Forecasting the Impact of Climate Change on Tetraclinis articulata Distribution in the Mediterranean Using MaxEnt and GIS-Based Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Species Occurrence Data
2.3. Climatic Data
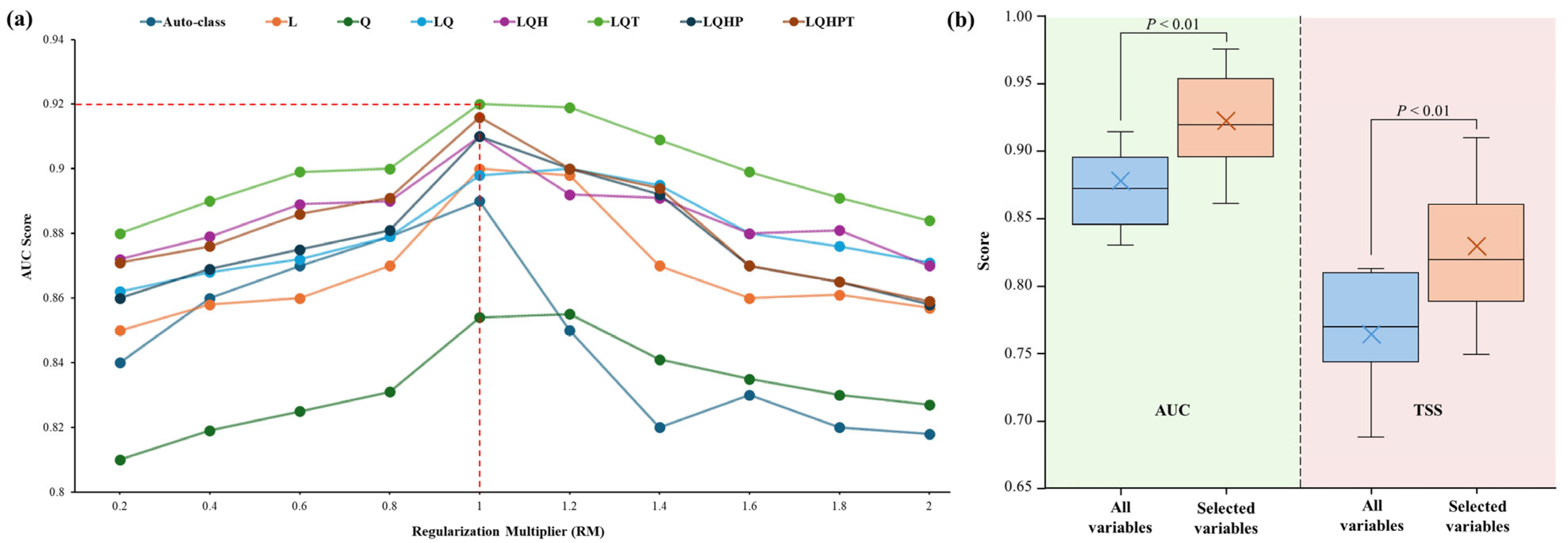
2.4. Species Distribution Model (SDM) and Variable Selection
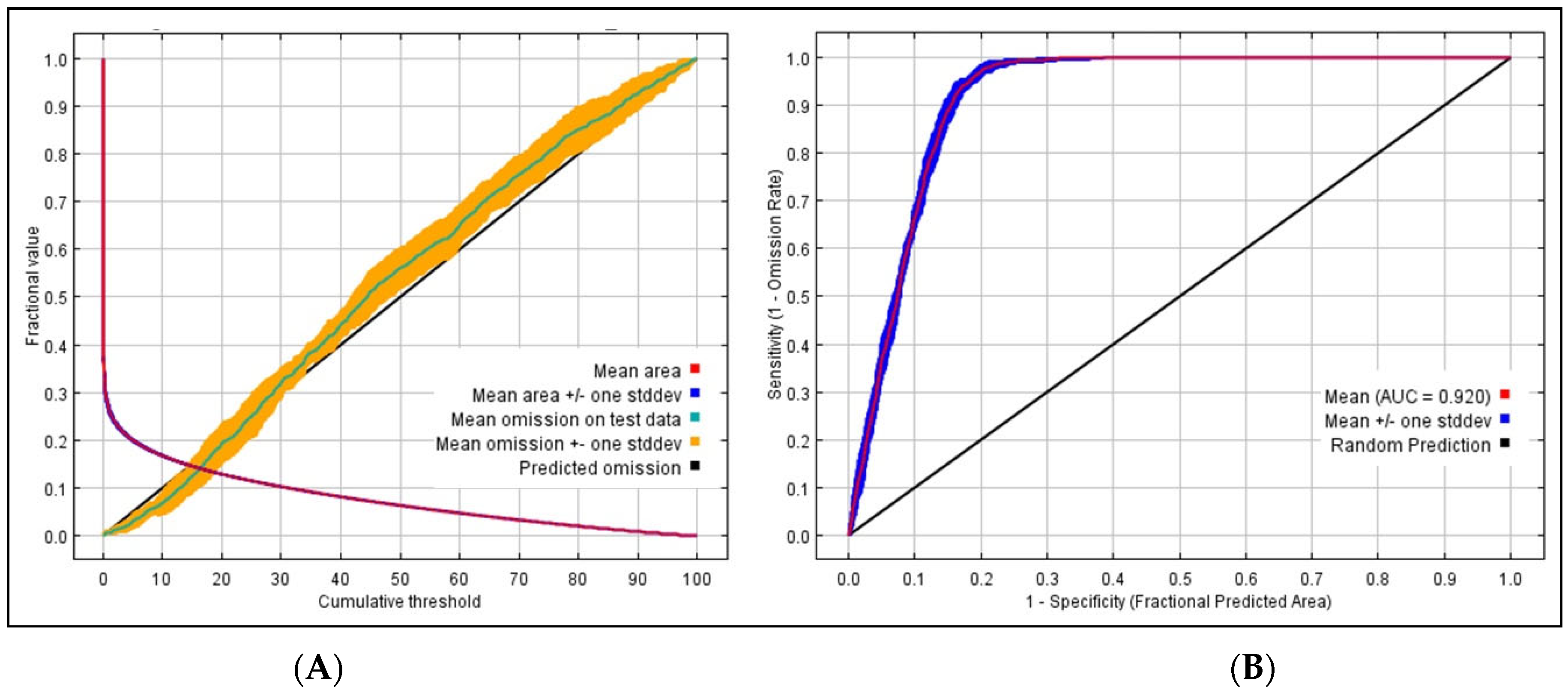
2.5. Model Accuracy Assessment and Map Generation
3. Results
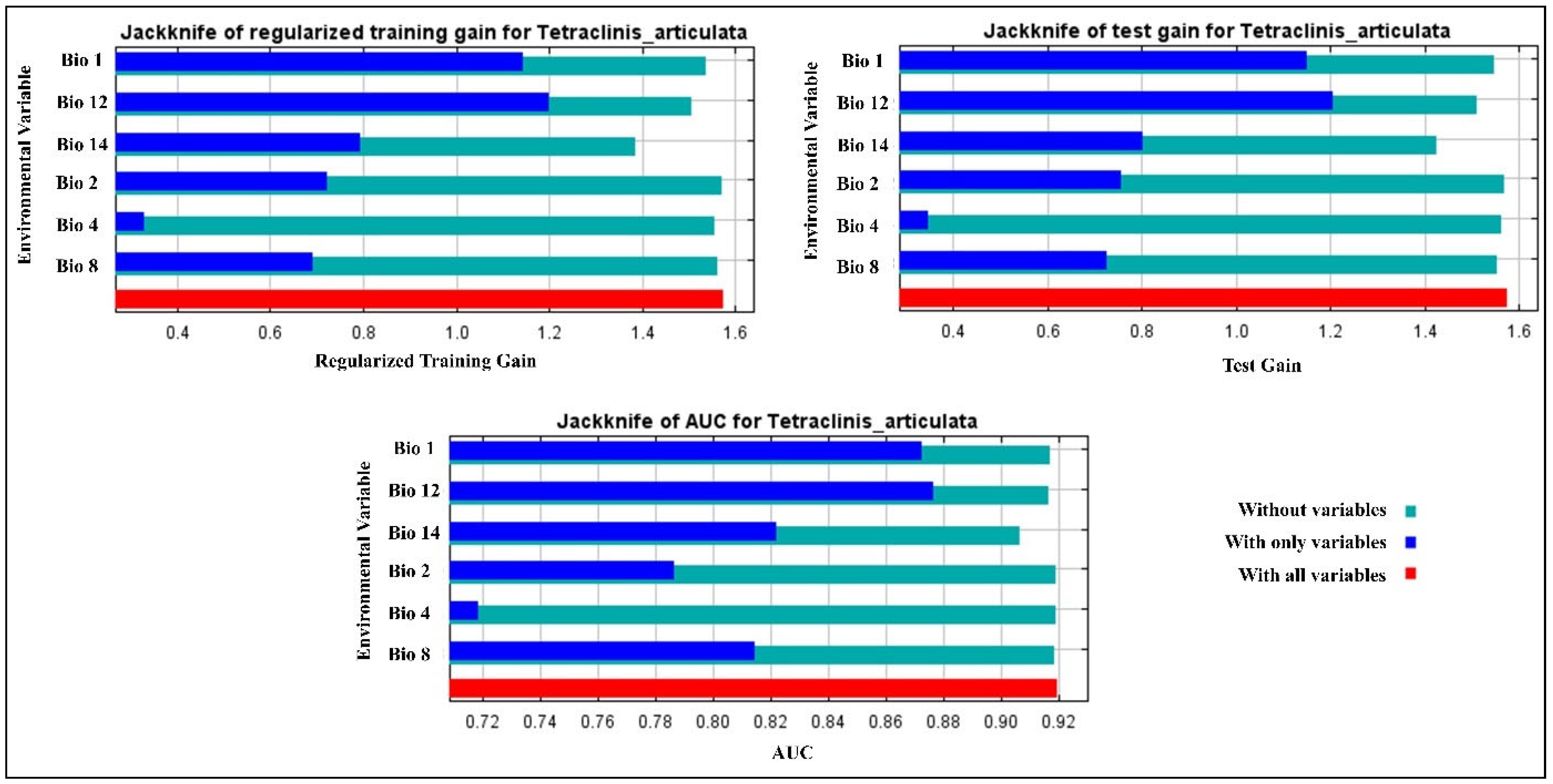
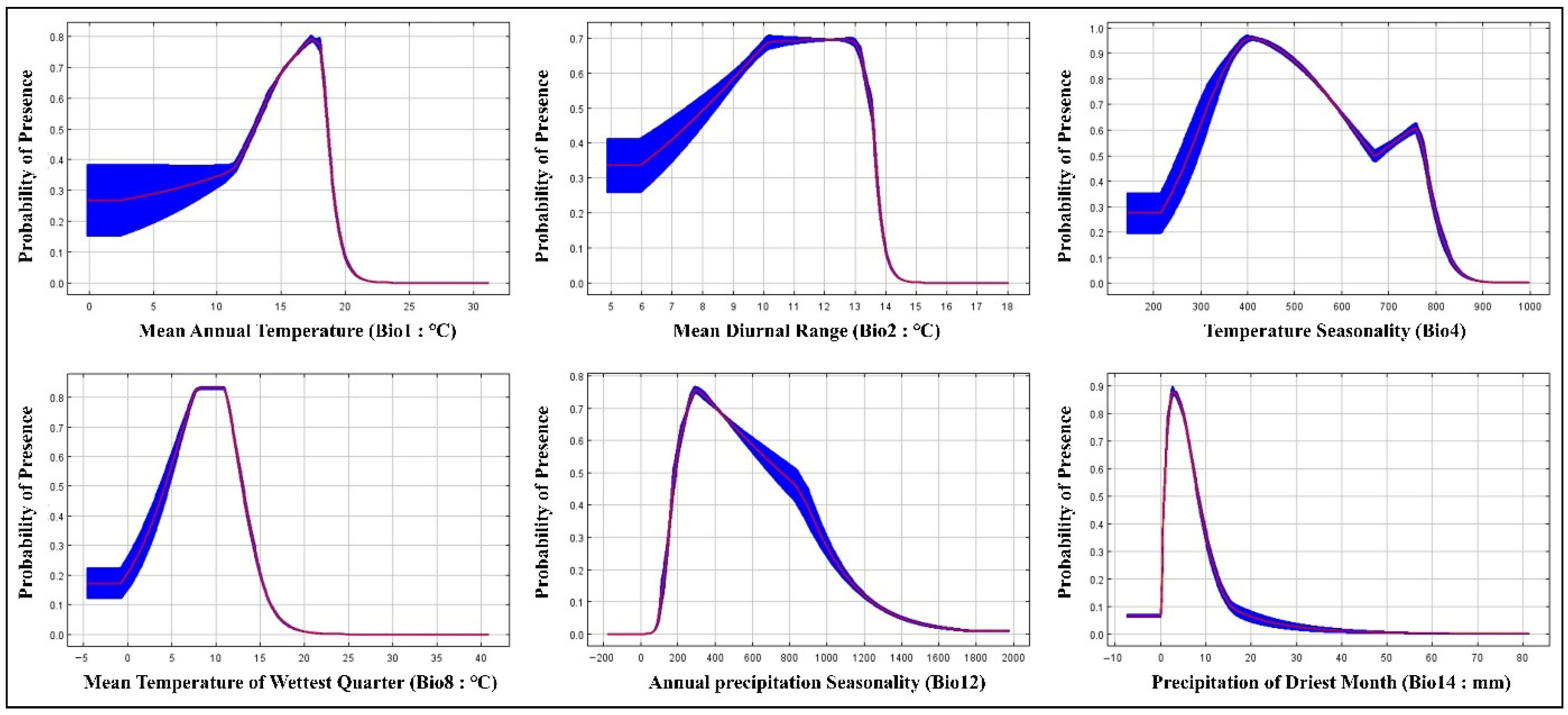
3.1. Model Accuracy and Contributions of Predictor Variable
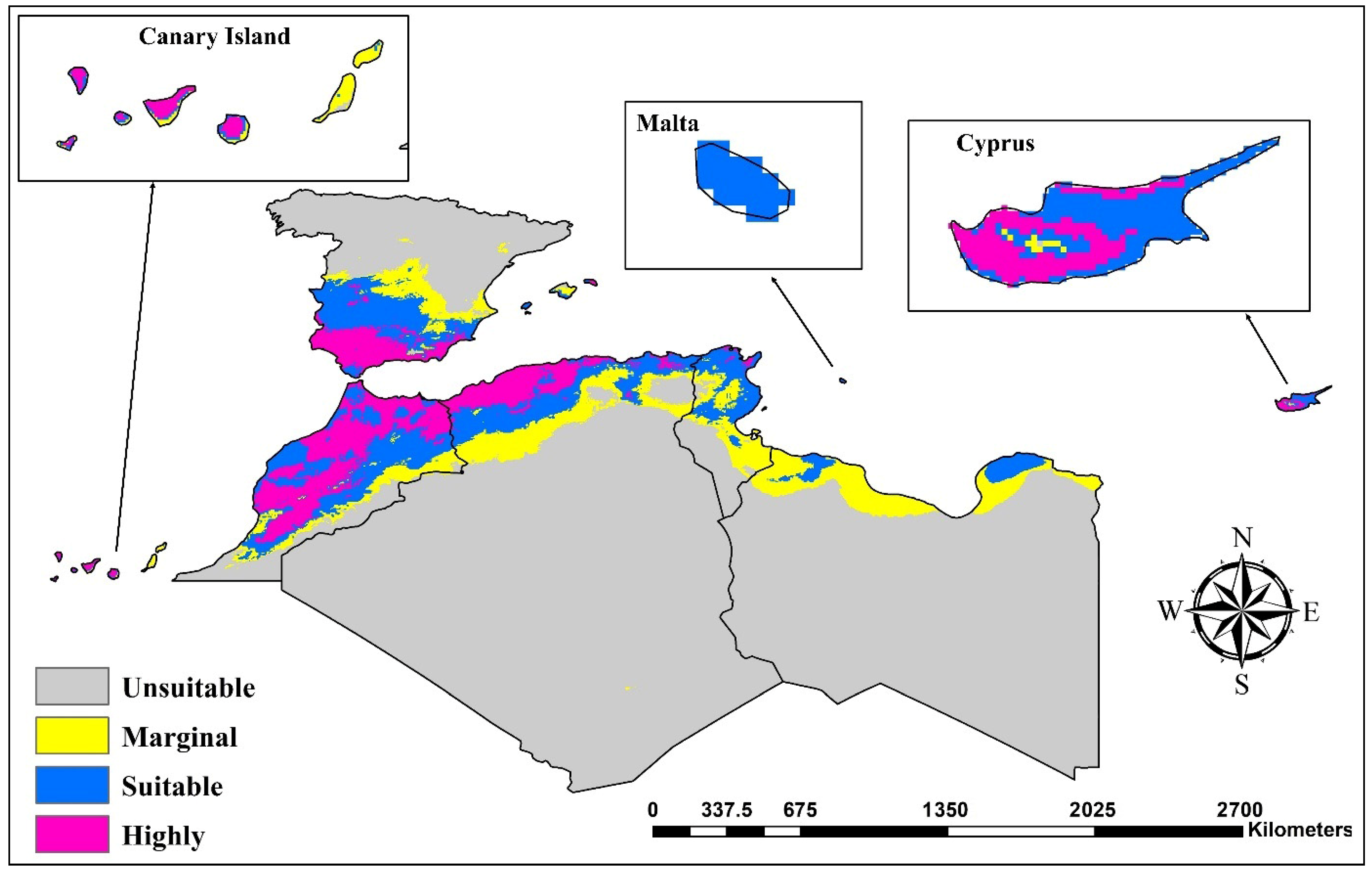
3.2. Current Suitable Habitats
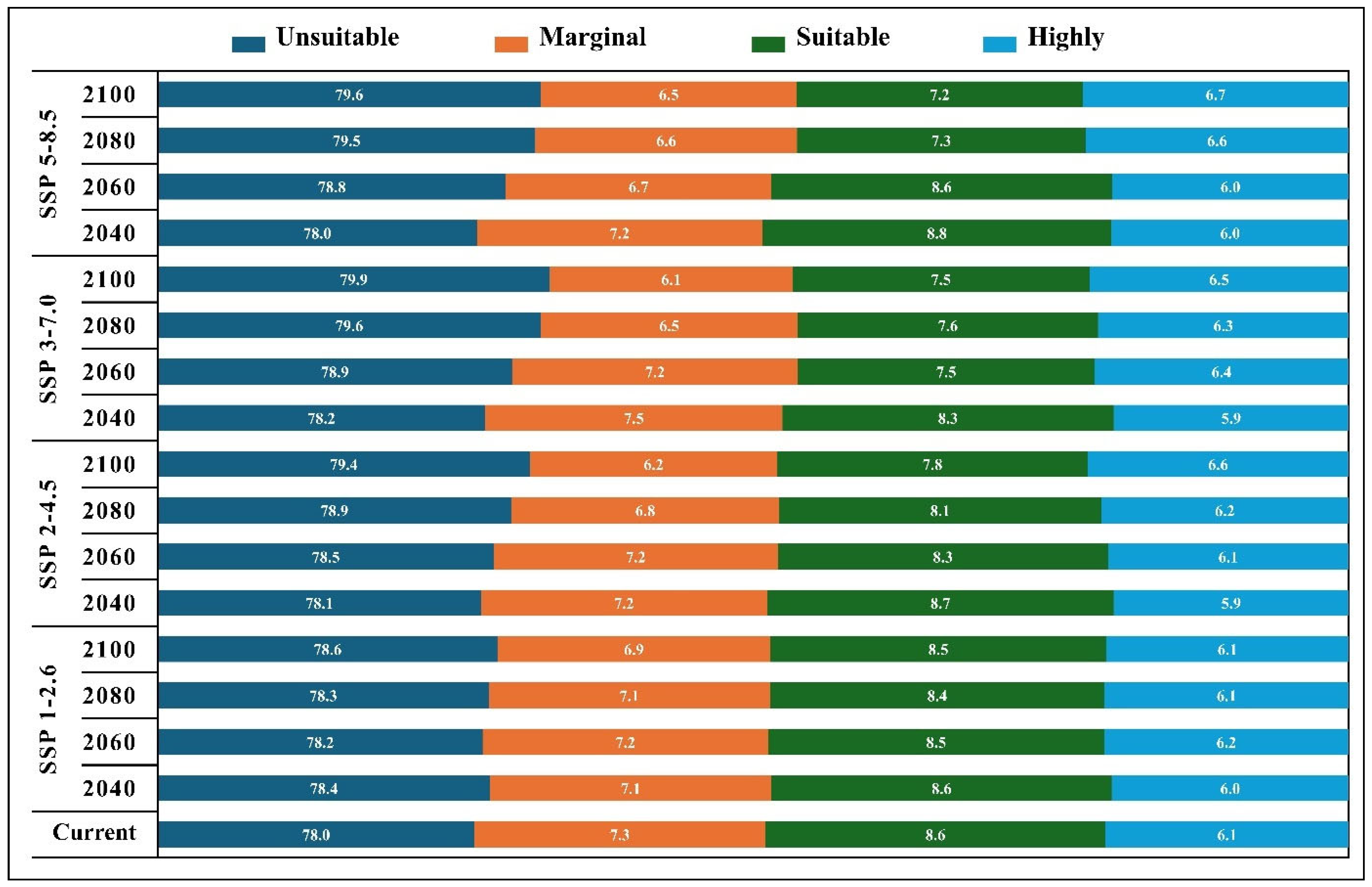
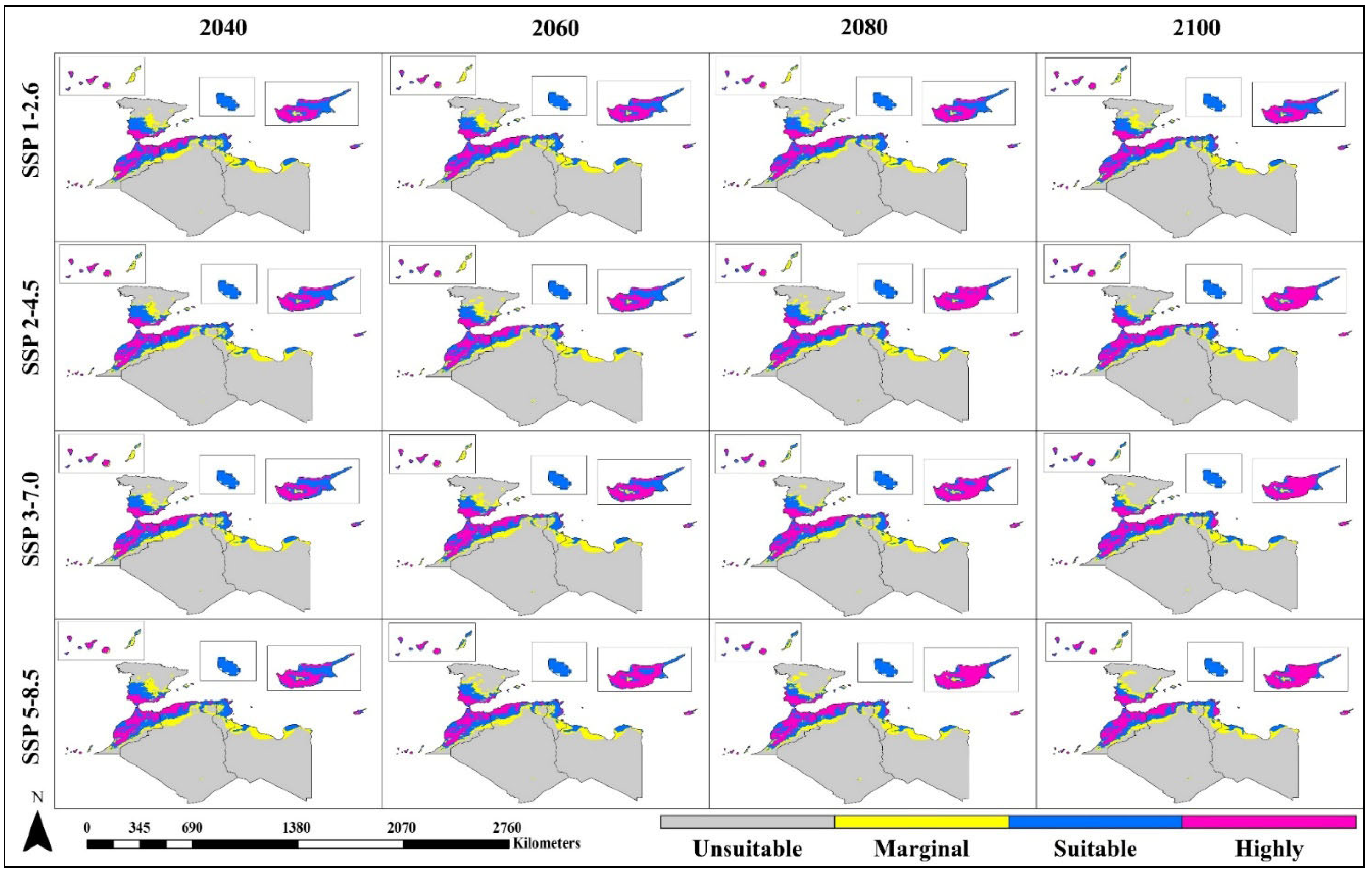
3.3. Future Suitable Habitat Under Climate Change Scenarios
3.4. Area-Wise Comparison Between Current and Future Projections
3.4.1. Algeria
3.4.2. Tunisia
3.4.3. Morocco
3.4.4. Libya
3.4.5. Cyprus
3.4.6. Malta
3.4.7. Spain and Canary Islands
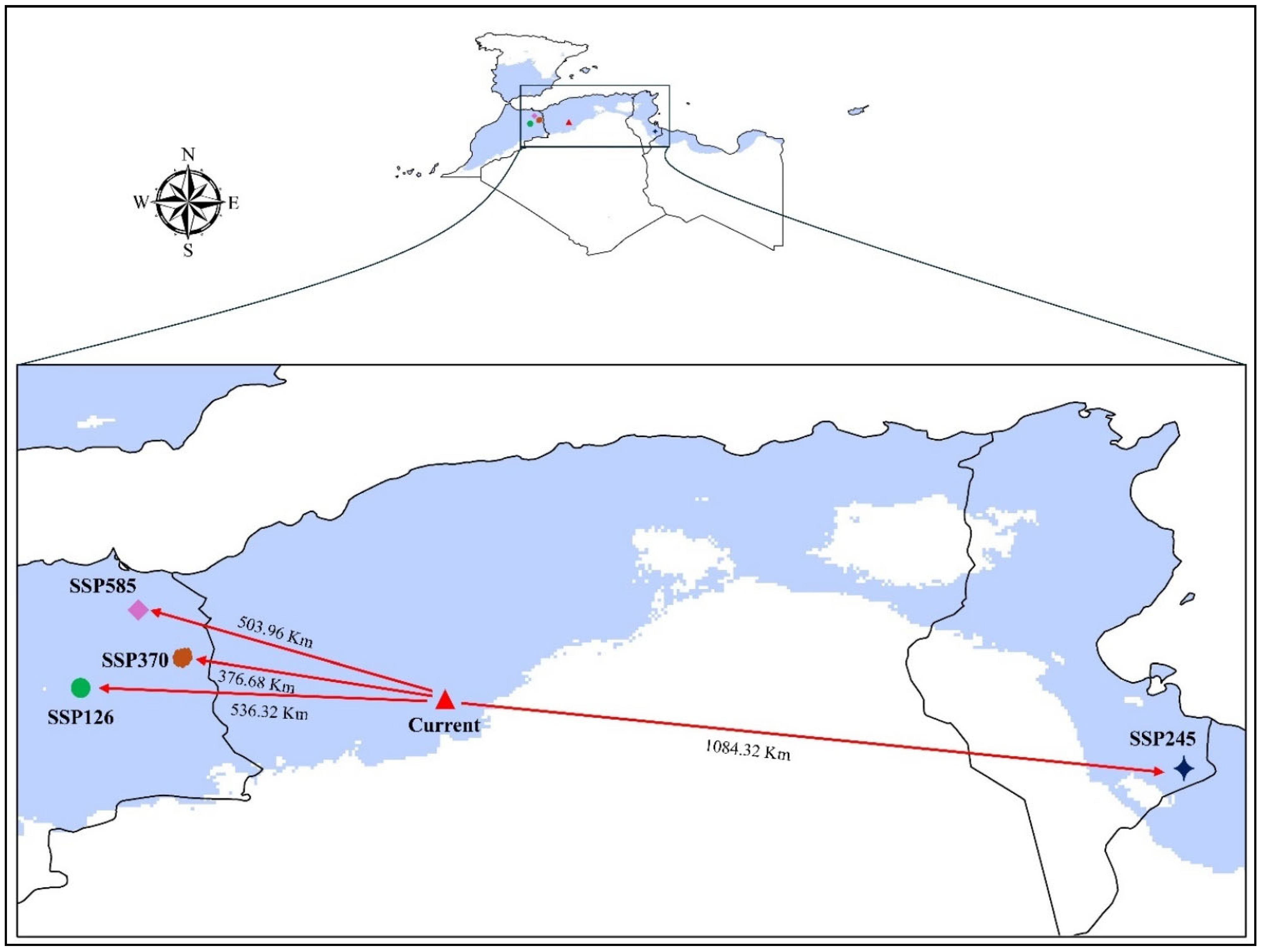
3.5. Tetraclinis Articulata Centroid Shift
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayat, U.; Akram, M.; Kour, S.; Arif, T.; Shi, J. Pest Risk Assessment of Aeolesthes sarta (Coleoptera: Cerambycidae) in Pakistan under Climate Change Scenario. Forests 2023, 14, 253. [Google Scholar] [CrossRef]
- Hayat, U.; Kour, S.; Akram, M.; Shi, J.; Wiarta, R. Assessing the Global Pest Risk of Aeolesthes sarta with Regards to the Host Specie Populus alba under Climate Change Scenarios. Forests 2023, 14, 1260. [Google Scholar] [CrossRef]
- Hayat, U. City longhorn beetle (Aeolesthes sarta): A review of the species, its distribution, ecology, damage, prevention and control. J. For. Sci. 2022, 68, 199–212. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Skov, F. Potential impact of climate change on the northern nemoral forest herb flora of Europe. Biodivers. Conserv. 2006, 15, 3341–3356. [Google Scholar] [CrossRef]
