Abstract
In June 2020, needle blight symptoms on Pinus thunbergii were discovered in Bazhong City, Sichuan Province, China. Fungal isolates were obtained from the pine needles of P. thunbergii. After examining morphological characteristics and conducting multi-locus (ITS, ACT, TUB2 and RPB2) phylogenetic analyses, the isolates SC1–SC5 were determined to be a new species, Biscogniauxia sinensis. Genealogical Concordance Phylogenetic Species Recognition with a pairwise homoplasy index test was used to further verify the results of the phylogenetic analyses. The morphology and phylogenetic relationships between this new species and other related Biscogniauxia species were discussed. To our knowledge, this is also the first report of Biscogniauxia sinensis associated with pine needle blight on P. thunbergii in China. The needle damage of P. thunbergii associated with Biscogniauxia sinensis will detrimentally affect the carbon absorption and photosynthetic efficiency of P. thunbergii, further reduce the absorption of nutrients by Japanese black pine and may lead to the imbalance of pine forest conditions, which will have a negative impact on the forest ecological system.
1. Introduction
Pinus thunbergii (Japanese black pine) is native to Japan and Korea and is currently widely distributed in China due to its evergreen attributes, fast growth, and salinity tolerance [1]. It plays essential roles in ecological restoration, such as sand fixation and afforestation [2,3,4]. Despite the ecological effects, P. thunbergii in China were infected by many pathogens, which caused the wilt and mortality of pine trees. For instance, pine wilt disease caused by the pine wood nematode (Bursaphelenchus xylophilus) is the most devastating pine disease in China [5]. Needle cast disease caused by Lophodermium pinastri was extremely infectious, with a high incidence in young and mature forests. At the early stage, infected needles exhibited yellow spots, then turned to light brown, leading to the abscission of needles. In the spring of the subsequent year, the fallen needles became thin; black horizontal lines emerged and divided the needles into smaller portions. An oval, black fruiting body was then formed between these lines. Upon maturation, these fruiting bodies absorbed moisture, expanded, and developed a central longitudinal fissure, through which ascospores were released [6,7,8]. Brown-spot needle blight on P. thunbergii caused by Lecanosticta acicola initially produced small, discolored spots on infected needles, turned brown and died off quickly. The disease started at the base of the crown and gradually spread upward [9]. Such diseases are common with pine needles and also have posed severe threats to the growing conditions of P. thunbergii. However, the symptoms of pine needle blight with sporadic black spots are different from these aforementioned diseases.
In June 2020, pine needle blight symptoms were found on P. thunbergii at Nanyang Forest Farm, Bazhong city, Sichuan Province, China. Initial observations of discoloration and wilting on black pine needles were made in May, with a significant increase in yellowing and wilting symptoms occurring in June. The incidence peaked during July and August, coinciding with rising summer temperatures and the onset of the rainy season, sometimes extending into September. This disease progressed rapidly, infecting black pines of various ages and ultimately caused the needles to turn brown, even causing the complete death of the trees. Surveys indicated a disease incidence rate of 30%, signifying a high severity of this disease.
The objectives of this study are to determine a fungus isolated from Pinus thunbergii in Sichuan Province with subsequent morphological and phylogenetic analyses. GCPSR with a PHI test was conducted to further justify the placement of this species. Its morphology and molecular differences were compared with known species in the genus.
2. Materials and Methods
2.1. Sampling and Isolation
In total, 20 needles with typical symptoms were collected from Japanese black pines in a pine forest in Bazhong, Sichuan (31°85′88.09″ N, 106°75′36.69″ E (DMS)). All 20 needles were soaked in 1.5% sodium hypochlorite solution for 90 s for surface disinfection and rinsed in sterile water 3 times. The disinfected material was removed using a sterilized scalpel, and disease/health junction parts were cut into 2–3 × 2–3 mm blocks. These tissue blocks were cultivated on 2% potato dextrose agar medium (PDA) and incubated at 25 °C without light [10]. Conidia were removed from colonies with a needle, and pure cultures were obtained by single spore isolation using serial dilution techniques [11]. These pure isolates were stored in the Forest Pathology Laboratory of Nanjing Forestry University. The holotype of the new fungal taxon from this study was deposited at the China Forestry Culture Collection Center (CFCC).
2.2. Colony Observations
Morphological descriptions of the colonies and colony growth rates of isolates were evaluated with PDA and OA. The mycelium block (6 mm in diameter) was placed at the plate center and incubated at 25 °C. Each treatment had five replicates. Colony diameters were measured in perpendicular directions on 5th and 7th days for calculating the growth rate. Color, shape, and texture of the colonies on both sides were recorded.
2.3. Genomic DNA Extraction, PCR, and Sequencing
DNA was extracted from all isolates using the CTAB method [12], and DNA quality and quantity were assessed using Nanodrop, Qubit (Thermo Fisher, Waltham, MA, USA). According to the study on phylogenetic relationship of Obolarina/Biscogniauxia by Mirabolfathy et al. [13], four loci of ITS, ACT, TUB2 and RPB2 were amplified and sequenced with primer sets of ITS-5/ITS-4 [14], ACT-512F/ACT-783R [15], Bt2a/Bt2b [16], and fPB2-5F/fPB2-7cR [14], respectively. The polymerase chain reaction (PCR) was performed in a total volume of 20 µL, which contained 1 µL of DNA sample, 1 µL sense primer, 1 µL antisense primer, 7 µL ddH2O and 10 µL of 2× Green Taq Mix (Table 1). Sequencing of the PCR amplicons was performed by Shanghai Sangon Biotechnology Company.

Table 1.
Primers and parameters used for PCR amplification.
