Predicting Forest Carbon Sequestration of Ecological Buffer Zone in Urban Agglomeration: Integrating Vertical Heterogeneity and Age Class Dynamics to Unveil Future Trajectories
Abstract
1. Introduction
2. Materials and Methods
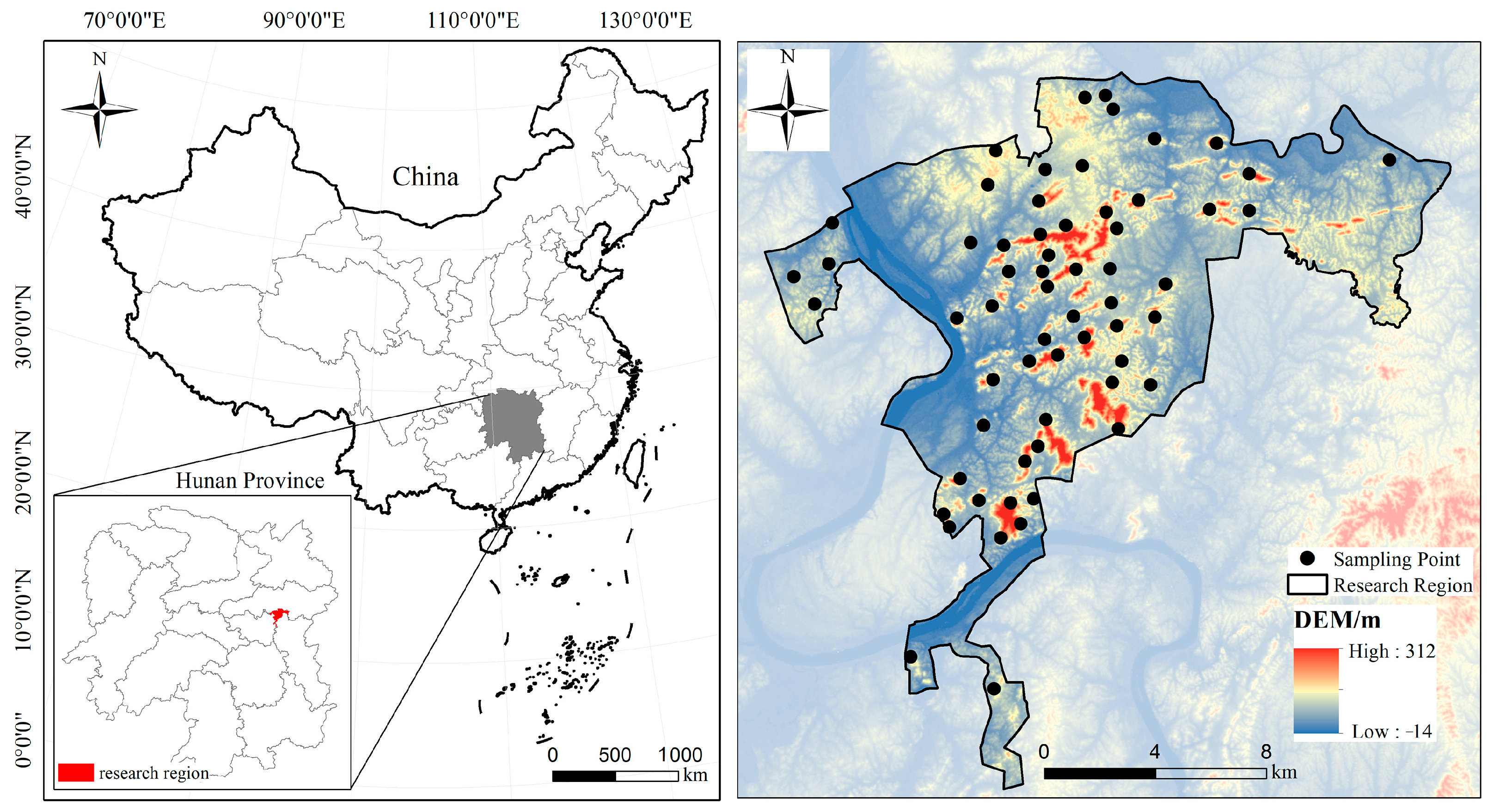
2.1. Study Site
2.2. Research Methods
2.2.1. Plant Community Survey
2.2.2. Determination of Biomass
2.2.3. Sample Collection and Analysis
2.2.4. Statistical Analysis
3. Results
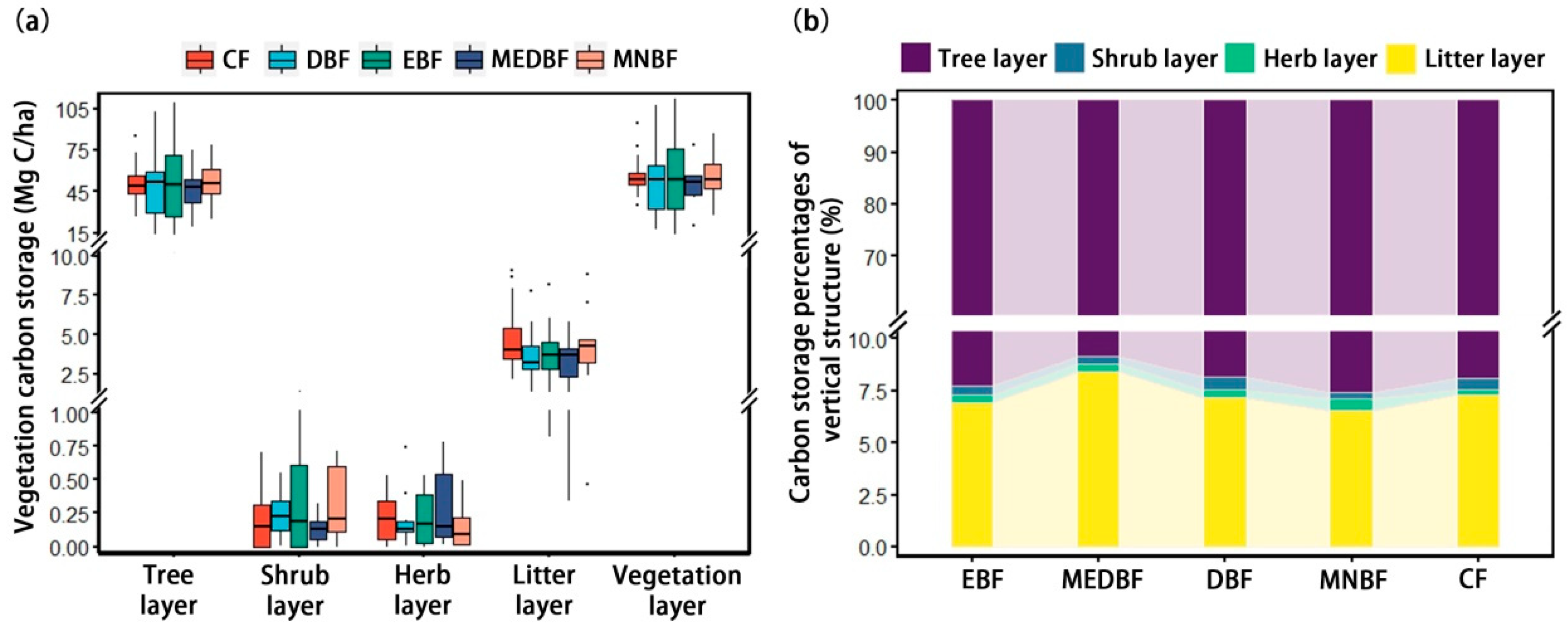
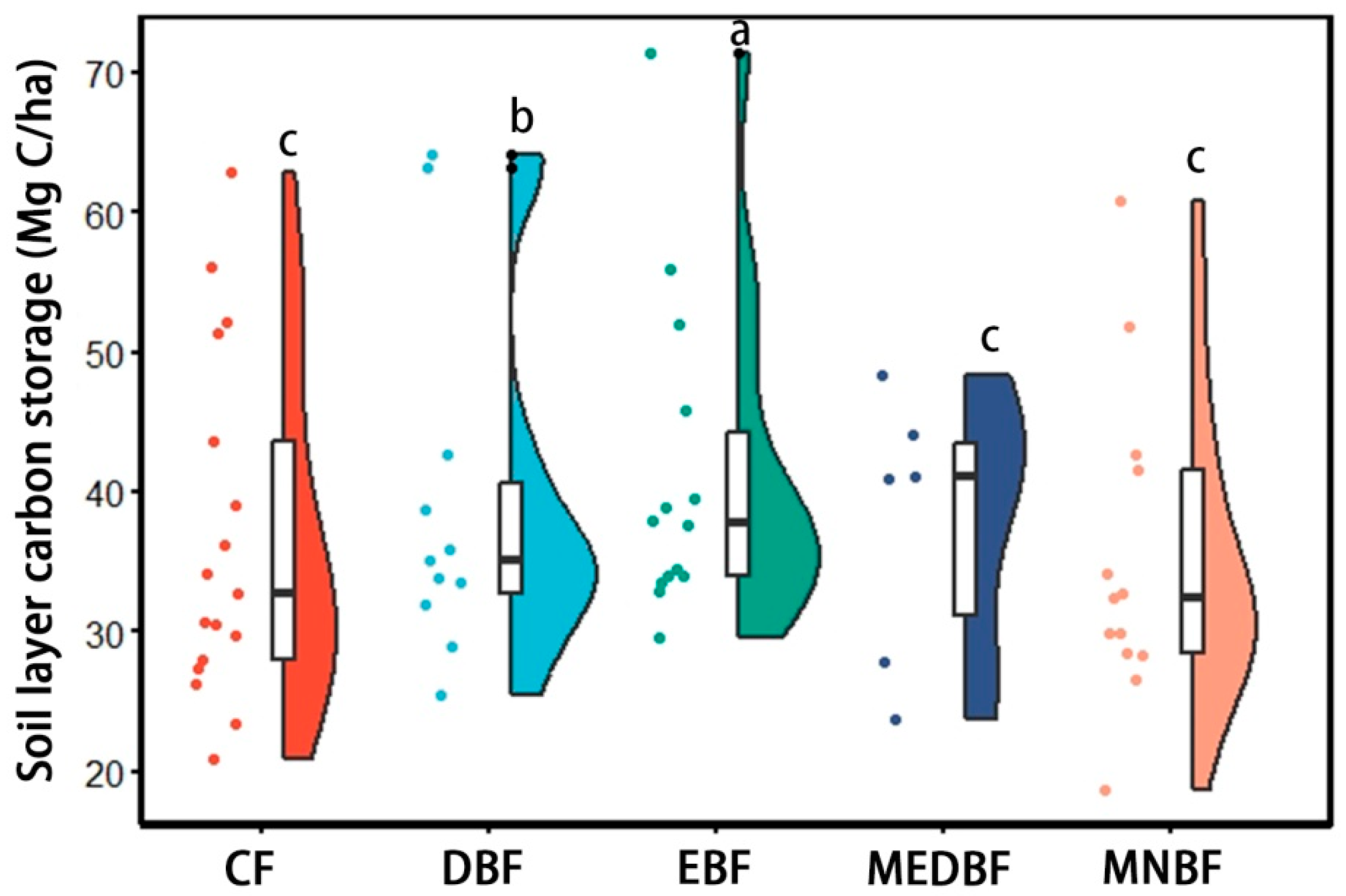
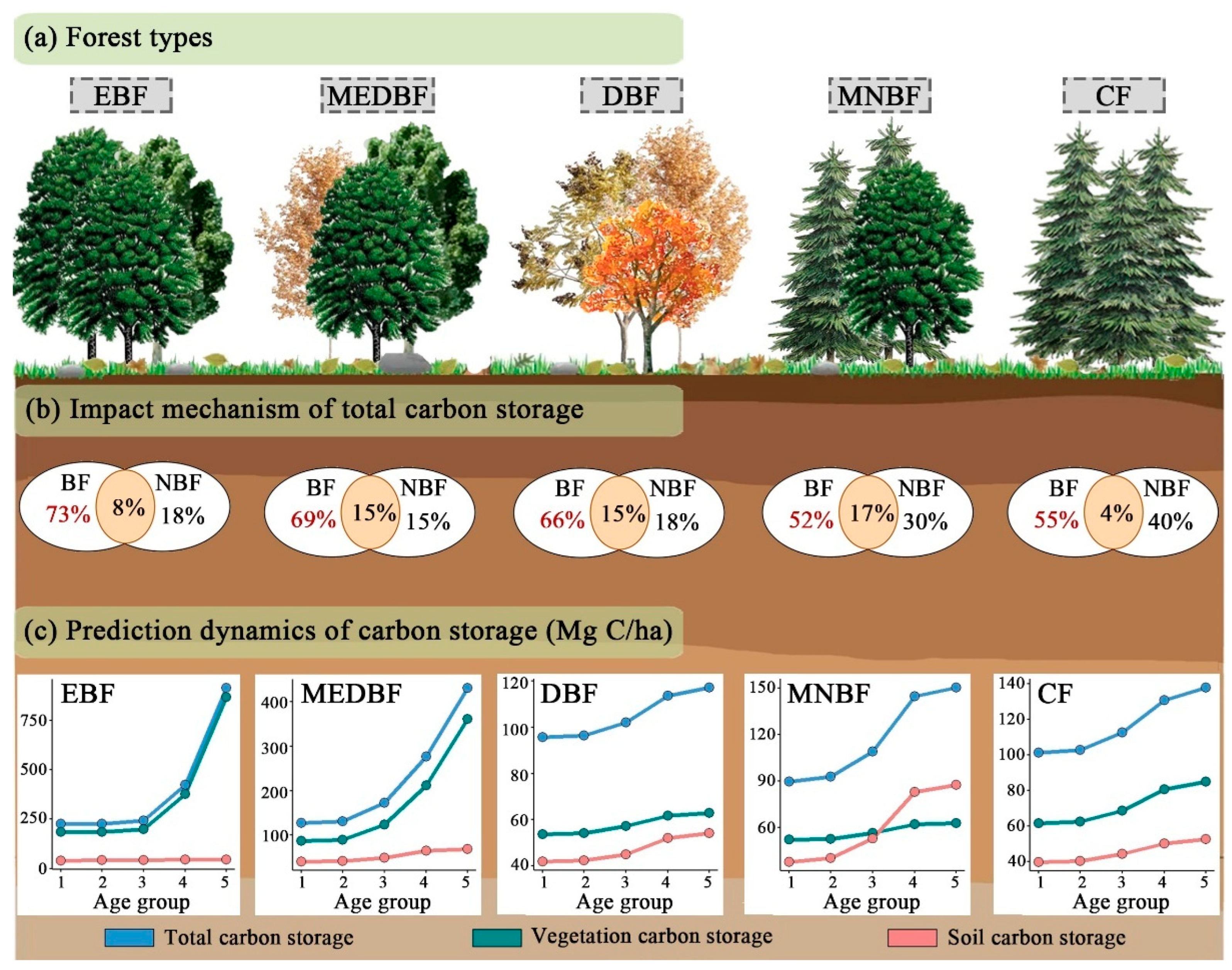
3.1. Changes in C Storage and Vertical Distribution Patterns of Different Forest Types
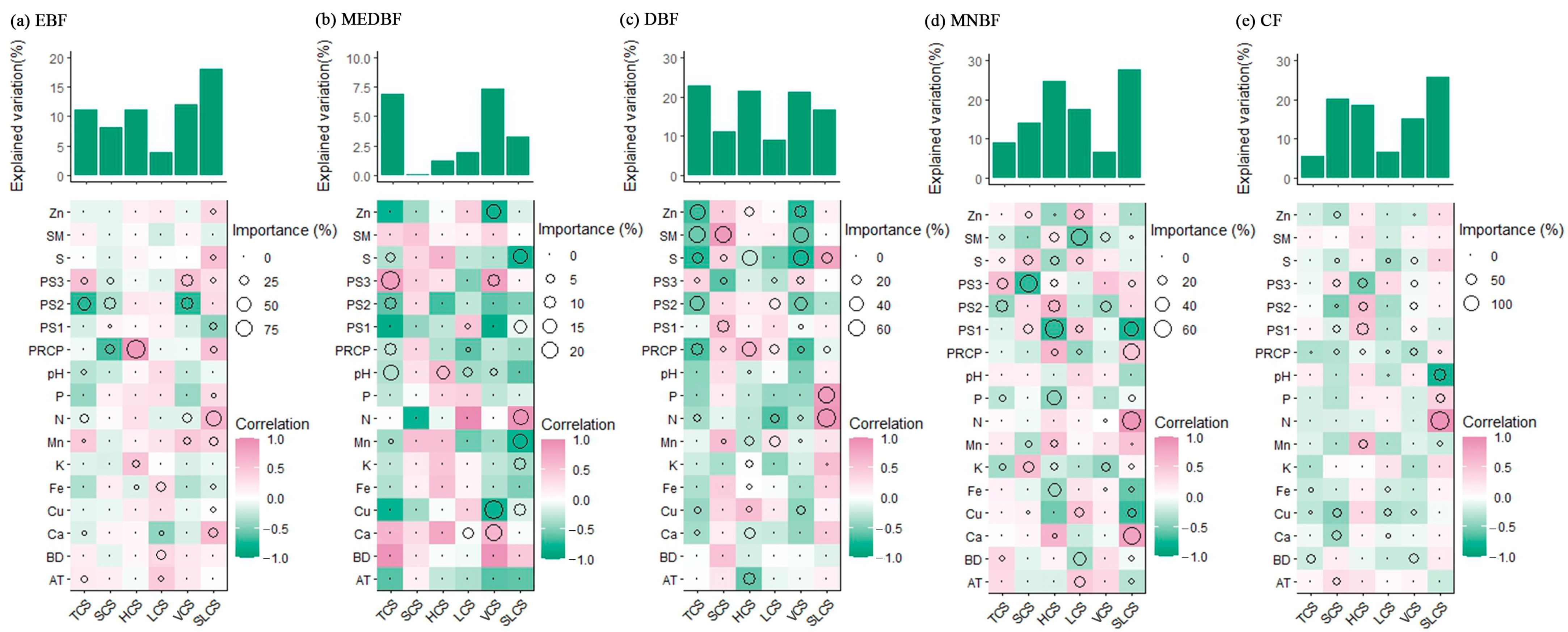
3.2. Biological Factors Influencing C Storage of Vegetation and Soil in Different Forest Types
3.3. Effects of Non-Biological Factors on C Storage of Vegetation and Soil in Different Forest Types
3.4. Synergy Effects of Biological and Non-Biological Factors on the Variation in C Storage of Different Layers
3.5. Prediction Dynamics of C Storage with Age Group
4. Discussion
4.1. Spatial Heterogeneity and Vertical Stratification of C Storage Between Forest Types
4.2. Biological and Non-Biological Regulation of Stratified C Allocation
4.3. Integrated Biotic–Abiotic Controls on C Allocation
4.4. Age-Modulated C Trajectories and Partitioning
4.5. Comparison of C Stores and Drivers Between Urban Buffer and Natural Forests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CZT-GHD | Chang-Zhu-Tan Green Heart District |
| C | Carbon |
| SOC | Soil organic carbon |
| EBF | Evergreen broad-leaved forest |
| MEDBF | Mixed evergreen and deciduous broad-leaved forest |
| DBF | Deciduous broad-leaved forest |
| MNBF | Mixed needle and broad-leaved forest |
| CF | Coniferous forest |
| N | Total nitrogen |
| P | Total phosphorus |
| K | Total potassium |
| Ca | Total calcium |
| S | Total sulfur |
| Mn | Total manganese |
| Fe | Total iron |
| Cu | Total copper |
| Zn | Total zinc |
| BD | Bulk density |
| SM | Soil moisture |
| PS1 | Clay particles (<2 um) |
| PS2 | Powder particles (2–50 um) |
| PS3 | Sand particles (>50 um) |
| AT | Air temperature |
| PRCP | Precipitation |
| SC | Shrub coverage |
| AHS | Average height of shrubs |
| HC | Herb coverage |
| AHC | Average height of herbs |
| TVC | Total vegetation coverage |
| NP | Number of plants |
| PD | Plant density |
| AAH | Average dominant height |
| DBH | Average diameter at breast height |
| TH | Average diameter at breast height |
| CD | Canopy density |
| TV | Total volume |
| TSV | Tree layer volume |
| TB | Tree layer biomass |
| SB | Shrub layer biomass |
| HB | Herb layer biomass |
| LB | Litter layer biomass |
| SI | Shannon–Wiener’s diversity index |
| TCS | Tree layer carbon storage |
