Photosynthetic Performance and Phytoremediation Potential of Narrow Crown Black-Cathay Poplar Under Combined Cadmium and Phenol Pollution
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experiment Design
2.3. Measurement of Photosynthetic Physiological Parameters
2.4. Determination of Residual Cd Content in Culture Medium
2.5. Determination of Plant Biomass and Cd Content in Various Organs
2.6. Data Processing and Analysis
3. Results
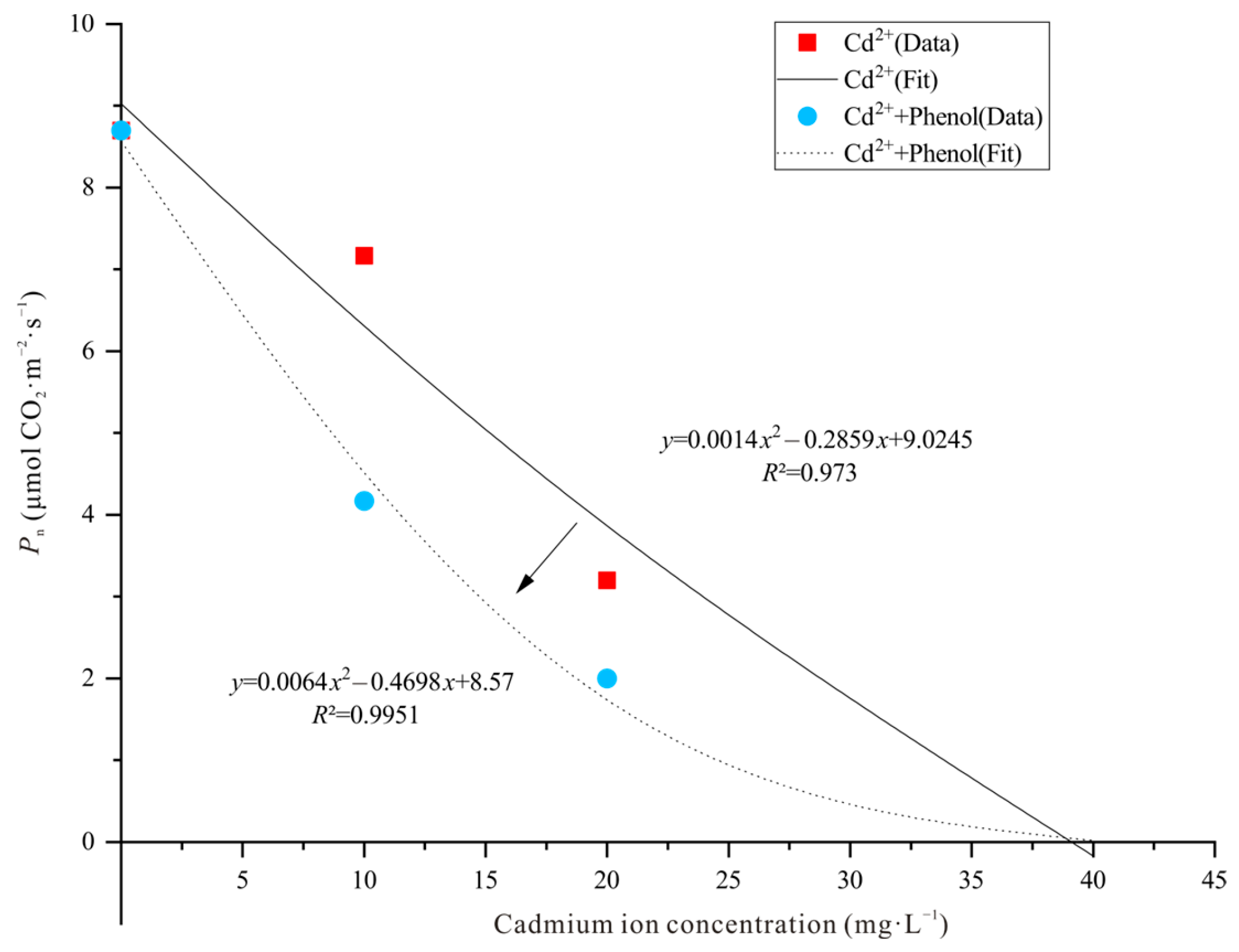
3.1. Photosynthetic Physiological Characteristics of Narrow Crown Black-Cathay Poplar
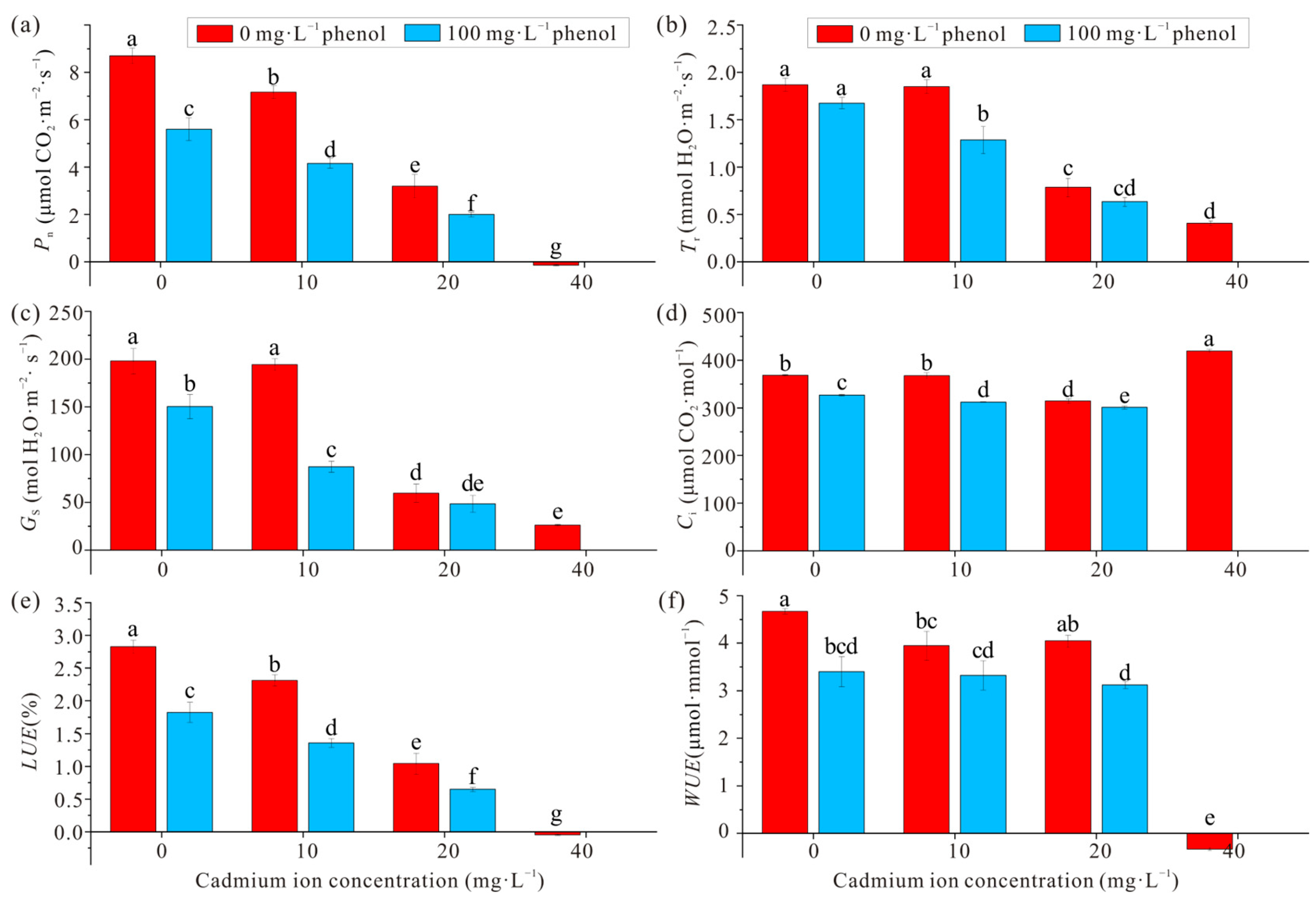
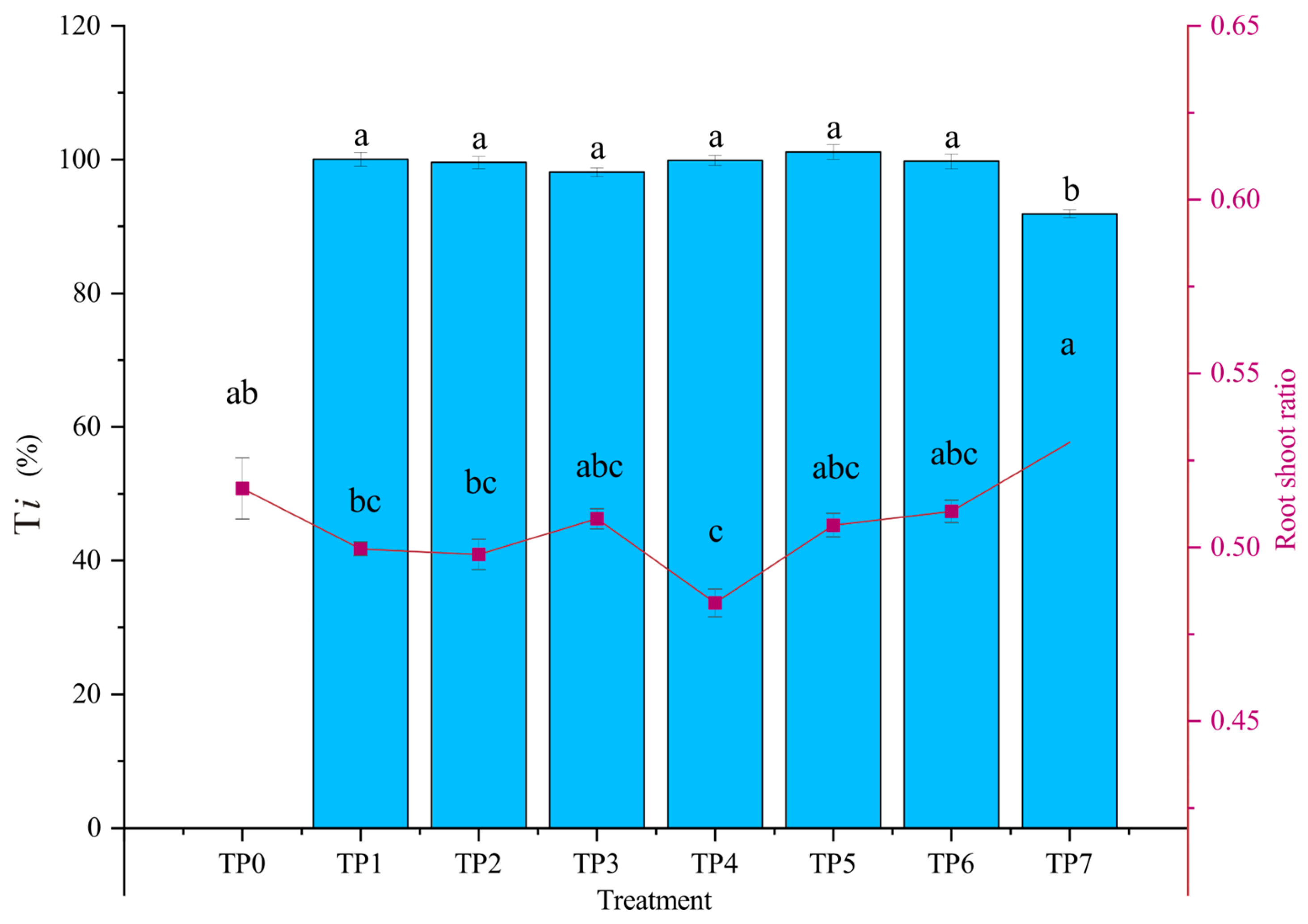
3.2. Biomass and Tolerance Index (Ti) of Narrow Crown Black-Cathay Poplar
3.3. Cd Content and Translocation Factor (Tf) in Different Organs
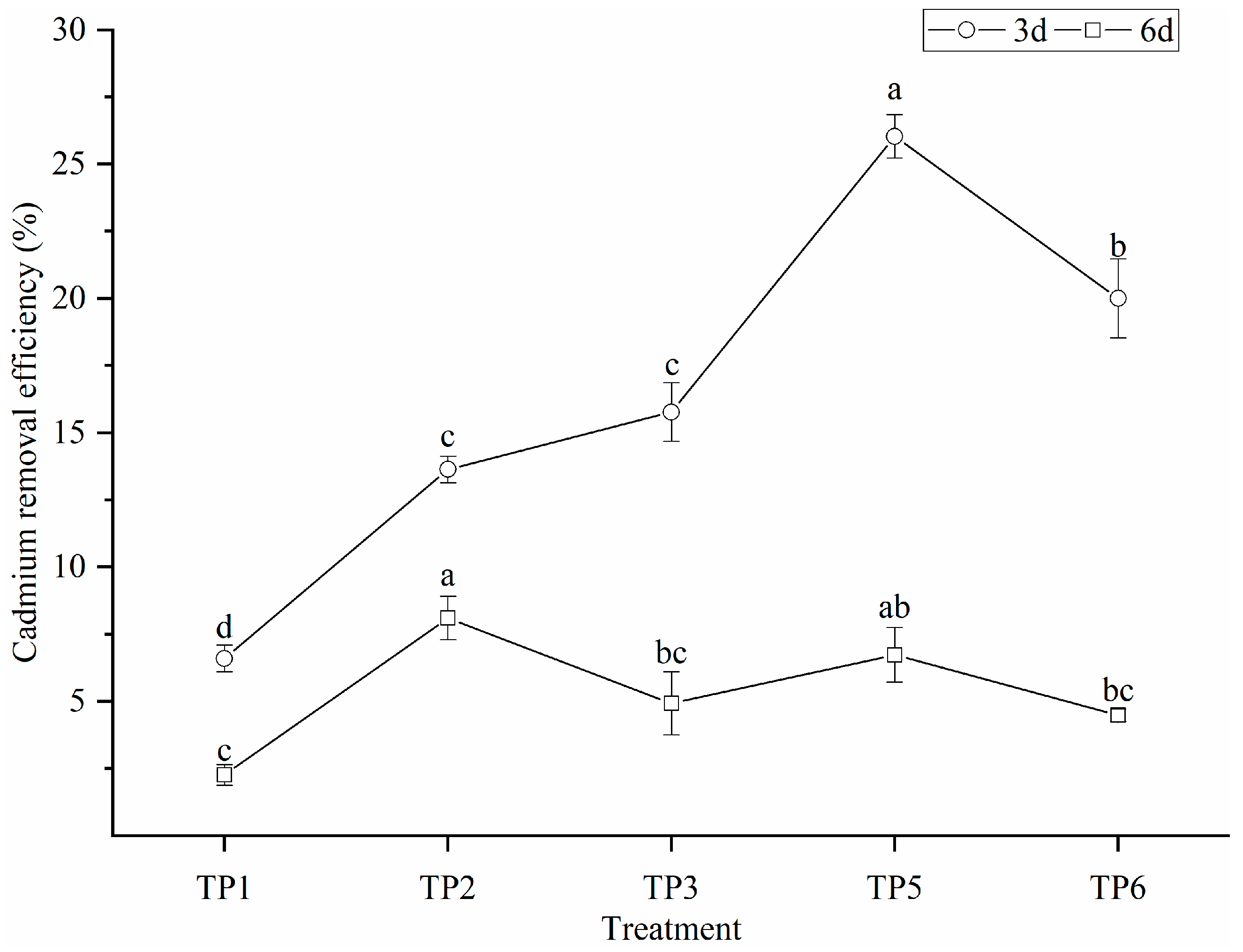
3.4. Removal Efficiency of Cd
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thavamani, P.; Megharaj, M.; Naidu, R. Multivariate analysis of mixed contaminants (PAHs and heavy metals) at manufactured gas plant site soils. Environ. Monit. Assess. 2012, 184, 3875–3885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, M.; An, S.; Lin, K.; Li, H.; Cui, C.; Fu, R.; Zhu, J. The combined effect of decabromodiphenyl ether (BDE-209) and copper (Cu) on soil enzyme activities and microbial community structure. Environ. Toxicol. Pharmacol. 2012, 34, 358–369. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Xia, H. Effect of cadmium on growth, photosynthesis, mineral nutrition and metal accumulation of bana grass and vetiver grass. Ecotoxicol. Environ. Saf. 2014, 106, 102–108. [Google Scholar] [CrossRef]
- Ontañon, O.M.; González, P.S.; Agostini, E. Biochemical and molecular mechanisms involved in simultaneous phenol and Cr (VI) removal by Acinetobacter guillouiae SFC 500-1A. Environ. Sci. Pollut. Res. 2015, 22, 13014–13023. [Google Scholar] [CrossRef] [PubMed]
- Paisio, C.E.; Fernandez, M.; González, P.; Talano, M.; Medina, M.; Agostini, E. Simultaneous phytoremediation of chromium and phenol by Lemna minuta Kunth: A promising biotechnological tool. Int. J. Environ. Sci. Technol. 2018, 15, 37–48. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Simultaneous removal of organics and heavy metals from industrial wastewater: A review. Chemosphere 2021, 262, 128379. