Characterization of the Expansin Gene Promoters in Populus trichocarpa
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Expression Analysis of Expansin Genes
2.3. Expansin Gene and Promoter Sequences
2.4. Phylogenetic Analysis
2.5. Sequence Characterization of the Expansin Gene Promoters
2.6. Association Analysis between Expansin Gene Expression and Promoter Elements
3. Results
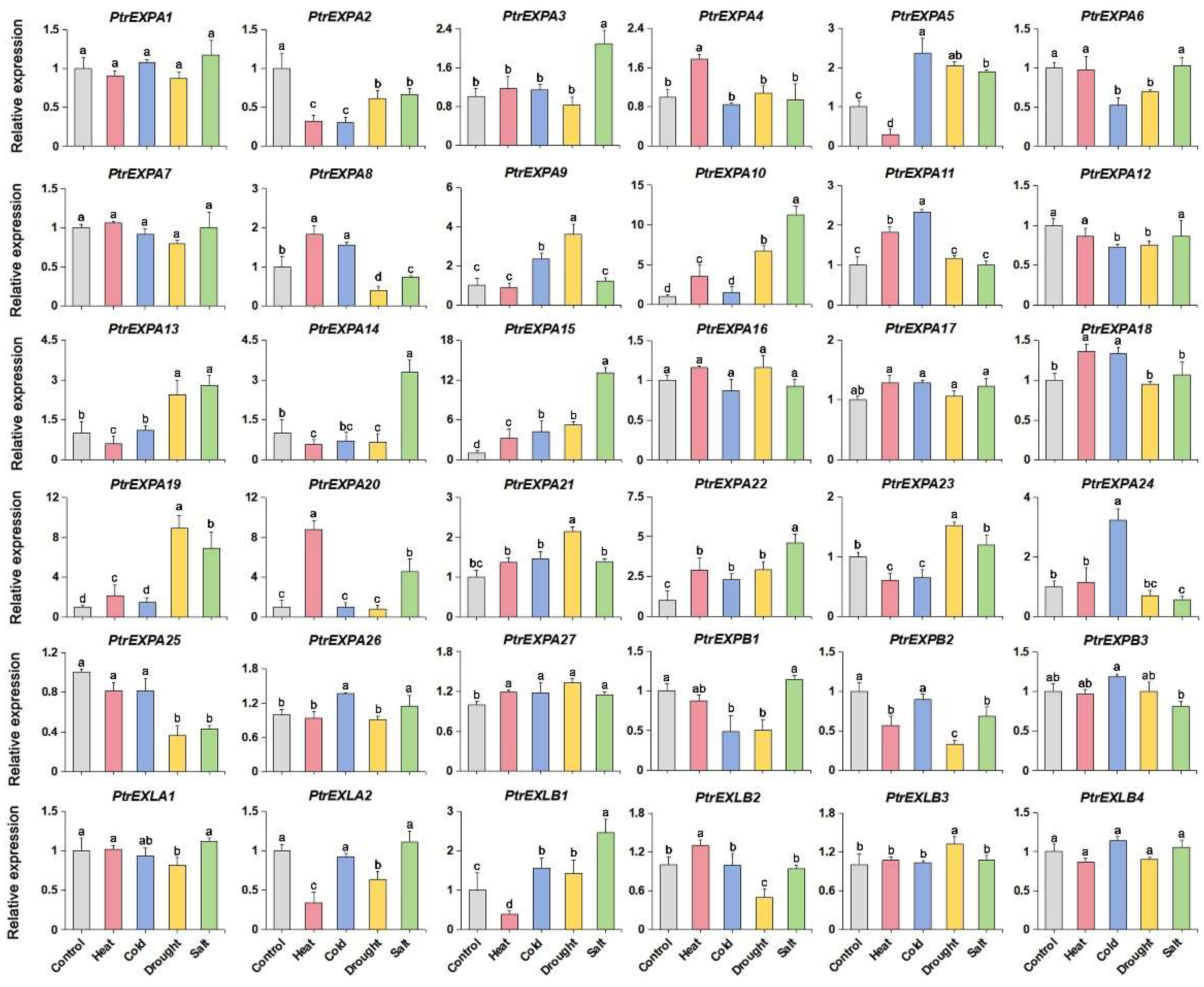
3.1. Expression Pattern of the Expansin Genes in Populus tomentosa under Stress Treatments
3.2. Base Usage Flanking the Start Codon
3.3. Base Usage in the Conserved Elements of Promoters
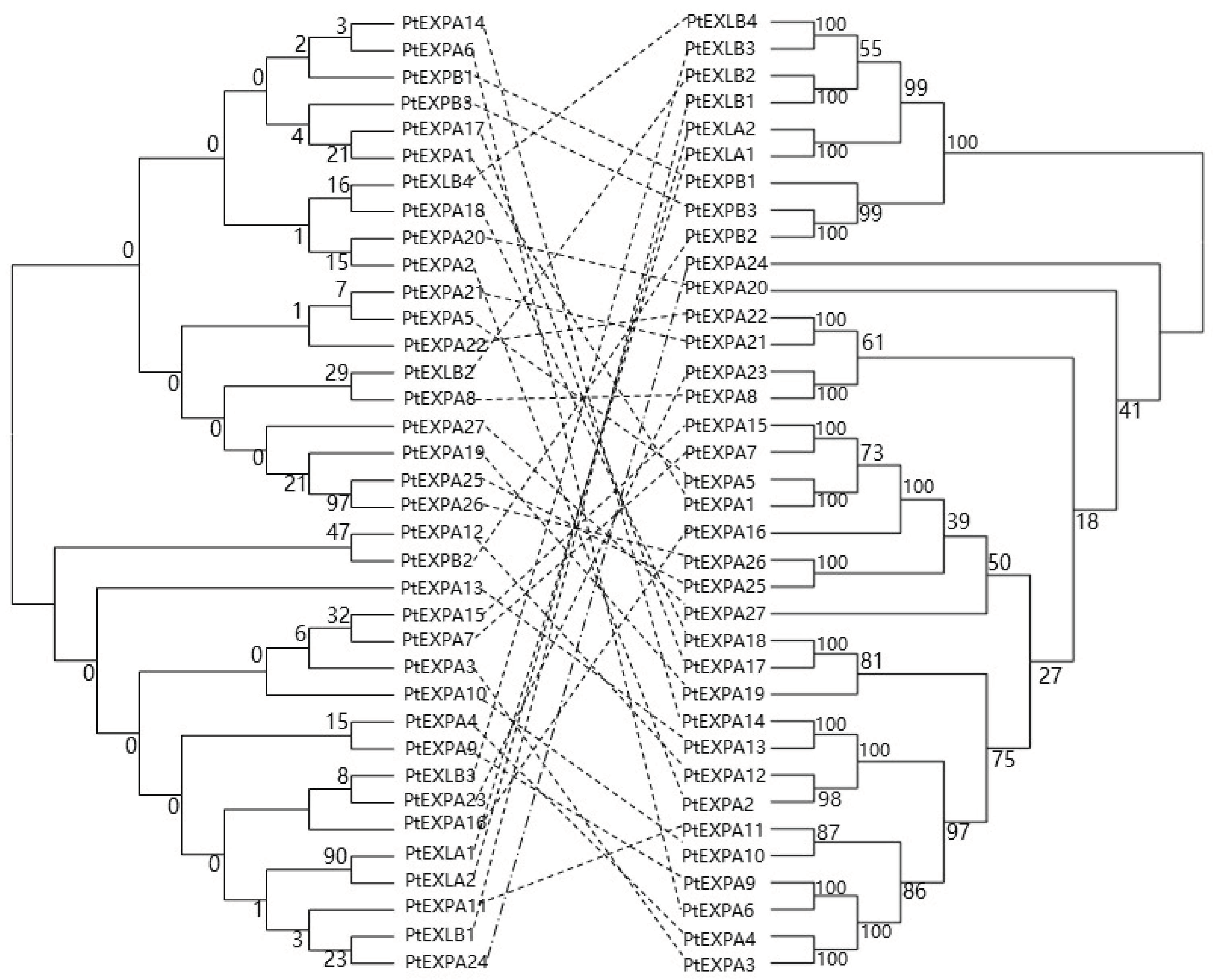
3.4. Sequence Similarity of the Promoters
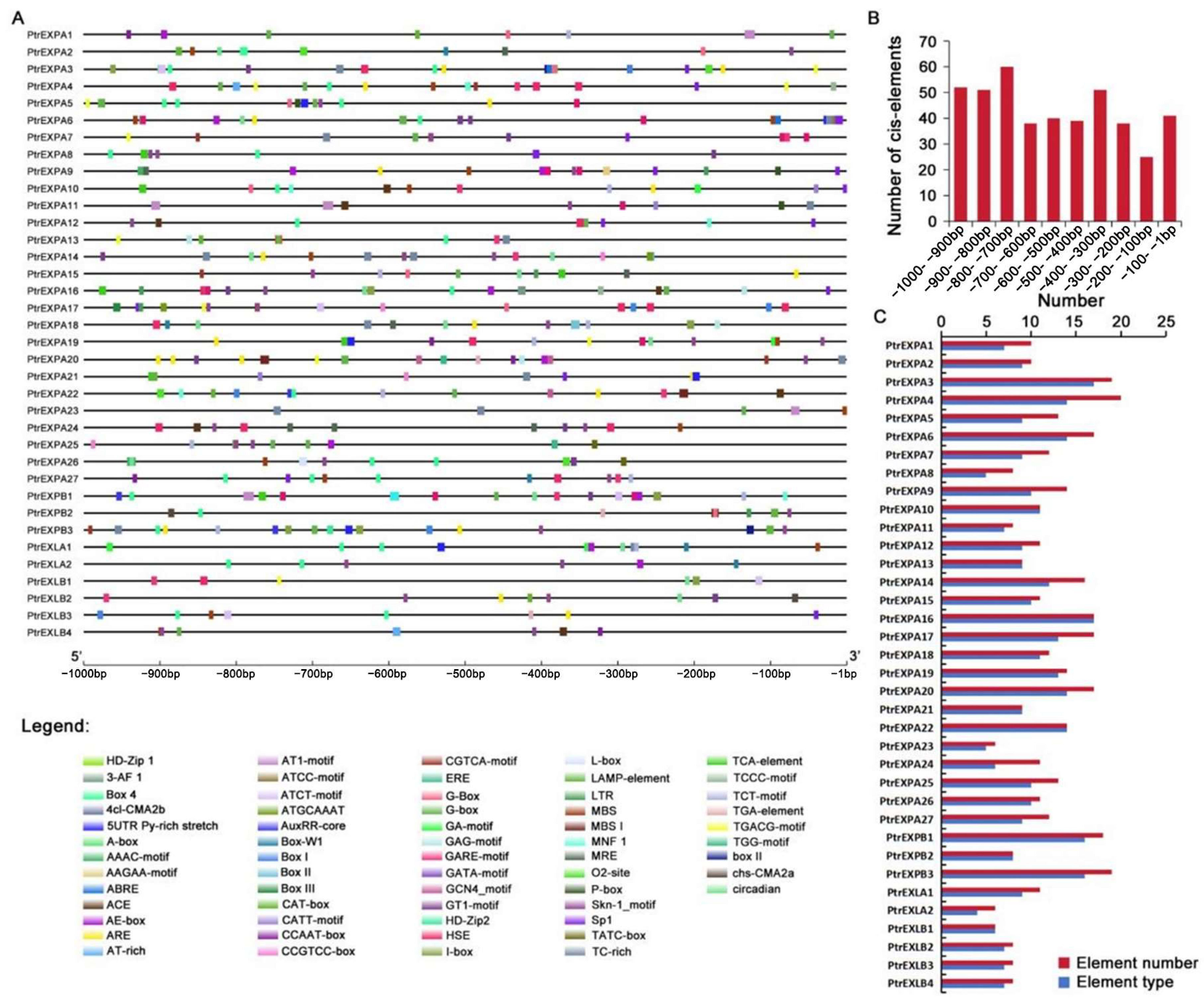
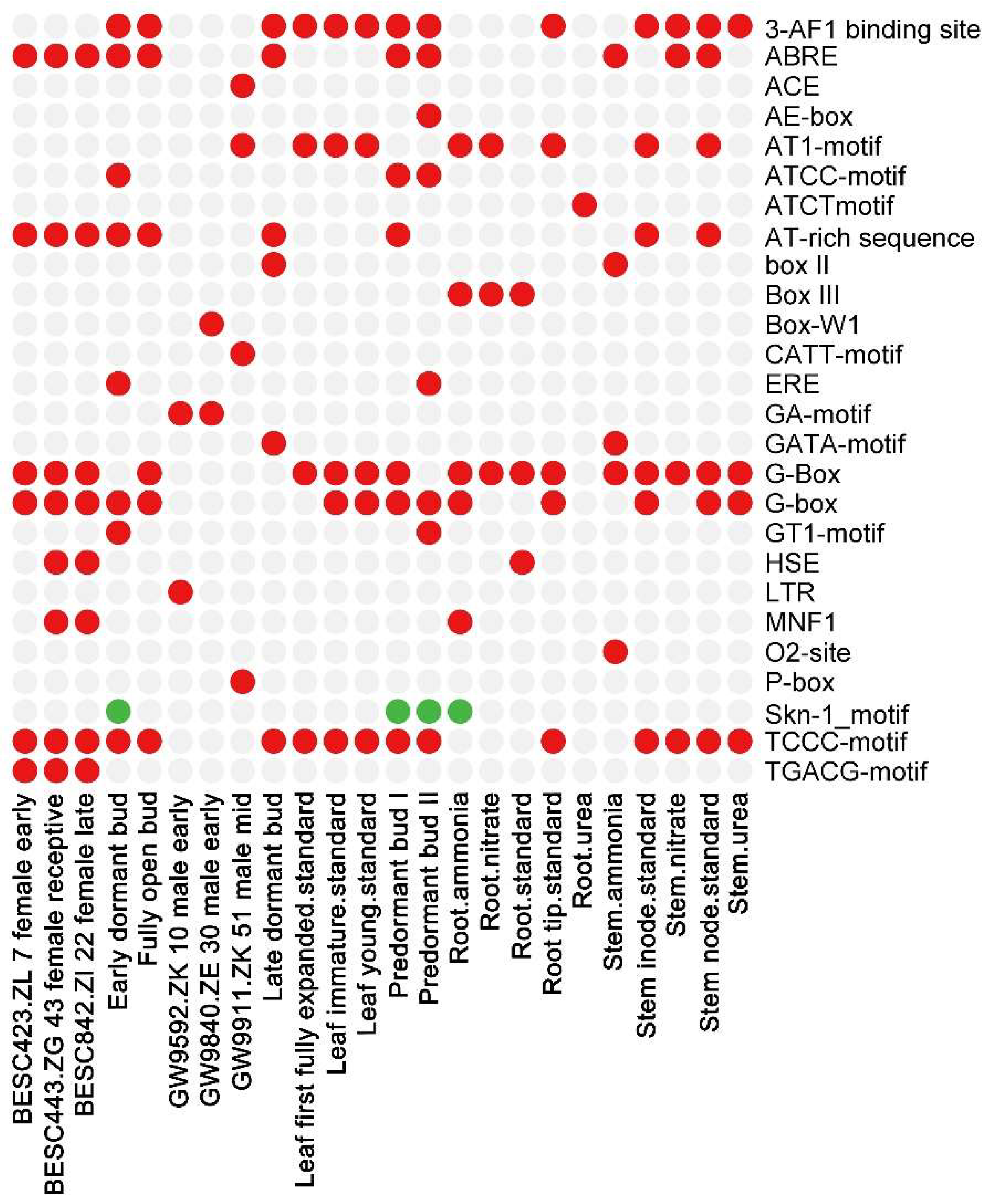
3.5. Regulatory Elements in Poplar Expansin Gene Promoters
3.6. Promoter Components in Response to Plant Tissues and Growth Conditions
4. Discussion
4.1. Phylogeny of Promoters and Coding Sequences
4.2. Promoter Characteristics in Association with Gene Expression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, T.J.; Kwon, T.H.; Kim, T.G.; Loc, N.H.; Yang, M.S. Comparing constitutive promoters using CAT activity in transgenic tobacco plants. Mol. Cells 2003, 16, 117–122. [Google Scholar] [CrossRef]
- Christensen, A.H.; Sharrock, R.A.; Quail, P.H. Maize polyubiquitin genes: Structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol. 1992, 18, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.A.; Fromm, M.E. Tissue-specific expression in transgenic maize of four endosperm promoters from maize and rice. Transgenic Res. 1997, 6, 157–168. [Google Scholar] [CrossRef]
- Yeon, M.H.; Park, C.H.; Lee, Y.E.; Roh, J.; Kim, S.K. Seed-specifically overexpressed Arabidopsis cytochrome P450 85A2 promotes vegetative and reproductive growth and development of Arabidopsis thaliana. J. Plant Biol. 2022, 65, 75–86. [Google Scholar] [CrossRef]