| SCS | Shrub layer carbon storage |
| HCS | Herb layer carbon storage |
| LCS | Litter layer carbon storage |
| VCS | Vegetation layer carbon storage |
| SLCS | Soil layer carbon storage |
| BF | Biological factor |
| NBF | Non-biological factor |
| ANOVA | A one-way analysis of variance |
| SEM | Structural equation modeling |
| IQR | Interquartile range |
Appendix A
Appendix A.1
| Forest Types | Number of Sample Plots | Dominant Tree Species | Age Group | Shrub Coverage (%) | Average Shrub Height (m) | Herb Coverage (%) | Average Herbaceous Height (m) | Total Vegetation Coverage (%) | Number of Plants | Plant Density (Plants/ha) | Average Dominant Height (m) | Average Breast Height Diameter (cm) | Average Tree Height (m) | Canopy Density | Soil Texture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EBF | 14 | Cinnamomum camphora Lithocarpus glaber Castanopsis sclerophylla Schima superba Ilex chinensis | Juvenile, middle-aged | 5~47 | 0.7~3.0 | 5~80 | 0.3~1.05 | 75~90 | 80~175 | 1199~2624 | 5.7~15.8 | 7.4~15.7 | 5.1~13.7 | 0.5~0.8 | Clay, loam, and clay loam soils |
| MEDBF | 6 | Choerospondias axillaris Cinnamomum camphora Toxicodendron vernicifluum Liquidambar formosana Paulownia | Juvenile, middle-aged | 7~40 | 1.1~1.2 | 7~55 | 0.32~0.90 | 75~92 | 98~144 | 1469~2159 | 10.3~11.5 | 9.2~10.9 | 6.5~8.2 | 0.5~0.7 | Loam and clay loam soils |
| DBF | 11 | Liquidambar formosana Quercus Paulownia Firmiana simplex Rhus chinensis | Juvenile, middle-aged | 8~51 | 0.5~2 | 5~73 | 0.17~0.86 | 85~95 | 26~248 | 390~3718 | 7.3~16.1 | 9.9~20.0 | 6.6~11.9 | 0.3~0.8 | Clay, loam, and clay loam soils |
| MNBF | 13 | Chinese Fir Cinnamomum camphora Pinus massoniana Castanopsis sclerophylla Pinus elliottii | Juvenile, middle-aged | 5~80 | 0.8~2.0 | 5~80 | 0.2~0.7 | 75~96 | 57~280 | 855~4198 | 8.2~13.9 | 8.4~17.8 | 5.4~10.5 | 0.6~0.9 | Clay, loam, and clay loam soils |
| CF | 17 | Chinese Fir Pinus massoniana | Juvenile, middle-aged | 2~50 | 0.8~1.8 | 6~78 | 0.2~0.78 | 75~97 | 94~388 | 1409~5817 | 9.7~17 | 8.3~20 | 6.5~13.9 | 0.6~0.9 | Clay, loam, clay loam, and sandy clay loam soils |
Appendix A.2
| Tree Species | Models |
|---|---|
| Chinese Fir | V = 0.000058777042D1.9699831H0.89646157 |
| Pinus massoniana | V = 0.000062341803D1.8551497H0.95682492 |
| Pinus elliottii | V = 0.000086791543DxHy x = 1.663800058 + 0.009429976(D + 10H) y = 0.969340486 − 0.029203083(D + 2.5H) |
| Broad-leaved tree | V = 0.000050479055D1.9085054H0.99076507 |
Appendix A.3
| Tree Species | a (Mg/m3) | b (Mg) | Carbon Content |
|---|---|---|---|
| Cinnamomum camphora | 0.9292 | 6.4940 | 0.4916 |
| Liquidambar (Other hard broadleaf varieties) | 0.9292 | 6.4940 | 0.4901 |
| Broad-leaved mixed forest | 0.9788 | 5.3764 | 0.4796 |
