Genetic Diversity and Population Structure of Bursaphelenchus xylophilus in Guangdong, Guangxi, and Jiangsu Provinces in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Geographical Origin and Preservation of Nematode Samples
2.2. DNA Extraction and High-Throughput Genome Re-Sequencing
2.3. Sequencing Data Processing
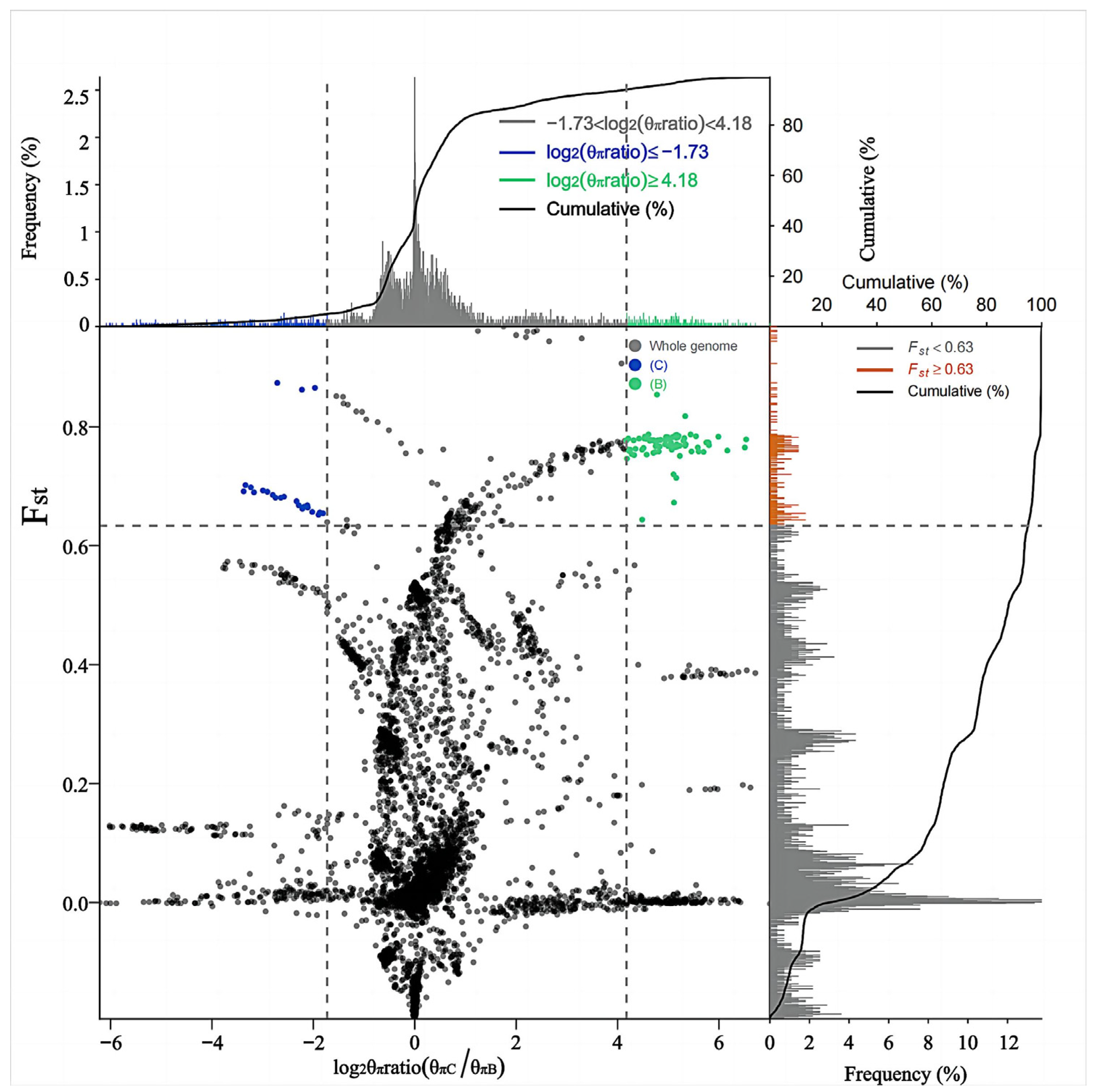
2.4. Analysis of Population Genetic Differentiation