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Effect of separate and combined toxicity of bisphenol A and zinc on the soil microbiome. Int. J. Mol. Sci. 2022, 23, 5937. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Chen, J.; Zhang, J.; Teng, H.H. Combined toxicity of Cd and aniline to soil bacteria varying with exposure sequence. Environ. Int. 2024, 190, 108916. [Google Scholar] [CrossRef]
- Gauthier, P.T.; Norwood, W.P.; Prepas, E.E.; Pyle, G.G. Metal–PAH mixtures in the aquatic environment: A review of co-toxic mechanisms leading to more-than-additive outcomes. Aquat. Toxicol. 2014, 154, 253–269. [Google Scholar] [CrossRef]
- Chen, F.; Tan, M.; Ma, J.; Zhang, S.; Li, G.; Qu, J. Efficient remediation of PAH-metal co-contaminated soil using microbial-plant combination: A greenhouse study. J. Hazard. Mater. 2016, 302, 250–261. [Google Scholar] [CrossRef]
- Wang, X.; Teng, Y.; Wang, X.; Xu, Y.; Li, R.; Sun, Y.; Hu, W.; Zhao, L.; Ren, W.; Luo, Y. Effects of combined pollution of organic pollutants and heavy metals on biodiversity and soil multifunctionality in e-waste contaminated soil. J. Hazard. Mater. 2022, 440, 129727. [Google Scholar] [CrossRef]
- Yang, Q.; Li, G.; Jin, N.; Zhang, D. Synergistic/antagonistic toxicity characterization and source-apportionment of heavy metals and organophosphorus pesticides by the biospectroscopy-bioreporter-coupling approach. Sci. Total Environ. 2023, 905, 167057. [Google Scholar] [CrossRef] [PubMed]
- Mathur, J.; Panwar, R. Synergistic effect of pyrene and heavy metals (Zn, Pb, and Cd) on phytoremediation potential of Medicago sativa L.(alfalfa) in multi-contaminated soil. Environ. Sci. Pollut. Res. 2024, 31, 21012–21027. [Google Scholar] [CrossRef]
- Rashid, I.; Murtaza, G.; Zahir, Z.A.; Farooq, M. Effect of humic and fulvic acid transformation on cadmium availability to wheat cultivars in sewage sludge amended soil. Environ. Sci. Pollut. Res. 2018, 25, 16071–16079. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, T.R.; Maier, R.M. Impact of metals on the biodegradation of organic pollutants. Environ. Health Perspect. 2003, 111, 1093–1101. [Google Scholar] [CrossRef]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef]