- Roh, J.; Lee, Y.E.; Park, C.H.; Kim, S.K. Usefulness and molecular mechanism of seed-specificity introduced by AtBZR1 and AtBES1 to improve seed yield and quality in Arabidopsis thaliana. J. Plant Biol. 2023, 66, 233–242. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, C.X.; Cheng, L.Z.; Yu, S.S.; Shen, C.J.; Pan, Y. Over-expression of OsPT2 under a rice root specific promoter Os03g01700. Plant Physiol. Biochem. 2019, 136, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Jenny, P.; Sakure, A.A.; Yadav, A.; Kumar, S. Molecular cloning and characterization of root-specific SlREO promoter of the Indian tomato (Solanum lycopersicum L.) cultivar. Funct. Plant Biol. 2024, 51, FP24063. [Google Scholar] [CrossRef]
- Vaughan, S.P.; James, D.J.; Lindsey, K.; Massiah, A.J. Characterization of FaRB7, a near root-specific gene from strawberry (Fragaria × Ananassa Duch.) and promoter activity analysis in homologous and heterologous hosts. J. Exp. Bot. 2006, 57, 3901–3910. [Google Scholar] [CrossRef]
- Zhang, X.C.; Li, M.Y.; Ruan, M.B.; Xia, Y.J.; Wu, K.X.; Peng, M. Isolation of AtNUDT5 gene promoter and characterization of its activity in transgenic Arabidopsis thaliana. Appl. Biochem. Biotechnol. 2013, 169, 1557–1565. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, J.H.; Poindexter, M.R.; Shao, Y.; Liu, W.; Lenaghan, S.C.; Ahkami, A.H.; Blumwald, E.; Stewart, C.N. Rational design and testing of abiotic stress-inducible synthetic promoters from poplar cis-regulatory elements. Plant Biotechnol. J. 2021, 19, 1354–1369. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shao, Y.; Chaffin, T.A.; Lee, J.H.; Poindexter, M.R.; Ahkami, A.H.; Blumwald, E.; Stewart, C.N. Performance of abiotic stress-inducible synthetic promoters in genetically engineered hybrid poplar (Populus tremula × Populus alba). Front. Plant Sci. 2022, 13, 1011939. [Google Scholar] [CrossRef]
- Yang, Y.G.; Tagaloguin, P.; Chaffin, T.A.; Shao, Y.H.; Mazarei, M.; Millwood, R.J.; Stewart, C.N. Drought stress-inducible synthetic promoters designed for poplar are functional in rice. Plant Cell Rep. 2024, 43, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Xiao, Y.X.; Tian, Y.K.; Wang, C.H.; Yang, S.L.; Li, Y. Sequence characters analysis of a β-Tubulin gene and its promoter in apple. Acta Agric. Boreali Sin. 2018, 33, 78–85. [Google Scholar] [CrossRef]
- Juven-Gershon, T.; Kadonaga, J.T. Regulation of gene expression via the core promoter and the basal transcriptional machinery. Dev. Biol. 2010, 339, 225–229. [Google Scholar] [CrossRef]