- Lenihan, J.M.; Bachelet, D.; Neilson, R.P.; Drapek, R. Response of vegetation distribution, ecosystem productivity, and fire to climate change scenarios for California. Clim. Chang. 2008, 87, 215–230. [Google Scholar] [CrossRef]
- López de Heredia, U.; Carrión, J.; Jiménez, P.; Collada, C.; Gil, L. Molecular and palaeoecological evidence for multiple glacial refugia for evergreen oaks on the Iberian Peninsula. J. Biogeogr. 2007, 34, 1505–1517. [Google Scholar] [CrossRef]
- García-Castaño, J.L.; Balao, F.; Lorenzo, M.T.; Véla, E.; Hadjadj-Aoul, S.; Mifsud, S.; Terrab, A. A complex genetic structure of Tetraclinis articulata (Cupressaceae) in the western Mediterranean. Bot. J. Linn. Soc. 2021, 197, 420–438. [Google Scholar] [CrossRef]
- Abdelwahed, F.; Mohamed, R.; Bousselham, K.; Mohsine, Z.; Fatima, E.B.-C.; De Marsan, M.; Abdelwahed, F.F.; Abderrahim, F. Natural durability of Tetraclinis articulata (Vahl) Masters woods against wood decay fungi: Laboratory test. Wood Res. 2015, 60, 865–872. [Google Scholar]
- Bourkhiss, M.; Hnach, M.; Bourkhiss, B.; Ouhssine, M.; Chaouch, A. Composition chimique et propriétés antimicrobiennes de l’huile essentielle extraite des feuilles de Tetraclinis articulata (Vahl) du Maroc. Afr. Sci. Rev. Int. Sci. Technol. 2007, 3, 61267. [Google Scholar] [CrossRef]
- Esteve-Selma, M. Bosques de Tetraclinis articulata. In Manual de Directrices Ecológicas Para la Conservación de Los Tipos de Habitat de Interés Comunitario en España; Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente y Medio Rural y: Madrid, Spain, 2009. [Google Scholar]
