Vertical Distribution of Mites (Acari) in a “Miniature Forest” of Sphagnum Mosses in a Forest Bog in Western Norway
Abstract
1. Introduction
2. Material and Methods
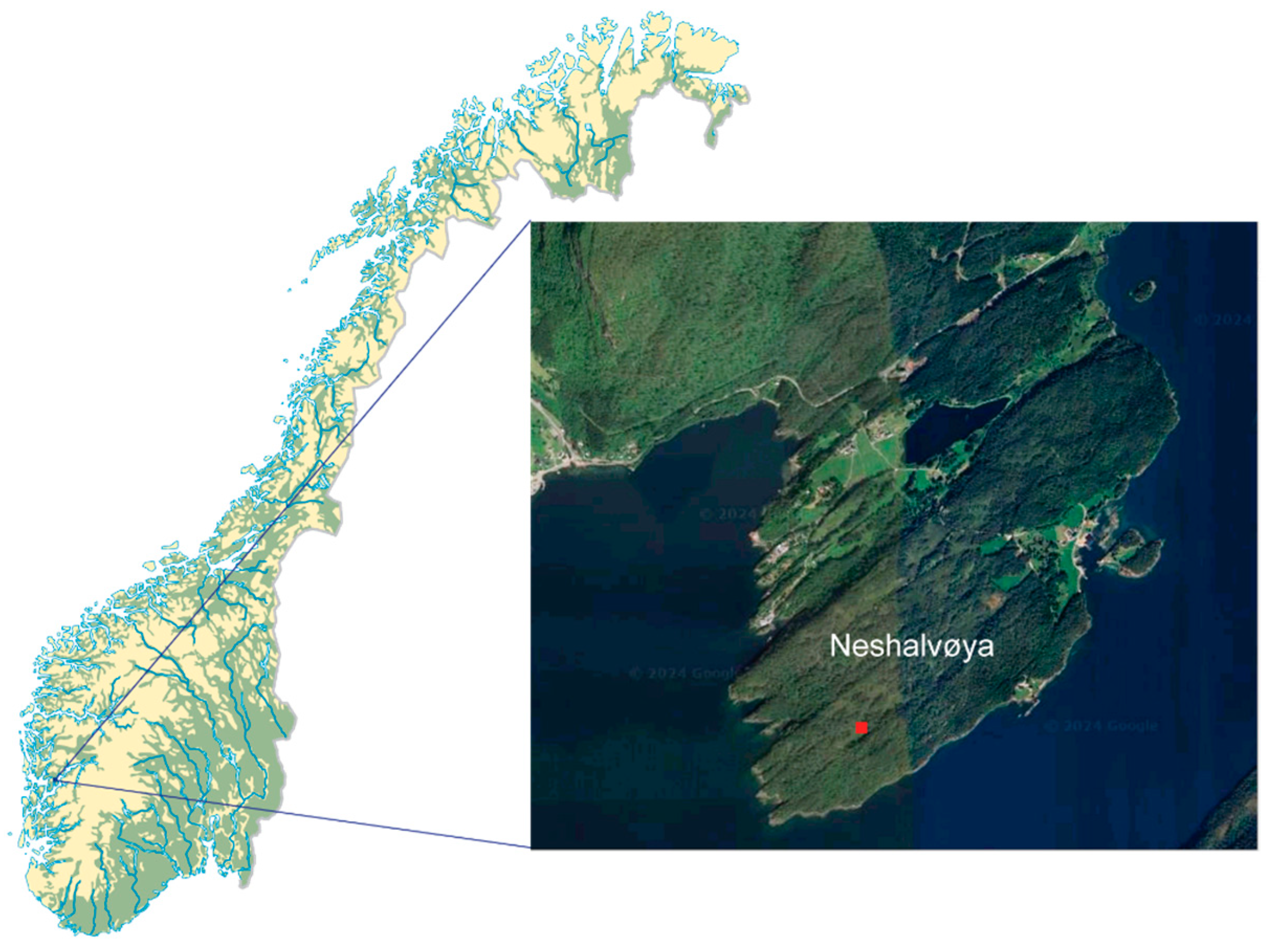
2.1. Study Area
2.2. Sampling and Identification
2.3. Statistical Analyses
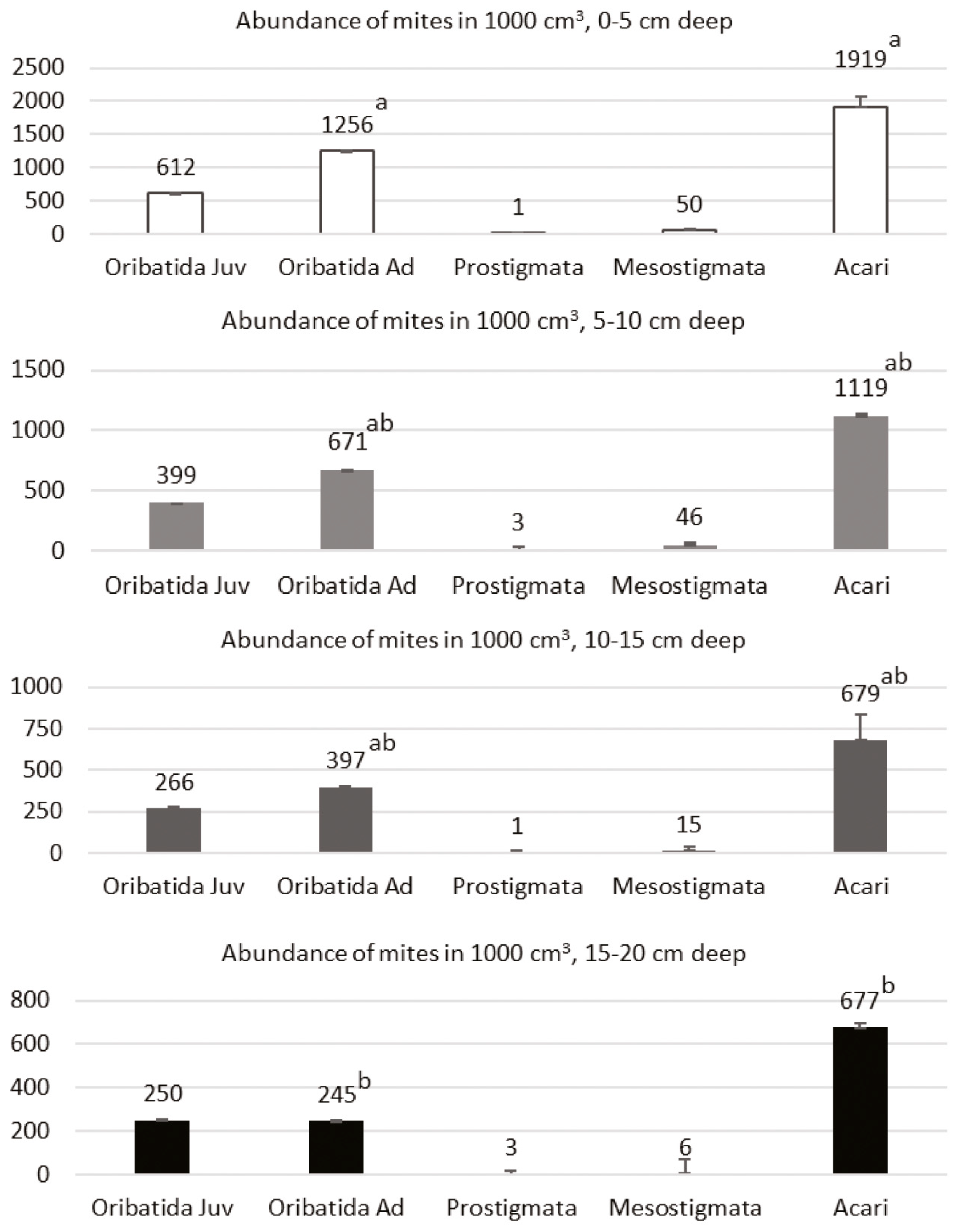
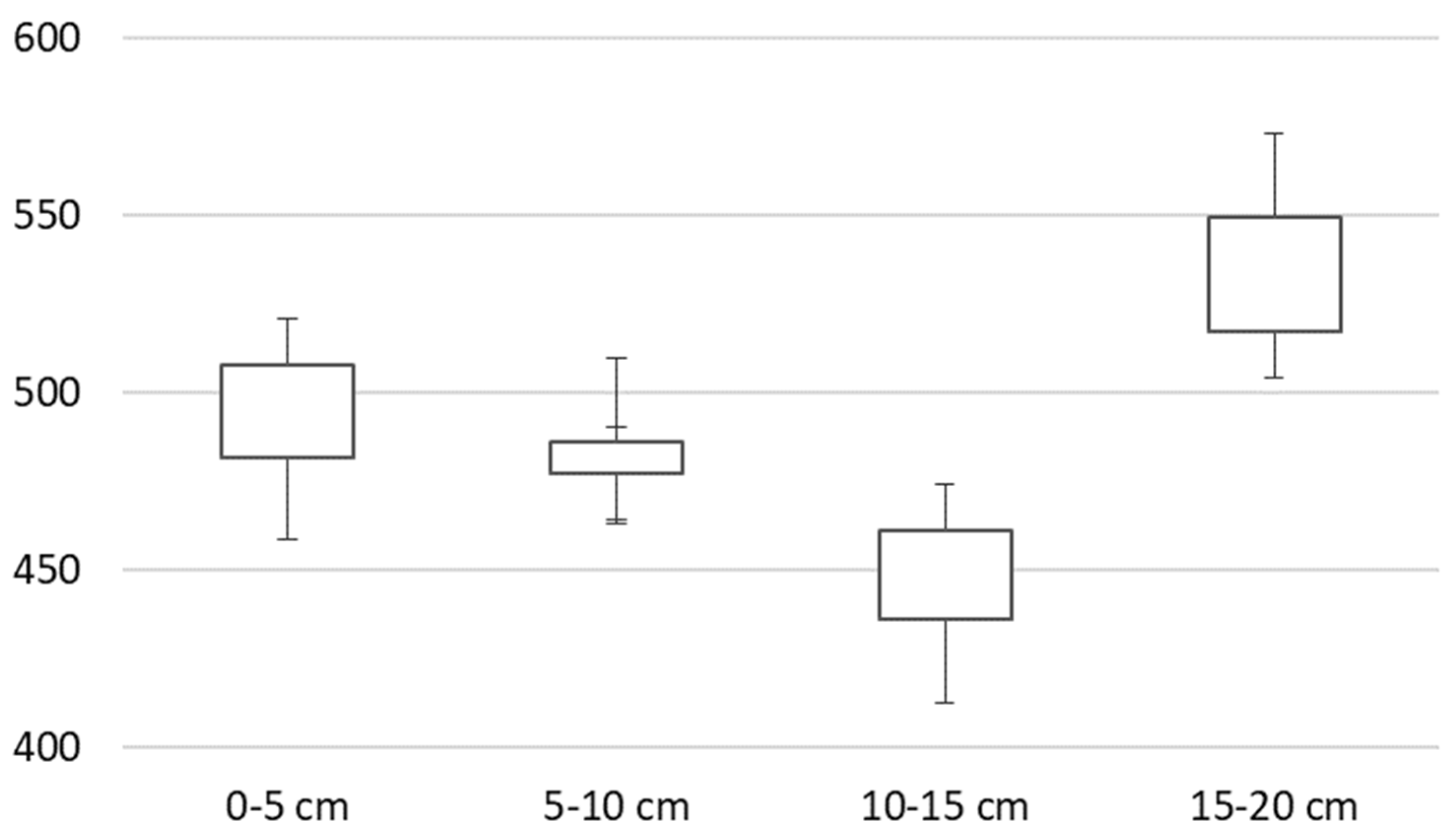
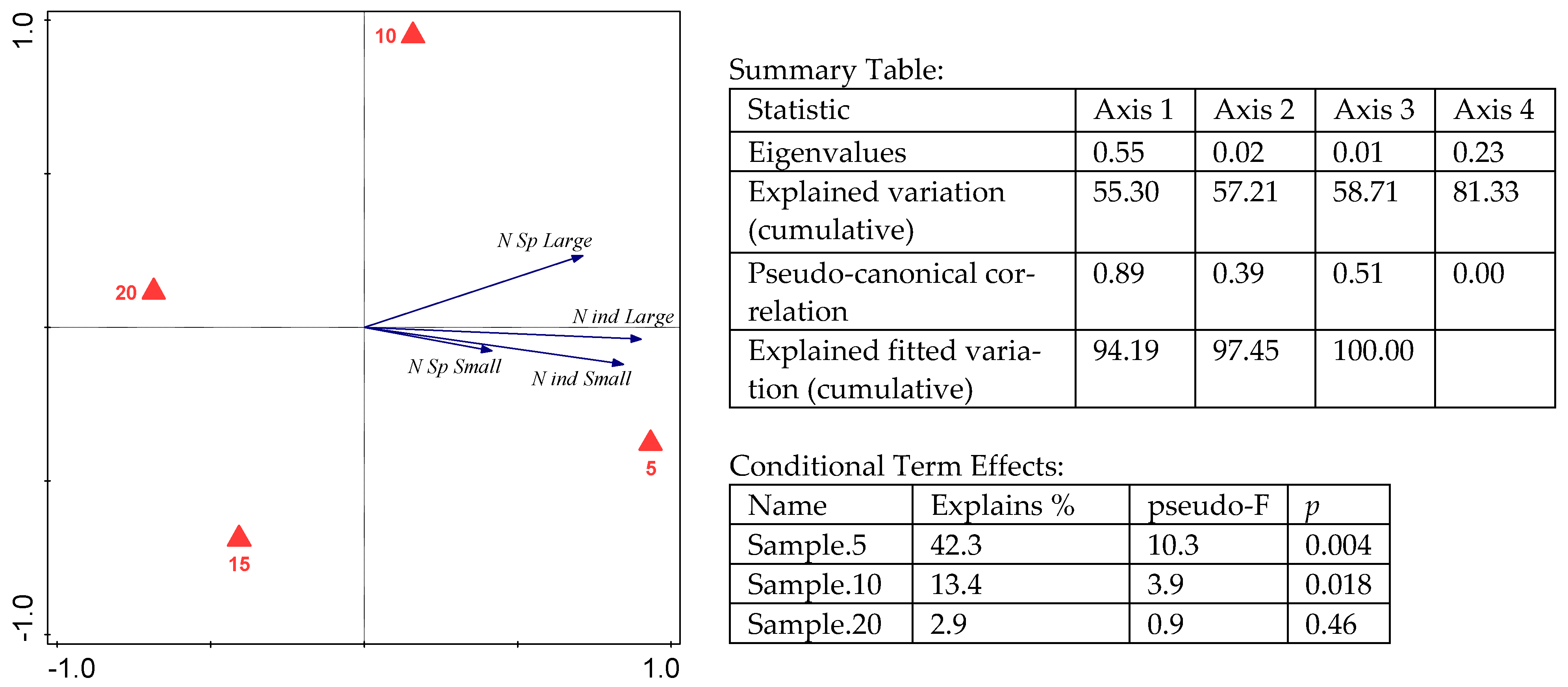
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barreto, C.; Lindo, Z. Drivers of decomposition and the detrital invertebrate community differ across a hummock-hollow microtopology in Boreal peatlands. Écoscience 2018, 25, 39–48. [Google Scholar] [CrossRef]
- Borcard, D. Les Oribates des tourbières du Jura suisse (Acari, Oribatei). Ecologie I. Quelques aspects de la communauté d’Oribates des sphaignes de la tourbière du Cachot. Rev. Suisse Zool. 1991, 98, 303–317. [Google Scholar] [CrossRef]
- Mumladze, L.; Murvanidze, M.; Behan-Pelletier, V. Compositional patterns in Holarctic peat bog inhabiting oribatid mite (Acari: Oribatida) communities. Pedobiologia 2013, 56, 41–48. [Google Scholar] [CrossRef]
- Borcard, D. Les Oribates des tourbières du Jura suisse (Acari, Oribatei). Ecologie IV. Distribution verticale. Rev. Suisse Zool. 1993, 100, 175–185. [Google Scholar]
- Seniczak, A.; Seniczak, S.; Iturrondobeitia, J.C.; Solhøy, T.; Flatberg, K.I. Diverse Sphagnum mosses support rich moss mite communities (Acari, Oribatida) in mires of Western Norway. Wetlands 2020, 40, 1339–1351. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S.; Iturrondobeitia, J.C.; Marciniak, M.; Kaczmarek, S.; Mąkol, J.; Kaźmierski, A.; Zawal, A.; Schwarzfeld, M.D.; Flatberg, K.I. Inclusion of juvenile stages improves diversity assessment and adds to our understanding of mite ecology—A case study from mires in Norway. Ecol. Evol. 2022, 12, e9530. [Google Scholar] [CrossRef]
