1. Introduction
Tamaulipan thornscrub forest (also known as thornforest) is the native ecological system in wetter, lower salinity portions of the Lower Rio Grande Valley (LRGV) of southernmost Texas and northern Mexico. Thornforests include densely wooded but highly variable shrublands and woodlands that were cleared for agriculture expansion aggressively in the twentieth century, leaving only 5% relatively undisturbed [
1]. Reestablishment of habitats suitable for native wildlife through ecological restoration of abandoned agricultural fields and other degraded areas has become a top management priority in the region, especially because Tamaulipan thornforests have been identified as a conservation hotspot due to their high biodiversity and high risk of human alteration [
2], and because they are central to a regional ecotourism industry that generates
$307.1 million annually [
3].
Motivated by their high conservation and economic value, many institutions have contributed to restore lost thornforest habitat in the LRGV, including governmental entities such as the U.S. Fish and Wildlife Service (USFWS) and Texas Parks and Wildlife Department (TPWD), NGOs such as The Nature Conservancy and American Forests, and even corporations such as the LandLife Company [
4]. However, habitat restoration projects in the LRGV have mostly focused on maximizing acres restored, which has led to insufficient funds being allocated for post-restoration monitoring. The lack of monitoring has created knowledge gaps of methodological and site acquisition best practices when restoring Tamaulipan thornforest habitats. Many studies on thornforest restoration have been conducted in the LRGV, and, in one example, the authors evaluated the potential of different species of native transplanted seedlings to achieve a closed canopy [
5]. However, the oldest restoration study plot examined in their study had been restored seven years prior, and long-term restoration responses may differ from short-term responses due to time-dependent changes as a result of restoration treatments [
6]. Moreover, short-term evaluation does not capture all of the environmental variability transplanted seedlings will experience, which could affect restoration outcomes and serial surveys are suggested [
6]. Another study evaluated the regeneration of woody thornforest species in areas that had different land use histories in northeastern Mexico, where the restoration sites were allowed to regenerate naturally over a period of 21 years. This study revealed that abundance, dominance, species richness, and diversity were influenced differently depending on land use history [
7].
Time since restoration, patch size, patch isolation, and restoration method (active or passive) have been identified as important factors that affect restoration outcomes, such as plant species richness, abundance, and diversity. While assessing time since restoration in relation to restoration outcomes, Lindborg and Erickson found a positive relationship between restoration time and plant species total richness [
8]. A similar study observed increased numbers of species with longer restoration times in a previously grazed grassland in Finland. Here, they suggest with sufficient time, species in the region will gain the opportunity to colonize the area, but rare species may require additional years for their populations to recover [
9].
Patch size and isolation have also been identified as important considerations affecting species richness in existing and restored habitats. Isolation has shown to have direct effects on species immigration rates and related ecological responses and the effects of patch size have varied between species [
10,
11]. Kolb and Diekmann observed a positive relationship between patch size and species richness and a negative relationship between species richness and isolation. They attribute the negative species richness and distribution effect associated with isolation to increased distances between suitable habitat patches [
10]. In another study conducted in the Yucatan Peninsula, the authors found that patch area and isolation significantly predicted species densities in fragmented forests that had achieved equilibrium, where the same relationships between patch size and isolation and species richness were observed [
12].
Restoration strategies employed in the region include both passive and active restoration, where passive restoration allows for natural succession to occur with minimal intervention or management and relies on recruitment of vegetation over time, whereas active restoration also relies on natural succession but additionally employs active management strategies to accelerate plant recruitment, such as seedling transplantation and/or direct seeding [
4]. Active or passive restoration methods have shown outcomes may vary in relation to biogeographic region. One such study conducted in southeast Queensland, Australia observed similar outcomes in passively and actively restored sites regarding biomass accrual, but actively restored sites contained fewer tree and shrub individuals, which may have resulted from limited recruitment in plantings [
13]. A literature review conducted in 2014 revealed active restoration can accelerate the recovery of species richness and indicates considerable benefits across diversity indicators [
14]. However, some evidence suggests passive restoration is more economical than active restoration and may produce similar outcomes [
15,
16].
The primary factors considered during site selection were biogeographic region, time since restoration, patch size, isolation, and restoration method due to their influences on species related markers observed in other studies. Additionally, there have not been any studies conducted in the LRGV that have specifically considered these factors when assessing long-term restoration outcomes. The relationships between these site characteristics and restoration outcomes may differ in the LRGV than what was identified in the literature due to differences in local species and environmental factors.
Physical landscape characteristics can be critical factors in plant community development, such as landscape hydrology, soil characteristics, and topography [
17]. Nagamatsu et al. observed different establishment rates of deciduous trees at different topographic positions across the landscape citing light availability, moisture regime, and surface material movement differences as significant factors [
18]. Durbecq et al. identified soil physiochemical properties, elevation, aspect, and annual solar radiation as significant factors for plant community establishment [
19]. Additionally, Reid et al. examined thornforest plant species distribution in northeastern Mexico. In their study, they included two climatic regions, three substrate types, and three topographic situations and found that the distributions of most plant species were related to the variations in the physical environment [
20].
Predictive environmental factors and geographic features that have been shown to affect the vegetation structure and composition of restored sites were incorporated in this study due to differences that occur in vegetation establishment. Soil moisture and temperature were collected at each survey location, but these values can be ephemeral, so long-term temperature and precipitation were additionally evaluated. As identified in the studies above, water holding/runoff and moisture availability affect vegetation community composition and factors such as slope, elevation, soil sand content, and vegetation survey distances to temporary and permanent water sources were also included in our assessment of factors that could influence restoration outcomes.
The objectives of this study are to (1) quantify the plant community composition of 12 restored Tamaulipan thornforest habitats in the eastern LRGV that vary in time since restoration, patch size, degree of isolation, restoration method (active vs. passive), and local environmental conditions and (2) examine and quantify the relationships between site and environmental variables and restoration outcomes at the 12 surveyed restoration sites.
