Shading Effects on the Growth and Physiology of Endangered Hopea hainanensis Merr. & Chun Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
2.2.1. Plant Materials
2.2.2. Experimental Design
2.2.3. Data Collection
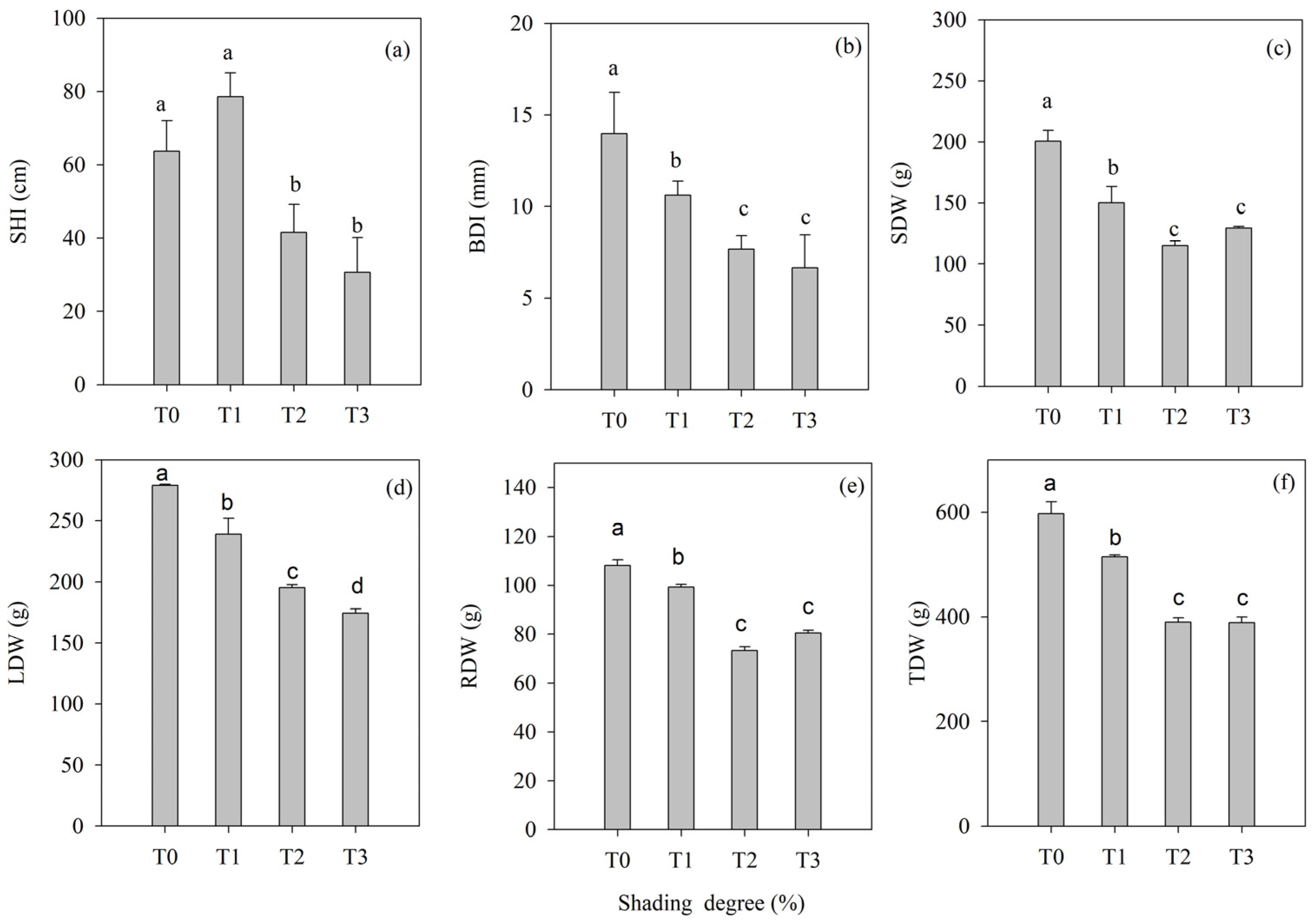
- Growth parameters: Five randomly selected seedlings were sampled per treatment at experiment initiation (June 2022) and termination (June 2023). The seedling height (SH) and basal diameter (BD; measured at the ground) were measured using steel tape (8208, Deli Group Co., Ltd., Ningbo, China) and digital calipers (SHG-200MM, Shanghai Tool Works Co., Ltd., Shanghai, China), respectively. Growth over the experimental period was calculated by subtracting the initial measurements from the final values. After thoroughly rinsing the harvested seedlings, the leaves, stems, and roots were separated. Tissues were oven-dried (105 °C for 30 min to ensure enzyme deactivation, then 80 °C to constant mass), and the dry weight of the leaves (LDW), stems (SDW), and roots (RDW) was recorded using an analytical balance, as well as the total biomass (TDW) [45].