- Verma, S.P.; Sarkar, B. Simultaneous removal of Cd (II) and p-cresol from wastewater by micellar-enhanced ultrafiltration using rhamnolipid: Flux decline, adsorption kinetics and isotherm studies. J. Environ. Manag. 2018, 213, 217–235. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Rinklebe, J.; Tsang, D.C.; Bashir, A.; Maqbool, A.; Tack, F.; Ok, Y.S. Cadmium phytoremediation potential of Brassica crop species: A review. Sci. Total Environ. 2018, 631, 1175–1191. [Google Scholar] [CrossRef]
- Wei, R.; Guo, Q.; Tian, L.; Kong, J.; Bai, Y.; Okoli, C.P.; Wang, L. Characteristics of cadmium accumulation and isotope fractionation in higher plants. Ecotoxicol. Environ. Saf. 2019, 174, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Adarme-Duran, C.; Ágreda, J.; Brandão, P.; Castillo, E. Cadmium availability in rhizosphere and non-rhizosphere soils in cacao farms in Santander, Colombia. Environ. Monit. Assess. 2024, 196, 1254. [Google Scholar] [CrossRef]
- Chen, P.; Yuan, L.; Zhou, Z.; Xu, G.; Chen, W.; Cao, Y.; Li, C.; Fu, Q.; Fan, W.; Hu, S. Moso bamboo alleviates Uranium/Cadmium stress through altering the rhizosphere micro-environment and regulating roots carbon and nitrogen metabolism. Environ. Res. 2025, 276, 121452. [Google Scholar]
- Park, J.-S.; Brown, M.T.; Han, T. Phenol toxicity to the aquatic macrophyte Lemna paucicostata. Aquat. Toxicol. 2012, 106, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Jobby, R.; Kudale, S.; Modi, N.; Dhaneshwar, A.; Desai, N. Biodegradation of phenol using hairy roots of Helianthus annuus L. Int. Biodeterior. Biodegrad. 2013, 77, 106–113. [Google Scholar]
- Zhou, M.; Zhang, J.; Sun, C. Occurrence, ecological and human health risks, and seasonal variations of phenolic compounds in surface water and sediment of a potential polluted river basin in China. Int. J. Environ. Res. Public Health 2017, 14, 1140. [Google Scholar]
- Hollanda, L.R.; de Souza, J.A.B.; Foletto, E.L.; Dotto, G.L.; Chiavone-Filho, O. Applying bottom ash as an alternative Fenton catalyst for effective removal of phenol from aqueous environment. Environ. Sci. Pollut. Res. 2023, 30, 120763–120774. [Google Scholar] [CrossRef]