- Jin, J.; Li, L.L.; Fan, D.Y.; Du, Y.W.; Jia, H.C.; Yang, L.; Jia, W.S.; Hao, Q. Budding mutation reprogrammed flavonoid biosynthesis in jujube by deploying MYB41 and bHLH93. Plant Physiol. Biochem. 2024, 211, 108665. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Z.X.; He, G.H.; Liu, M.N.; Liu, C.; Liu, M.M.; Lv, T.T.; Wang, A.M.; Wang, Y.; Zhao, M.Z. Genome-wide identification and integrated analysis of the FAR1/FHY3 gene family and genes expression analysis under methyl jasmonate treatment in Panax ginseng C. A. Mey. BMC Plant Biol. 2024, 24, 549. [Google Scholar] [CrossRef]
- Hendelman, A.; Zebell, S.; Rodriguez-Leal, D.; Dukler, N.; Lippman, Z.B.; Robitaille, G.; Wu, X.L.; Kostyun, J.; Tal, L.; Wang, P.P.; et al. Conserved pleiotropy of an ancient plant homeobox gene uncovered by cis-regulatory dissection. Cell 2021, 184, 1724–1739. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Wang, Y.; Wang, X.; Wang, L.; Xu, J. The expansin gene SmEXPA13 in Salix matsudana in association with plant salt tolerance. Plant Cell Tiss. Org. 2023, 154, 219–225. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Yang, L.; Wang, X.; Zhang, J.; Xu, J. Characterization and functional analysis of the PtEXLA1 gene from poplar. Plant Biotechnol. Rep. 2024, 18, 119–128. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Ding, Y.; Xu, J. Bioinformatics analysis of the expansin gene family in poplar genome. J. Beijing For. Univ. 2014, 36, 59–67. [Google Scholar] [CrossRef]
- Dharmawardhana, P.; Brunner, A.M.; Strauss, S.H. Genome-wide transcriptome analysis of the transition from primary to secondary stem development in Populus trichocarpa. BMC Genom. 2010, 11, 150. [Google Scholar] [CrossRef]
- Yin, Z.H.; Zhou, F.W.; Chen, Y.N.; Wu, H.T.; Yin, T.M. Genome-Wide analysis of the expansin gene family in Populus and characterization of expression changes in response to phytohormone (abscisic acid) and abiotic (low-temperature) stresses. Int. J. Mol. Sci. 2023, 24, 7759. [Google Scholar] [CrossRef]
- Geisler-Lee, J.; Geisler, M.; Coutinho, P.M.; Segerman, B.; Nishikubo, N.; Takahashi, J.; Aspeborg, H.; Djerbi, S.; Master, E.; Andersson-Gunneras, S.; et al. Poplar carbohydrate-active enzymes.: Gene identification and expression analyses. Plant Physiol. 2006, 140, 946–962. [Google Scholar] [CrossRef] [PubMed]