- Sghaier, T.; Cañellas, I.; Calama, R.; Sánchez-González, M. Modelling diameter distribution of Tetraclinis articulata in Tunisia using normal and Weibull distributions with parameters depending on stand variables. iforest-Biogeosci. For. 2016, 9, 702. [Google Scholar] [CrossRef]
- Kouider, H.; Assia, L. Synthèse bibliographique sur le thuya de berbérie [Tetraclinis articulata (Vahl) Mast.]. Geo-Eco-Trop 2017, 41, 13–27. [Google Scholar]
- Ayache, F. Les Résineux Dans la Région de Tlemcen: Aspect Écologique et Cartographie (Thesis). Magister en Ecologie et Environnement. Master’s Thesis, Université abou Bekr Belkaid Tlemcen, Tlemcen, Algérie, 2014. Available online: http://dspace.univ-tlemcen.dz/handle/112/6642 (accessed on 25 May 2025).
- Esteve-Selma, M.; Martínez-Fernández, J.; Hernández, I.; Montávez, J.; Lopez, J.; Calvo, J.; Robledano, F. Effects of climatic change on the distribution and conservation of Mediterranean forests: The case of Tetraclinis articulata in the Iberian Peninsula. Biodivers. Conserv. 2010, 19, 3809–3825. [Google Scholar] [CrossRef]
- Ghallab, A.; Boubekraoui, H.; Laaribya, S.; Ben-Said, M. Potential natural vegetation pattern based on major tree distribution modeling in the western Rif of Morocco. iforest-Biogeosci. For. 2024, 17, 405. [Google Scholar] [CrossRef]
- Khatib, S.; Sobeh, M.; Bouissane, L. Tetraclinis articulata (vahl) masters: An insight into its ethnobotany, phytochemistry, toxicity, biocide and therapeutic merits. Front. Pharmacol. 2022, 13, 977726. [Google Scholar] [CrossRef] [PubMed]
- Marini, K. Climate and environmental change in the Mediterranean–main facts. MedEC Erişim 2018, 1, 2019. [Google Scholar]
- Esteve-Selma, M.; Martínez-Fernández, J.; Hernández-García, I.; Montávez, J.; López-Hernández, J.; Calvo, J. Potential effects of climatic change on the distribution of Tetraclinis articulata, an endemic tree from arid Mediterranean ecosystems. Clim. Chang. 2012, 113, 663–678. [Google Scholar] [CrossRef]