- Li, X.; Zeng, G.-M.; Huang, J.-H.; Zhang, D.-M.; Shi, L.-J.; He, S.-B.; Ruan, M. Simultaneous removal of cadmium ions and phenol with MEUF using SDS and mixed surfactants. Desalination 2011, 276, 136–141. [Google Scholar] [CrossRef]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Ann. Agric. Sci. 2018, 63, 123–127. [Google Scholar]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hou, H.; Zhao, L.; Sun, Z.; Li, H. Protective Effect of foliar application of sulfur on photosynthesis and antioxidative defense system of rice under the stress of Cd. Sci. Total Environ. 2020, 710, 136230. [Google Scholar]
- Liu, Z.; He, X.; Chen, W.; Yuan, F.; Yan, K.; Tao, D. Accumulation and tolerance characteristics of cadmium in a potential hyperaccumulator—Lonicera japonica Thunb. J. Hazard. Mater. 2009, 169, 170–175. [Google Scholar] [CrossRef]
- Kang, W.; Bao, J.; Zheng, J.; Xu, F.; Wang, L. Phytoremediation of heavy metal contaminated soil potential by woody plants on Tonglushan ancient copper spoil heap in China. Int. J. Phytoremediat. 2018, 20, 1–7. [Google Scholar] [CrossRef]
- Ouyang, L.; Chen, S.; Yang, W.; Zheng, J.; Ye, L.; Liu, Q.; Yang, J. Organic fertilizer improved the lead and cadmium metal tolerance of Eucalyptus camaldulensis by enhancing the uptake of potassium, phosphorus, and calcium. Front. Plant Sci. 2024, 15, 1444227. [Google Scholar] [CrossRef]
- Unterbrunner, R.; Puschenreiter, M.; Sommer, P.; Wieshammer, G.; Tlustoš, P.; Zupan, M.; Wenzel, W. Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environ. Pollut. 2007, 148, 107–114. [Google Scholar] [CrossRef]
- Marmiroli, M.; Pietrini, F.; Maestri, E.; Zacchini, M.; Marmiroli, N.; Massacci, A. Growth, physiological and molecular traits in Salicaceae trees investigated for phytoremediation of heavy metals and organics. Tree Physiol. 2011, 31, 1319–1334. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Olguín, E.J.; Sánchez-Galván, G. Heavy metal removal in phytofiltration and phycoremediation: The need to differentiate between bioadsorption and bioaccumulation. N. Biotechnol. 2012, 30, 3–8. [Google Scholar] [PubMed]
- Liu, M.; Sun, J.; Li, Y.; Xiao, Y. Nitrogen fertilizer enhances growth and nutrient uptake of Medicago sativa inoculated with Glomus tortuosum grown in Cd-contaminated acidic soil. Chemosphere 2017, 167, 204–211. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Wei, Z.; Van Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef] [PubMed]