- Gray-Mitsumune, M.; Mellerowicz, E.G.; Abe, H.; Schrader, J.; Winzell, A.; Sterky, F.; Blomqvist, K.; McQueen-Mason, S.; Teeri, T.T.; Sundberg, B. Expansins abundant in secondary xylem belong to subgroup a of the α-expansin gene family. Plant Physiol. 2004, 135, 1552–1564. [Google Scholar] [CrossRef]
- Zhou, P.; Zhu, Q.; Xu, J.; Huang, B. Cloning and characterization of a gene, AsEXP1, encoding expansin proteins inducible by heat stress and hormones in creeping bentgrass. Crop Sci. 2011, 51, 333–341. [Google Scholar] [CrossRef]
- Ogden, T.H.; Rosenberg, M.S. Multiple sequence alignment accuracy and phylogenetic inference. Syst. Biol. 2006, 55, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Shahmuradov, I.A.; Solovyev, V.V.; Gammerman, A.J. Plant promoter prediction with confidence estimation. Nucleic Acids Res. 2005, 33, 1069–1076. [Google Scholar] [CrossRef]
- Sampedro, J.; Lee, Y.; Carey, R.E.; dePamphilis, C.; Cosgrove, D.J. Use of genomic history to improve phylogeny and understanding of births and deaths in a gene family. Plant J. 2005, 44, 409–419. [Google Scholar] [CrossRef]
- Sampedro, J.; Cosgrove, D.J. The expansin superfamily. Genome Biol. 2005, 6, 242. [Google Scholar] [CrossRef]
- Deng, S.; Cheng, N.; Ding, R.; Liu, Y.; Zhang, Y.H. Cloning, sequence alignment and functional analysis of AFS gene promoter in apple and pear fruits. Acta Hortic. Sin. 2015, 42, 2353–2361. [Google Scholar] [CrossRef]
- Massin, P.; Rodrigues, P.; Marasescu, M.; van der Werf, S.; Naffakh, N. Cloning of the chicken RNA polymerase I promoter and use for reverse genetics of influenza A viruses in avian cells. J. Virol. 2005, 79, 13811–13816. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.P.; Hao, Z.; Huang, X.; Chiang, V.L. Context sequences of translation initiation codon in plants. Plant Mol. Biol. 1997, 35, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Dabi, T.; Beeche, A.; Yamamoto, R.; Lawton, M.A.; Lamb, C. Cloning and properties of a rice gene encoding phenylalanine ammonia-lyase. Plant Mol. Biol. 1995, 29, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Wray, G.A.; Hahn, M.W.; Abouheif, E.; Balhoff, J.P.; Pizer, M.; Rockman, M.V.; Romano, L.A. The evolution of transcriptional regulation in eukaryotes. Mol. Biol. Evol. 2003, 20, 1377–1419. [Google Scholar] [CrossRef]
