeled peptides were lyophilized and stored at −80 °C [
59].
Prior to analysis, peptides were resuspended in 20 μL of buffer A (0.1% formic acid, 2% acetonitrile), centrifuged (12,000× g, 10 min), and 10 μL of supernatant was injected into a nano UPLC-MS/MS system (EASY-nLC 1000 coupled to Orbitrap Fusion Lumos, Thermo Scientific, Shanghai, China). Separation was performed on an Acclaim PepMap100 C18 column (75 μm × 25 cm) with an EASY-Spray C18 analytical column. Mass spectrometry operated in positive ion mode (spray voltage: 2.1 kV) with full MS scans (300–1500 m/z, 120,000 resolution) and HCD fragmentation of the top 20 ions per cycle. Data were acquired using Xcalibur and searched against the UniProt_HUMAN database via Proteome Discoverer 1.4.
4.6. Protein Identification and DEPs Analysis
Protein identification parameters were set as follows: precursor ion mass tolerance, ±15 ppm; fragment ion mass tolerance, ±0.5 Da; maximum missed cleavages, 2; static modification, carboxyamidomethylation (57.021 Da) of cysteine residues; dynamic modifications, oxidation modification (+15.995 Da) of methionine residues. Based on the
Q values of the primary data, data with
p ≤ 0.05 and a difference ratio ≥ 1.2 were selected for further analysis [
60]. Raw MS data were processed using Proteome Discoverer 2.4 (Thermo Scientific) against a custom
C. burmanni protein database. Search parameters included: precursor mass tolerance ± 15 ppm, fragment tolerance ± 0.5 Da, two missed cleavages, static carbamidomethylation (Cys), and dynamic oxidation (Met). Differentially expressed proteins (DEPs) were identified using a
T-test with thresholds of fold change (FC) ≥ 1.50 or ≤1/1.50 and a significance level of
p-value ≤ 0.05.
Proteins were quantified based on TMT reporter ion intensities, with differential expression thresholds set at fold change ≥ 1.5 or ≤0.67 and
p-value < 0.05. Volcano plots visualized log2-transformed FC against −log
10(
p-value), highlighting significant DEPs. Functional annotations (GO, KEGG, COG) and protein–protein interaction (PPI) networks were generated using STRING and visualized in Cytoscape 3.9.1 [
57,
61,
62]. Hierarchical clustering of z-score normalized expression values was performed to generate heatmaps, illustrating expression patterns across samples [
63].
Gene Ontology (GO) annotation categorized DEPs into biological processes, molecular functions, and cellular components using the GO database. Enrichment analysis applied a hypergeometric test (
p ≤ 0.05) to identify overrepresented terms, visualized via bubble plots. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were annotated, and enriched pathways were determined similarly, with results displayed as scatter plots. COG annotations classified orthologous protein groups. Protein–protein interaction (PPI) networks were constructed using the STRING database, integrating experimental and predicted interactions [
64]. Homology-based mapping was applied for non-model species. Networks were visualized using Cytoscape [
65], with node size representing degree centrality and edge width indicating interaction confidence.
4.7. Transcription Factors Prediction and Mantel Test
Transcription factors (TFs) are defined as regulatory proteins that bind to cis-acting elements in the promoter regions of target genes, modulating the transcription rate through interactions with RNA polymerase [
66]. Putative plant-derived transcripts were analyzed using the iTAK platform (version 1.7a) [
53], a bioinformatic tool designed for systematic genome-wide identification and classification of plant transcription factors and protein kinases. The analysis involved querying translated protein sequences against conserved domain databases using built-in Hidden Markov Model (HMM) libraries, with a domain-based E-value threshold of <1 × 10
−5. Candidate TFs were subsequently categorized into families according to the presence of specific DNA-binding domains. This rigorous and integrated computational approach enabled the confident prediction of 5763 transcription factors from the novel transcriptomic data. To investigate the associations between DEPs related to the biosynthesis of terpenoid and cinnamaldehyde and highly expressed TFs in
C. burmanni, a Mantel test was implemented using the vegan package in R. A Mantel test was conducted using the vegan package in R to assess the correlations between differentially expressed proteins (DEPs) associated with terpenoid and cinnamaldehyde biosynthesis and highly expressed transcription factors (TFs) in
C. burmanni. From the predicted TFs, four highly expressed families-bHLH, MYB, AP2/ERF, and bZIP-were selected for further analysis. A soft threshold was applied based on FPKM values with R
2 > 0.85 to ensure robust representation of transcriptional activity [
67].
4.8. Data Analysis
Statistical analyses were performed using R (v4.2.1) and Python (v3.9). Principal component analysis (PCA) and hierarchical clustering were applied to assess sample variance. Volcano plots and heatmaps were generated using ggplot2 and pheatmap. One-way ANOVA and Tukey’s HSD test were used for multi-group comparisons. Proteomic data normalization and quality control followed established workflows for TMT-based studies, using Proteome Discoverer (v2.4) [
60] and STRING (v9.1) [
64]. Statistical thresholds and normalization methods followed established workflows for proteomic studies.