2.4. Morphological Identification
Conidia, conidiogenous cells, and conidiophores were visualized and measured with a Zeiss Axio Imager A2m microscope equipped with Differential Interference Contrast optics (DIC) (Carl Zeiss, Oberkochen, Germany). Microphotographs were taken with ZEN 2 (blue edition, Axio Cam HR R3). The morphological characteristics were described based on the morphology of conidia, conidiogenous cells, and conidiophores [13].
2.5. Phylogenetic Analyses
All nucleotide sequences were searched in NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 15 June 2023) using BLAST toolkit. The BLASTn search based on ITS sequences indicated Biscogniauxia sp. SC3 had the highest sequence identity (100%) with Biscogniauxia maritima (MN844502). Based on the TUB2 and RPB2 sequences, Biscogniauxia sp. SC3 showed highest sequence identity with Biscogniauxia atropunctata (AY951673, 93.65%) and Biscogniauxia atropunctata (JX507778, 96.03%), respectively. While BLASTing with ACT sequence, Biscogniauxia sp. SC3 had the highest sequence identity (94.98%) with Obolarina persica (JX507798). The 72 available ITS sequences of all Biscogniauxia taxa were retrieved from the GenBank and a preliminary phylogenetic analysis was conducted using ITS sequences only. Based on the phylogenetic analysis of ITS, 21 closely related taxa with all multi-locus sequences (ITS + ACT + TUB2 + RPB2) were determined and retrieved from GenBank to construct a more reliable phylogenetic tree. The most closely related species outside the study group like Annulohypoxylon cohaerens [17] and Hypoxylon rubiginosum [18] were chosen as the outgroup [13,19]. The members of the genus Biscogniauxia included in phylogenetic analyses were listed in Table 2. BioEdit 7.0.5.3 [20] was used for multiple sequence alignment, clipping and merging to concatenated ITS + ACT + TUB2 + RPB2 sequence dataset [21]. The concatenated dataset was analyzed in PhyloSuite v1.2.2 [22,23]. ModelFinder was used to choose the most suitable model based on AIC and BIC criteria [24]. Maximum likelihood (ML) analysis was implemented with sequences of multi-loci using IQtree ver. 1.6.8. Bootstrap with 1000 replicates and the GTR + F+I + G4 model were used to improve the assessment of evolutionary branch stability and confidence level in the phylogenetic trees [25,26]. GTR + F + I + G4 model adjusted branch length distribution and the model parameters, until the likelihood value reached maximum. Bayesian Inference (BI) phylogenies were inferred using MrBayes 3.2.6 [27] with SYM + I + G model, 2 parallel runs and 2,000,000 generations. The initial 25% of sampled data were discarded as burn-in. The remaining trees were pooled, and the posterior probabilities (PP) of each branch system as a monophyletic system were calculated. Phylogenetic trees were visualized in FigTree v. 1.4.0 [28].

Table 2.
List of Biscogniauxia strains used in phylogenetic analysis.
2.6. Genealogical Concordance Phylogenetic Species Recognition Analysis
The Genealogical Concordance Phylogenetic Species Recognition (GCPSR) model and paired homology index (PHI or Φw) tests were used to make comparisons between new species and closely related taxa [47]. The PHI tests were applied in SplitsTree4 [48] to identify the level of recombination among closely related species using a four-locus dataset. A PHI index lower than 0.05 threshold (Φw < 0.05) indicated remarkable recombination in the dataset. The association between this new taxon and closely related species was visualized with splits graphs using the LogDet transformation and splits decomposition options [26].
3. Results
3.1. Sampling and Isolation
In June 2020, pine needle blight symptoms were found on P. thunbergii at Nanyang Forest Farm, Bazhong city, Sichuan Province, China. The incidence of the disease was ca. 30%, but severe. All infected pine needles showed brownish-yellow necrotic lesions at the tips, then the symptoms gradually spread to the entire pine needles. The pine needles became light brown, chlorotic, dry, and necrotic. In the late stage, infected pine needles defoliated.
Five fungal isolates (SC1, SC2, SC3, SC4 and SC5) with similar colony morphology were obtained from the infected pine needle tissues. The above isolates were used for subsequent phylogenetic analyses, and isolate SC3 was employed for morphology study.
3.2. Phylogenetic Analyses
The phylogenetic analysis using ITS sequences only showed that five fungal isolates (SC1, SC2, SC3, SC4 and SC5) were clustered in an independent clade, and they were closely related with B. atropunctata (G411 and SGSGf17), B. mediterranea var. microspora and B. nummularia var. merrillii (Figure 1).
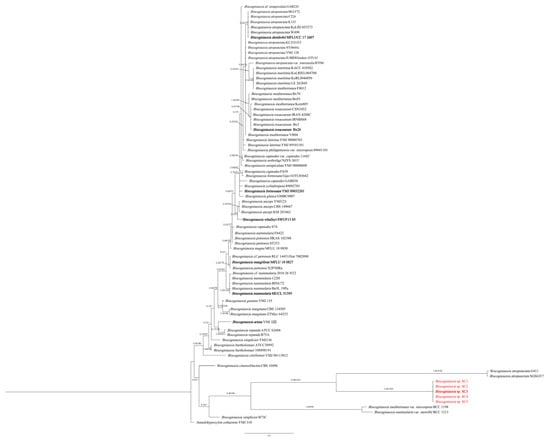
Figure 1.
Phylogenetic relationship of SC1, SC2, SC3, SC4 and SC5 with related taxa in Biscogniauxia derived from ITS. The tree is rooted with Annulohypoxylon cohaerens (YMJ 310). The values on the branches are Bayesian posterior probability (BI-PP) ≥ 0.90 and the ML bootstrap value (BS) ≥ 70, respectively. Isolates in this study are highlighted in red and holotype isolates are in bold.