| Mixed broadleaf–conifer forest | 0.8136 | 18.4660 | 0.4893 |
| Chinese Fir | 0.4652 | 19.1410 | 0.5127 |
| Pinus massoniana | 0.5034 | 20.5470 | 0.5271 |
| Coniferous mixed forest | 0.5292 | 25.087 | 0.5168 |
Appendix A.4
| Tree Species | Equations | a | b |
|---|---|---|---|
| Camellia oleifera | M = a(D2H)b | 0.0563 | 0.9291 |
| Gardenia jasminoides | M = a + b × D2H | 0.0019 | 0.0223 |
| Loropetalum chinense | M = a + b × D2H | 0.0101 | 0.0341 |
| Rhododendron simsii | M = a(D2H)b | 0.0298 | 0.8484 |
| Vaccinium bracteatum | M = a(D2H)b | 0.0494 | 0.7627 |
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Luijkx, I.T.; Peters, G.P.; et al. Global Carbon Budget 2023. Earth Syst. Sci. Data 2023, 15, 5301–5369. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Houghton, R.A.; Fang, J.; Kauppi, P.E.; Liao, Y.; Zhou, G.; Poorter, L.; Zhou, M.; et al. The enduring world forest carbon sink. Nature 2024, 631, 563–569. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2022: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Lorenz, K. Carbon Sequestration in Forest Ecosystems; Springer: Berlin, Germany, 2010. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Mensah, S.; Veldtman, R.; Assogbadjo, A.E.; Glèlè Kakaï, R.; Seifert, T. Tree species diversity promotes aboveground carbon storage through functional diversity and functional dominance. Ecol. Evol. 2016, 6, 7546–7557. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Jiang, M.; Liu, H.; Liu, G. How environmental and vegetation factors affect spatial patterns of soil carbon and nitrogen in a subtropical mixed forest in Central China. J. Soils Sediments 2017, 17, 2296–2304. [Google Scholar] [CrossRef]
- Liu, X.; Trogisch, S.; He, J.S.; Niklaus, P.A.; Bruelheide, H.; Tang, Z.; Erfmeier, A.; Scherer-Lorenzen, M.; Pietsch, K.A.; Yang, B.; et al. Tree species richness increases ecosystem carbon storage in subtropical forests. Proc. R. Soc. B 2018, 285, 20181240. [Google Scholar] [CrossRef]
- Augusto, L.; Boča, A. Tree functional traits, forest biomass, and tree species diversity interact with site properties to drive forest soil carbon. Nat. Commun. 2022, 13, 1097. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Aguila, L.C.R.; Wu, D.; Lie, Z.; Xu, W.; Tang, X.; Liu, J. Carbon sequestration and storage capacity of Chinese fir at different stand ages. Sci. Total Environ. 2023, 904, 166962. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Du, Z.; Zhou, L.; Zhou, G.; Coco, G.; Gao, J.; Zhou, X. The stand age governs forest root: Shoot ratios across northeast China. Agric. For. Meteorol. 2025, 370, 110595. [Google Scholar] [CrossRef]