- Moshood, A.Y.; Abdulraheem, M.I.; Li, L.; Zhang, Y.; Zhang, W.; Gen, K.; Zang, Y.; Raghavan, V.; Hu, J. Sustainable forest soil management for remediation of heavy metals contamination: Integrating technologies and ecosystem health. Antonie van Leeuwenhoek 2025, 118, 95. [Google Scholar] [CrossRef]
- Gupta, A.; Balomajumder, C. Removal of Cr (VI) and phenol using water hyacinth from single and binary solution in the artificial photosynthesis chamber. J. Water Process Eng. 2015, 7, 74–82. [Google Scholar]
- Zacchini, M.; Pietrini, F.; Scarascia Mugnozza, G.; Iori, V.; Pietrosanti, L.; Massacci, A. Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water Air Soil Pollut. 2009, 197, 23–34. [Google Scholar]
- Liu, Z.; Chen, W.; He, X.; Jia, L.; Yu, S.; Zhao, M. Hormetic responses of Lonicera japonica Thunb. to cadmium stress. Dose-Response 2015, 13, 1–10. [Google Scholar]
- Xu, S.; Li, B.; Li, P.; He, X.; Chen, W.; Yan, K.; Li, Y.; Wang, Y. Soil high Cd exacerbates the adverse impact of elevated O3 on Populus alba ’Berolinensis’ L. Ecotoxicol. Environ. Saf. 2019, 174, 35–42. [Google Scholar]
- Panwar, R.; Mathur, J. Comparative analysis of remediation efficiency and ultrastructural translocalization of polycyclic aromatic hydrocarbons in Medicago sativa, Helianthus annuus, and Tagetes erecta. Int. J. Phytoremediat. 2023, 25, 1743–1761. [Google Scholar] [CrossRef]
- Pietrini, F.; Di Baccio, D.; Iori, V.; Veliksar, S.; Lemanova, N.; Juškaitė, L.; Maruška, A.; Zacchini, M. Investigation on metal tolerance and phytoremoval activity in the poplar hybrid clone “Monviso” under Cu-spiked water: Potential use for wastewater treatment. Sci. Total Environ. 2017, 592, 412–418. [Google Scholar] [CrossRef]
- Pilipović, A.; Zalesny, R.S., Jr.; Rončević, S.; Nikolić, N.; Orlović, S.; Beljin, J.; Katanić, M. Growth, physiology, and phytoextraction potential of poplar and willow established in soils amended with heavy-metal contaminated, dredged river sediments. J. Environ. Manag. 2019, 239, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Zalesny, R.S., Jr.; Calagari, M. Effects of urban wastewater application on growth, biomass, nutrition, and heavy-metal accumulation of Populus nigra L. “62/154,” P. alba L. “20/45,” P. euramericana (Dode) Guinier “92/40,” and Salix excelsa S.G. Gmel grown in heavy-metal contaminated soil. Int. J. Phytoremediat. 2023, 25, 1371–1383. [Google Scholar]