The phylogenetic analyses using a four-locus (ITS, ACT, TUB2, RPB2) dataset of Biscogniauxia were conducted to verify the above classification result. This data set consisted of 84 sequences, including 21 ITS sequences, 21 ACT sequences, 21 TUB2 sequences and 21 RPB2 sequences from 17 taxa. Meanwhile, 20 novel sequences from 5 isolates (SC1-SC5) of Biscogniauxia sp. were generated from our collections and analyzed. Consequently, phylogenetic analyses were performed with a total of 1911 characters, including gaps that were ACT: 1–320, ITS: 321–654, RPB2: 655–1507, TUB2: 1508–1911. In this phylogenetic tree, five isolates (SC1-SC5) were clustered in a distinct clade with well-supported values (BI/ML = 1/94) (Figure 2), and these isolates differed from all other known species (Figure 2). Notably, this clade had a close relationship with B. atropunctata YMJ128. Based on the two aforementioned phylogenetic analyses, the five fungal isolates represented a new species, Biscogniauxia sinensis sp. nov.
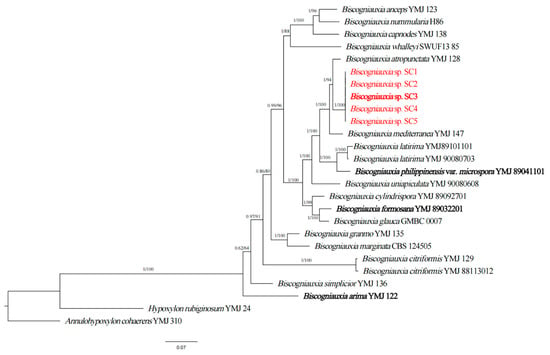
Figure 2.
Phylogenetic relationship of five isolates (SC1, SC2, SC3, SC4 and SC5) with related taxa in Biscogniauxia derived from completed squences of ITS, ACT, TUB and RPB2. The tree is rooted with Annulohypoxylon cohaerens (YMJ 310) and Hypoxylon rubiginosum (YMJ 24). The values on the branches are Bayesian posterior probability (BI-PP) ≥ 0.90 and the ML bootstrap value (BS) ≥ 70, respectively. Isolates in this study are highlighted in red and holotype isolates are in bold.
3.3. Genealogical Concordance Phylogenetic Species Recognition
The GCPSR principle was applied to evaluate the boundary of different species. The PHI test (Figure 3) on Biscogniauxia sinensis showed that there was no significant recombination (Φw = 1.0) between Biscogniauxia sinensis and its closely related species, B. atropunctata (SGSGf17, B5A, YMJ128).
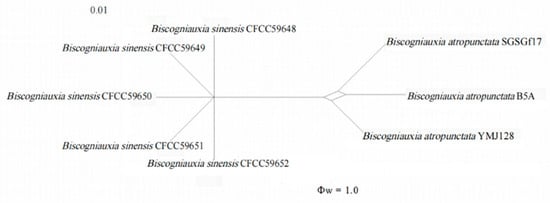
Figure 3.
Pairwise homoplasy index test among Biscogniauxia sinensis and their closely related taxa.
3.4. Taxonomy
Biscogniauxia sinensis Xiao-Lei Ding, Chang-Xia Qiao and D.W. Li, sp. nov. Figure 3.
Index Fungorum Number: IF 900185.
Etymology: The epithet refers to China where the holotype was collected.
Diagnosis: Differs from B. atropunctata in its shorter conidiogenous cells, is light brown and has smaller conidia. Differs from B. mediterranea in its smaller conidiogenous cells and shorter conidia. Differs from B. rosacearum in its shorter and wider conidia.
Description: The sexual state was not observed in vitro. Conidiophores, which are hyaline to yellowish, often occur on the aerial hyphae, composing of a principal axis and one or more branches, (7.5-) 8.2–11.8 (-13.7) × (1.9-) 2.2–3.0 (-3.6) µm, (mean ± SD = 10.0 ± 1.8 × 2.6 ± 0.4 µm, n = 40), with conidiogenous cells developing terminally, generally two to three. Conidiogenous cells are hyaline, oval, (3.8-) 4.5–6.5 (-7.9) × (2.9-) 3.3–4.1 (-4.5) µm, (mean ± SD = 5.5 ± 1.0 × 3.7 ± 0.4 µm, n = 40), and the apical area has remnants of conidial secession, with the apical end distorted due to producing a large number of conidia. The conidia are hyaline to light brown, globose or subglobose, smooth, and aggregated, (3.3-) 3.5–4.3 (-5.0) × (2.9-) 3.3–3.9 (-4.4) µm, (mean ± SD = 3.9 ± 0.4 × 3.6 ± 0.3 µm, n = 40).
Culture characteristics: Colonies on PDA grow fast, reaching the whole Petri dish in 5 d at 25 °C; they are initially white, cottony, and radial with abundant aerial mycelia and diffuse margins, and become floccose with the reverse side being reddish-brown with brown pigmentation after about 14 d (Figure 4G), even showing no sporulation. Colonies of Biscogniauxia sinensis that cover the entire OA Petri dish in 9 d at 25 °C, are regular and round. The mycelia changed from translucent at first to white and turned pale gray with aging. After 15 d, the reverse side became translucent to yellowish (Figure 4H). The mycelium was tight, and the aerial hyphae were raised, white and flocculent. Sporulation formed more frequently on aerial hyphae.