We hypothesized that the time since restoration, isolation, patch size, and intervention level (restoration method) will influence plant community composition, species richness, total plant abundance, diversity, and physical forest structure, both overall and within different forest layers. We expect invasive grass prevalence will affect plant community structure, especially in the ground layer. Geographic features and environmental factors will additionally affect restoration outcomes.
2. Methods
2.1. Description of Lower Rio Grande Valley Landscape and Site Selection
The Lower Rio Grande Valley (LRGV) is located at the southernmost region of Texas which encompasses 240 km along the Texas–Mexico border ending at the Gulf of Mexico and represents a transition area from temperate to tropical conditions [
1]. Precipitation varies across the landscape as eastern and coastal regions receive about 59 cm of precipitation and western regions receive about 48 cm, annually. Soil composition consists of clay, clay flat, and clay loam due to the region’s location within the Rio Grande River delta, and landforms are generally level or gently rolling [
21]. The LRGV hosts various types of native habitats including thornforest (thornscrub), flood plains, wetlands, tidal marshes, coastal prairies, and grasslands [
1,
22]. Collectively, the four counties (Cameron, Hidalgo, Willacy, and Starr county) of the LRGV contain 1200 plant species, 300 butterfly species, and about 700 vertebrates, of which at least 520 are birds [
23]. Not only is a high diversity maintained in the area but, some species are found in few or no other areas, such as
Leopardus pardalis albescens (northern ocelot) and
Falco femoralis septentrionalis (northern aplomado falcon), both of which are federally endangered [
1].
Thornforest canopy cover is commonly dense or open depending on component species and accompanying understory or shrub layer is usually dense ranging from 1 to 3 m in height, where species composition can vary to just a few species in recently restored sites to diverse assemblages in more mature sites. Herbaceous layer in mixed deciduous thornscrub is usually sparse consisting of native grasses and forbs, but invasive grasses may additionally be present and dominate the herbaceous layer [
21].
Twelve Tamaulipan thornforest sites in the eastern LRGV with a documented history of restoration efforts were selected for this study based on the time since restoration began at each site, habitat patch size, degree of isolation, and restoration strategy (active planting vs. passive revegetation;
Figure 1). The sites selected occupied two of the six ecoregions of the LRGV including the lower Rio Grande Valley and the lower Rio Grande alluvial floodplain (
Figure S1). Most sites selected, 11 out of 12, were located throughout Cameron County, TX with the exception of Goat Island, which was located in southern Willacy County, TX. The goal of site selection was to maximize the variance among the four focal site characteristics to allow the independent assessment of each variable.
However, this proved to be a difficult task considering the history of restoration efforts in the LRGV and availability of restored sites. All the sites that had been restored longer than 35 years ago are also larger and were passively restored and the newly restored sites are smaller and were actively restored. The partial confoundedness of site size, restoration method, and time since restoration made assessment of these characteristics not entirely possible. Patch size and interior-to-edge ratio additionally shared the same issue of confoundedness, where larger patches also had a higher interior-to-edge ratio. The 12 sites selected varied in time since restoration from 15 years to over 70 years, where six had been actively restored with either direct seeding or seedling transplantation and six were passively restored. Two restored sites did have some seedling transplantation for restoration, but the amount transplanted was very small and was not considered the primary means of restoration. Therefore, these two sites were classified as passively restored for this study.
Degree of isolation was determined using Google Earth version 7.3.6.10441 (Google, Mountain View, CA, USA) and the Texas Ecological Mapping System and calculated as the percentage of the area within a one-kilometer buffer from the perimeter of a study site that was not composed of thorn forest habitat. Additionally, it was important to incorporate environmental factors in our assessment to examine how relevant environmental and geographic factors may have affected each site’s current plant community. This includes climatic considerations given that Cameron County’s situation along the Texas coast results in significant east to west variation in temperature, precipitation, slope, and elevation that may affect plant species recruitment and habitat establishment.
2.2. Field Data Collection
Data collection for the reforested sites occurred in July of 2022 and was performed concurrently with some aspects of faunal assessments within the same study sites and survey points that utilized mammal, lepidoptera, herptile, and avian surveys [
24]. Prior to vegetation evaluation, three survey points per site were established for faunal assessment with a minimum distance of 200 m per site. Two vegetation sampling points were then selected per faunal survey point that were vegetatively representative of that survey point and spaced 50–80 m from each other, for a total of six vegetation sampling points per study site. A central point was haphazardly selected for each sampling point where we measured soil moisture and soil temperature, and captured a canopy photo directed at zenith, which we later used to determine canopy cover percentage via ImageJ image processing software version 1.44 (National Institutes of Health: Bethesda, MD, USA).
The point quarter method was utilized for canopy tree site characterization, where the closest tree in each quadrant was identified and its proximity to the central point, estimated height, and diameter at breast height (DBH) were recorded. A canopy tree was defined as any woody species with a DBH of greater than five centimeters in order to differentiate canopy trees from understory vegetation. Understory was evaluated by delineating a circle around the central point with a radius of 1.8 m for an approximate understory sampling area of 10 m
2. Species, height, cover area, and DBH were recorded for understory vegetation (DBH < 5 cm) when the main stem fell within the sampling area. Overhanging understory vegetation into the sampling area at a height of about 1.4 m was recorded as an estimate of area occupied by species within the designated understory sampling area. Ground cover was determined by placing a one-meter quadrat in each quadrant, defined by point quarter method, about 1.8 m from the central point and estimating the percentage of ground covered by each species present. As with overhanging understory vegetation, the same practice of recording overhanging ground cover vegetation was utilized with a different height requirement of 10 to 15 cm.
Figure S2 shows a graphical representation of the data collection plan.