- Leaf morphometrics: For each seedling, five mature leaves from the upper canopy were analyzed using a leaf area meter (LI-3000C, LI-COR Biosciences, Lincoln, NE, USA) to determine the leaf length (LL), width (LW), and area (LA). A digital micrometer (SYNTEK ST-124, Zhejiang Deqing Shengtai Core Electronics Technology Co., Ltd., Deqing, China) was used to quantify leaf thickness (LT). A SPAD chlorophyll meter (JN-4N, Zhengzhou Jinnong Technology Co., Ltd., Zhengzhou, China) was used to record the leaf chlorophyll content. After measurement, leaves were dried (80 °C, 72 h) and weighed (0.01 mg precision) to calculate the specific leaf area (SLA = LA/dry mass) [46].
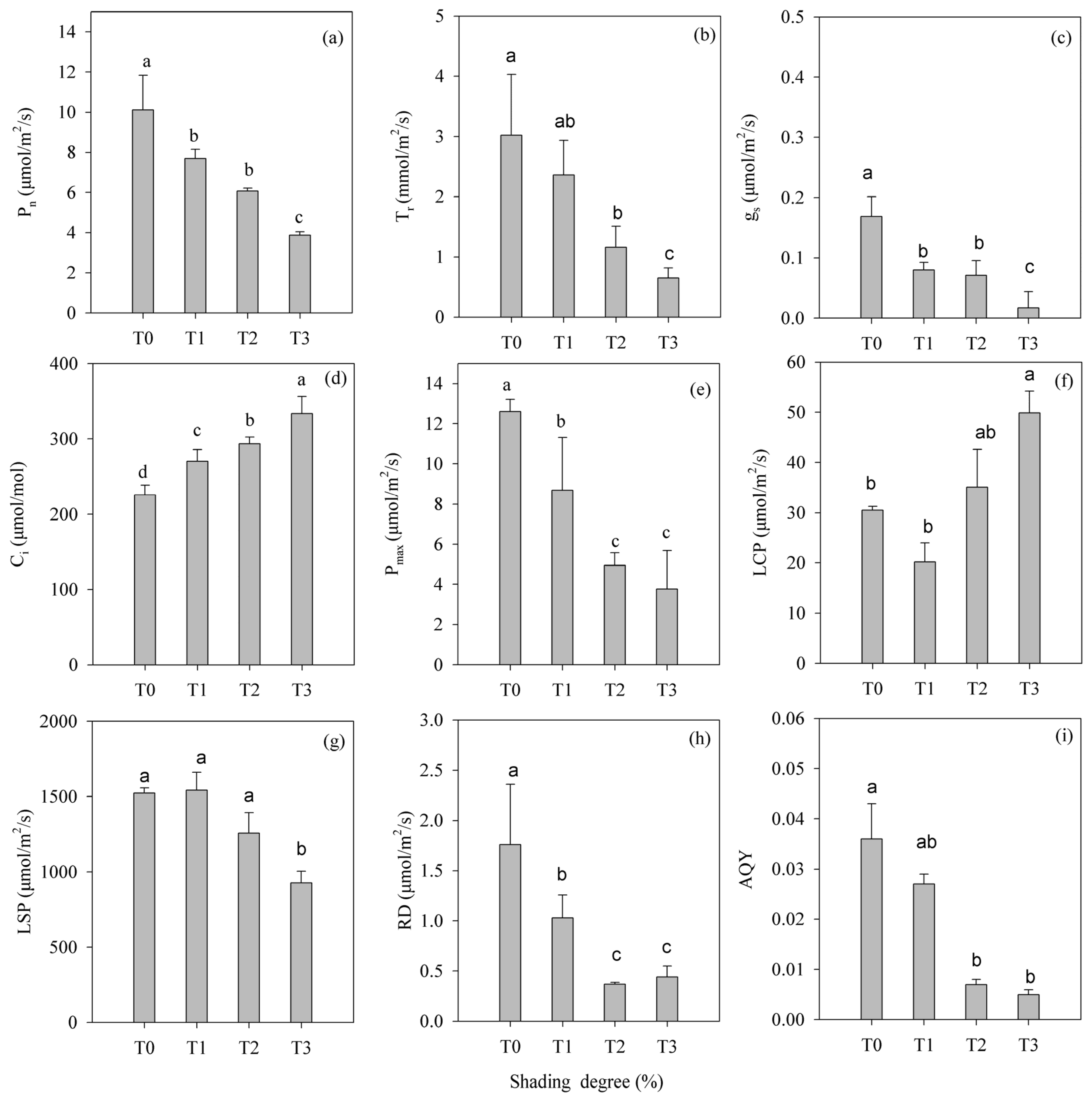
- Photosynthetic characterization: Photosynthetic light-response curves were generated over a photosynthetically active radiation (PAR) gradient of 0–1800 μmol/m2/s (12-step protocol) using a Li-6800 Portable Photosynthesis System (LI-COR Biosciences, Lincoln, NE, USA) for five mature leaves per treatment. The following settings were used: 500 μmol/s airflow, 400 μmol·mol−1 reference CO2 level, 25 °C leaf chamber temperature, 50% relative humidity, and 10,000 rpm cooling fan speed. Real-time measurements captured the intercellular CO2 concentration (Ci), net photosynthetic rate (Pn), stomatal conductance (gs), and transpiration rate (Tr). A non-linear regression analysis of the light-response data was implemented to determine the apparent quantum yield (AQY), dark respiration rate (RD), light compensation point (LCP), light saturation point (LSP), and maximum photosynthetic capacity (Pmax) following established protocols [29].