- Holt, J.A. The vertical distribution of cryptostigmatic mites, soil organic matter and macroporosity in three North Queensland rainforest soils. Pedobiologia 1981, 22, 202–209. [Google Scholar] [CrossRef]
- Anderson, J.M. Observations on the vertical distribution of Oribatei (Acarina) in two woodland soils. Ann. Zool. Ecol. Anim. Suppl. 1971, 127, 257–272. [Google Scholar]
- Luxton, M. Studies on the oribatid mites of a Danish beech wood soil. V. Vertical distribution. Pedobiologia 1981, 21, 365–386. [Google Scholar] [CrossRef]
- Karasawa, S.; Hijii, N. Vertical stratification of oribatid (Acari: Oribatida) communities in relation to their morphological and life-history traits and tree structures in a subtropical forest in Japan. Ecol. Res. 2008, 23, 57–69. [Google Scholar] [CrossRef]
- Guidi, C.; Frey, B.; Brunner, I.; Meusburger, K.; Vogel, M.E.; Chen, X.; Stucky, T.; Gwiazdowicz, D.J.; Skubała, P.; Bose, A.K.; et al. Soil fauna drives vertical redistribution of soil organic carbon in a long-term irrigated dry pine forest. Glob. Chang. Biol. 2022, 28, 3145–3160. [Google Scholar] [CrossRef]
- Wood, T.G. Acari and Collembola of moorland soils from Yorkshire, England. II. Vertical distribution in four grassland soils. Oikos 1967, 18, 137–140. [Google Scholar] [CrossRef]
- Curry, J.P. Seasonal and vertical distribution of the arthropod fauna of an old grassland soil. Sci. Proc. R. Dublin Soc. Ser. B 1971, 3, 49–71. [Google Scholar]
- Whelan, J. Seasonal fluctuations and vertical distribution of the acarine fauna of three grassland sites. Pedobiologia 1985, 28, 191–201. [Google Scholar]
- Önen, Ö.; Koç, K. Seasonal and vertical distribution of Acarina fauna of grassland. Çankaya Univ. J. Sci. Eng. 2011, 8, 277–289. [Google Scholar]
- Price, D.W.; Benham, G.S., Jr. Vertical distribution of soil-inhabiting microarthropods in an agricultural habitat in California. Environ. Entomol. 1977, 6, 575–580. [Google Scholar] [CrossRef]