2.3. GIS Quantification of Environmental and Geographic Factors
Environmental and geographic factors can also influence plant recruitment and establishment [
18,
25], so we used ArcMap Geographic Information System (GIS) software version 10.8.2 (ESRI: Redlands, CA, USA) to extract relevant attributes for each vegetation sampling point we surveyed in the field. Long-term precipitation and temperature values were extracted from interpolated raster data for 30-year annual average precipitation and 30-year daily average temperature developed by the PRISM Climate Group (PRISM Climate Group 2021). LiDAR-based elevation raster data were retrieved from the United States Geologic Survey (USGS) National Map (USGS 2021). The National Wetlands Inventory (NWI) shapefile was used to quantify proximity to both temporary and permanent water sources (USFW 2022). United States Department of Agriculture (USDA) Web Soil Survey soil data shapefiles were used to determine soil type and percentage of sand content (as a proxy for drainage) in the top 30 cm of soil for each vegetation survey point (USDA 2019). Analysis of vector and raster data were performed in the Texas state plane-south projection, with the exception of the National Wetlands Inventory data. This data set was assigned to North American Datum 1983 (NAD 1983) and analysis was performed unprojected.
2.4. Statistical Analyses
Statistical analyses were performed in R version 4.1.1 (R Foundation for Statistical Computing: Vienna, Austria). Prior to analysis, we calculated average percent cover values for understory and ground layer species and dominance values for canopy layer species for each sampling point (n = 36, 3 per study site). Dominance values were calculated from our point quarter data as the product of the density and the average basal area.
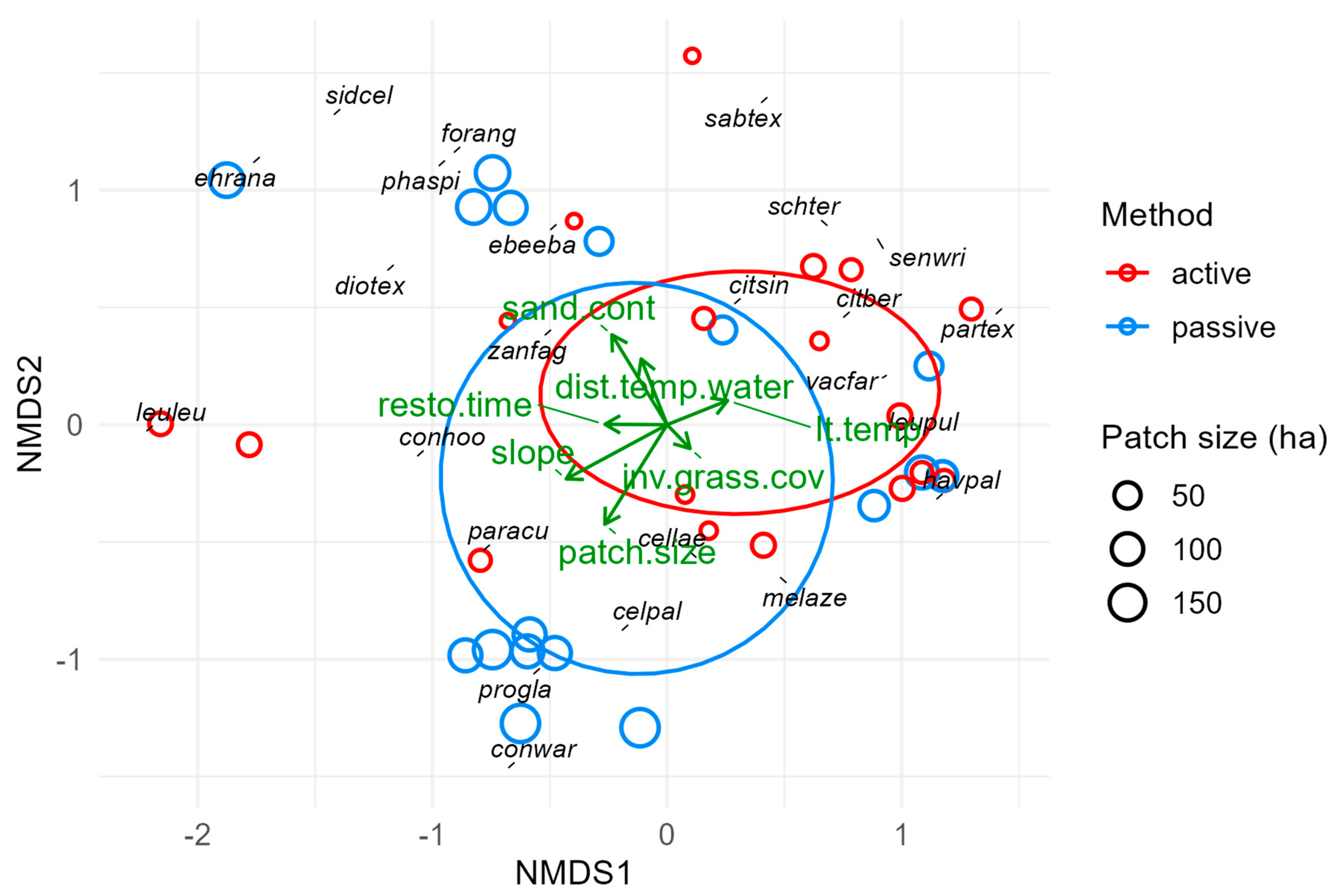
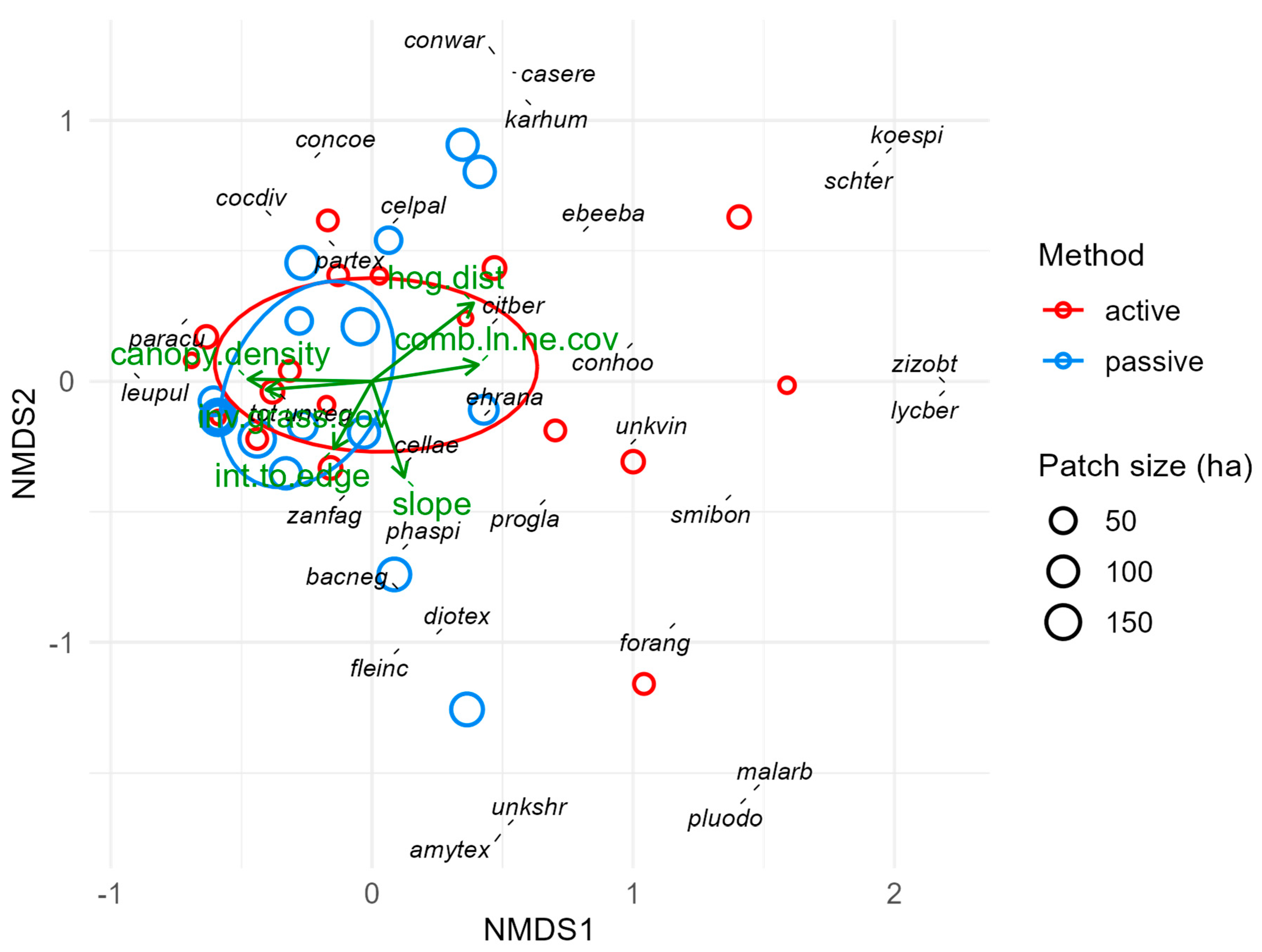
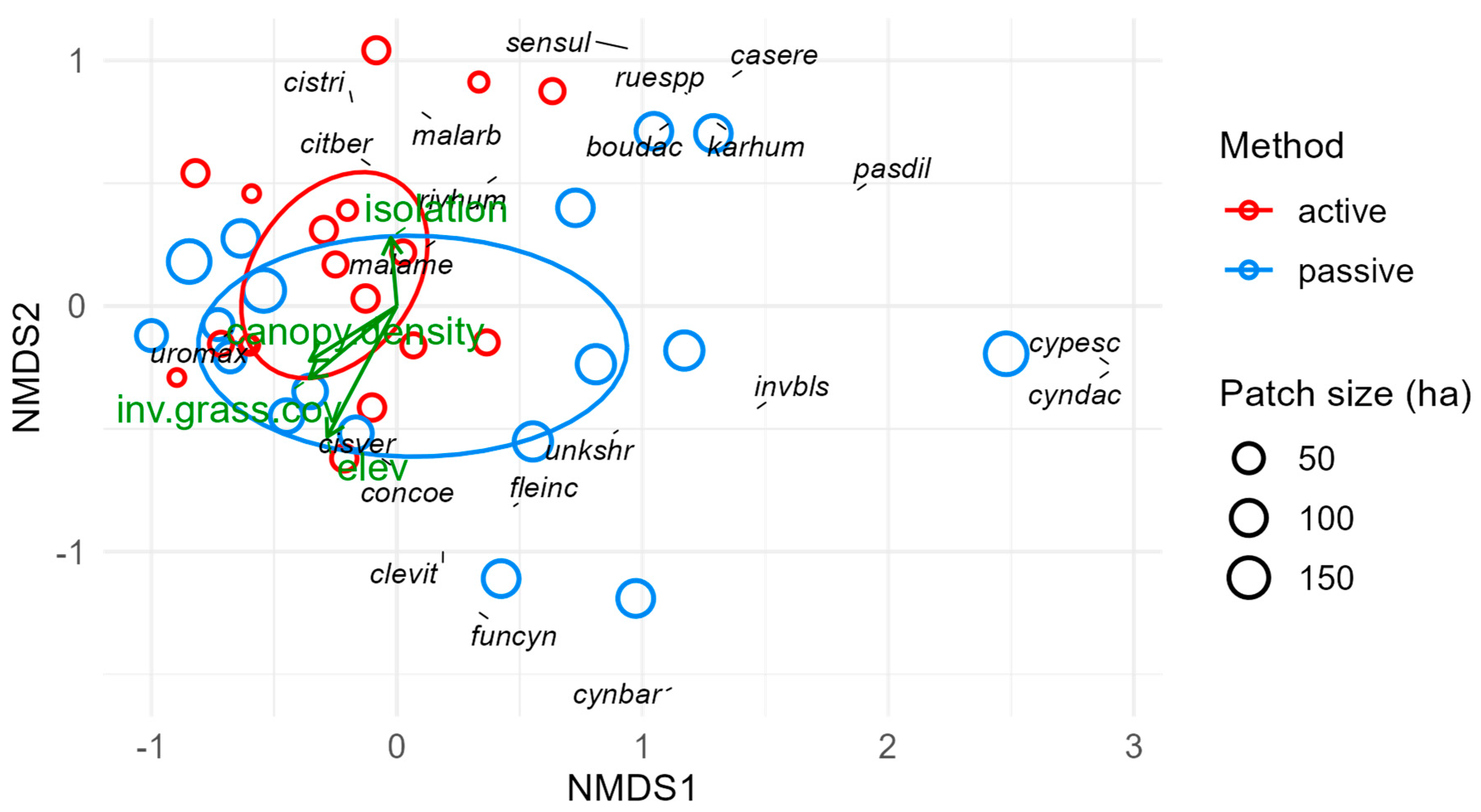
We performed a principal component analysis (PCA) to elucidate and visualize how 19 different sites, environmental, and geographic factors were related to one another and how they varied across our study sites and sampling points. We also anticipated that some environmental factors were correlated (such as patch size and edge-to-interior ratio), and 19 variables is a relatively large number of potentially predictive factors to consider; for both reasons, PCA is a logical first step in clarifying the overall environmental context.