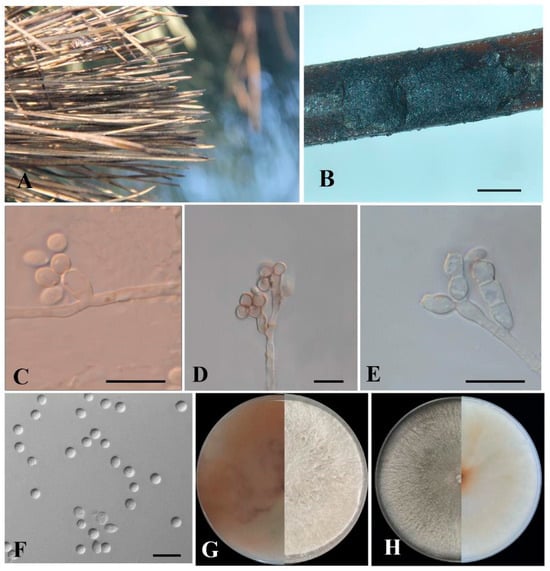
Figure 4.
Morphological characteristics of Biscogniauxia sinensis from Pinus thunbergii. (A) Diseased needles in the field. (B) Conidiomata on needles. (C–E) Conidiophores, conidiogenous cells and conidia. (F) Conidia. (G) Cultures on PDA from above (right) and reverse (left). (H) Cultures on OA from above (left) and reverse (right). Scale bars: (B) = 500 µm; (C–G) = 10 µm.
Holotype: China, Sichuan Province, Bazhong city, Nanyang Forest Farm, 31°85′88.09″ N, 106°75′36.69″ E (DMS), isolated from pine needles of Pinus thunbergii, June 2020, Xiao-Lei Ding, holotype CFCC59650. The holotype is a living specimen being maintained via lyophilization at the China Forestry Culture Collection Center (CFCC), Chinese Academy of Forestry, Beijing, China, and the ex-type SC3 is preserved at the Forest Pathology Laboratory, Nanjing Forestry University.
Habitat and host: On the pine needles of Pinus thunbergii with blight.
Known distribution: Bazhong, Sichuan Province, China.
Additional specimens examined: China, Sichuan Province, Bazhong city, Nanyang Forest Farm, 31°85′88.09″ N, 106°75′36.69″ E (DMS), isolated from a pine needle of Pinus thunbergii, June 2020, Xiao-Lei Ding, CFCC 59,648 (=SC1), CFCC 59,649 (=SC2), CFCC 59,651 (=SC4), CFCC 59,652 (=SC5).
Note: In the multi-locus phylogenetic tree of the Biscogniauxia species, five strains of Biscogniauxia sinensis formed a single clade. Biscogniauxia sinensis can be distinguished from the closely related species B. atropunctata, based on the base-pair differences in ITS (36 out of 469), ACT (17 out of 278), TUB2 (45 out of 520), RPB2 (47 out of 1133). Morphologically, Biscogniauxia sinensis has shorter conidiogenous cells than those of B. atropunctata (4.5–6.5 × 3.3–4.1 vs. 5–10 × 3.5–4.5) µm [35]. Conidia are colorless to light brown, which are distinguished from the colorless conidia of B. atropunctata [49]. Moreover, Biscogniauxia sinensis has smaller conidia (3.5–4.3 × 3.3–3.9 µm vs. 4–5.5 × 3–4.5 µm) than those of B. atropunctata [49].
4. Discussion
Based on the morphological features, multi-locus phylogeny, and GCPSR analyses, the five isolates obtained from wilting needles of P. thumbergii were identified as a new species. According to the available information, this is also the first report of pine needle blight on P. thunbergii associated with Biscogniauxia sinensis in China.
It is worth noting that the new species associated with pine needle blight on P. thunbergii was closely related with B. atropunctata in the multi-locus and ITS-only phylogenetic analyses. By comparing the sequences, there are obvious differences between B. atropunctata and Biscogniauxia sinensis. To further compare the morphological characteristics of these two species [49], we found that the conidia size of isolates SC1 to SC5 are different to those of B. atropunctata; they also differed remarkably in their colony morphology and growth rates [49]. The radial growth rate of B. atropunctata on OA was 12.8 mm/d, and it was white felty, azonate, had diffuse and ropy margins and became floccose, with greenish-olivaceous or dull green patches. The reverse of B. atropunctata was dull green or greenish-olivaceous [49]. Nevertheless, the isolates SC1–SC5 had a growth rate of 9.8 mm/d, which is much slower than that of B. atropunctata. The colony of SC3 on OA (Figure 3) was different from that of B. atropunctata on morphology and color. The identification results showed that the fungus on P. thunbergii differed from B. atropunctata and a new species, Biscogniauxia sinensis.
Ju et al. (1998) wrote a monograph on the genus Biscogniauxia, in which 49 taxa were covered worldwide [50]. In their study, morphological differences among similar genera were discussed, and the critical identifying features among Biscogniauxia species were provided. This genus presents a synteny relationship with other related but morphologically different genera, such as Graphostroma, Camillea and Obolarina, in Xylariaceae [39,51,52], while, Wendt et al. (2018) reinstate and amended the family Graphostromataceae [44].
Graphostromataceae comprised Biscogniauxia, Graphostroma, Camillea, Obolarina, Theissenia, and Vivantia [53]. Biscogniauxia has been reported from various woody hosts around the world; more than 10 new hosts have been found in relevant studies recorded in the fungi database of the USDA [54]. Biscogniauxia was found in Malus, Mangifera, Prunus, Pyrus, and Vitis fruit trees. Most of these hosts are angiosperms, but B. atropunctata has been isolated from Juniperus virginiana in the United States [55]. Meanwhile, B. mediterranea usually occurs on Quercus suber in the Mediterranean basin [56,57]. But B. mediterranea has been reported as a pathogen of Pinus sylvestris in Spain [58]. In 2020, it was also isolated from a twig of Abies alba with needle browning symptoms in Poland [59]. This fungus has long been considered as an endophyte in all aerial organs (rarely in leaves) of oak trees and has an incubation period without symptoms [60]. However, when the host is weakened or stressed by other pathogens, B. mediterranea can become a facultative pathogen; it can accelerate the decline of the tree and eventually lead to the death of the hosts [61,62,63]. Similar study also indicates that, as an endophyte, it lies dormant within Scots pine (Pinus sylvestris) and can influence host growth, leading to slower growth rates in the pine trees [64]. Biscogniauxia nummularia is recognized as an endophyte in dicotyledonous angiosperms and some grass species [49]. In 2019, B. nummularia was first recorded in southern Poland as an endophyte of white fir (Abies alba) and Pinus × rhaetica Brugger [65,66]. Research has illuminated the endophytic characteristics of B. nummularia, enabling it to swiftly transition from a benign endophyte to a primary pathogen [67,68,69]. Similarly, Biscogniauxia, typically found in woody plants, has also been isolated from pines such as Pinus koraiensis, firs such as Abies nephrolepis, and Douglas fir, as well as from Taxus cuspidata as an endophyte [70].