2.3. Data Statistics and Analysis
3. Results
3.1. Shading Intensity Effects on Hopea hainanensis Merr. & Chun Seedling Growth
3.2. Shading Intensity Effects on Leaf Morphology in H. hainanensis Seedlings
3.3. Shading Intensity Effects on Photosynthetic Parameters in H. hainanensis Seedlings
3.4. Comprehensive Evaluation of Shade Tolerance in H. hainanensis Seedlings
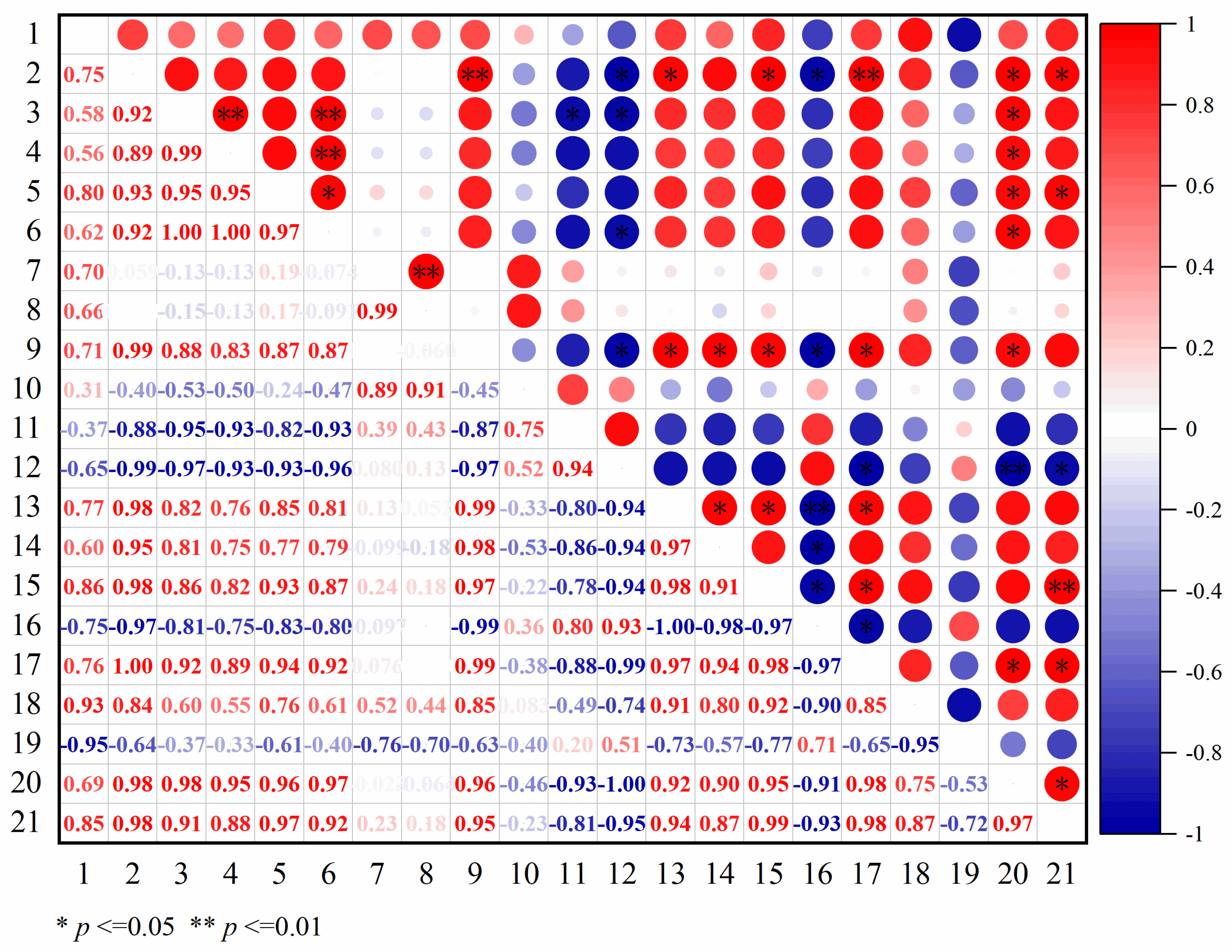
3.4.1. Trait Correlations in the Shading Experiment
3.4.2. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, D.L.; Jin, Y.Q.; Cui, M.F.; Wang, H.; Jiang, H.; Zhu, Y.Y. Effects of shading on photosynthetic characteristics and leaf anatomical structure of Cercidiphyllum japonicum seedlings. Acta Bot. Boreal.-Occident. Sin. 2019, 39, 1053–1063. [Google Scholar]
- Umesh, M.R.; Angadi, S.; Begna, S.; Gowda, P.; Prasad, P.V.V. Shade tolerance response of legumes in terms of biomass accumulation, leaf photosynthesis, and chlorophyll pigment under reduced sunlight. Crop Sci. 2023, 63, 278–292. [Google Scholar] [CrossRef]
- Chai, S.F.; Tang, J.M.; Mallik, A.; Shi, Y.C.; Zou, R.; Li, J.T.; Wei, X. Eco-physiological basis of shade adaptation of Camellia nitidissima, a rare and endangered forest understory plant of Southeast Asia. BMC Ecol. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.R.; Zhang, Z.H.; Zhang, C.L.; Zhang, J.Q.; Ran, A. Ecophysiological responses of three tree species to a high altitude environment in the southeastern Tibetan plateau. Forests 2018, 9, 48. [Google Scholar] [CrossRef]
- Lee, K.-A.; Kim, Y.-N.; Kantharaj, V.; Lee, Y.B.; Woo, S.Y. Seedling growth and photosynthetic response of Pterocarpus indicus L. to shading stress. Plant Signal. Behav. 2023, 18, 2245625. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.C.; Hu, H.L.; Chen, H.; Hu, T.X.; Zhang, J.; Tie, D.X.; Zhang, C.H.; Liu, X.J. Effects of shading on growth and photosynthetic physiological characteristics of Phoebe zhennan seedlings. J. Northwest A&F Univ. (Nat. Sci. Ed.) 2020, 48, 56–64. [Google Scholar]
- Chen, C.; Jin, Z.X.; Yuan, M.; Luo, G.Y.; Li, Y.L.; Shan, F.Q. Seasonal variation of photosynthetic characteristics in endangered Magnolia sinostellata seedlings under different light intensities. J. Zhejiang A&F Univ. 2022, 39, 950–959. [Google Scholar]