We then performed separate multivariate analyses for each forest layer and the ensemble plant community (all layers combined) to characterize the observed plant communities and evaluate the relationships between community composition and environmental variables. We fit nonmetric multidimensional scaling (NMS) ordinations using the metaMDS() function in the ‘vegan’ package with Bray–Curtis dissimilarity values for each sampling point. We used relative abundance values to reduce fit stress to acceptable levels (below 0.2) where necessary. Understory vegetation was relatively sparse and absent from five sampling points, so we had to include a percentage for open (unvegetated) understory in our abundance values to reduce fit stress to acceptable levels. Next, we fit site characteristics (time since restoration, patch size, isolation, and restoration method) and environmental variables (e.g., soil moisture and temperature, long-term temperature and precipitation, slope, elevation, distance to temporary and permanent water sources, and soil sand content) to each NMS ordination to quantify and visualize how these factors were associated with different sites and species. Some variables, such as metrics of invasive plant prevalence, can be interpreted as both predictors and response variables and thus are only included in some models below.
To examine the effects of our categorical (restoration method) and continuous environmental variables on community composition, we used the adonis2() function in the ‘vegan’ package to perform permutational multiple analysis of covariance (PerMANCOVA) for the canopy, understory, ground, and ensemble plant communities. First, however, we reduced any sets of correlated environmental variables to a single variable by omitting all but the factor that explained the most variance. Our PerMANCOVAs used a bootstrapping procedure to generate 10,000 randomized datasets by sampling with replacement from the pool of observed values and then compared the F statistics generated using the randomized datasets to the F statistics generated using the actual observed values to calculate p-values for each model term.