Endophytes usually reside within the internal tissues of plants without causing apparent harm to the host [71]. Due to imbalances in nutrient exchange, environmental changes, and change in host, endophytes can transit into pathogens under such circumstances [72]. Biscogniauxia is globally distributed, usually acting as an ubiquitous wood decomposer and a common endophyte [69]. There is substantial evidence that Biscogniauxia species exist as endophytes within healthy trees and later become pathogenic to plants when adverse stress occurs [70,73]. Although Biscogniauxia has been characterized as an endophytic fungus, evidence from several studies indicate that these endophytes, akin to pathogenic bacteria, possess intrinsic virulence factors and exhibit prolonged latent periods. This virulence potential facilitates the establishment of infections in host plants, particularly when they are located in senescent branches or are subjected to environmental stressors [74]. One of the most significant influence factors on the occurrence of Biscogniauxia is climate change. It has been proved that warm temperatures and a prolonged summer drought favor the growth of Biscogniauxia species [75]. Most species of Biscogniauxia are particularly inclined to affect trees under water stress [76]. Biscogniauxia sinensis might act as an endophyte in many plants, potentially existing symbiotically within them. However, it could also function as a plant pathogen, particularly under stressful conditions such as drought. Thus, the application of sanitation and scarification practices along with appropriate fungicides can effectively prevent and reduce the occurrence of the Biscogniauxia on oak trees [77]. However, the control of fungi of this genus on Japanese black pine remains to be studied. Next, biocontrol agents and other control measures will be studied for effectively reducing the impact of Biscogniauxia sinensis on forests and ecological system.The climatic conditions during summer in Bazhong City, Sichuan Province, are high temperatures and drought, which enhance plant transpiration and trigger stress conditions. These conditions may induce the expression of pathogenic traits of endophytic fungi. Although the isolation of endophytic fungi was not the focus of our study during the initial field survey, it is necessary to conduct further comprehensive research on this phenomenon.
Generally, Biscogniauxia is not frequently reported to cause plant diseases in China. In 2008, B. mandshurica was found on dead branches of Malus sp. in Changbai Mountain, Jilin Province, China [78]. In March 2014, a new species, B. glaucae, was found on the dead bark of an unknown plant in Guizhou Province, China [36]. In 2014, B. petrensis was found on rock from two karst caves in Guizhou Province, China [79]. In November 2015, B. dendrobii was found in Dendrobium aphyllum stems from a nursery in Guizhou Province, China [35]. This is the first report that the new species identified here is associated with discolored and withered needles on P. thunbergii in forest. The symptoms have affected the potential value of pine trees, influenced the balance of the forest environment, and caused consequential economic and ecological losses [10].
The discovery and classification of the new species Biscogniauxia sinensis not only enhances the diversity of the genus but also provides new perspectives on its genetic diversity and evolutionary relationships with other species [80]. It will help us better understand its ecological role and interactions with host plants. Additionally, it aids in research on how these fungi adapt to various environments and hosts, which is crucial for maintaining the health and stability of forest ecosystems.
5. Conclusions
The morphological features, multi-locus phylogeny, and GCPSR analysis were employed to determine Biscogniauxia sinensis as a novel species associated with pine needle blight on P. thunbergii. The discovery and classification of Biscogniauxia sinensis will expand the current knowledge of this genus. It is necessary to conduct further studies on this disease from multiple perspectives (mycology, epidemiology, ecology and evolution, as well as economy). From the practical aspect, future studies should also focus on reducing its potential damage to P. thunbergii and forest ecosystems.