- Besnard, S.; Heinrich, V.H.A.; Carvalhais, N.; Ciais, P.; Herold, M.; Luijkx, I.; Peters, W.; Suarez, D.R.; Santoro, M.; Yang, H. Global covariation of forest age transitions with the net carbon balance. Nat. Ecol. Evol. 2025, 9, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Besnard, S.; Santoro, M.; Herold, M.; Cartus, O.; Gütter, J.; Heinrich, V.; Herault, B.; Kassi, J.; Koirala, S.; N’Guessan, A.; et al. Global Age Mapping Integration (GAMI); V. 2.0; GFZ Data Services: Potsdam, Germany, 2023. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Z.; Piao, S.; Peng, C.; Ciais, P.; Wang, Q.; Li, X.; Zhu, X. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef]
- Li, C.; Zhang, R.; Li, T.; Guo, H.; Guo, R. Dynamic Changes and Influencing Factors of Vegetation in the “Green Heart” Zone of the Chang-Zhu-Tan Urban Agglomeration during the Past 21 Years. Int. J. Environ. Res. Public Health 2023, 20, 4517. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Li, S.; Wu, B. Study on the variation of carbon storage in the Chang-Zhu-Tan urban agglomeration in China based on topographic relief. Front. Environ. Sci. 2024, 12, 1481540. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X. Spatio-temporal evolution and multi-scenario prediction of ecosystem carbon storage in Chang-Zhu-Tan Urban agglomeration based on the FLUS-InVEST Model. Sustainability 2024, 16, 7025. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry: An Analysis of Global Change, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 419–444. [Google Scholar]
- Luo, Y.; Ahlström, A.; Allison, S.D.; Batjes, N.H.; Brovkin, V.; Carvalhais, N.; Chappell, A.; Ciais, P.; Davidson, E.A.; Finzi, A.; et al. Toward more realistic projections of soil carbon dynamics by Earth system models. Glob. Biogeochem. Cycles 2016, 30, 40–56. [Google Scholar] [CrossRef]
- Malhi, Y.; Baldocchi, D.D.; Jarvis, P.G. The carbon balance of tropical, temperate and boreal forests. Plant Cell Environ. 1999, 22, 715–740. [Google Scholar] [CrossRef]
- Tikuye, B.G.; Ray, R.L.; Gurau, S. Modeling carbon stock change and carbon dioxide emissions under different ecosystems in the Brazos River Basin, USA. Environ. Chall. 2025, 19, 101138. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Gough, C.M.; Halperin, A.; Hofmeister, K.L.; Nave, L.E.; Bohrer, G.; Curtis, P.S. Maintaining high rates of carbon storage in old forests: A mechanism linking canopy structure to forest function. For. Ecol. Manag. 2013, 298, 111–119. [Google Scholar] [CrossRef]
- Atkins, J.W.; Fahey, R.T.; Hardiman, B.S.; Gough, C.M. Forest canopy structural complexity and light absorption relationships at the subcontinental scale. J. Geophys. Res. Biogeosci. 2018, 123, 1387–1405. [Google Scholar] [CrossRef]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The ecology of soil carbon: Pools, vulnerabilities, and biotic and abiotic controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, G.; Zhang, J.; Qi, Y. Contributions of Biotic and Abiotic Factors to the Spatial Heterogeneity of Aboveground Biomass in Subtropical Forests: A Case Study of Guizhou Province. Sustainability 2022, 14, 10771. [Google Scholar] [CrossRef]