Finally, to examine how site characteristics related to restoration outcomes, we performed a series of univariate analyses for the same four plant community groups. For each, we fit linear or permutational linear models and performed ANCOVAs or multiple regressions using marginal (Type III) sums of squares to examine the effects of our environmental variables on species richness, overall plant abundance, and Shannon–Weiner diversity. Our full models included all the site, environmental, and geographic variables considered in our PerMANCOVAs, but we purged the less explanatory variables from correlated sets (as above) and pruned model terms that explained little variance to increase our statistical power. We performed Shapiro–Wilk tests of normality on model residuals and Breusch–Pagan tests of homoscedasticity for all linear models and calculated the variance inflation factor for all model terms in each model to quantify multicollinearity and confirm that our models met all relevant assumptions. A p-value of 0.05 was used to determine significance and test model assumptions.
4. Discussion
Our goals were to (1) describe and compare the plant communities in restored thornforests in the Lower Rio Grande Valley (LRGV) of southern Texas and (2) identify which site, environmental, or geographic factors have driven thornforest restoration outcomes.
Table S19 summarizes the model results shown in
Table 1,
Table 2,
Table 3 and
Table 4, with factors ordered by the count of response variables for which each factor had a statistically significant or marginal effect (
p < 0.1). Numerically, invasive species influenced the most outcome metrics, with ground cover by invasive grasses and the log ratio of native to exotic cover influencing 12 and 9 (out of 17) response variables, respectively. Notably, all 17 response variables were influenced by at least one of these metrics of invasive plant prevalence. Invasive species are discussed below. Next, restoration time and slope each influenced 9 response variables, and canopy density and distance to temporary water influenced 8 variables. Restoration method influenced relatively many (6) but fewer response variables than expected. Time and method are frequently important to restoration outcomes in the literature, but the effects of restoration method are not as universal and often more complex and nuanced than those of time. The two are also related in key ways: here, time and method are partially confounded, and, generally, most methods aim to accelerate processes that naturally occur over time. Notably, restoration method affected several outcomes time did not, so 12 of 17 responses were influenced by restoration time or method. Below, we explore some of the key hypotheses and conceptual frameworks in restoration ecology to explain why some factors may have played larger roles than others.