- Shi, K.; Li, Z.; Zhang, W.J.; He, X.; Zeng, Y.L.; Tan, X.F. Effects of different light conditions on growth, photosynthetic diurnal variation and chlorophyll fluorescence parameters of Vernicia fordii seedlings. J. Cent. South Univ. For. Technol. 2018, 38, 35–42+50. [Google Scholar]
- Liu, J.C.; Zhao, L.J.; Zhu, L.Q. Effects of shading on growth and photosynthetic characteristics of three Magnoliaceae seedlings. Guihaia 2020, 40, 1159–1168. [Google Scholar]
- Xue, G.Y.; Wu, J.D.; Zhou, B.J.; Zhu, X.P.; Zeng, J.; Ma, Y.; Wang, Y.N.; Jia, H.Y. Effects of shading on the growth and photosynthetic fluorescence characteristics of Castanopsis hystrix seedlings of top community-building species in southern subtropical China. Forests 2023, 14, 1659. [Google Scholar] [CrossRef]
- Li, X.M.; Jiang, M.; Ren, Y.C.; Pang, J.S.; Ren, J.J.; Li, G.F.; Yuan, Y.C.; Xing, X.D.; Zhou, M.M.; Wang, J.M.; et al. Growth, physiological, and transcriptome analyses reveal Mongolian Oak seedling responses to shading. Forests 2024, 15, 538. [Google Scholar] [CrossRef]
- Tang, X.L.; Liu, G.Z.; Jiang, J.; Liu, B.; Zhang, Y.X.; Di, L. Effects of shading on chlorophyll fluorescence characteristics and energy allocation in one-year and three-year-old Phoebe bournei seedlings. Chin. J. Ecol. 2020, 39, 3247–3254. [Google Scholar]
- Azevedo, G.F.C.; Marenco, R.A. Growth and physiological changes in saplings of Minquartia guianensis and Swietenia macrophylla during acclimation to full sunlight. Photosynthetica 2012, 50, 86–94. [Google Scholar] [CrossRef]
- Xu, S.S.; Tang, Y.; Zhong, M.H.; Li, L.Y.; Wu, L.H.; Lin, K.M.; Cao, G.Q.; Ye, Y.Q. Effects of shading on growth, photosynthetic characteristics and nutrient content of Aglaonema spp. Pratac. Sci. 2022, 39, 2083–2094. [Google Scholar]
- Wang, Z.X.; Zhu, J.M.; Wang, J.; Wang, Y.; Lu, Y.X.; Zheng, Q.R. Responses of photosynthetic characteristics and biomass allocation of Phoebe bournei saplings to light environment. Acta Ecol. Sin. 2012, 32, 3841–3848. [Google Scholar] [CrossRef]
- Kanga, D.B.; Sungb, J.W.; Leec, D.H. Effects of shading and fertilizer treatments on the growth characteristicsof Chamaecyparis obtusa (S. et Z.) Endlicher seedlings. For. Sci. Technol. 2021, 17, 125–134. [Google Scholar]