- Sulman, B.N.; Moore, J.A.M.; Abramoff, R.; Averill, C.; Kivlin, S.; Georgiou, K.; Sridhar, B.; Hartman, M.D.; Wang, G.; Wieder, W.R.; et al. Multiple models and experiments underscore large uncertainty in soil carbon dynamics. Biogeochemistry 2018, 141, 109–123. [Google Scholar] [CrossRef]
- Yu, Z.; Ciais, P.; Piao, S.; Houghton, R.A.; Lu, C.; Tian, H.; Agathokleous, E.; Kattel, G.R.; Sitch, S.; Goll, D.; et al. Forest expansion dominates China’s land carbon sink since 1980. Nat. Commun. 2022, 13, 5374. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guan, D.S. Carbon stock of the ecosystem of lower subtropical broadleaved evergreen forests of different ages in pearl river delta, China. J. Trop. For. Sci. 2014, 26, 249–258. [Google Scholar]
- Maier, C.A.; Johnsen, K.H.; Anderson, P.H.; Palmroth, S.; Kim, H.S.; McCarthy, H.R.; Oren, R. The response of coarse root biomass to long-term CO2; enrichment and nitrogen application in a maturing Pinus taeda stand with a large broadleaved component. Glob. Change Biol. 2022, 28, 1458–1476. [Google Scholar] [CrossRef]
- Joshi, S.R.; Tfaily, M.M.; Young, R.P.; McNear, D.H., Jr. Root exudates induced coupled carbon and phosphorus cycling in a soil with low phosphorus availability. Plant Soil 2024, 498, 371–390. [Google Scholar] [CrossRef]
- Song, Y.; Gu, X.Y.; Yan, H.Y.; Mao, W.T.; Wu, X.L.; Wang, Y.X. Dynamics of microbes and enzyme activities during litter decomposition of Pinus massoniana forest in mid-subtropical area. Environ. Sci. 2014, 35, 1151–1158. [Google Scholar]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, B.; Xu, Q.; Gao, D.; Zuo, H.; Ren, R.; Zhang, Y.; Chen, Z. Enhanced Carbon Storage in Mixed Coniferous and Broadleaf Forest Compared to Pure Forest in the North Subtropical-Warm Temperate Transition Zone of China. Forests 2024, 15, 1520. [Google Scholar] [CrossRef]
- Guo, L.B.; Wang, M.; Gifford, R.M. The change of soil carbon stocks and fine root dynamics after land use change from a native pasture to a pine plantation. Plant Soil 2007, 299, 251–262. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; Paré, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Change Biol. 2009, 16, 439–453. [Google Scholar] [CrossRef]
- Ekblad, A.; Wallander, H.; Godbold, D.L.; Cruz, C.; Johnson, D.; Baldrian, P.; Björk, R.G.; Epron, D.; Kieliszewska-Rokicka, B.; Kjøller, R.; et al. The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: Role in carbon cycling. Plant Soil 2013, 366, 1–27. [Google Scholar] [CrossRef]