- Kharouba, H.M.; Nadeau, J.L.; Young, E.; Kerr, J.T. Using species distribution models to effectively conserve biodiversity into the future. Biodiversity 2008, 9, 39–46. [Google Scholar] [CrossRef]
- Hayat, U.; Shi, J.; Wu, Z.; Rizwan, M.; Haider, M.S. Which SDM Model, CLIMEX vs. MaxEnt, Best Forecasts Aeolesthes sarta Distribution at a Global Scale under Climate Change Scenarios? Insects 2024, 15, 324. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4 July 2004; p. 83. [Google Scholar]
- Zhang, X.; Zhao, J.; Wang, M.; Li, Z.; Lin, S.; Chen, H. Potential distribution prediction of Amaranthus palmeri S. Watson in China under current and future climate scenarios. Ecol. Evol. 2022, 12, e9505. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Sánchez-Gómez, P.; Jiménez, J.F.; Vera, J.B.; Sánchez-Saorín, F.J.; Martínez, J.F.; Buhagiar, J. Genetic structure of Tetraclinis articulata, an endangered conifer of the western Mediterranean basin. Silva Fenn. 2013, 47, 1073. [Google Scholar] [CrossRef]
- Makkaoui, M.; Abbas, Y.; EL ANTRY-TAZY, S.; Medraoui, L.; Alami, M.; Rabani, S.; Filali-Maltouf, A. Genetic diversity of ten Moroccan populations of Tetraclinis articulata as revealed by inter simple sequence repeat (ISSR) markers. BOIS For. DES Trop. 2020, 345, 15–25. [Google Scholar] [CrossRef]
- Rozas, V.; Garcia-Cervigon, A.I.; Garcia-Hidalgo, M.; Rodriguez-Garcia, E.; Olano, J.M. Living on the edge: Legacy of water availability on Tetraclinis articulata secondary growth under semiarid conditions in Morocco. Dendrochronologia 2021, 68, 125853. [Google Scholar] [CrossRef]