- Duan, R.Y.; Ma, Y.H.; Yang, L.M. Effects of shading on photosynthetic pigments and photosynthetic parameters of Lespedeza buergeri seedlings. IOP Conf. Ser. Mater. Sci. Eng. 2018, 452, 022158. [Google Scholar] [CrossRef]
- Luo, G.Y.; Chen, C.; Li, Y.L.; Jin, Z.X. Effects of light intensity on photosynthetic characteristics of endangered Ulmus elongata. Chin. J. Ecol. 2021, 40, 980–988. [Google Scholar]
- Yin, D.S.; Shen, H.L. Shade tolerance of forest plants and their morphological and physiological adaptations: A review. Chin. J. Appl. Ecol. 2016, 27, 2687–2698. [Google Scholar]
- Tang, Y.; Yang, P.R.; Lü, N.N.; Liu, Z.H.; Zhong, S.F.; Huang, J.H.; Shen, Z.Y.; Zheng, X.Y.; Xu, S.S.; Cao, G.Q.; et al. Effects of shading on growth and photosynthetic characteristics of Cunninghamia lanceolata seedlings. Chin. J. Appl. Environ. Biol. 2023, 29, 1084–1092. [Google Scholar]
- Lv, N.N.; Liu, Z.H.; Yang, P.R.; Zhong, S.F.; Zheng, X.Y.; Tang, Y.; Ye, Y.Q.; Cao, G.Q.; Xu, S.S. Effects of different shading treatments on growth and soil carbon and nitrogen metabolic enzyme activities of Cunninghamia lanceolata seedlings. Acta Ecol. Sin. 2024, 44, 3600–3611. [Google Scholar]
- Puértolas, J.; Benito, L.F.; Peñuelas, J.L. Effects of nursery shading on seedling quality and post-planting performance in two mediterranean species with contrasting shade tolerance. New For. 2009, 38, 295–308. [Google Scholar] [CrossRef]
- Abiem, I.; Kenfack, D.; Chapman, H. Assessing the impact of abiotic and biotic factors on seedling survival in an African montane forest. Front. For. Glob. Change 2023, 6, 1108257. [Google Scholar] [CrossRef]
- Martini, F.; Zou, C.; Goodale, U.M. Intrinsic biotic factors and microsite conditions drive seedling survival in a species with masting reproduction. Ecol. Evol. 2019, 9, 14261–14272. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.J.; Condit, R.; Hubbell, S.P.; Comita, L.S. Abiotic niche partitioning and negative density dependence drive tree seedling survival in a tropical forest. Proc. R. Soc. B Biol. Sci. 2017, 284, 20172210. [Google Scholar] [CrossRef] [PubMed]
- Ssali, F.; Moe, S.; Sheil, D. Damage to artificial seedlings across a disturbed Afromontane forest landscape. Biotropica 2019, 51, 652–663. [Google Scholar] [CrossRef]