- Salehi, A.; Shariat, A. Comparative performance of Populus spp. and Salix spp. for growth, nutrition, and heavy metal uptake in a wastewater hydroponic system. Int. J. Phytoremediat. 2024, 26, 1369–1378. [Google Scholar] [CrossRef]
- Wilkins, D. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978, 80, 623–633. [Google Scholar] [CrossRef]
- Lux, A.; Šottníková, A.; Opatrná, J.; Greger, M. Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiol. Plant. 2004, 120, 537–545. [Google Scholar] [CrossRef]
- Tufail, M.A.; Iltaf, J.; Zaheer, T.; Tariq, L.; Amir, M.B.; Fatima, R.; Asbat, A.; Kabeer, T.; Fahad, M.; Naeem, H. Recent advances in bioremediation of heavy metals and persistent organic pollutants: A review. Sci. Total Environ. 2022, 850, 157961. [Google Scholar] [CrossRef]
- Wang, Y.; Imran, M.A.; Zhao, J.; Sultan, M.; Li, M. Single/joint effects of pyrene and heavy metals in contaminated soils on the growth and physiological response of maize (Zea mays L.). Front. Plant Sci. 2024, 15, 1505670. [Google Scholar] [CrossRef]
- Elhadj, Z.; Brahim-Tazi, N.A.; Belguermi, A.; Haddad, F.Z.; Bekkay, Y.; Meghabar, R. Organic and heavy metal pollutants in dredged sediment of Oran Harbor, Algeria. Environ. Monit. Assess. 2024, 196, 834. [Google Scholar] [CrossRef]
- Li, C.; Yao, Y.; Liu, X.; Chen, H.; Li, X.; Zhao, M.; Zhao, H.; Wang, Y.; Cheng, Z.; Wang, L. Integrated metabolomics, transcriptomics, and proteomics analyses reveal co-exposure effects of polycyclic aromatic hydrocarbons and cadmium on ryegrass (Lolium perenne L.). Environ. Int. 2023, 178, 108105. [Google Scholar] [CrossRef]
- Mediavilla, S.; Santiago, H.; Escudero, A. Stomatal and mesophyll limitations to photosynthesis in one evergreen and one deciduous Mediterranean Oak species. Photosynthetica 2002, 40, 553–559. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Guo, K.; Huo, Y.; He, G.; Sun, H.; Guan, Y.; Xu, N.; Yang, W.; Sun, G. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol. Environ. Saf. 2020, 202, 110856. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.A.; Hayat, S.; Ahmad, A. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 2011, 84, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef]