4.1. Island Biogeography Theory and Plant Community Assembly
Forest patches in a matrix of non-forest habitats and human land use often behave enough like islands, e.g., in terms of colonization and local extinction, that principles of island biogeography theory (IBT) apply; namely, that species richness increases with patch size and over time and decreases with isolation [
26,
27]. Relevant environmental variables in our analyses include patch size, restoration time, isolation, and interior-to-edge ratio. All of these except isolation form a vector cluster in our PCA that corresponds to the direction of separation between restoration method groups (
Figures S4 and S5). This means passively restored sites collectively had longer times since restoration, larger patch sizes, and higher interior-to-edge ratios than actively restored sites. Therefore, despite efforts to select sites with independent variability in these factors, the variables for restoration time, patch size, isolation, and restoration method are partially confounded. We did disentangle these factors enough to identify which explained the most variance in each response variable, but we could not model all these factors together without violating assumptions of multicollinearity. Thus, we cannot rule out factors omitted to avoid multicollinearity as unimportant, but we can say they are less important because we retained the factors that explained the most variance. This has implications for the interpretations of other results.
Patch size influenced only three response variables: canopy composition, canopy richness, and (marginally) ensemble forest composition. This suggests patch size was relatively unimportant, contrary to IBT. Even if its importance to restoration outcomes is masked by its association with restoration time and method, those factors still explained more variance in outcomes. Alternatively, the sizes of surveyed patches ranged from 4.5 to 175 ha, which does not adequately represent what are often considered large patches in other restoration studies. This, too, could mask the importance of patch size, but the lack of very large patches does reflect the reality of forest restoration in south Texas.
Time since restoration was one of the most influential factors, impacting nine response variables, namely richness and diversity in the canopy, understory, and ground layers and overall forest, plus a marginal effect on canopy composition. These effects on richness and abundance were all positive except in the canopy layer, where, in mature forests, dominant climax trees may eventually displace early and mid-successional species, depending on disturbance regimes. These patterns are consistent with both IBT the restoration literature.
Isolation had an intermediate degree of influence, impacting seven response variables, with positive effects on understory richness and diversity and overall forest richness, abundance, and diversity, plus marginal effects on ground and forest composition. Isolation results in less connectivity to nearby thornforest habitats and may reflect a smaller contiguous forest complex where distinct forest patches are adjacent. IBT holds that isolation reduces colonization and thus richness and diversity [
28], and this applies to thornforests if distances are sufficient to reduce zoochory, anemochory, and/or hydrochory. Animal dispersers may not be present in isolated areas due to range and vegetation cover requirements [
29]. Thus, the positive relationships observed contradict IBT. However, none of our survey sites were particularly isolated; values ranged from 23 to 92%, indicating each site had at least part of a neighboring thornforest patch within 1 km. Like with patch size, perhaps the range of isolation values observed was insufficient to fully detect its effects. Even so, this would not explain the consistently positive relationships observed. Future analyses, including metrics of absolute distance to the nearest patch [
11], are merited.
Edge effects, considered in this study via the interior-to-edge ratio, impacted only four outcome metrics but can similarly influence colonization and richness due to windbreaking and animal habitat requirements [
30]. A low interior-to-edge ratio will reduce occurrence of species that regularly inhabit edges, e.g., due to microclimate specialization [
31]. Generally, increased light availability at forest edges influences recruitment of herbaceous plant species, and gradients of microclimates from forest edges to their interiors affect plant species distributions [
32]. The rarity of significant edge effects in this study could suggest their strength was relative uniform across study sites, which may relate to the lack of any very large patches. Alternatively, variation owing to edge effects would be diluted if there was significant variation in forest structure across study sites, which there was.
Restoration method relates to IBT two ways: (1) method is partially confounded with patch size, restoration time, and interior-to-edge ratio, and (2) the difference between active and passive methods was the intentional planting of thornforest species, which directly promotes colonization. Active restoration increased canopy cover, understory abundance and (marginally) diversity, and overall forest richness, abundance, and diversity. These patterns likely mean efforts to facilitate colonization and accelerate succession were successful because, although we saw positive relationships between restoration outcomes and the factors confounded with method (like patch size), actively restored sites were associated with lower values for those factors.
4.2. Abiotic Filters, Especially Water Availability
Community assembly is regulated by biotic and abiotic mechanisms and requirements that filter which species can occupy a habitat based on their functional traits [
33]. Abiotic filters are factors that prevent species establishment because their traits make them unable to cope with environmental conditions, acquire necessary resources, or perform required functions [
34]. Factors we considered that may serve as or correlate with abiotic filters included slope, elevation, soil moisture, soil temperature, long-term precipitation, long-term temperature, distances to temporary and permanent water bodies, soil sand content, and, for understory and ground layers only, canopy density and cover. Water availability is likely the principal abiotic filter in the hot and semi-arid LRGV. Most thornscrub plant species have adaptations for drought tolerance or avoidance. Water availability varies in space and time and with edaphic properties, which makes it hard to quantify, hence the many water-related factors we considered.
Slope influenced nine restoration outcomes: canopy, understory, ground, and forest richness, canopy composition and cover, and forest diversity, plus marginal effects on understory composition and diversity (
Table S19). Slope influences water runoff and retention, thus greater slope reduces water availability [
35] and the likelihood of successful seed dispersal [
36]. Slope had a negative effect on canopy cover but all its effects on richness and diversity were positive. This is counterintuitive but logical if greater water stress reduced the advantage invasive grasses had over more stress tolerant native plants or precluded invasion entirely. In contrast, higher soil sand content increases soil drainage and reduces water availability [
37], but sand content affected only canopy composition.