- Fang, Z.S.; Zhong, C.R.; Cheng, C.; Lü, X.B.; Chen, X. Effects of shading on the growth and biomass allocation of Hernandia nymphaeifolia seedlings. Mol. Plant Breed. 2024, 22, 7837–7844. [Google Scholar]
- Rao, D.D.; Han, Y.; Wu, E.H.; Chen, Y. Growth and photosynthetic characteristics of Populus qiongdaoensis seedlings under different light intensities. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2024, 44, 61–69. [Google Scholar]
- Zhou, H.; Wei, R.P.; Li, J.Y.; Su, Y.; Hu, D.H.; Zheng, H.Q. Effects of light intensity on growth and photosynthetic characteristics of Michelia chapensis seedlings. Chin. J. Ecol. 2024, 43, 709–715. [Google Scholar]
- Liu, B.E.; Liao, B.W. Physiological and ecological responses of Acanthus ilicifolius seedlings to different light intensities under tidal environments. For. Res. 2013, 26, 192–199. [Google Scholar]
- Gao, Z.; Khalid, M.; Jan, F.; Saeed-ur-Rahman; Jiang, X.; Yu, X. Effects of light-regulation and intensity on the growth, physiological and biochemical properties of Aralia elata (miq.) seedlings. S. Afr. J. Bot. 2019, 121, 456–462. [Google Scholar] [CrossRef]
- Xiao, Y.X.; Yin, L.H.; Yu, W.B.; Tang, J.W. Study on population structure and seedling regeneration of ex-situ cultivated Hopea hainanensis. Plant Sci. J. 2023, 41, 604–612. [Google Scholar]
- Luo, W.; Xu, H.; Li, Y.P.; Xie, C.P.; Lu, C.Y.; Liang, C.S.; Su, H.H.; Li, Z.L. Population structure and quantitative dynamics of Hopea hainanensis, a wild plant with extremely small populations. For. Res. 2023, 36, 169–177. [Google Scholar]
- Zang, R.G.; Dong, M.; Li, J.Q.; Chen, X.Y.; Zeng, S.J.; Jiang, M.X.; Li, Z.Q.; Huang, J.H. Research on conservation and restoration techniques for typical wild plants with extremely small populations. Acta Ecol. Sin. 2016, 36, 7130–7135. [Google Scholar]
- Song, Y.B.; Shen-Tu, X.L.; Dong, M. Intraspecific variation of samara dispersal traits in the endangered tropical tree Hopea hainanensis (Dipterocarpaceae). Front. Plant Sci. 2020, 11, 599764. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.J.; Ma, J.M.; Huang, R.L.; Wang, Y.; Jiang, Y.; Ling, Y.M.; Yang, J.S.; Liang, H.Z.; Liu, X.S.; Liao, N.Y. The effects of shading on the photosynthetic performance of endangered plant Horsfieldia Hainanensis seedlings. Forests 2024, 15, 3. [Google Scholar] [CrossRef]