Author Contributions
C.Q.: conceptualization, writing—original draft and review and editing, data curation, investigation, methodology and visualization. R.Z.: writing—review and editing, investigation. X.D.: writing—review and editing, project administration, resources and supervision. D.L.: writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This project is supported by the National Natural Science Foundation of China 31800543.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are very grateful to Mao-Jiao Zhang for her assistance in morphological and phylogenetic analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, H.; Wang, Z.; Song, W.; Zhao, Z.; Zhao, Y. Isolation of proanthocyanidins from Pinus thunbergii needles and tyrosinase inhibition activity. Process Biochem. 2021, 100, 245–251. [Google Scholar] [CrossRef]
- Tanaka, S.; Tomita, R.; Saijo, H.; Takahashi, K.; Ashitani, T. Growth-inhibitory activity of components in Cryptomeria japonica leaves against Robinia pseudoacacia. J. For. Res. 2020, 25, 192–197. [Google Scholar] [CrossRef]
- Masaka, K.; Torita, H.; Sato, H.; Kon, H.; Fukuchi, S.M. Decline of Pinus thunbergii Parlat. stands due to excess soil moisture caused by a buried andosol layer at a coastal sand site in Hokkaido, northern Japan. J. For. Res. 2010, 15, 341–346. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Lee, J. Studies on the characteristics of growth of Pinus thunbergii planted in a Costal Sand Zone. J. Korean For. Soc. 2012, 101, 656–662. [Google Scholar]
- Beccaccioli, M.; Grottoli, A.; Scarnati, L.; Faino, L.; Reverberi, M. Nanopore hybrid assembly of Biscogniauxia mediterranea isolated from Quercus cerris affected by charcoal disease in an endangered coastal wood. Microbiol. Resour. Announc. 2021, 10, e0045021. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G. Control Measures against Main Pinus koraiensis Diseases in Liaoning Province. Plant Dis. Pests 2022, 13, 5–6. [Google Scholar] [CrossRef]
- Hirose, D.; Osono, T. Development and seasonal variations of Lophodermium populations on Pinus thunbergii needle litter. Mycoscience 2006, 47, 242–247. [Google Scholar] [CrossRef]
- Du, Z. Common diseases of pine needles and comprehensive prevention and control measures. Mod. Agric. Technol. 2022, 31–32. [Google Scholar]
- Li, C.D.; Zhu, X.Q.; Han, Z.M. Investigation on brown-spot needle blight of pines in China. J. Nanjing For. Univ. 1986, 29, 11–18. [Google Scholar]
- Ding, X.L.; Zhao, R.W.; Lin, S.X.; Ye, J.R. Morphological and phylogenetic resolution of Diplodia neojuniperi emerging Diplodia top dieback of Pinus thunbergii Parl. in China. Phyton 2022, 91, 2813–2825. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.C.; Zhang, Y.; Li, D.W.; Ye, J.R. Colletotrichum gloeosporioides sensu stricto is a pathogen of leaf anthracnose on evergreen spindle tree (Euonymus japonicus). Plant Dis. 2015, 100, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Damm, U.; Mostert, L.; Crous, P.W.; Fourie, P.H. Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Persoonia 2008, 20, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Mirabolfathy, M.; Ju, Y.M.; Hsieh, H.M.; Rogers, J.D. Obolarina persica sp. nov., associated with dying Quercus in Iran. Mycoscience 2013, 54, 315–320. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Lousie, G.N.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from Filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.M.; Ju, Y.M.; Rogers, J.D. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 2005, 97, 844–865. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.M.; Rogers, J.D. A Revision of the Genus Hypoxylon; APS Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Sohrabi, M.; Mohammadi, H.; Armengol, J.; León, M. New report of Biscogniauxia rosacearum as a pathogen on almond trees in Iran. J. Plant Dis. Prot. 2022, 129, 411–417. [Google Scholar] [CrossRef]
- Rajpoot, A.; Kumar, V.; Kumari, R.; Singh, T. Molecular Characteristic of Indian Pangolin with Existing Manidae Species Based on Mitochondrial Genes: A Wildlife Forensic Approach. In Proceedings of the 4th International Arab Forensic Sciences & Forensic Medicine Conference, Riyadh, Saudi Arabia, 27–29 November 2018. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Tang, X.Y.; Hu, S.; Zhu, W.Y.; Wu, X.P.; Sang, W.J.; Ding, H.X.; Peng, L.J. First report of gray spot on tobacco caused by Alternaria alstroemeriae in China. Plant Dis. 2023, 107, 2546. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Kalyaanamoorthy; Subha; Minh, B.Q.; Wong; Thomas, K.F.; Haeseler, V.; Arndt; Jermiin; Lars, S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Dong, H.-P.; Hou, L.-J.; Liu, Y.; Ou, Y.-F.; Zheng, Y.-L.; Han, P.; Liang, X.; Yin, G.-Y.; Wu, D.-M.; et al. Newly discovered Asgard archaea Hermodarchaeota potentially degrade alkanes and aromatics via alkyl/benzyl-succinate synthase and benzoyl-CoA pathway. ISME J. 2021, 15, 1826–1843. [Google Scholar] [CrossRef]
- Wan, Y.; Li, D.W.; Si, Y.Z.; Li, M.; Huang, L.; Zhu, L.H. Three new species of Diaporthe causing leaf blight on Acer palmatum in China. Plant Dis. 2023, 107, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1. 4.0. A Graphical Viewer of Phylogenetic Trees; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2012. [Google Scholar]
- Hsieh, H.M.; Lin, C.R.; Fang, M.J.; Rogers, J.D.; Ju, Y.M. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol. Phylogenetics Evol. 2010, 54, 957–969. [Google Scholar] [CrossRef]