- Huihui, Z.; Xin, L.; Zisong, X.; Yue, W.; Zhiyuan, T.; Meijun, A.; Yuehui, Z.; Wenxu, Z.; Nan, X.; Guangyu, S. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol. Environ. Saf. 2020, 195, 110469. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.L.G.; Marques, L.G.; Colepicolo, P. Antioxidant enzymes are induced by phenol in the marine microalga Lingulodinium polyedrum. Ecotoxicol. Environ. Saf. 2015, 116, 84–89. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Lin, Y.; Wang, G. Toxicological effects of phenol on four marine microalgae. Environ. Toxicol. Pharmacol. 2017, 52, 170–176. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, Z.; Yang, W.; Guo, J.; Yan, Z.; Li, J.; Lin, J.; Cao, X.; Tang, J.; Liu, Z. Unraveling the metabolic effects of benzophenone-3 on the endosymbiotic dinoflagellate Cladocopium goreaui. Front. Microbiol. 2023, 13, 1116975. [Google Scholar] [CrossRef]
- Krupa, Z.; Siedlecka, A.; Kleczkowski, L.A. Cadmium-affected level of inorganic phosphate in rye leaves influences Rubisco subunits. Acta Physiol. Plant. 1999, 21, 257–261. [Google Scholar] [CrossRef]
- Ahmadi, H.; Corso, M.; Weber, M.; Verbruggen, N.; Clemens, S. CAX1 suppresses Cd-induced generation of reactive oxygen species in Arabidopsis halleri. Plant Cell Environ. 2018, 41, 2435–2448. [Google Scholar]
- Brugnoli, E.; Björkman, O. Growth of cotton under continuous salinity stress: Influence on allocation pattern, stomatal and non-stomatal components of photosynthesis and dissipation of excess light energy. Planta 1992, 187, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, D.-L.; Wang, L.; Yang, Y.-F. Response of transpiration characteristics and water use efficiency of Setaria viridis to the enhancement of simulated photosynthetic radiation and CO2 enrichment. Chin. J. Plant Ecol. 2003, 27, 448. [Google Scholar]
- Sharma, S.S.; Kumar, V. Responses of wild type and abscisic acid mutants of Arabidopsis thaliana to cadmium. J. Plant Physiol. 2002, 159, 1323–1327. [Google Scholar] [CrossRef]

| Treatment | Composition | Average Inhibition Rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cd (mg·L−1) | Phenol (mg·L−1) | Pn | Tr | GS | Ci | WUE | LUE | |
| TP0 | 0 | 0 | - | - | - | - | - | - |
| TP1 | 10 | 0 | 17.6 | 1.1 | 1.9 | 0.2 | 18.2 | 15.5 |