- Lu, X.X.; Zang, R.G.; Ding, Y.; Huang, J.H.; Xu, Y. Habitat characteristics of Hopea hainanensis, a wild plant with extremely small populations, and their effects on seedling abundance. Biodivers. Sci. 2020, 28, 289–295. [Google Scholar]
- Laurance, W.F. Reflections on the tropical deforestation crisis. Biol. Conserv. 1999, 91, 109–117. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.B.; Nong, S.Q.; Li, D.H.; Li, Y.L.; Song, J.Y. Developmental characteristics of Hopea hainanensis population under two different protection modes. Acta Ecol. Sin. 2019, 39, 3740–3748. [Google Scholar]
- Wang, S.B.; Liang, H.P.; Wang, H.L.; Li, L.Z.; Xu, Y.; Liu, Y.; Liu, M.; Wei, J.P.; Ma, T.; Le, C.; et al. The chromosome-scale genomes of Dipterocarpus turbinatus and Hopea hainanensis (Dipterocarpaceae) provide insights into fragrant oleoresin biosynthesis and hardwood formation. Plant Biotechnol. J. 2021, 20, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Zhang, H.L.; Zhang, L.; Nizamani, M.M.; Zhou, T.X.; Zhang, H.Y.; Liu, T.T. Genetic diversity assessment of Hopea hainanensis in Hainan Island. Front. Plant Sci. 2022, 14, 1075102. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.Y.; Jiang, X.C.; Song, S.Q.; Lei, Y.B.; Yin, S.H. Changes in germinability and desiccation-sensitivity of recalcitrant Hopea hainanensis Merr. et Chun seeds during development. Seed Sci. Technol. 2007, 35, 21–31. [Google Scholar] [CrossRef]
- Li, G.Y.; Chen, F.F.; Yang, Z.L. Study on seedling raising techniques of Hopea hainanensis seeds. Trop. For. 2015, 43, 7–8+13. [Google Scholar]
- Wen, X.Y.; Huang, F.F.; Gan, X.H.; Zhang, W.Q.; Huang, Y.H.; Xu, X.Y.; Zhou, Y. Introduction performance of Hopea hainanensis and Vatica mangachapoi in Guangdong Arboretum. For. Environ. Sci. 2017, 33, 52–56. [Google Scholar]
- Zhang, P.; Pang, S.J.; Liu, S.L.; Chen, H.H.; Duan, R.M.; Zeng, Q.Y. Effects of shading on growth and chlorophyll fluorescence parameters of Keteleeria cyclolepis seedlings. Acta Bot. Boreal.-Occident. Sin. 2023, 43, 1716–1722. [Google Scholar]
- Yao, Y.H.; Li, L.; Yu, L.; Wang, W.G.; Chen, K.M.; Fu, W.Y.; Zhou, X.Y.; Xia, G.H. Effects of shading on growth and physiological characteristics of Photinia glabra var. magnoliaefolia seedlings. J. Northeast. For. Univ. 2025, 53, 67–78. [Google Scholar]