- Setälä, H.; Aarnio, T. Vertical stratification and trophic interactions among organisms of a soil decomposer food web—A field experiment using 15N as a tool. Eur. J. Soil Biol. 2002, 38, 29–34. [Google Scholar] [CrossRef]
- Bengtsson, F.; Granath, G.; Rydin, H. Photosynthesis, growth, and decay traits in Sphagnum—A multispecies comparison. Ecol. Evol. 2016, 6, 3325–3341. [Google Scholar] [CrossRef]
- Rajski, A. Studium ekologczno-faunistyczne nad mechowcami (Acari-Oribatei) w kilku zespołach roślinnych. I. Ekologia. Poznańskie Tow. Przyj. Nauk Poznań 1961, 25, 123–287. [Google Scholar]
- Solhøy, T. Oribatids (Acari) from an oligotrophic bog in western Norway. Fauna Nor. 1979, 26B, 91–94. [Google Scholar]
- Popp, E. Semiaquatile Lebensräume (Bülten) in Hoch- und Niedermooren. (2. Teil: Die Milbenfauna). Int. Rev. Gesamten Hydrobiol. 1962, 47, 533–579. [Google Scholar] [CrossRef]
- Kuriki, G. Vertical distribution of oribatid mites in Akaiyachi Moor, Northeast Japan. Edaphologia 1998, 60, 11–16. [Google Scholar]
- Markkula, I. Vertical distribution of soil animals in a virgin and drained raised bog. SUO 1981, 32, 126–129. [Google Scholar]
- Price, D.W. Vertical distribution of small arthropods in a California pine forest soil. Ann. Entomol. Soc. Am. 1975, 68, 174–180. [Google Scholar] [CrossRef]
- Caterino, M.S.; Recuero, E. Overcoming life stage-centric biases illuminates arthropod diversity, systematics and biology. Syst. Entomol. 2024. online version of record. [Google Scholar] [CrossRef]
- Denegri, G.M. Review of oribatid mites as intermediate hosts of tapeworms of the Anoplocephalidae. Exp. Appl. Acarol. 1993, 17, 567–580. [Google Scholar] [CrossRef]
- Mullen, G.R.; Oconnor, B.M. Chapter 26—Mites (Acari). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 533–602. [Google Scholar]
- Evjen, K.; Hareland, A.; Kirkemo, M.; Grjotheim, I.; Jørgensen, Å.M. Neshalvøya. Prosjekt 5—LAA 234 Områdeanalyse Kvam. 2006. Available online: https://herland.net/amund/pdf_filer/analyse2.pdf (accessed on 4 March 2024).