- Dai, W.; Xiao, R.; Wei, C.; Yang, F. Plant litter traits control the accumulation of mineral-associated organic carbon by influencing its molecular composition and diversity. Soil Tillage Res. 2025, 253, 106667. [Google Scholar] [CrossRef]
- Yang, L.L.; Wen, S.Z.; Xing, Y.J. The Planning Strategies of Changsha-Zhuzhou-Xiangtan Urban Agglomeration Based on” Two-Oriented Society”. J. Cent. South Univ. For. Technol. 2018, 38, 81–87. [Google Scholar] [CrossRef]
- GB/T 38590-2020; Technical Regulations for Continuous Forest Inventory. Standards Press of China: Beijing, China, 2020.
- Liu, Q.J. Tree Volume Tables of China; China Forestry Publishing House: Beijing, China, 2017; ISBN 978-7-5038-9152-3. [Google Scholar]
- State Forestry Administration. National Technical Guidelines for Forest Carbon Sink Monitoring and Accounting (Trial); China Forestry Publishing House: Beijing, China, 2011; ISBN 978-7-5038-6110-6.
- Xie, Z.Q.; Wang, Y.; Tang, Z.Y.; Xu, W.T. Biomass Models of Common Shrubs in China: A Handbook; Science Press: Beijing, China, 2019; ISBN 978-7-03-062123-8. [Google Scholar]
- Zheng, L.; Lu, L.H. Standing crop and nutrient characteristics of forest floor litter in China. J. Northwest For. Univ. 2012, 27, 63–69. [Google Scholar]
- Martin-Vertedor, A.I.; Dodd, I.C. Root-to-shoot signalling when soil moisture is heterogeneous: Increasing the proportion of root biomass in drying soil inhibits leaf growth and increases leaf abscisic acid concentration. Plant Cell Environ. 2011, 34, 1164–1175. [Google Scholar] [CrossRef]
- Imada, S.; Yamanaka, N.; Tamai, S. Water table depth affects Populus alba fine root growth and whole plant biomass. Funct. Ecol. 2008, 22, 1018–1026. [Google Scholar] [CrossRef]
- GB/T 42490-2023; Soil Quality—Determination of Total Content and Isotope Ratio of Organic Carbon and Nitrogen of Soil and Biological Samples—Stable Isotope Ratio Mass Spectrometry. Standards Press of China: Beijing, China, 2023.
- Dong, B.; Liu, M.; Jiang, J.; Shi, C.; Wang, X.; Qiao, Y.; Liu, Z.; Zhao, M.; Si, F. Growth, grain yield, and water use efficiency of rain-fed spring hybrid millet (Setaria italica) in plastic-mulched and unmulched fields. Agric. Water Manag. 2014, 143, 93–101. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Ed.; SSSA: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar] [CrossRef]
- US EPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils; United States Environmental Protection Agency: Washington, DC, USA, 1996. [Google Scholar]
- Peng, S. 1-km Monthly Mean Temperature Dataset for China (1901–2023). National Tibetan Plateau/Third Pole Environment Data Center. 2019. Available online: https://cstr.cn/18406.11.Meteoro.tpdc.270961 (accessed on 1 March 2025).