- Li, X.Q.; Zhang, F.L.; Yang, T.; Zhe, G.X.; Mao, C.L.; Wu, Y. Effects of shading on leaf morphology and photosynthetic parameters of endangered Horsfieldia amygdalina seedlings. Plant Physiol. J. 2019, 55, 80–90. [Google Scholar]
- Hu, X.; Xu, R.J.; Shu, Q.; Guo, W.; Zhang, J.; Shang, Z.A.; Qi, L.H. Population structure and dynamics of Hopea reticulata, an endemic plant in Ganshiling, Hainan Island. Chin. J. Trop. Crops 2020, 41, 1939–1945. [Google Scholar]
- Li, Y.P.; Xu, H.; Chen, J.; Lei, J.; Luo, W. Research status and prospects of Hopea hainanensis, a wild plant with extremely small populations. J. Trop. Subtrop. Bot. 2024, 32, 685–694. [Google Scholar]
- Jin, X.J.; Yin, X.S.; Xu, Y.T. Effects of different light and soil moisture on seed germination of three Dipterocarpaceae species. J. West China For. Sci. 2015, 44, 36–40. [Google Scholar]

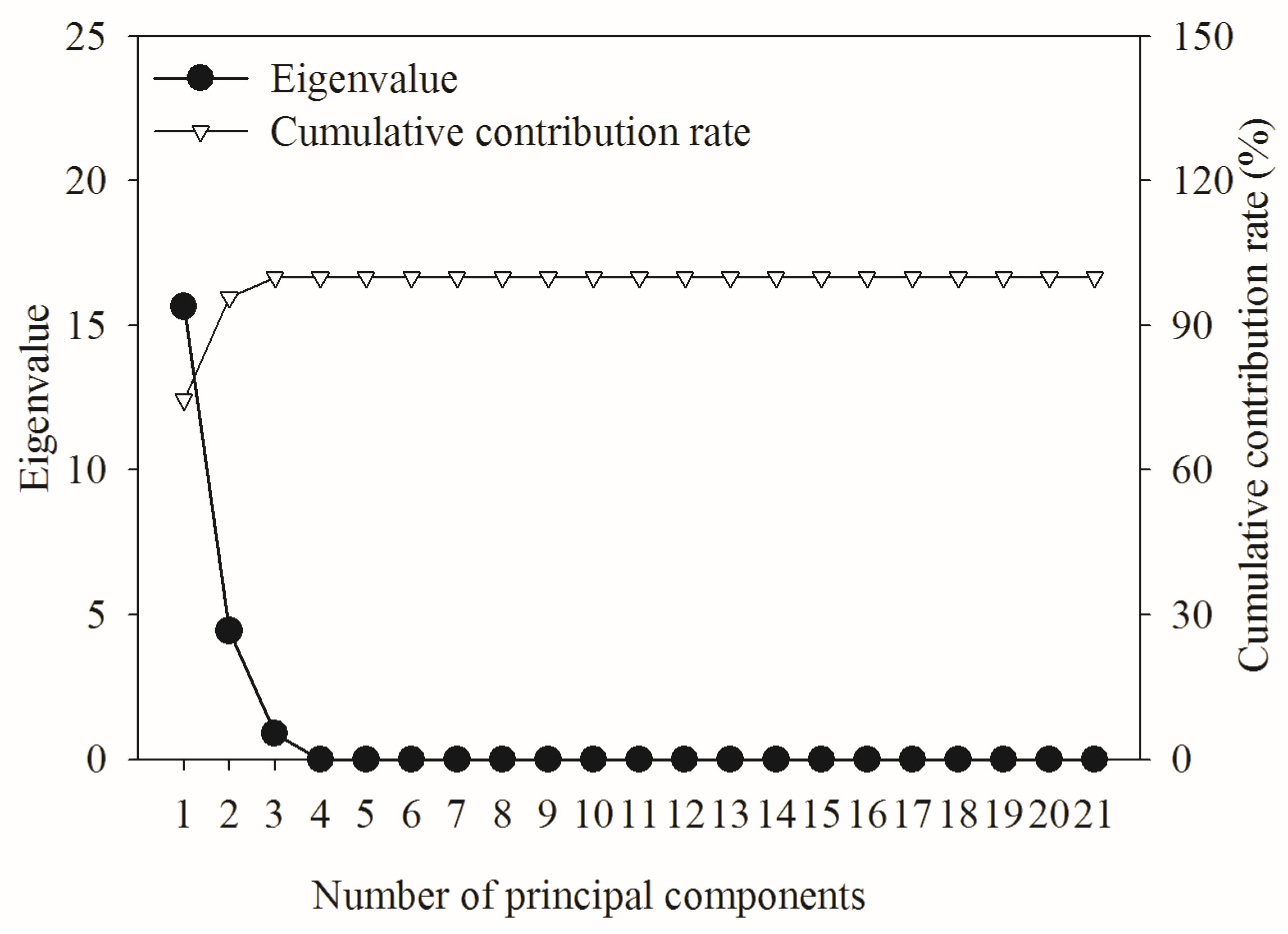
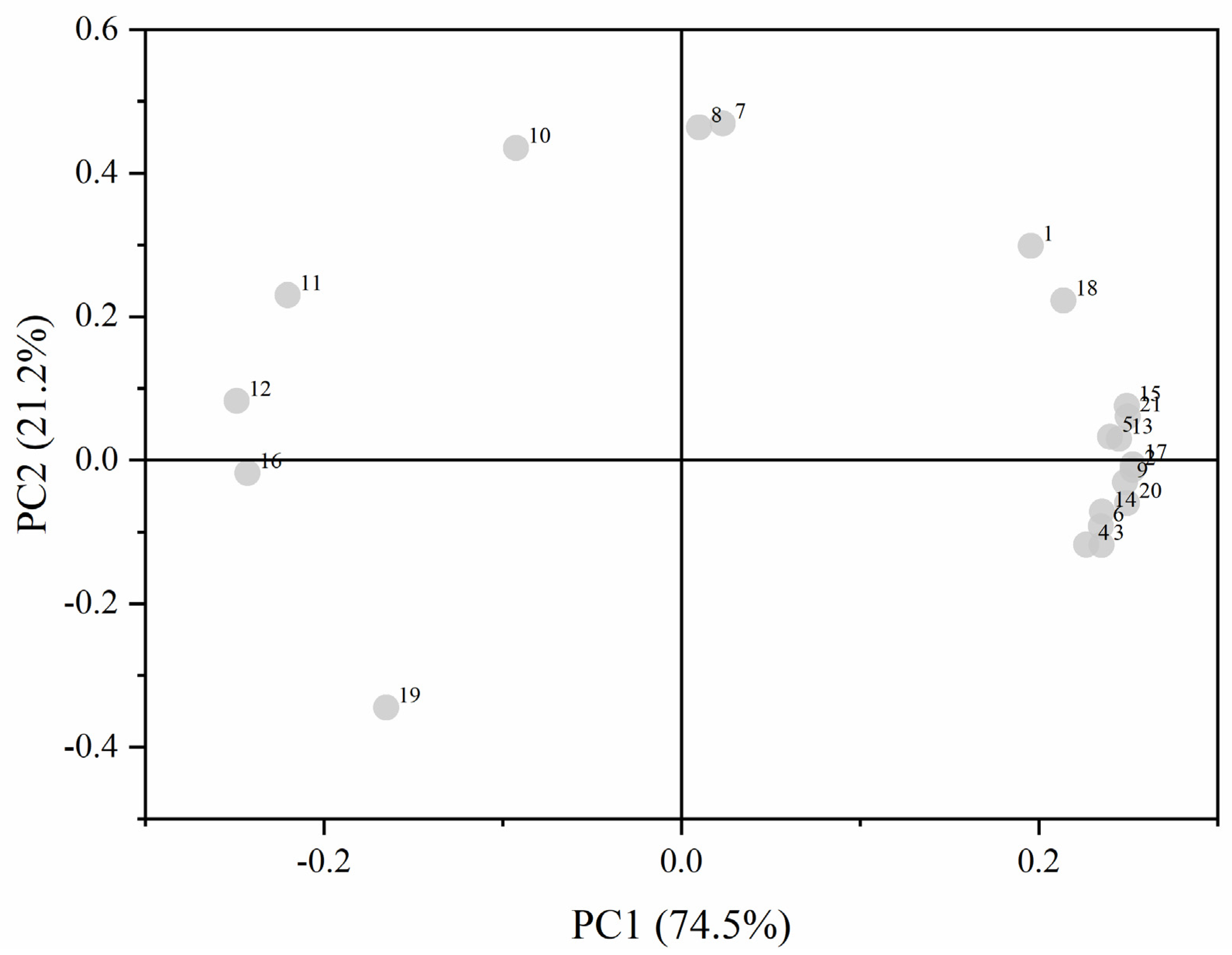
| Shading Degree | 1st Principal Component Score (Y1) | 2nd Principal Component Score (Y1) | Aggregate Score (Y) |
|---|---|---|---|
| T0 | 5.001 | −1.677 | 3.373 |
| T1 | 1.298 | 2.908 | 1.584 |
| T2 | −2.690 | 0.200 | −1.963 |
| T3 | −3.610 | −1.430 | −2.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Lin, L.; Chen, F.; Wang, X.; Shi, M.; Chen, L.; Yang, X.; Dong, X.; Zhang, M. Shading Effects on the Growth and Physiology of Endangered Hopea hainanensis Merr. & Chun Seedlings. Forests 2025, 16, 1193. https://doi.org/10.3390/f16071193
Huang C, Lin L, Chen F, Wang X, Shi M, Chen L, Yang X, Dong X, Zhang M. Shading Effects on the Growth and Physiology of Endangered Hopea hainanensis Merr. & Chun Seedlings. Forests. 2025; 16(7):1193. https://doi.org/10.3390/f16071193
Chicago/Turabian StyleHuang, Chuanteng, Ling Lin, Feifei Chen, Xuefeng Wang, Mengmeng Shi, Lin Chen, Xiaoli Yang, Xiaona Dong, and Mengwen Zhang. 2025. "Shading Effects on the Growth and Physiology of Endangered Hopea hainanensis Merr. & Chun Seedlings" Forests 16, no. 7: 1193. https://doi.org/10.3390/f16071193
APA StyleHuang, C., Lin, L., Chen, F., Wang, X., Shi, M., Chen, L., Yang, X., Dong, X., & Zhang, M. (2025). Shading Effects on the Growth and Physiology of Endangered Hopea hainanensis Merr. & Chun Seedlings. Forests, 16(7), 1193. https://doi.org/10.3390/f16071193