- Lynch, M.; Scofield, D.G.; Hong, X. The evolution of transcription-initiation sites. Mol. Biol. Evol. 2005, 22, 1137–1146. [Google Scholar] [CrossRef]
- Beaudoin, J.D.; Perreault, J.P. 5′-UTR G-quadruplex structures acting as translational repressors. Nucleic Acids Res. 2010, 38, 7022–7036. [Google Scholar] [CrossRef]
- Calvo, S.E.; Pagliarini, D.J.; Mootha, V.K. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc. Natl. Acad. Sci. USA 2009, 106, 7507–7512. [Google Scholar] [CrossRef]
- Jores, T.; Tonnies, J.; Wrightsman, T.; Buckler, E.S.; Cuperus, J.T.; Fields, S.; Queitsch, C. Synthetic promoter designs enabled by a comprehensive analysis of plant core promoters. Nat. Plants 2021, 7, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Lv, Y.D.; Wang, Y.; Rose, J.K.C.; Shen, F.; Han, Z.Y.; Zhang, X.Z.; Xu, X.F.; Wu, T.; Han, Z.H. TATA box insertion provides a selection mechanism underpinning adaptations to Fe deficiency. Plant Physiol. 2017, 173, 715–727. [Google Scholar] [CrossRef]
- Joshi, C.P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987, 15, 6643–6653. [Google Scholar] [CrossRef] [PubMed]
- Bucher, P. Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J. Mol. Biol. 1990, 212, 563–578. [Google Scholar] [CrossRef]
- Sreenivasulu, G.; Senthilkumaran, B.; Sudhakumari, C.C.; Guan, G.; Oba, Y.; Kagawa, H.; Nagahama, Y. 20β-hydroxysteroid dehydrogenase gene promoter: Potential role for cyclic AMP and xenobiotic responsive elements. Gene 2012, 509, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Bezhani, S.; Sherameti, I.; Pfannschmidt, T.; Oelmuller, R. A repressor with similarities to prokaryotic and eukaryotic DNA helicases controls the assembly of the CAAT box binding complex at a photosynthesis gene promoter. J. Biol. Chem. 2001, 276, 23785–23789. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M. An analysis of 5′-noncoding sequences from 699 vertebrates messenger RNAs. Nucleic Acids Res. 1987, 15, 8125–8148. [Google Scholar] [CrossRef]
- Kozak, M. Recognition of AUG and alternative initiator codons is augmented by G in position +4 but is not generally affected by the nucleotides in positions +5 and +6. Embo J. 1997, 16, 2482–2492. [Google Scholar] [CrossRef]