Distance to a temporary water body affected eight response variables. Most temporary water bodies in the region are seasonally flooded oxbow lakes known as resacas, which are relatively common in the region and critically important for wildlife. Proximity to such a feature increases the likelihood a sampling point is in a historic riparian zone or has a high water table, which should increase water availability. Temporary water distance had negative relationships with understory, ground, and forest richness and diversity, meaning these variables were higher where we expect water availability was higher. This pattern could contradict the notion that low water availability suppressed invasive grasses and benefitted drought-tolerant natives, but we think it is more likely that this reflects the fact that some sampling points were within or very near seasonal resacas, where thornforests are generally significantly more diverse than in drier areas.
Distance to a permanent water body, however, impacted only four restoration outcomes. Permanent water distance influenced forest composition and had positive effects on ground and forest richness and a negative effect on ground abundance. This means, unlike temporary water, richness was higher where water availability was likely lower. This most likely reflects the invasive grass suppression mechanism hypothesized above, which would also be consistent with the observed negative relationship with plant abundance in the ground layer, where more drought-sensitive herbaceous species occur.
Elevation influenced six response variables and, in the generally flat LRGV region within the Texas coastal plain, signifies proximity to the coast or riparian areas. Local hills and valleys, though infrequent and subtle, are also reflected by elevation and influence water availability. Either way, lower elevations should have higher water availability, so the positive relationships between elevation and canopy cover, ground abundance, and forest richness suggest these variables were higher where water availability was lower. This may again reflect the invasive grass suppression mechanism and could complement the pattern seen for temporary water distance, namely, higher (drier) elevations promote thornforest diversity unless a patch is in or very near a low-lying seasonal resaca. Alternatively, increased flood disturbance at low elevations could drive this pattern [
38]. The negative relationship between elevation and canopy diversity probably reflects the fact that riparian thornforests generally support the tallest, densest canopy layers and include riparian specialist species absent in drier thornforests, like
Taxodium mucronatum.
Long-term temperature also affected six restoration outcomes; it had negative relationships with canopy richness and ground richness, abundance, and diversity but a positive relationship with canopy abundance. Temperatures are typically high and freezes are rare in the LRGV, so they mainly function as an abiotic filter by imposing heat stress. One key plant response to heat stress is increased transpiration, which exacerbates water scarcity. Thus, these negative relationships with temperature are consistent with the notion that water availability is driving restoration outcomes, especially because fewer ground layer species possess the level of stress tolerance typical of most woody thornforest species. Importantly, this stress tolerance strategy may render many thornforest species more susceptible to competitive displacement where abiotic conditions are less restrictive [
39].
Long-term precipitation is central to water availability, but it only had one significant negative relationship with canopy richness and a marginal negative effect on canopy diversity. These negative relationships are consistent with our hypothesis that water stress may indirectly benefit stress-tolerant natives by suppressing invasive grasses, which is not mutually exclusive with water scarcity limiting restored thornforest richness, abundance, or diversity. Long-term precipitation probably had few significant effects because it was relatively consistent and exhibited little spatial variation across the focal region.
Soil moisture content is the most fundamental representation of water availability for plants, yet it only had positive relationships with understory diversity, forest abundance, and (marginally) understory abundance. Similarly, soil temperature only had negative relationships with canopy diversity and understory abundance. Rather than suggesting soil moisture and temperature were unimportant, these infrequent effects more likely reflect the high variability and abrupt changes due to stochastic weather events characteristic of both variables. Moreover, soil moisture and temperature were measured only once per sampling point, which confers limited predictive power to such transitory variables. We suggest soil moisture and temperature valuers were more indicative of shading effects produced by local vegetation; namely, higher canopy or understory cover decreased soil temperatures and reduced evapotranspiration, which increased soil moisture.
Concerning light availability, canopy density and cover were excluded from canopy layer analyses, but density still influenced eight response variables out of the remaining 12, making it one of the most influential factors. Canopy cover, however, only had positive relationships with understory richness and (marginally) forest abundance. Canopy density influenced understory, ground, and forest composition and had negative relationships with understory richness, abundance, and diversity and with forest richness and diversity. These patterns likely reflect greater reductions in resources for the understory and ground layers where canopies are denser [
40]. Although most understory and ground species are adapted for lower light levels, there is a limit to their shade tolerances, and water use by canopy trees may be reducing soil water content in their rhizospheres. Alternatively, this may simply reflect initial development of forest canopies. Especially in younger forests with less time since restoration, slow growing thornforest species may not have reached canopy heights but are still growing taller. Additionally, mature thornforest canopy heights reflect local resource availability and are often low due to water scarcity.
4.3. Biotic Filters, Especially Invasive Species
In addition to abiotic filters, community assembly is regulated by biotic mechanisms and the presence or absence of interactions that govern local establishment of species [
33]. Competitive exclusion, a principal biotic filter, limits trait similarity of coexisting species in an environment, and invasive species are often superior competitors [
41]. Abiotic filters restrict trait diversity among occupying species, especially in stressful environments like the LRGV, which results in trait similarities between species and can lead to biotic filtering via competitive exclusion [
33]. Native plant communities that do not exhibit trait dispersal across available niche space create local resource availabilities that allow incoming species to become established, including invasive species (although having saturated niche space does not preclude invasion by superior competitors) [
33].
In the LRGV, after overgrazing depleted native grasses, numerous exotic grass species with drought tolerance were introduced (principally from Africa) as cattle forage, and several are now invasive and pose major threats to regional biodiversity, especially
Bothriochloa ischaemum (King Ranch bluestem),
Dichanthium annulatum (Kleberg bluestem),
Pennisetum ciliare (buffel grass), and
Urochloa maxima (Guinea grass) [
42,
43]. We considered two metrics of invasive species prevalence: ground cover by invasive grasses and the log ratio of native to exotic cover, which considered all forest layers and all exotic plant species. As noted above, these metrics influenced 12 and 9 response variables, respectively, and all 17 response variables were influenced by at least one of these metrics.