- Overvoll, O. Industriområde for skipsverft i Hansvågen, Kvam herad: Konsekvensutgreiing for biologisk mangfald og naturressursar. Rådgivende Biol. 2007, 34, 1017. [Google Scholar]
- Førland, E.J. Precipitation normals, normal period 1961–1990. Det Nor. Meterologiske Inst. Klima 1993, 39, 1–63. [Google Scholar]
- Aune, B. Air temperature normals, normalperiod 1961–1990. Det Nor. Meteorol. Inst. Klima 1993, 2, 1–63. [Google Scholar]
- Weigmann, G. Hornmilben (Oribatida). Die Tierwelt Deutschlands; Goecke. & Evers: Keltern, Germany, 2006; Volume 76, p. 520. [Google Scholar]
- Grandjean, F. Étude sur le développement des Oribates. Bull. Société Zool. Fr. 1933, 58, 30–61. [Google Scholar]
- Chistyakov, M.P. Biology and postembryonic development of Oppia nova (Oudem., 1902) (Oribatei), being a dominating species on turbaries from Gorky region. In Proceedings of the Fourth Scientific Conference of Zoologists, Boston, MA, USA, 29 December 1969; Gorky State Pedagogical Institute: Gorky, Russia, 1970; Volume 114, pp. 51–64. [Google Scholar]
- Subbotina, I.A. On the biology of Scheloribates laevigatus (C.L. Koch, 1836)—An armored mite of the family Scheloribatidae. Sci. Notes Gorky State Pedagog. Inst. 1967, 66, 33–50. [Google Scholar]
- Sitnikova, L.G. Postembryonic development of the mite Eupelops torulosus (C.L. Koch, 1839) (Oribatei, Pelopidae). Parazitol. Sb. 1978, 28, 53–72. [Google Scholar]
- Webb, N.R. Observations on Steganacarus magnus: Differences between the instars. Acarologia 1979, 20, 138–146. [Google Scholar] [PubMed]
- Behan-Pelletier, V.M. Ceratozetes (Acari: Ceratozetidae) of Canada and Alaska. Can. Entomol. 1984, 116, 1449–1517. [Google Scholar] [CrossRef]
- Seniczak, S.; Klimek, A. The morphology of juvenile stages of moss mites of the family Camisiidae (Acari: Oribatida). Part I. Zool. Anz. 1990, 225, 71–86. [Google Scholar]
- Norton, R.A.; Behan-Pelletier, V.M.; Wang, H.-F. The aquatic oribatid mite genus Mucronothrus in Canada and the western U.S.A. (Acari: Trhypochthoniidae). Can. J. Zool. 1996, 74, 926–949. [Google Scholar] [CrossRef]
- Seniczak, S.; Seniczak, A. Morphology of three species of Crotonioidea Thorel, 1876 (Acari: Oribatida), and relations between some genera. Zool. Anz. 2009, 248, 195–211. [Google Scholar] [CrossRef]
- Seniczak, S.; Seniczak, A. Morphological ontogeny and ecology of a common peatland mite, Nanhermannia coronata (Acari, Oribatida, Nanhermanniidae). Animals 2023, 13, 3590. [Google Scholar] [CrossRef] [PubMed]
- Seniczak, S.; Żelazna, E. The morphology of juvenile stages of moss mites of the family Nothridae (Acari: Oribatida). II. Zool. Anz. 1992, 229, 149–162. [Google Scholar]
- Seniczak, S.; Norton, R.A.; Seniczak, A. Morphology of Eniochthonius minutissimus (Berlese, 1904) and Hypochthonius rufulus C. L. Koch, 1835 (Acari: Oribatida: Hypochthonioidea). Ann. Zool. 2009, 59, 373–386. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S.; Kaczmarek, S. Morphology, distribution and ecology of Eupelops curtipilus and Eupelops plicatus (Acari, Oribatida, Phenopelopidae). Int. J. Acarol. 2015, 41, 77–95. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S.; Kaczmarek, S.; Chachaj, B. Morphological ontogeny and ecology of Adoristes ovatus (Acari: Oribatida: Liacaridae), with comments on Adoristes Hull. Syst. Appl. Acarol. 2017, 22, 2038–2056. [Google Scholar] [CrossRef]
- Pfingstl, T.; Krisper, G. No difference in the juveniles of two Tectocepheus species (Acari: Oribatida, Tectocepheidae). Acarologia 2011, 51, 199–218. [Google Scholar] [CrossRef]