- Peng, S. 1-km Monthly Precipitation Dataset for China (1901–2023). National Tibetan Plateau/Third Pole Environment Data Center. 2020. Available online: https://data.tpdc.ac.cn/zh-hans/data/faae7605-a0f2-4d18-b28f-5cee413766a2 (accessed on 1 March 2025).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. R Package Version 3.4.4. 2023. Available online: https://ggplot2.tidyverse.org (accessed on 1 September 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 1 September 2024).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Rigatti, S.J. Random forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Pedersen, T.L. Patchwork: The Composer of Plots. R Package Version 1.2.0, 2024. Available online: https://CRAN.R-project.org/package=patchwork (accessed on 1 September 2024).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4, 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 September 2024).
- GB/T 26424-2010; Technical Regulations for Inventory for Forest Management Planning and Design. China Forestry Publishing House: Beijing, China, 2011.
- Mu, Y.X.; Lin, W.Y.; Diao, X.L.; Zhang, Z.; Wang, J.; Lu, Z.J.; Guo, W.C.; Wang, Y.; Hu, C.X.; Zhao, C.Y. Implementation of the visual aesthetic quality of slope forest autumn color change into the configuration of tree species. Sci. Rep. 2022, 12, 1034. [Google Scholar] [CrossRef]
- Li, Y.; Bao, W.; Bongers, F.; Chen, B.; Chen, G.; Guo, K.; Jiang, M.; Lai, J.; Lin, D.; Liu, C.; et al. Drivers of tree carbon storage in subtropical forests. Sci. Total Environ. 2019, 654, 684–693. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, B.; Lin, J. A Basin-Scale Estimation of Carbon Stocks of a Forest Ecosystem Characterized by Spatial Distribution and Contributive Features in the Liuxihe River Basin of Pearl River Delta. Forests 2016, 7, 299. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Qiao, Y.; Yuan, H.; Xu, W.; Xia, X. Effects of Microtopography on Neighborhood Diversity and Competition in Subtropical Forests. Plants 2025, 14, 870. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Wu, J.; Shah, J.A.; Wang, X.; Lyu, Y.; Guo, Z.; Sun, H. Tree diversity drives understory carbon storage rather than overstory carbon storage across forest types. J. For. Res. 2024, 35, 125. [Google Scholar] [CrossRef]
- Conforti, M.; Lucà, F.; Scarciglia, F.; Matteucci, G.; Buttafuoco, G. Soil carbon stock in relation to soil properties and landscape position in a forest ecosystem of southern Italy (Calabria region). Catena 2016, 144, 23–33. [Google Scholar] [CrossRef]
- Borah, P. Role of Soil Properties on Ecosystem Carbon Flux in a Semi-Evergreen Forest of North East India. Ph.D. Thesis, Tezpur University, Tezpur, India, 2023. [Google Scholar]
- Bird, S.B.; Herrick, J.E.; Wander, M.M.; Wright, S.F. Spatial heterogeneity of aggregate stability and soil carbon in semi-arid rangeland. Environ. Pollut. 2002, 116, 445–455. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Wei, L.; Gosselin, F.; Rao, X.; Lin, Y.; Wang, J.; Jian, S.; Ren, H. Overstory and niche attributes drive understory biomass production in three types of subtropical plantations. For. Ecol. Manag. 2021, 482, 118894. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration, 4th ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Peh, K.S.H.; Lewis, S.L.; Lloyd, J. Mechanisms of monodominance in diverse tropical tree-dominated systems. J. Ecol. 2011, 99, 891–898. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Malhi, S. Effects of fertilization and other agronomic measures on nutritional quality of crops. J. Sci. Food Agric. 2008, 88, 7–23. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Hemingway, J.D.; Rothman, D.H.; Grant, K.E.; Rosengard, S.Z.; Eglinton, T.I.; Derry, L.A.; Galy, V.V. Mineral protection regulates long-term global preservation of natural organic carbon. Nature 2019, 570, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Steinweg, J.M.; Dukes, J.S.; Paul, E.A.; Wallenstein, M.D. Microbial responses to multi-factor climate change: Effects on soil enzymes. Front. Microbiol. 2013, 4, 146. [Google Scholar] [CrossRef]
- Gomez, E.J.; Delgado, J.A.; Gonzalez, J.M. Environmental factors affect the response of microbial extracellular enzyme activity in soils when determined as a function of water availability and temperature. Ecol. Evol. 2020, 10, 10105–10115. [Google Scholar] [CrossRef] [PubMed]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.B.; Nunan, N. Dynamic interactions at the mineral-organic matter interface. Nat. Rev. Earth Environ. 2021, 2, 402–416. [Google Scholar] [CrossRef]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.M.; Jandt, U. Global trait-environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef]
- Blouin, M.; Dubs, F.; Ponge, J.F. Harnessing trait-environment interactions to predict ecosystem functions. Front. Ecol. Environ. 2025, 23, e2826. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, X.; Hao, D.; Bond-Lamberty, B.; Daigneault, A.; Patel, P.L.; Chen, M. Role of forest biomass change in shaping future land use and land cover change. ESS Open Arch. 2024. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, D.; Dong, W.; Cai, W.; Yuan, W. Accurate representation of leaf longevity is important for simulating ecosystem carbon cycle. Basic Appl. Ecol. 2016, 17, 396–407. [Google Scholar] [CrossRef]
- Baum, C.; Fienemann, M.; Glatzel, S.; Gleixner, G. Overstory-specific effects of litter fall on the microbial carbon turnover in a mature deciduous forest. For. Ecol. Manag. 2009, 258, 109–114. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, Y.J.; Chun, J.H.; Kim, A.R.; Lee, C.B. Ecosystem services and multifunctionality are co-regulated by biotic and abiotic factors along with forest types in a temperate forest of South Korea. Glob. Ecol. Conserv. 2025, 61, e03683. [Google Scholar] [CrossRef]