- Liu, Q.P.; Tan, J.; Xue, Q.Z. Effect of the flanking sequence architecture of AUG, a initiator codon on gene expression level in rice. Sci. Agric. Sin. 2004, 37, 625–629. [Google Scholar]
- Chen, F.; Bradford, K.J. Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol. 2000, 124, 1265–1274. [Google Scholar] [CrossRef]
- Wang, Y. Construction of Castor (Ricinus communis L.) Yeast-One Hybrid cDNA Library and Screening of Skn-1 Binding Protein. Master’s thesis, Inner Mongolia Minzu University, Inner Mongolia, China, 2018. [Google Scholar]
- Guo, W.; Zhao, J.; Li, X.; Qin, L.; Yan, X.L.; Liao, H. A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J. 2011, 66, 541–552. [Google Scholar] [CrossRef]
- Ookawara, R.; Satoh, S.; Yoshioka, T.; Ishizawa, K. Expression of α-expansin and xyloglucan endotransglucosylase/hydrolase genes associated with shoot elongation enhanced by anoxia, ethylene and carbon dioxide in arrowhead (Sagittaria pygmaea Miq.) tubers. Ann. Bot. 2005, 96, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.; Bourdais, G.; Reidy, B.; Bencivenni, C.; Massonneau., A.; Condamine, P.; Rolland, G.; Conéjéro, G.; Rogowsky, P.; Tardieu, F. Association of specific expansins with growth in maize leaves is maintained under environmental, genetic, and developmental sources of variation. Plant Physiol. 2007, 143, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.C.; Tian, J.; Belanger, F.C.; Huang, B.R. Identification and characterization of an expansin gene AsEXP1 associated with heat tolerance in C3Agrostis grass species. J. Exp. Bot. 2007, 58, 3789–3796. [Google Scholar] [CrossRef]
- Chen, S.K.; Ren, H.Y.; Luo, Y.X.; Feng, C.Z.; Li, H.F. Genome-wide identification of wheat (Triticum aestivum L.) expansin genes and functional characterization of TaEXPB1A. Environ. Exp. Bot. 2021, 182, 104307. [Google Scholar] [CrossRef]
- Rose, J.K.C.; Lee, H.H.; Bennett, A.B. Expression of a divergent expansin gene is fruit-specific and ripening-regulated. Proc. Natl. Acad. Sci. USA 1997, 94, 5955–5960. [Google Scholar] [CrossRef] [PubMed]