- Norton, R.A.; Ermilov, S.G. Catalogue and historical overview of juvenile instars of oribatid mites (Acari: Oribatida). Zootaxa 2014, 3833, 1–132. [Google Scholar] [CrossRef]
- Norton, R.A.; Ermilov, S.G. Catalogue of juvenile instars of oribatid mites (Acari: Oribatida)—The next decade (2014–2023). Zootaxa 2024, 5419, 451–494. [Google Scholar] [CrossRef]
- Ermilov, S.G.; Tolstikov, A.V.; Weigmann, G. Morphology of adult and juvenile instars of Galumna obvia (Acari, Oribatida, Galumnidae), with discussion of its taxonomic status. ZooKeys 2013, 357, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Subías, L.S. Listado sistemático, sinonímico y biogeográfico de los Ácaros Oribátidos (Acariformes, Oribatida) del mundo (1748–2002). Graellsia 2004, 60, 3–305. [Google Scholar] [CrossRef]
- Subías, L.S. Listado sistematico, sinonimico y biogeografico de los acaros oribatidos (Acariformes, Oribatida) del mundo (except fosiles). Monogr. Electrón. SEA 2022, 12, 1–538. [Google Scholar]
- Brückner, A.; Heethoff, M.; Norton, R.A.; Wehner, K. Body size structure of oribatid mite communities in different microhabitats. Int. J. Acarol. 2018, 44, 367–373. [Google Scholar] [CrossRef]
- Stanisz, A. Easy Course of Statistic Using Statistica PL and Medicine Examples, 1. In Basic Statistic; StatSoft Polska: Kraków, Poland, 2006; p. 532. [Google Scholar]
- Braak, C.J.F.T. CANOCO—A FORTRAN Program for Canonical Community Ordination by (Partial) (Detrended) (Canonical) Correspondence Analysis, Principle Component Analysis and Redundancy Analysis; TNO Inst: Wageningen, The Netherlands, 1988. [Google Scholar]
- Braak, C.J.F.T.; Jongman, R.H.G.; Tongeren, O.F.R.V. Data Analysis in Community and Landscape Ecology; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Łomnicki, A. Wprowadzenie do Statystyki dla Przyrodników; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2024; p. 282. [Google Scholar]
- Lebrun, P. Ecologie et biocenotique de quelques peuplements d’arthropodes edaphiques. Mémoires Musée R. D’histoire Nat. Belg. 1971, 165, 1–203. [Google Scholar]
- Perdue, J.C.; Crossley, D.A. Vertical distribution of soil mites (Acari) in conventional and no-tillage agricultural systems. Biol. Fertil. Soils 1990, 9, 135–138. [Google Scholar] [CrossRef]
- Revelo Tobar, H.; Estrada-Venegas, E.; Equihua-Martínez, A.; Valdez-Carrasco, J. Oribatid mites in agricultural and natural soils: A case study of vertical distribution. Entomol. Commun. 2022, 4, ec04015. [Google Scholar] [CrossRef]
- Urhan, R.; Katilmis, Y.; Kahveci, A.Ö. Vertical distribution of soil mites (Acari) on Dalaman (Mugla Prov.—Turkey). Munis Entomol. Zool. 2008, 3, 333–341. [Google Scholar]
- Pollierer, M.M.; Dyckmans, J.; Scheu, S.; Haubert, D. Carbon flux through fungi and bacteria into the forest soil animal food web as indicated by compound-specific 13C fatty acid analysis. Funct. Ecol. 2012, 26, 978–990. [Google Scholar] [CrossRef]
- Lehmitz, R.; Maraun, M. Small-scale spatial heterogeneity of stable isotopes signatures (d15N, d13C) in Sphagnum sp. transfers to all trophic levels in oribatid mites. Soil Biol. Biochem. 2016, 100, 242–251. [Google Scholar] [CrossRef]
- Behan-Pelletier, V.; Hill, S. Feeding habits of sixteen species of Oribatei (Acari) from an acid peat bog, Glenamoy, Ireland. Rev. D’ecologie Biol. Sol. 1983, 20, 221–267. [Google Scholar]
- Smrž, J. Internal anatomy of Hypochthonius rufulus (Acari, Oribatida). J. Morphol. Zool. 1989, 200, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Siepel, H.; Ruiter-Dijkman, E.M.D. Feeding guilds of oribatid mites based on their carbohydrase activities. Soil Biol. Biochem. 1993, 25, 1491–1497. [Google Scholar] [CrossRef]