- Mikutta, R.; Turner, S.; Schippers, A.; Gentsch, N.; Meyer-Stüve, S.; Condron, L.M.; Peltzer, D.A.; Richardson, S.J.; Eger, A.; Hempel, G.; et al. Microbial and abiotic controls on mineral-associated organic matter in soil profiles along an ecosystem gradient. Sci. Rep. 2019, 9, 10294. [Google Scholar] [CrossRef]
- Sokol, N.W.; Whalen, E.D.; Jilling, A.; Kallenbach, C.; Pett-Ridge, J.; Georgiou, K. Global distribution, formation and fate of mineral-associated soil organic matter under a changing climate: A trait-based perspective. Funct. Ecol. 2022, 36, 1411–1429. [Google Scholar] [CrossRef]
- Bramble, D.S.E.; Ulrich, S.; Schöning, I.; Mikutta, R.; Brandt, L.; Poll, C.; Schrumpf, M. Formation of mineral-associated organic matter in temperate soils is primarily controlled by mineral type and modified by land use and management intensity. Glob. Change Biol. 2024, 30, e17024. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.C.; Zou, C.; Zhao, Y.; Wei, P.; Liu, Y.Y.; Zhu, G.Y. Effects of iron/aluminum mineral phases on soil organic carbon storage in different clay soils of subtropical acidic forests. Catena 2025, 252, 108853. [Google Scholar] [CrossRef]
- Laurans, M.; Hérault, B.; Vieilledent, G.; Vincent, G. Vertical stratification reduces competition for light in dense tropical forests. For. Ecol. Manag. 2014, 329, 79–88. [Google Scholar] [CrossRef]
- Deng, J.; Fang, S.; Fang, X.; Jin, Y.; Kuuid, Y.; Lin, F.; Liu, C. Forest understory vegetation study: Current status and future trends. For. Res. 2023, 3, 6. [Google Scholar] [CrossRef]
- Daba, M.H.; Dejene, S.W. The role of biodiversity and ecosystem services in carbon sequestration and its implication for climate change mitigation. Environ. Sci. Nat. Resour. 2018, 11, 555810. [Google Scholar] [CrossRef]
- Bertini, S.C.B.; Azevedo, L.C.B. Soil microbe contributions in the regulation of the global carbon cycle. In Microbiome Under Changing Climate; Woodhead Publishing: Sawston, UK, 2022; pp. 69–84. [Google Scholar] [CrossRef]
- Szanser, M.; Ilieva-Makulec, K.; Kajak, A.; Górska, E.; Kusińska, A.; Kisiel, M.; Wojewoda, D. Impact of litter species diversity on decomposition processes and communities of soil organisms. Soil Biol. Biochem. 2011, 43, 9–19. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Liu, A.; Lei, F.; Liu, R.; Li, S. Intraspecific transitioning of ecological strategies in Pinus massoniana trees across restoration stages. Ecol. Evol. 2024, 14, e11305. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.W.; Long, J.N. Age-related decline in forest growth: An emergent property. For. Ecol. Manag. 2001, 144, 175–181. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Burgess-Conforti, J.R.; Moore, P.A.; Owens, P.R.; Miller, D.M.; Ashworth, A.J.; Hays, P.D.; Anderson, K.R. Are soils beneath coniferous tree stands more acidic than soils beneath deciduous tree stands? Environ. Sci. Pollut. Res. 2019, 26, 14920–14929. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, W.; Sun, Y.; Wang, F.; Miu, Y. Simulation of Spatial and Temporal Distribution of Forest Carbon Stocks in Long Time Series-Based on Remote Sensing and Deep Learning. Forests 2023, 14, 483. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, J.; Gao, J.; He, X.; Liu, X.; Chen, Y.; Li, C. Functional diversity explains ecosystem carbon storage in subtropical forests. Glob. Change Biol. 2025, 31, e70120. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xiang, W.H.; Liu, C. Storage and Distribution of Soil Organic Carbon and Nitrogen in Four Subtropical Forests in Central Southern China. J. Soil Water Conserv. 2012, 26, 169–173. [Google Scholar]
- Chen, X.; Reich, P.B.; Taylor, A.R.; An, Z.; Chang, S.X. Resource availability enhances positive tree functional diversity effects on carbon and nitrogen accrual in natural forests. Nat. Commun. 2024, 15, 8615. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.M.; Rashid, I.; Waheed, M.; Khuroo, A.A. From forest floor to tree top: Partitioning of biomass and carbon stock in multiple strata of forest vegetation in Western Himalaya. Environ. Monit. Assess. 2023, 195, 812. [Google Scholar] [CrossRef]
- Mora, F.; Jaramillo, V.J.; Bhaskar, R.; Gavito, M.; Siddique, I.; Byrnes, J.E.K.; Balvanera, P. Carbon accumulation in neotropical dry secondary forests: The roles of forest age and tree dominance and diversity. Ecosystems 2018, 21, 536–550. [Google Scholar] [CrossRef]
- Luo, Z.; Viscarra-Rossel, R.A.; Qian, T. Similar importance of edaphic and climatic factors for controlling soil organic carbon stocks of the world. Biogeosciences 2021, 18, 2063–2073. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Liao, J.; Liu, Y.; Huang, Y.; Li, Q.; Yi, X.; Wang, L.; Wu, L.; Shi, Z. Predicting Forest Carbon Sequestration of Ecological Buffer Zone in Urban Agglomeration: Integrating Vertical Heterogeneity and Age Class Dynamics to Unveil Future Trajectories. Forests 2025, 16, 1648. https://doi.org/10.3390/f16111648
Chen C, Liao J, Liu Y, Huang Y, Li Q, Yi X, Wang L, Wu L, Shi Z. Predicting Forest Carbon Sequestration of Ecological Buffer Zone in Urban Agglomeration: Integrating Vertical Heterogeneity and Age Class Dynamics to Unveil Future Trajectories. Forests. 2025; 16(11):1648. https://doi.org/10.3390/f16111648
Chicago/Turabian StyleChen, Chan, Juyang Liao, Yan Liu, Yaqi Huang, Qiaoyun Li, Xinyu Yi, Ling Wang, Linshi Wu, and Zhao Shi. 2025. "Predicting Forest Carbon Sequestration of Ecological Buffer Zone in Urban Agglomeration: Integrating Vertical Heterogeneity and Age Class Dynamics to Unveil Future Trajectories" Forests 16, no. 11: 1648. https://doi.org/10.3390/f16111648
APA StyleChen, C., Liao, J., Liu, Y., Huang, Y., Li, Q., Yi, X., Wang, L., Wu, L., & Shi, Z. (2025). Predicting Forest Carbon Sequestration of Ecological Buffer Zone in Urban Agglomeration: Integrating Vertical Heterogeneity and Age Class Dynamics to Unveil Future Trajectories. Forests, 16(11), 1648. https://doi.org/10.3390/f16111648