- Charco, J. Guía de los Árboles y Arbustos del Norte de África; Agencia Española de Cooperación Internacional: Madrid, Spain, 2001. [Google Scholar]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]
- Meryem, M.; Younes, A.; Leila, M.; Mohammed, A.; Salwa, E.A.-T.; Abdelkarim, F.-M. Development of a Core Collection for Tetraclinis articulata Using ISSR Markers and Maximization Strategy. Plant Mol. Biol. Report. 2023, 41, 427–439. [Google Scholar] [CrossRef]
- El Mechri, O.; Belgherbi, B.; Benaradj, A.; Berkane, I. Spatial distribution of Tetraclinis articulata (Vahl) Mast. formations in north-western Algeria. Biodivers. Res. Conserv. 2024, 74, 43–52. [Google Scholar] [CrossRef]
- Asbabou, A.; Hanane, T.; Gourich, A.A.; Siddique, F.; Drioiche, A.; Remok, F.; Saidi, S.; Adadi, I.; Khamar, H.; Almaary, K.S. Phytochemical profile, physicochemical, antioxidant and antimicrobial properties of Juniperus phoenicea and Tetraclinis articulate: In vitro and in silico approaches. Front. Chem. 2024, 12, 1397961. [Google Scholar] [CrossRef]
- Khatib, S.; Mahdi, I.; Drissi, B.; Fahsi, N.; Bouissane, L.; Sobeh, M. Tetraclinis articulata (Vahl) Mast.: Volatile constituents, antioxidant, antidiabetic and wound healing activities of its essential oil. Heliyon 2024, 10, e24563. [Google Scholar] [CrossRef]
- Tatebe, H.; Ogura, T.; Nitta, T.; Komuro, Y.; Ogochi, K.; Takemura, T.; Sudo, K.; Sekiguchi, M.; Abe, M.; Saito, F. Description and basic evaluation of simulated mean state, internal variability, and climate sensitivity in MIROC6. Geosci. Model Dev. 2019, 12, 2727–2765. [Google Scholar] [CrossRef]