- Eissfeller, V.; Beyer, F.; Valtanen, K.; Hertel, D.; Maraun, M.; Polle, A.; Scheu, S. Incorporation of plant carbon and microbial nitrogen into the rhizosphere food web of beech and ash. Soil Biol. Biochem. 2013, 62, 76–81. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S.; Maraun, M.; Graczyk, R.; Mistrzak, M. Oribatid mite species numbers increase, densities decline and parthenogenetic species suffer during bog degradation. Exp. Appl. Acarol. 2016, 68, 409–428. [Google Scholar] [CrossRef]
- Hartenstein, R. Soil Oribatei. 1. Feeding specificity among forest soil Oribatei (Acarina). Ann. Entomol. Soc. Am. 1962, 55, 202–206. [Google Scholar] [CrossRef]
- Schatz, H.; Bruckner, A. Hornmilben (Acari: Oribatida) aus dem Wildnisgebiet Dürrenstein und dem Urwald Rothwald (Österreich). Silva Fera 2021, 8, 42–62. [Google Scholar]
- Corral-Hernández, E.; Iturrondobeitia, J.C. Effects of cattle and industries on oribatid mite communities of grassland soil in the Basque Country (Spain). Int. J. Acarol. 2012, 38, 217–229. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S.; Graczyk, R.; Waldon-Rudzionek, B.; Nowicka, A.; Pacek, S. Seasonal dynamics of oribatid mites (Acari, Oribatida) in a bog in Poland. Wetlands 2019, 39, 853–864. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S.; Iturrondobeitia, J.C.; Gwiazdowicz, D.J.; Waldon-Rudzionek, B.; Flatberg, K.I.; Bolger, T. Mites (Oribatida and Mesostigmata) and vegetation as complementary bioindicators in peatlands. Sci. Total Environ. 2022, 851, 158335. [Google Scholar] [CrossRef] [PubMed]
- Skubała, P.; Rola, K.; Osyczka, P. Oribatid communities and heavy metal bioaccumulation in selected species associated with lichens in a heavily contaminated habitat. Environ. Sci. Pollut. Res. Int. 2016, 23, 8861–8871. [Google Scholar] [CrossRef] [PubMed]
- Ľuptáčik, P.; Čuchta, P.; Jakšová, P.; Miklisová, D.; Kováč, Ľ.; Alatalo, J.M. Cushion plants act as facilitators for soil microarthropods in high alpine Sweden. Biodivers. Conserv. 2021, 30, 3243–3264. [Google Scholar] [CrossRef]
- Mitchell, M.J. Vertical and horizontal distribution of oribatid mites (Acari: Cryptostigmata) in an aspen woodland soil. Ecology 1978, 59, 516–525. [Google Scholar] [CrossRef]
- Norton, R.A.; Ermilov, S.G. Paedomorphosis and sexuality in Eulohmanniidae (Acari, Oribatida): Surprising diversity in a relictual family of oribatid mites. Acarologia 2022, 62, 989–1069. [Google Scholar] [CrossRef]
- Salt, G.; Hollick, F.S.; Raw, F.; Brian, M.V. The arthropod population of pasture soil. J. Anim. Ecol. 1948, 17, 139–150. [Google Scholar] [CrossRef]
- Evans, G.O.; Macfarlane, D.; Sheals, J.G. The Terrestrial Acari of the British Isles: An Introduction to Their Morphology, Biology and Classification. Trustess of the British Museum: London, UK, 1968. [Google Scholar]
- Schneider, K.; Migge, S.; Norton, R.A.; Scheu, S.; Langel, R.; Reineking, A.; Maraun, M. Trophic niche differentiation in soil microarthropods (Oribatida, Acari): Evidence from stable isotope ratios (15N/14N). Soil Biol. Biochem. 2004, 36, 1769–1774. [Google Scholar] [CrossRef]

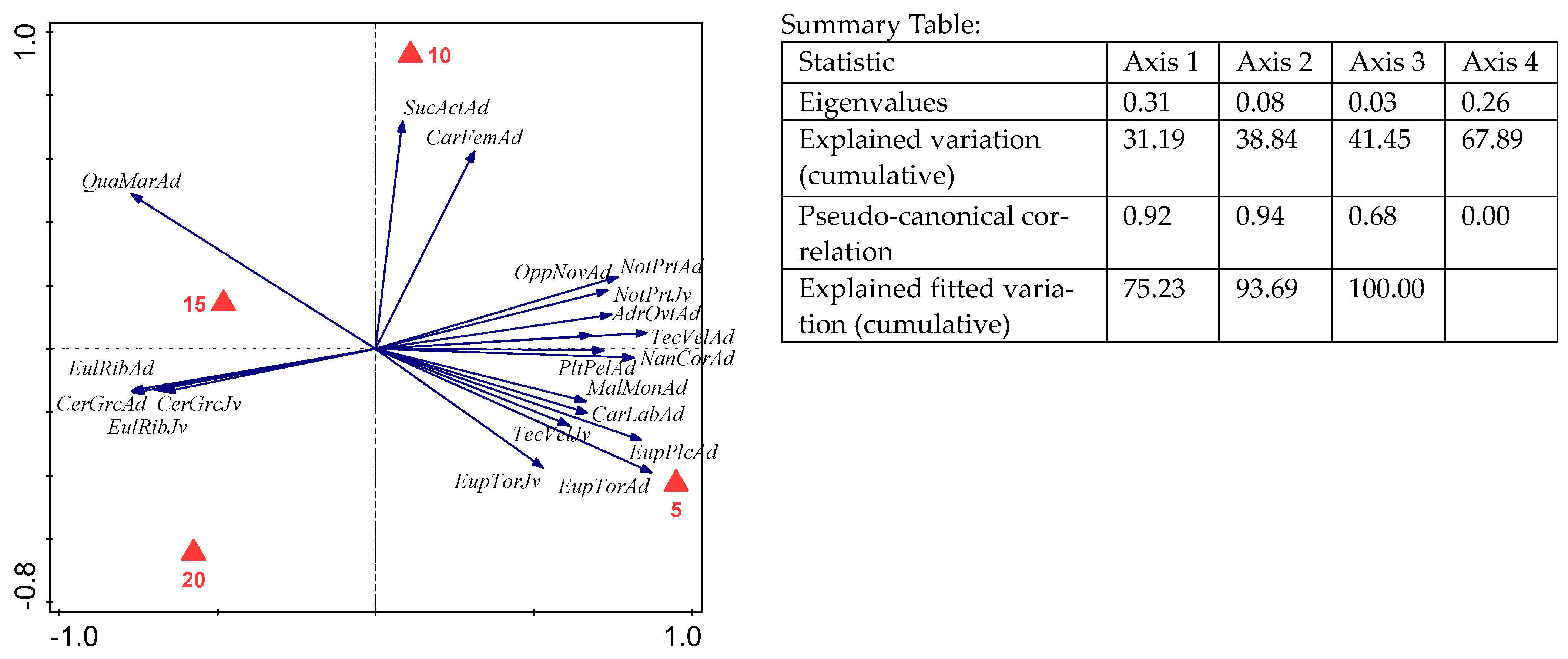
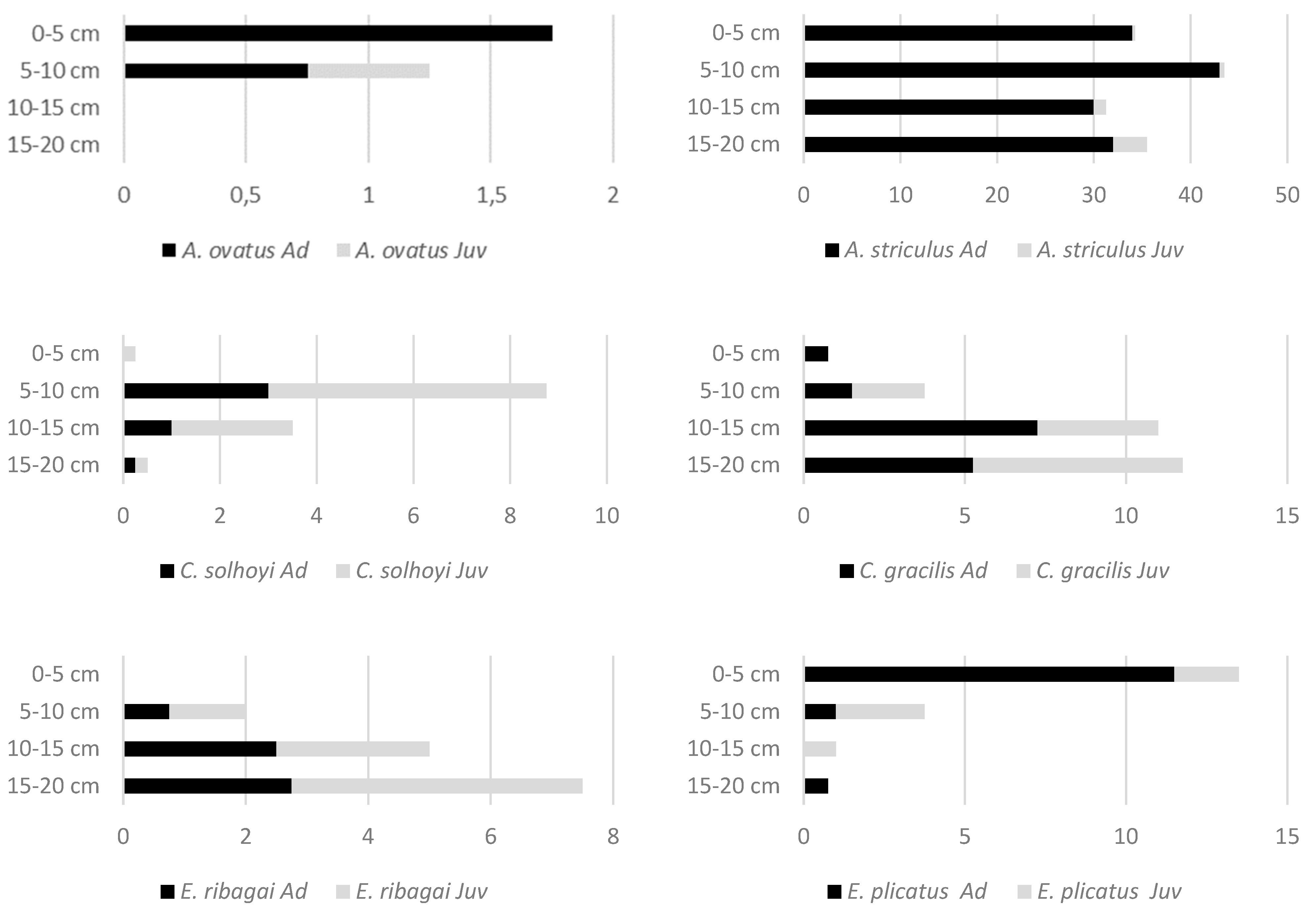
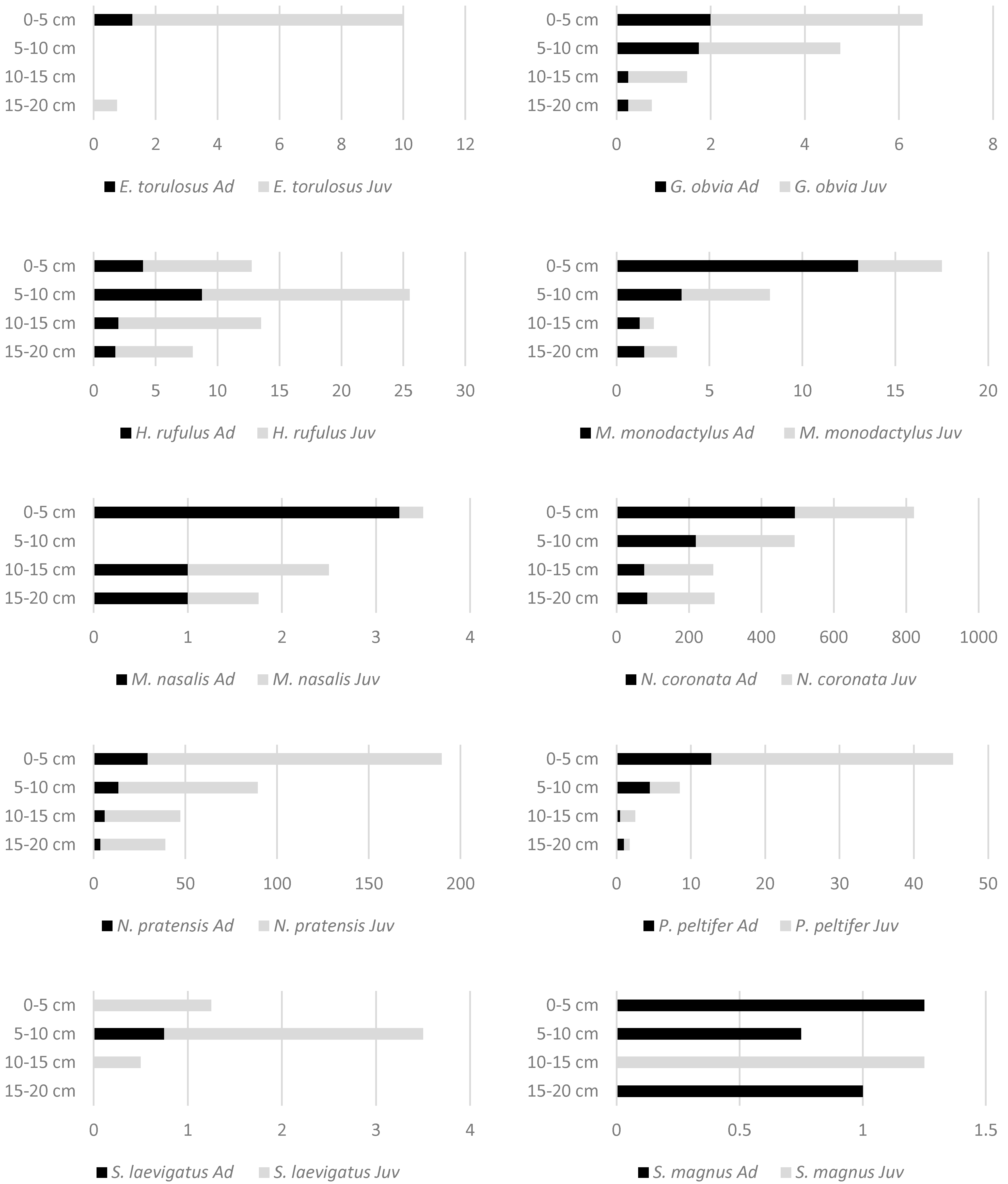

| Species | Abbreviation for RDA | Length (µm) | 0–5 cm | 5–10 cm | 10–15 cm | 15–20 cm |
|---|---|---|---|---|---|---|
| Adoristes ovatus (C.L. Koch, 1839) * | AdrOvt | 590 | 1.75 (±1.71) | 1.25 (±1.89) | 0.00 | 0.00 |
| Achipteria coleoptrata (Linnaeus, 1758) * | 590 | 0.25 (±0.50) | 0.00 | 0.00 | 0.00 | |