- Crous, P.W.; Akulov, A.; Balashov, S.; Boers, J.; Braun, U.; Castillo, J.; Delgado, M.A.; Denman, S.; Erhard, A.; Gusella, G.; et al. New and Interesting Fungi. Fungal Syst. Evol. 2023, 11, 109–156. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ballesteros, J.; González, V.; Salazar, O.; Acero, J.; Portal, M.A.; Julián, M.; Rubio, V.; Bills, G.F.; Polishook, J.D.; Platas, G.; et al. Phylogenetic study of Hypoxylon and related genera based on ribosomal ITS sequences. Mycologia 2000, 92, 964–977. [Google Scholar] [CrossRef]
- Pinto, T. The Phyloecology of Hypoxylon sensu lato. Master’s Thesis, University of California, Berkeley, CA, USA, 2001. [Google Scholar]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef]
- Ju, Y.M.; Rogers, J.D. New and interesting Biscogniauxia taxa, with a key to the world species. Mycol. Res. 2001, 105, 1123–1133. [Google Scholar] [CrossRef]
- Ma, X.Y.; Nontachaiyapoom, S.; Hyde, K.D.; Jeewon, R.; Doilom, M.; Chomnunti, P.; Kang, J.C. Biscogniauxia dendrobii sp. nov. and B. petrensis from Dendrobium orchids and the first report of cytotoxicity (towards A549 and K562) of B. petrensis (MFLUCC 14-0151) in vitro. S. Afr. J. Bot. 2020, 134, 382–393. [Google Scholar] [CrossRef]
- Li, Q.R.; Gong, X.F.; Zhang, X.; Pi, Y.H.; Long, S.H.; Wu, Y.P.; Shen, X.C.; Kang, Y.Q.; Kang, J.C. Phylogeny of Graphostromatacea with two new species (Biscogniauxia glaucae sp. nov. and Graphostroma guizhouensis sp. nov.) and new record of Camillea broomeana isolated in China. Arch. Microbiol. 2021, 203, 6119–6129. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, M.C.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Stadler, M.; Gareth Jones, E.B.; Promputtha, I.; Suwannarach, N.; Camporesi, E.; Bulgakov, T.S.; Liu, J.K. Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Divers. 2022, 112, 1–88. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.; Hyde, K.; Jones, E.; McKenzie, E.; Bhat, D.J.; Dayarathne, M.; Huang, S.-K.; Norphanphoun, C.; Senanayake, I.; Perera, R.; et al. Families of Sordariomycetes. Fungal Divers. 2016, 79, 1–317. [Google Scholar] [CrossRef]
- Raimondo, M.L.; Lops, F.; Carlucci, A. Charcoal canker of pear, plum, and quince trees caused by Biscogniauxia rosacearum sp. nov. in Southern Italy. Plant Dis. 2016, 100, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Travadon, R.; Lawrence, D.P.; Moyer, M.M.; Fujiyoshi, P.T.; Baumgartner, K. Fungal species associated with grapevine trunk diseases in Washington wine grapes and California table grapes, with novelties in the genera Cadophora, Cytospora and Sporocadus. Frontiers 2022, 3, 1018140. [Google Scholar] [CrossRef]
- Okane, I.; Srikitikulchai, P.; Toyama, K.; Læssøe, T.; Sivichai, S.; Hywel-Jones, N.; Nakagiri, A.; Potacharoen, W.; Suzuki, K.-i. Study of endophytic Xylariaceae in Thailand: Diversity and taxonomy inferred from rDNA sequence analyses with saprobes forming fruit bodies in the field. Mycoscience 2008, 49, 359–372. [Google Scholar] [CrossRef]
- Jankowiak, R.; Bilański, P.; Zając, J.; Jobczyk, A.; Taerum, S.J. The culturable leaf mycobiome of Viscum album subsp. austriacum. For. Pathol. 2023, 53, e12821. [Google Scholar] [CrossRef]
- Vandegrift, R.; Newman, D.S.; Dentinger, B.T.M.; Batallas-Molina, R.; Dueñas, N.; Flores, J.; Goyes, P.; Jenkinson, T.S.; McAlpine, J.; Navas, D.; et al. Richer than Gold: The fungal biodiversity of Reserva Los Cedros, a threatened Andean cloud forest. Bot. Stud. 2023, 64, 17. [Google Scholar] [CrossRef] [PubMed]
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkämper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsa-ard, J.J.; Srikitikulchai, P.; Peršoh, D.; et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018, 17, 115–154. [Google Scholar] [CrossRef]
- Bashiri, S.; Abdollahzadeh, J.; Evidente, A. Diagnosing and pathogenicity of Biscogniauxia species, the causal agents of oak charcoal canker and decline in Zagros forests of Iran. J. Plant Pathol. 2022, 104, 1011–1025. [Google Scholar] [CrossRef]
- Spies, C.F.J.; Mostert, L.; Carlucci, A.; Moyo, P.; van Jaarsveld, W.J.; du Plessis, I.L.; van Dyk, M.; Halleen, F. Dieback and decline pathogens of olive trees in South Africa. Persoonia 2020, 45, 196–220. [Google Scholar] [CrossRef]
- Quaedvlieg, W.; Binder, M.; Groenewald, J.Z.; Summerell, B.A.; Carnegie, A.J.; Burgess, T.I.; Crous, P.W. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia 2014, 33, 1–40. [Google Scholar] [CrossRef]
- Royse, D.; Baars, J.J.P.; Tan, Q. Current overview of Mushroom production in the world. In Edible and Medicinal Mushrooms; Diego, C.Z., Pardo-Giménez, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar]
- Ju, Y.M.; Rogers, J.D.; Martín, F.S.; Granmo, A. The genus Biscogniauxia. Mycotaxon 1998, 66, 1–98. [Google Scholar]
- Bahmani, Z.; Abdollahzadeh, J.; Amini, J.; Evidente, A. Biscogniauxia rosacearum the charcoal canker agent as a pathogen associated with grapevine trunk diseases in Zagros region of Iran. Sci. Rep. 2021, 11, 14098. [Google Scholar] [CrossRef] [PubMed]
- Pažoutová, S.; Šrůtka, P.; Holuša, J.; Chudíčková, M.; Kolařík, M. The phylogenetic position of Obolarina dryophila (Xylariales). Mycol. Prog. 2010, 9, 501–507. [Google Scholar] [CrossRef]
- Peláez, F.; Platas, G.; Sánchez-Ballesteros, J. Molecular phylogenetic studies within the Xylariaceae based on ribosomal DNA sequences. Fungal Divers. 2008, 31, 111–134. [Google Scholar]