- Babaousmail, H.; Ayugi, B.; Rajasekar, A.; Zhu, H.; Oduro, C.; Mumo, R.; Ongoma, V. Projection of extreme temperature events over the Mediterranean and Sahara using bias-corrected CMIP6 models. Atmosphere 2022, 13, 741. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Souza, P.G.C.; da Silva, R.S.; Santana, P.A., Jr.; Picanço, M.C.; Kyerematen, R.; Sètamou, M.; Ekesi, S.; Borgemeister, C. Climate-induced range shifts of invasive species (Diaphorina citri Kuwayama). Pest Manag. Sci. 2022, 78, 2534–2549. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Q.; Cheng, L.; Zhao, L.; Zhang, H.; Wei, J. The potential global distribution of the white peach scale Pseudaulacaspis pentagona (Targioni tozzetti) under climate change. Forests 2020, 11, 192. [Google Scholar] [CrossRef]
- Hayat, U.; Ahmad, M.H.; Sattar, W.; Zhu, Z. A host availability-driven model for predicting the distribution of Red Palm Weevil in China. Crop Prot. 2025, 192, 107176. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Zare, M.; Moameri, M.; Ghorbani, A.; Sahragard, H.P.; Mostafazadeh, R.; Dadjou, F.; Biswas, A. Modeling habitat suitability of Dorema ammoniacum D Don. in the rangelands of central Iran. Sci. Rep. 2024, 14, 16185. [Google Scholar] [CrossRef] [PubMed]
- Monzón, J.; Moyer-Horner, L.; Palamar, M.B. Climate change and species range dynamics in protected areas. Bioscience 2011, 61, 752–761. [Google Scholar] [CrossRef]
- Hacket-Pain, A.J.; Cavin, L.; Friend, A.D.; Jump, A. Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Eur. J. For. Res. 2016, 135, 897–909. [Google Scholar] [CrossRef]
- Castaño-Santamaría, J.; López-Sánchez, C.A.; Obeso, J.R.; Barrio-Anta, M. Modelling and mapping beech forest distribution and site productivity under different climate change scenarios in the Cantabrian Range (North-Western Spain). For. Ecol. Manag. 2019, 450, 117488. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Matías, L.; Jump, A.S. Interactions between growth, demography and biotic interactions in determining species range limits in a warming world: The case of Pinus sylvestris. For. Ecol. Manag. 2012, 282, 10–22. [Google Scholar] [CrossRef]