| Atropacarus striculus (C.L. Koch, 1835) | 695 | 34.25 (±14.29) | 43.50 (±3.79) | 31.25 (±9.98) | 35.50 (±6.24) | |
| Banksinoma lanceolata (Michael, 1885) | 330 | 0.75 (±0.96) | 0.00 | 0.00 | 0.00 | |
| Camisia solhoyi Colloff, 1993 | 840 | 0.25 (±0.50) | 8.75 (±11.81) | 3.50 (±2.65) | 0.50 (±1.00) | |
| Carabodes femoralis (Nicolet, 1855) * | CarFem | 658 | 0.75 (±0.96) | 2.00 (±1.15) | 0.50 (±1.00) | 0.00 |
| Carabodes labyrinthicus (Michael, 1879) * | CarLab | 505 | 3.00 (±1.15) | 0.75 (±0.96) | 0.50 (±0.58) | 1.00 (±2.00) |
| Carabodes reticulatus Berlese, 1913 * | 755 | 0.25 (±0.50) | 0.00 | 0.00 | 0.00 | |
| Carabodes rugosior Berlese, 1916 * | 585 | 0.75 (±1.50) | 0.25 (±0.50) | 0.00 | 0.00 | |
| Ceratoppia sexpilosa (L. Koch, 1879) | 700 | 0.50 (±0.58) | 0.00 | 0.75 (±0.50) | 0.25 (±0.50) | |
| Ceratozetes sp. | 340 | 0.00 | 0.00 | 0.25 (±0.50) | 0.00 | |
| Ceratozetes gracilis (Michael, 1884) * | CerGrc | 545 | 0.75 a (±0.96) | 3.75 ab (±2.75) | 11.00 ab (±4.08) | 11.75 b (±5.68) |
| Chamobates borealis (Trägårdh 1902) | 370 | 0.00 | 0.00 | 0.00 | 0.25 (±0.50) | |
| Chamobates pusillus (Berlese 1895) | 420 | 1.00 (±1.41) | 0.00 | 0.00 | 0.00 | |
| Cultroribula bicultrata (Berlese, 1905) | 238 | 0.25 (±0.50) | 0.75 (±0.96) | 0.25 (±0.50) | 0.00 | |
| Dameobelba minutissima (Sellnick, 1929) | 260 | 0.00 | 0.00 | 0.50 (±1.00) | 0.75 (±0.96) | |
| Eniochthonius minutissimus (Berlese, 1904) | 375 | 0.50 (±0.58) | 0.00 | 0.50 (±0.58) | 0.00 | |
| Eulohmannia ribagai (Berlese, 1910) | EulRib | 665 | 0.00 a | 2.00 ab (±1.63) | 5.00 ab (±1.41) | 7.50 b (±5.07) |
| Eupelops plicatus (C.L. Koch, 1835) | EupPlc | 590 | 13.50 a (±5.45) | 3.75 ab (±3.30) | 1.00 ab (±1.41) | 0.75 b (±1.50) |
| Eupelops torulosus (C.L. Koch, 1839) | EupTor | 690 | 10.00 (±14.17) | 0.00 | 0.00 | 0.75 (±1.50) |
| Galumna obvia (Berlese, 1914) * | 775 | 6.50 (±6.03) | 4.75 (±5.62) | 1.50 (±1.29) | 0.75 (±1.50) | |
| Hemileius initialis (Berlese, 1908) | 500 | 0.25 (±0.50) | 0.00 | 0.00 | 0.00 | |
| Hoplophthiracarus illinoisensis (Ewing 1909) | 550 | 84.00 (±100.68) | 28.25 (±33.23) | 7.75 (±10.01) | 1.50 (±2.38) | |
| Hydrozetes lacustris (Michael, 1882) | 480 | 0.00 | 0.00 | 0.00 | 0.25 (±0.50) | |
| Hypochthonius rufulus C.L. Koch, 1835 | 675 | 12.75 (±10.37) | 25.50 (±24.85) | 13.50 (±16.66) | 8.00 (±6.68) | |
| Lagenobates lagenulus (Berlese, 1904) | 300 | 0.00 | 0.00 | 0.00 | 0.25 (±0.50) | |
| Liebstadia humerata Sellnick, 1928 | 345 | 0.25 (±0.50) | 0.25 (±0.50) | 0.00 | 0.00 | |
| Limnozetes ciliatus (Schrank, 1803) | 300 | 0.00 | 0.00 | 0.00 | 0.50 (±1.00) | |
| Limnozetes rugosus (Sellnick, 1923) | 358 | 0.25 (±0.50) | 0.00 | 0.00 | 0.25 (±0.50) | |
| Liochthonius alpestris (Forsslund, 1958) | 190 | 38.75 (±35.42) | 26.50 (±22.49) | 5.00 (±8.12) | 0.75 (±0.96) | |
| Liochthonius neglectus Moritz, 1976 | 194 | 0.50 (±1.00) | 0.00 | 0.00 | 1.50 (±1.91) | |
| Liochthonius sp. | 182 | 0.50 (±1.00) | 0.25 (±0.50) | 0.00 | 0.00 | |
| Liochthonius strenzkei Forsslund, 1963 | 185 | 4.25 (±7.85) | 12.50 (±25.00) | 1.50 (±3.00) | 0.25 (±0.50) | |
| Malaconothrus monodactylus (Michael, 1888) | MalMon | 415 | 17.50 (±11.47) | 8.25 (±9.54) | 2.00 (±2.71) | 3.25 (±3.77) |
| Mortizoppia translamellata (Willmann, 1923) | 295 | 50.00 (±37.73) | 59.50 (±57.23) | 24.25 (±29.83) | 10.25 (±16.17) | |
| Mucronothrus nasalis (Willmann, 1929) | 690 | 3.50 (±6.35) | 0.00 | 2.50 (±3.00) | 1.75 (±1.71) | |
| Nanhermannia coronata Berlese, 1913 | NanCor | 525 | 821.25 (±310.76) | 491.75 (±275.73) | 267.00 (±175.38) | 270.75(±73.71) |
| Nothrus pratensis Sellnick, 1928 | NotPrt | 850 | 189.75 a (±60.67) | 89.50 ab (±20.87) | 47.25 b (±34.06) | 39.00 b (±25.60) |