- Daranagama, D.A.; Hyde, K.D.; Sir, E.B.; Thambugala, K.M.; Stadler, M. Towards a natural classification and backbone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae. Fungal Divers. 2018, 88, 1–165. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Systematic Mycology and Microbiology Laboratory-Fungal Databases; ARS, USDA: Washington, DC, USA, 2023. [Google Scholar]
- Hoffman, M.T.; Arnold, A.E. Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycol. Res. 2008, 112, 331–344. [Google Scholar] [CrossRef]
- Yangui, I.; Boutiti, M.Z.; Vettraino, A.M.; Bruni, N.; Vannini, A.; Jamaâ, M.L.B.; Boussaid, M.; Messaoud, C. Biscogniauxia mediterranea associated with cork oak (Quercus suber) in Tunisia: Relationships between phenotypic variation, genetic diversity and ecological factors. Fungal Ecol. 2019, 41, 224–233. [Google Scholar] [CrossRef]
- Bußkamp, J.; Bien, S.; Neumann, L.; Blumenstein, K.; Terhonen, E.; Langer, G.J. Endophytic community in juvenile Acer pseudoplatanus and pathogenicity of Cryptostroma corticale and other associated fungi under controlled conditions. J. Plant Pathol. 2024, 106, 565–577. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. United States National Fungus Collections Fungus-Host Dataset; ARS, USDA: Washington, DC, USA, 2021. [Google Scholar]
- Pusz, W.; Baturo-Ciesniewska, A.; Kaczmarek-Pienczewska, A.; Zwijacz-Kozica, T.; Patejuk, K. The mycobiota of needles and shoots of silver fir (Abies alba Mill.) with symptoms of Herpotrichia needle browning in the Tatra Mts. (Poland). Ann. For. Res. 2020, 63, 45–56. [Google Scholar] [CrossRef]
- Wilson, D.C. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Sirca, C.; Spano, D.; Franceschini, A. Variation of endophytic cork oak associated fungal communities in relation to plant health and water stress. For. Pathol. 2011, 41, 193–201. [Google Scholar] [CrossRef][Green Version]
- Marie-Laure, D.L.; Benoit, M.; Louis-Michel, N.; Dominique, P.; Andrea, V. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar]
- Vannini, A.; Lucero, G.; Anselmi, N.; Vettraino, A.M. Response of endophytic Biscogniauxia mediterranea to variation in leaf water potential of Quercus cerris. For. Pathol. 2008, 39, 8–14. [Google Scholar] [CrossRef]
- Sanz-Ros, A.V.; Müller, M.M.; San Martín, R.; Diez, J.J. Fungal endophytic communities on twigs of fast and slow growing Scots pine (Pinus sylvestris L.) in northern Spain. Fungal Biol. 2015, 119, 870–883. [Google Scholar] [CrossRef]
- Patejuk, K.; Kaczmarek-Pieńczewska, A.; Baturo-Ciesniewska, A.; Pusz, W. Mycobiota of peat-bog pine (Pinus × rhaetica) needles in the Stołowe Mountains National Park, Poland. Nova Hedwig. 2021, 112, 253–265. [Google Scholar] [CrossRef]
- Pusz, W.; Baturo-Ciesniewska, A.; Kaczmarek-Pieńczewska, A.; Zwijacz-Kozica, T. The health status of sliver fir (Abies alba Mill.) in selected locations of the Tatra national park and the Karkonosze national park. For. Wood Technol. 2020, 19, 145–152. [Google Scholar] [CrossRef]
- Petrini, L.E.; Petrini, O. Xylariaceous fungi as endophytes. Sydowia 1985, 38, 201–265. [Google Scholar] [CrossRef]
- Patejuk, K.; Baturo-Ciesniewska, A.; Pusz, W.; Kaczmarek-Pienczewska, A. Biscogniauxia charcoal canker-A new potential threat for Mid-European forests as an effect of climate change. Forests 2022, 13, 89. [Google Scholar] [CrossRef]
- Petrović, E.; Godena, S.; Cosic, J.; Vrandečić, K. Identification and pathogenicity of Biscogniauxia and Sordaria species isolated from Olive trees. Horticulturae 2024, 10, 243. [Google Scholar] [CrossRef]
- Amand, S.; Langenfeld, A.; Blond, A.; Dupont, J.; Nay, B.; Prado, S. Guaiane sesquiterpenes from Biscogniauxia nummularia featuring potent antigerminative activity. J. Nat. Prod. 2012, 75, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.M.; Nie, F.X.; Zhao, Y.Q.; Zhang, D.W.; Zhou, D.G.; Wu, J.F.; Qu, L.; Xiao, L.; Liu, L.L. A review on plant endophytes in response to abiotic stress. Environ. Pollut. Bioavailab. 2024, 36. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phukhamsakda, C.; Hyde, K.D.; McKenzie, E.H.C.; Saxena, R.K.; Li, Q. Do all fungi have ancestors with endophytic lifestyles? Fungal Divers. 2024, 125, 73–98. [Google Scholar] [CrossRef]
- Nugent, L.K.; Sihanonth, P.; Thienhirun, S.; Whalley, A.J.S. Biscogniauxia: A genus of latent invaders. Mycologist 2005, 19, 40–43. [Google Scholar]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109 Pt 6, 661–686. [Google Scholar] [CrossRef]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Granata, G.; Sidoti, A. Biscogniauxia nummularia: Pathogenic agent of a beech decline. For. Pathol. 2004, 34, 363–367. [Google Scholar] [CrossRef]
- Karami, J.; Kavosi, M.R.; Babanezhad, M.; Kiapasha, K. Integrated management of the charcoal disease by silviculture, chemical and biological methods in forest parks. J. Sustain. For. 2017, 37, 429–444. [Google Scholar] [CrossRef]
- Vasilyeva, L.; Li, Y.; Stephenson, S. Some pyrenomycetous fungi new to China. Mycotaxon 2009, 109, 415–428. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Liu, F.; Zhou, X.; Liu, X.Z.; Liu, S.J.; Cai, L. Culturable mycobiota from Karst caves in China, with descriptions of 20 new species. Persoonia 2017, 39, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Zhang, J.Y.; Xiao, Y.P.; Lu, Y.Z. Biscogniauxia dicranopteridis sp. nov. and B. petrensis isolated from Dicranopteris dichotoma in China. Phytotaxa 2024, 641, 255–266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).