As expected, invasive grass cover had predominantly negative effects on restoration outcomes, exhibiting negative relationships with canopy and ground richness and diversity, and understory and overall forest diversity. Invasive grasses, especially
Urochloa maxima, have regularly displaced native ground vegetation via competitive exclusion to create monodominant plant communities and, in some cases, invasive monocultures [
43,
44]. Crucially, these effects extend beyond the ground layer, with invasive grasses reducing light and water availability at the soil surface enough to reduce or preclude recruitment by woody canopy and understory species as well [
42,
43,
44]. Indeed, invasive grass cover influenced community composition in each forest layer and overall. Where invasive grasses dominated, trees with growth rates fast enough to escape the extreme shade of the grass canopy (more than those with the highest shade tolerance) were strongly favored, such
Leucaena leucocephala (also invasive),
Leucaena pulverulenta, and
Prosopis glandulosa. Similar impacts were seen in forests in south central Texas after invasion by
Bothriochloa ischaemum, one of the other regionally prevalent invasive grasses [
45].
Invasive grass cover also had positive relationships with canopy and ground abundance. The latter is expected, as cover in invasive monocultures and monodominant communities is often higher than in mixed native communities [
41]. The positive relationship with canopy abundance is harder to explain.
Urochloa maxima tolerates and may even favor some shade, but it is consistently absent from mature thornforests with dense closed canopies, suggesting an attainable limit to its shade tolerance [
46]. However, exceedingly few, if any, of the restored thornforests we surveyed had sufficient canopy closure to preclude
Urochloa maxima establishment. We suspect the surveyed forests with lower canopy abundances generated too little shade to significantly affect most ground layer species, while those with higher canopy abundances produced enough shade to suppress native ground species, but the effect on invasive grasses was weaker, which favored invasive grass cover and lead to the observed pattern [
46]. Alternatively, but not mutually exclusive, because invasive grasses are superior resource competitors and we also saw tradeoffs between canopy and understory abundance, and if canopy abundance was not high enough to suppress invasive grasses, this positive relationship could simply reflect local resource availability, with both canopy trees and invasive grasses being less abundant where water and/or nutrients were scarce and more abundant where plentiful.
Whereas invasive grass cover quantifies the raw abundance of the most important invasive species in this system, the log ratio of native to exotic plant cover quantifies the relative dominance by native species; so, by its mathematical definition, it decreases as invasive grass cover increases and it is not sensitive to differences in overall plant abundance. The log ratio of native to exotic cover had exclusively positive relationships with restoration outcomes, including understory and forest richness, overall forest diversity, and plant abundance in all layers and overall. This was expected since higher prevalences of invasive species generally have negative impacts on diversity and the abundance of native species, especially during restoration [
41]. However, given the positive relationships between invasive grass cover and canopy and ground abundances discussed above, the positive relationships between plant abundances and the log ratio of native to exotic cover merit explanation. We expect that, regardless of the degree of invasion or overall abundance of plants, having a greater proportion of native plants benefitted richness, diversity, and even abundance, which is consistent with the positive diversity–productivity relationships commonly observed in forests and grasslands due to trait complementarity (resource partitioning) and selection effects (of highly productive species) [
47].
Finally, hog disturbance was our sole animal-based biotic factor and our sole disturbance metric; it was quantified as the percentage of the ground where foraging behavior characteristic of invasive feral hogs or native javelina resulted in identifiable soil disturbance. Hog disturbance had few significant effects but influenced understory and forest composition and had a positive relationship with ground plant abundance. Some species require disturbance for recruitment, and this is likely reflected in the effects on understory and forest species composition; however, intermediate levels of disturbance often promote diversity by allowing disturbance- and stability-adapted species to coexist, but we saw no significant relationships between hog disturbance and richness or diversity [
48]. Beyond promoting disturbance-adapted species, feral hogs can also alter forest composition by grazing on seedlings [
49,
50] and selectively consuming seeds of species with larger propagules, which often favors smaller-seeded invasive species in addition to promoting invasion by disturbing the soil [
51]. The positive relationship with ground plant abundance mostly likely arose because soil disturbance promotes invasion by
Urochloa maxima and other invasives grasses.
5. Conclusions
We identified numerous factors that influenced thornforest restoration outcomes and could be used to inform future land acquisition and management decisions. Foremost was invasive grass cover, which reduced diversity and influenced species composition in each forest layer and overall, having a broad and powerful homogenizing effect (
Figure 6). Presence of invasive grasses, especially
Urochloa maxima (Guinea grass), significantly hindered restoration progress and should be considered during site selection because its control is sufficiently challenging and costly to be impractical in most circumstances. However, given invasive grass prevalence, avoidance is often impossible, in which case–control efforts at the time of native planting can be viable and effective in facilitating initial establishment. Additional research into invasive grass control is merited. Second, water availability and the many factors that influence it must be considered. The most diverse and abundant reforestation sites were in riparian areas or seasonal wetlands (e.g., resacas); otherwise, generally, higher water availability increased plant abundance and diversity, unless invasive grasses were present, where the oppositive was true. Third, the isolation, shape (interior vs. edge), and size of reforestation sites were relatively unimportant but were still influential. Given that legal and economic aspects of land acquisition overwhelmingly govern the dimensions and locations of restoration sites, considerations of isolation, shape, and size may be most appropriate and impactful when developing post-acquisition reintroduction and management plans.
Future thornforest restoration research should consider sites farther west in Willacy, Hidalgo, and Starr Counties where conditions become considerably drier and different species of invasive grasses are dominant. Ideally, any study would leverage a greater number of sites; we could assess only a small fraction of the thousands of hectares of restored thornforests, and the LRGV landscape is highly variable and heterogeneous. Finally, human impacts in the region are similarly diverse and heterogeneous and, crucially, ongoing, so greater consideration of land use history is merited, as are assessments of current human impacts on forest community structure.