- Salvà-Catarineu, M.; Romo, A.; Mazur, M.; Zielińska, M.; Minissale, P.; Dönmez, A.A.; Boratyńska, K.; Boratyński, A. Past, present, and future geographic range of the relict Mediterranean and Macaronesian Juniperus phoenicea complex. Ecol. Evol. 2021, 11, 5075–5095. [Google Scholar] [CrossRef]
- Elmahdy, S.I.; Mohamed, M.M. Factors controlling the changes and spatial variability of Junipers phoenicea in Jabal Al Akhdar, Libya, using remote sensing and GIS. Arab. J. Geosci. 2016, 9, 478. [Google Scholar] [CrossRef]
- Paparrizos, S.; Maris, F.; Matzarakis, A. Integrated analysis and mapping of aridity over Greek areas with different climate conditions. Glob. NEST J. 2016, 18, 131–145. [Google Scholar]
- Walas, Ł.; Sobierajska, K.; Ok, T.; Dönmez, A.A.; Kanoğlu, S.S.; Dagher-Kharrat, M.B.; Douaihy, B.; Romo, A.; Stephan, J.; Jasińska, A.K. Past, present, and future geographic range of an oro-Mediterranean Tertiary relict: The Juniperus drupacea case study. Reg. Environ. Chang. 2019, 19, 1507–1520. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Long-distance plant dispersal and habitat fragmentation: Identifying conservation targets for spatial landscape planning under climate change. Biol. Conserv. 2005, 123, 389–401. [Google Scholar] [CrossRef]
- Heller, N.E.; Zavaleta, E.S. Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biol. Conserv. 2009, 142, 14–32. [Google Scholar] [CrossRef]
- Gómez, P.S.; Stevens, D.; Fennane, M.; Tahar, S.; Gardner, M.F.; Thomas, P.I. Tetraclinis Articulata. Available online: https://threatenedconifers.rbge.org.uk/conifers/tetraclinis-articulata (accessed on 18 May 2025).