- Catala, C.; Rose, J.K.; Bennett, A.B. Auxin-regulated genes encoding cell wall-modifying proteins are expressed during early tomato fruit growth. Plant Physiol. 2000, 122, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Wang, Y.; Liu, M.Y.; Yan, P.W.; Niu, F.; Ma, F.Y.; Hu, J.; He, S.C.; Cui, J.H.; Yuan, X.Y.; et al. OsEXPA7 encoding an expansin affects grain size and quality traits in rice (Oryza sativa L.). Rice 2024, 17, 36. [Google Scholar] [CrossRef]
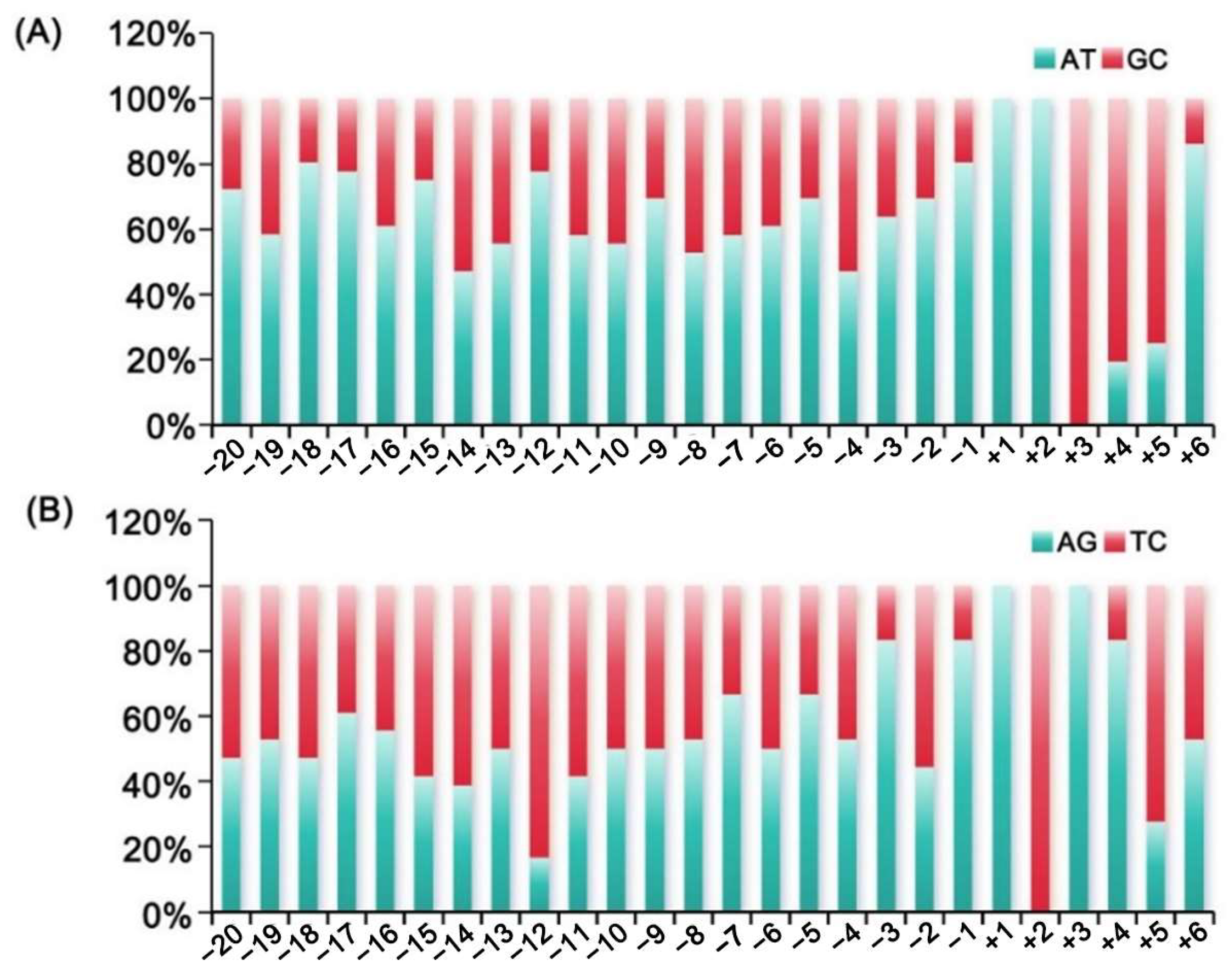
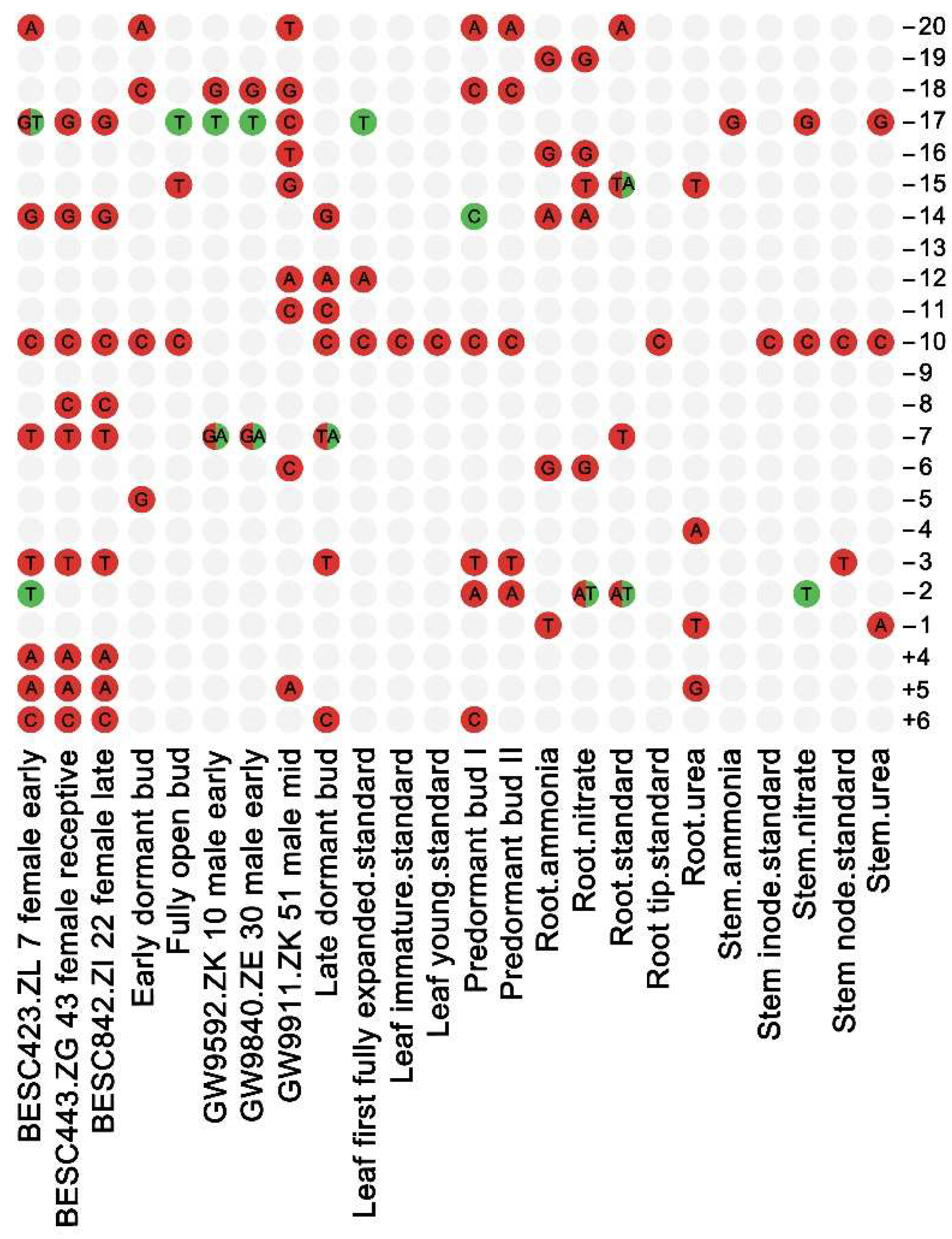
| Location | A | C | G | T |
|---|---|---|---|---|
| −20 | 36.11% | 16.67% | 11.11% | 36.11% |
| −19 | 27.78% | 16.67% | 25.00% | 30.56% |
| −18 | 30.56% | 2.78% | 16.67% | 50.00% |
| −17 | 44.44% | 5.56% | 16.67% | 33.33% |
| −16 | 38.89% | 22.22% | 16.67% | 22.22% |
| −15 | 33.33% | 16.67% | 8.33% | 41.67% |
| −14 | 19.44% | 33.33% | 19.44% | 27.78% |
| −13 | 27.78% | 22.22% | 22.22% | 27.78% |
| −12 | 16.67% | 22.22% | 0.00% | 61.11% |
| −11 | 25.00% | 25.00% | 16.67% | 33.33% |
| −10 | 27.78% | 22.22% | 22.22% | 27.78% |
| −9 | 25.00% | 5.56% | 25.00% | 44.44% |
| −8 | 25.00% | 19.44% | 27.78% | 27.78% |
| −7 | 44.44% | 19.44% | 22.22% | 13.89% |
| −6 | 33.33% | 22.22% | 16.67% | 27.78% |
| −5 | 50.00% | 13.89% | 16.67% | 19.44% |
| −4 | 25.00% | 25.00% | 27.78% | 22.22% |
| −3 | 50.00% | 2.78% | 33.33% | 13.89% |
| −2 | 38.89% | 25.00% | 5.56% | 30.56% |
| −1 | 75.00% | 11.11% | 8.33% | 5.56% |
| +1 | 100.00% | 0.00% | 0.00% | 0.00% |
| +2 | 0.00% | 0.00% | 0.00% | 100.00% |
| +3 | 0.00% | 0.00% | 100.00% | 0.00% |
| +4 | 16.67% | 13.89% | 66.67% | 2.78% |
| +5 | 11.11% | 58.33% | 16.67% | 13.89% |
| +6 | 44.44% | 5.56% | 8.33% | 41.67% |
| Gene | Gene # | TSS | 5′ UTR Length (bp) | Gene | Gene # | TSS | 5′ UTR Length (bp) |
|---|---|---|---|---|---|---|---|
| PtrEXLA1 | Potri.004G181700 | C | 77 | PtrEXPA13 | Potri.016G135200 | A | 85 |
| PtrEXLA2 | Potri.007G083400 | C | 88 | PtrEXPA14 | Potri.006G108000 | C | 120 |
| PtrEXLB1 | Potri.003G083200 | C | 120 | PtrEXPA15 | Potri.010G202500 | C | 65 |
| PtrEXLB2 | Potri.001G151500 | A | 76 | PtrEXPA16 | Potri.006G086100 | C | 61 |
| PtrEXLB3 | Potri.001G147200 | A | 56 | PtrEXPA17 | Potri.002G017900 | A | 659 |
| PtrEXLB4 | Potri.003G087000 | A | 55 | PtrEXPA18 | Potri.005G244100 | A | 58 |
| PtrEXPA1 | Potri.001G240900 | C | 368 | PtrEXPA19 | Potri.002G184700 | T | 888 |