| Nothrus silvestris Nicolet, 1855 | 760 | 0.25 (±0.50) | 0.25 (±0.50) | 0.00 | 0.00 | |
| Odontocepheus elongatus (Michael, 1879) | 655 | 0.00 | 0.50 (±0.58) | 0.50 (±0.58) | 0.00 | |
| Oppiella nova (Oudemanns, 1902) * | OppNov | 270 | 200.00 a (±82.25) | 99.00 ab (±14.31) | 70.50 ab (±35.05) | 46.00 b (±37.73) |
| Rhinoppia subpectinata (Oudemans, 1900) | 333 | 0.75 (±0.96) | 8.25 (±11.32) | 4.75 (±8.22) | 1.25 (±1.89) | |
| Paleacarus hystricinus Trägårdh, 1932 | 300 | 0.00 | 0.75 (±1.50) | 0.00 | 0.00 | |
| Phthiracarus boresetosus Jacot, 1930 | 703 | 0.25 (±0.50) | 0.25 (±0.50) | 0.00 | 0.00 | |
| Phthiracarus clavatus Parry, 1979 | 965 | 0.25 (±0.50) | 0.25 (±0.50) | 0.00 | 2.00 (±4.00) | |
| Phthiracarus italicus (Oudemans, 1900) | 895 | 0.00 | 0.00 | 0.25 (±0.50) | 0.00 | |
| Platynothrus peltifer (C.L. Koch, 1839) * | PltPel | 875 | 45.25 (±48.46) | 8.50 (±11.93) | 2.50 (±2.52) | 1.75 (±2.36) |
| Quadroppia maritalis Lions, 1982 | QuaMar | 190 | 0.25 a (±0.50) | 50.50 ab (±45.21) | 115.25 b (±114.60) | 18.75 ab (±8.26) |
| Rhysotritia ardua (C.L. Koch, 1841) | 818 | 3.25 (±4.57) | 1.50 (±2.38) | 3.00 (±3.56) | 2.75 (±3.20) | |
| Rhysotritia duplicata Grandjean, 1953) | 876 | 0.25 (±0.50) | 0.50 (±1.00) | 0.00 | 0.00 | |
| Scheloribates laevigatus (C.L. Koch, 1835) * | 595 | 1.25 (±2.50) | 3.50 (±4.36) | 0.50 (±1.00) | 0.00 | |
| Steganacarus magnus (Nicolet, 1855) | 1380 | 1.25 (±1.89) | 0.75 (±0.50) | 1.25 (±2.50) | 1.00 (±0.82) | |
| Suctobelbella acutidens (Forsslund, 1941) | SucAct | 208 | 0.00 | 8.50 (±7.94) | 0.00 | 0.00 |
| Suctobelbella arcana Moritz, 1970 | 203 | 47.50 (±33.36) | 25.50 (±20.07) | 7.50 (±6.45) | 5.00 (±4.24) | |
| Suctobelbella falcata (Forsslund, 1941) | 240 | 0.50 (±1.00) | 0.00 | 1.50 (±2.38) | 0.00 | |
| Suctobelbella forsslundi (Strenzke, 1950) | 208 | 0.00 | 3.50 (±4.12) | 0.25 (±0.50) | 2.00 (±2.31) | |
| Suctobelbella latirostris (Strenzke, 1950) | 245 | 0.25 (±0.50) | 0.00 | 0.00 | 0.00 | |
| Suctobelbella longirostris (Forsslund, 1941) | 300 | 1.50 (±1.73) | 7.25 (±8.38) | 13.25 (±12.71) | 5.25 (±6.40) | |
| Tectocepheus velatus (Michael, 1880) | TecVel | 300 | 266.50 a (±211.42) | 37.50 ab (±29.03) | 13.50 ab (±9.26) | 10.50 b (±11.03) |
| Tyrphonothrus foveolatus (Willmann, 1931) | 410 | 0.00 | 0.00 | 1.00 (±2.00) | 0.25 (±0.50) | |
| Tyrphonothrus maior (Berlese, 1910) | 568 | 0.00 | 0.00 | 0.00 | 0.25 (±0.50) | |
| Malaconothrus vietsi (Willmann, 1925) | 335 | 0.25 (±0.50) | 0.00 | 0.00 | 0.00 | |
| S | 48 | 38 | 37 | 35 | ||
| Hs | 1.966 | 2.149 | 2.116 | 1.768 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seniczak, A.; Iturrondobeitia, J.C.; Seniczak, S. Vertical Distribution of Mites (Acari) in a “Miniature Forest” of Sphagnum Mosses in a Forest Bog in Western Norway. Forests 2024, 15, 957. https://doi.org/10.3390/f15060957
Seniczak A, Iturrondobeitia JC, Seniczak S. Vertical Distribution of Mites (Acari) in a “Miniature Forest” of Sphagnum Mosses in a Forest Bog in Western Norway. Forests. 2024; 15(6):957. https://doi.org/10.3390/f15060957
Chicago/Turabian StyleSeniczak, Anna, Juan Carlos Iturrondobeitia, and Stanisław Seniczak. 2024. "Vertical Distribution of Mites (Acari) in a “Miniature Forest” of Sphagnum Mosses in a Forest Bog in Western Norway" Forests 15, no. 6: 957. https://doi.org/10.3390/f15060957
APA StyleSeniczak, A., Iturrondobeitia, J. C., & Seniczak, S. (2024). Vertical Distribution of Mites (Acari) in a “Miniature Forest” of Sphagnum Mosses in a Forest Bog in Western Norway. Forests, 15(6), 957. https://doi.org/10.3390/f15060957