| TP2 | 20 | 0 | 63.2 | 57.9 | 69.9 | 14.6 | 63.2 | 13.3 |
| TP3 | 40 | 0 | 100.0 | 36.1 | 86.9 | 0.0 | 100.0 | 100.0 |
| TP4 | 0 | 100 | 35.6 | 10.3 | 24.1 | 11.2 | 35.5 | 27.2 |
| TP5 | 10 | 100 | 52.1 | 31.2 | 55.9 | 15.1 | 52.1 | 28.8 |
| TP6 | 20 | 100 | 77.0 | 66.0 | 75.6 | 18.1 | 77.1 | 33.1 |
| TP7 | 40 | 100 | nd | nd | nd | nd | nd | nd |
| Treatment | Aboveground Parts Biomass (mg) | Underground Part Biomass (mg) | Total Biomass (mg) | |
|---|---|---|---|---|
| Stem | Leaf | Root | ||
| TP0 | 299.20 ± 0.59 a | 297.33 ± 8.29 a | 302.17 ± 1.73 bc | 898.70 ± 8.93 a |
| TP1 | 298.47 ± 1.32 a | 300.85 ± 1.24 a | 299.37 ± 0.43 cd | 898.68 ± 2.98 a |
| TP2 | 300.53 ± 0.56 a | 296.80 ± 2.90 a | 297.40 ± 0.69 d | 894.73 ± 3.30 a |
| TP3 | 300.53 ± 0.18 a | 284.00 ± 4.04 a | 297.03 ± 0.58 d | 881.57 ± 4.08 a |
| TP4 | 299.87 ± 1.48 a | 304.90 ± 2.83 a | 292.70 ± 1.67 e | 897.47 ± 2.69 a |
| TP5 | 301.13 ± 0.39 a | 299.40 ± 2.55 a | 308.23 ± 1.65 a | 908.77 ± 0.99 a |
| TP6 | 301.13 ± 1.39 a | 290.95 ± 0.38 a | 304.07 ± 1.24 b | 896.15 ± 1.65 a |
| TP7 | 285.43 ± 0.37 b | 254.78 ± 5.73 b | 285.43 ± 0.32 f | 825.65 ± 7.49 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, H.; Zheng, K.; Lu, Q.; Sun, S.; Li, C.; Xie, H. Photosynthetic Performance and Phytoremediation Potential of Narrow Crown Black-Cathay Poplar Under Combined Cadmium and Phenol Pollution. Forests 2025, 16, 1531. https://doi.org/10.3390/f16101531
Tian H, Zheng K, Lu Q, Sun S, Li C, Xie H. Photosynthetic Performance and Phytoremediation Potential of Narrow Crown Black-Cathay Poplar Under Combined Cadmium and Phenol Pollution. Forests. 2025; 16(10):1531. https://doi.org/10.3390/f16101531
Chicago/Turabian StyleTian, Huimei, Kaixin Zheng, Qiyun Lu, Siyuan Sun, Chuanrong Li, and Huicheng Xie. 2025. "Photosynthetic Performance and Phytoremediation Potential of Narrow Crown Black-Cathay Poplar Under Combined Cadmium and Phenol Pollution" Forests 16, no. 10: 1531. https://doi.org/10.3390/f16101531
APA StyleTian, H., Zheng, K., Lu, Q., Sun, S., Li, C., & Xie, H. (2025). Photosynthetic Performance and Phytoremediation Potential of Narrow Crown Black-Cathay Poplar Under Combined Cadmium and Phenol Pollution. Forests, 16(10), 1531. https://doi.org/10.3390/f16101531