- Grimshaw, J.; Bayton, R. Tetraclinis articulata. Available online: https://www.treesandshrubsonline.org/articles/tetraclinis/tetraclinis-articulata/ (accessed on 18 May 2025).
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]


| Variable | Variable Description | Variable Retained | p-Value |
|---|---|---|---|
| Bio1 | Annual mean temperature | ✔ | <0.8 |
| Bio2 | Mean diurnal range | ✔ | <0.8 |
| Bio3 | Isothermality (Bio2/Bio7) (×100) | >0.8 | |
| Bio4 | Temperature seasonality | ✔ | <0.8 |
| Bio5 | Max temperature of the warmest month | >0.8 | |
| Bio6 | Min temperature of coldest month | >0.8 | |
| Bio7 | Temperature Annual Range (Bio5–Bio6) | >0.8 | |
| Bio8 | Mean temperature of the wettest quarter | ✔ | <0.8 |
| Bio9 | Mean temperature of the driest quarter | >0.8 | |
| Bio10 | Mean temperature of warmest quarter | >0.8 | |
| Bio11 | Mean temperature of the coldest quarter | >0.8 | |
| Bio12 | Annual precipitation | ✔ | <0.8 |
| Bio13 | Precipitation of Wettest Month | >0.8 | |
| Bio14 | Precipitation of driest month | ✔ | <0.8 |
| Bio15 | Precipitation Seasonality (Coefficient of Variation) | >0.8 | |
| Bio16 | Precipitation of Wettest Quarter | >0.8 | |
| Bio17 | Precipitation of Driest Quarter | >0.8 | |
| Bio18 | Precipitation of Warmest Quarter | >0.8 | |
| Bio19 | Precipitation of Coldest Quarter | >0.8 |
| SSP1-2.6 | SSP2-4.5 | |||||||||
| Current-2040 | 2040-2060 | 2060-2080 | 2080-2100 | Current-2100 | Current-2040 | 2040-2060 | 2060-2080 | 2080-2100 | Current-2100 | |
| Unsuitable | 0.31 | −0.15 | 0.12 | 0.17 | 0.46 | −0.33 | 0.26 | 0.35 | 0.38 | 1.12 |
| Marginal | −0.19 | 0.09 | −0.09 | −0.17 | −0.36 | 0.27 | −0.04 | −0.32 | −0.42 | −0.87 |
| Suitable | 0.00 | −0.09 | −0.03 | 0.03 | −0.09 | 0.21 | −0.33 | −0.17 | −0.23 | −0.61 |
| Highly Suitable | −0.12 | 0.15 | −0.01 | −0.04 | −0.02 | −0.15 | 0.11 | 0.14 | 0.27 | 0.37 |
| SSP3-7.0 | SSP5-8.5 | |||||||||
| Current-2040 | 2040-2060 | 2060-2080 | 2080-2100 | Current-2100 | Current-2040 | 2040-2060 | 2060-2080 | 2080-2100 | Current-2100 | |
| Unsuitable | −0.90 | 0.54 | 0.58 | 0.19 | 1.52 | −1.48 | 0.59 | 0.59 | 0.11 | 1.34 |
| Marginal | 1.01 | −0.23 | −0.59 | −0.29 | −0.98 | 0.87 | −0.41 | −0.07 | −0.11 | −0.70 |
| Suitable | 0.43 | −0.70 | 0.08 | −0.07 | −0.88 | 1.05 | −0.16 | −1.07 | −0.05 | −1.10 |
| Highly Suitable | −0.53 | 0.39 | −0.07 | 0.18 | 0.33 | −0.45 | −0.02 | 0.54 | 0.06 | 0.46 |
| Scenario | Latitude | Longitude | Region | Shift Direction |
|---|---|---|---|---|
| Current | 1.394° N | 33.538° E | Algeria | Northwest |
| SSP1-2.6–2100 | −3.429° S | 33.588° E | Morocco | West |
| SSP2-4.5–2100 | 11.091° N | 32.501° E | Tunisa | East |
| SSP3-7.0–2100 | −1.947° S | 34.098° E | Morocco | Northwest |
| SSP5-8.5–2100 | −2.985° S | 34.707° E | Morocco | Northwest |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechergui, K.; Hayat, U.; Ahmad, M.H.; Alamri, S.M.; Alamery, E.R.; Faqeih, K.Y.; Aldubehi, M.A.; Jaouadi, W. Forecasting the Impact of Climate Change on Tetraclinis articulata Distribution in the Mediterranean Using MaxEnt and GIS-Based Analysis. Forests 2025, 16, 1600. https://doi.org/10.3390/f16101600
Mechergui K, Hayat U, Ahmad MH, Alamri SM, Alamery ER, Faqeih KY, Aldubehi MA, Jaouadi W. Forecasting the Impact of Climate Change on Tetraclinis articulata Distribution in the Mediterranean Using MaxEnt and GIS-Based Analysis. Forests. 2025; 16(10):1600. https://doi.org/10.3390/f16101600
Chicago/Turabian StyleMechergui, Kaouther, Umer Hayat, Muhammad Hammad Ahmad, Somayah Moshrif Alamri, Eman Rafi Alamery, Khadeijah Yahya Faqeih, Maha Abdullah Aldubehi, and Wahbi Jaouadi. 2025. "Forecasting the Impact of Climate Change on Tetraclinis articulata Distribution in the Mediterranean Using MaxEnt and GIS-Based Analysis" Forests 16, no. 10: 1600. https://doi.org/10.3390/f16101600
APA StyleMechergui, K., Hayat, U., Ahmad, M. H., Alamri, S. M., Alamery, E. R., Faqeih, K. Y., Aldubehi, M. A., & Jaouadi, W. (2025). Forecasting the Impact of Climate Change on Tetraclinis articulata Distribution in the Mediterranean Using MaxEnt and GIS-Based Analysis. Forests, 16(10), 1600. https://doi.org/10.3390/f16101600