| PtrEXPA2 | Potri.013G154700 | A | 91 | PtrEXPA20 | Potri.001G401700 | T | 633 |
| PtrEXPA3 | Potri.010G167200 | A | 168 | PtrEXPA21 | Potri.009G169500 | A | 356 |
| PtrEXPA4 | Potri.008G088300 | A | 117 | PtrEXPA22 | Potri.004G208300 | T | 713 |
| PtrEXPA5 | Potri.009G031800 | C | 596 | PtrEXPA23 | Potri.017G140000 | A | 195 |
| PtrEXPA6 | Potri.004G123200 | C | 199 | PtrEXPA24 | Potri.001G112866 | A | 320 |
| PtrEXPA7 | Potri.008G057100 | A | 595 | PtrEXPA25 | Potri.016G097500 | A | 90 |
| PtrEXPA8 | Potri.004G080200 | A | 573 | PtrEXPA26 | Potri.016G098700 | G | 96 |
| PtrEXPA9 | Potri.017G085300 | C | 185 | PtrEXPA27 | Potri.017G092700 | A | 88 |
| PtrEXPA10 | Potri.001G001100 | A | 230 | PtrEXPB1 | Potri.014G066300 | A | 68 |
| PtrEXPA11 | Potri.013G060800 | C | 162 | PtrEXPB2 | Potri.019G101900 | A | 207 |
| PtrEXPA12 | Potri.019G057500 | A | 72 | PtrEXPB3 | Potri.013G134300 | A | 205 |
| Subfamily | EXPA | EXPB | EXLA | EXLB |
|---|---|---|---|---|
| EXPA | 23.20–39.00(30.87) | |||
| 47.60–94.30(59.05) | ||||
| EXPB | 26.00–38.50(32.47) | 32.80–42.60(36.47) | ||
| 35.60–43.20(39.92) | 48.40–92.20(63.10) | |||
| EXLA | 26.10–39.20(31.90) | 32.10–34.90(33.03) | 55.00(55.00) | |
| 35.80–42.60(38.42) | 45.00–46.80(45.82) | 89.50(89.50) | ||
| EXLB | 24.50–36.00(30.20) | 29.10–33.60(31.58) | 29.40–33.50(31.00) | 27.50–35.10(31.82) |
| 33.10–42.60(37.90) | 41.40–46.50(44.01) | 49.80–53.40(51.31) | 52.40–89.70(68.93) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, X.; Wang, L.; Gong, L.; Li, M.; Xu, J. Characterization of the Expansin Gene Promoters in Populus trichocarpa. Forests 2024, 15, 1485. https://doi.org/10.3390/f15091485
Zhang J, Li X, Wang L, Gong L, Li M, Xu J. Characterization of the Expansin Gene Promoters in Populus trichocarpa. Forests. 2024; 15(9):1485. https://doi.org/10.3390/f15091485
Chicago/Turabian StyleZhang, Junkang, Xiaoyu Li, Lei Wang, Longfeng Gong, Mengtian Li, and Jichen Xu. 2024. "Characterization of the Expansin Gene Promoters in Populus trichocarpa" Forests 15, no. 9: 1485. https://doi.org/10.3390/f15091485
APA StyleZhang, J., Li, X., Wang, L., Gong, L., Li, M., & Xu, J. (2024). Characterization of the Expansin Gene Promoters in Populus trichocarpa. Forests, 15(9), 1485. https://doi.org/10.3390/f15091485