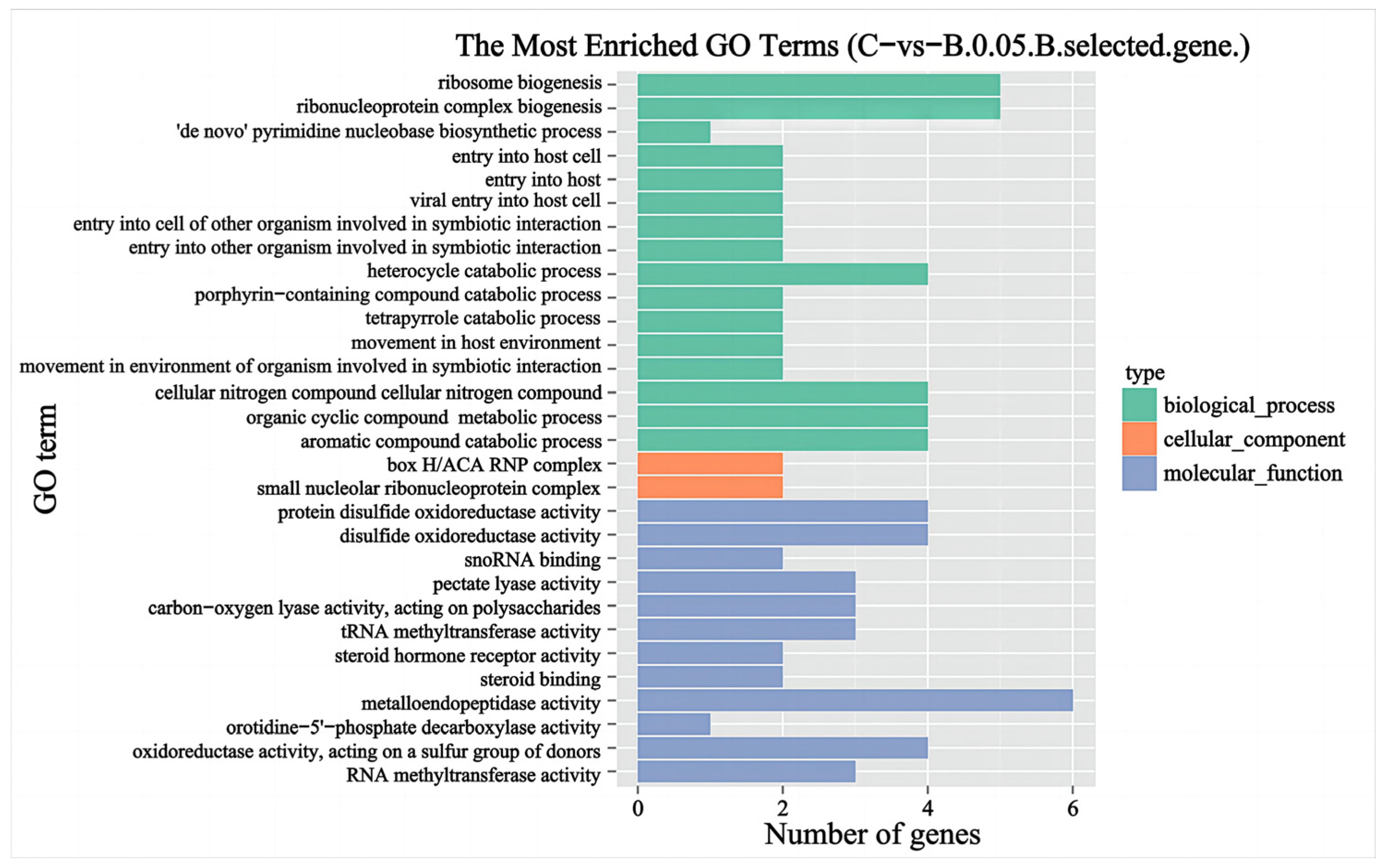
2.5. Selective Sweep and GO Enrichment Analysis
3. Results
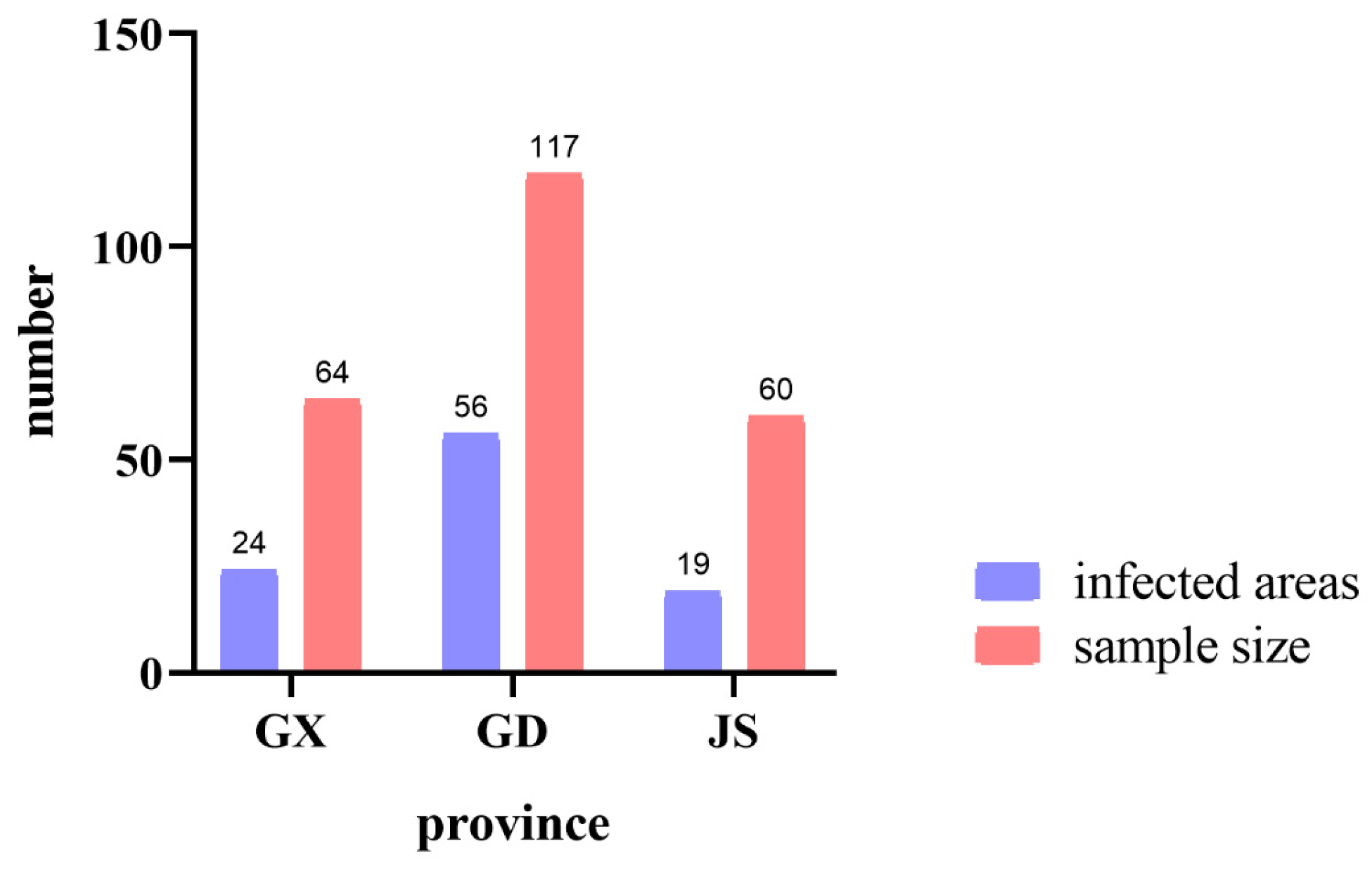
3.1. Sampling of PWN Strains
3.2. Statistics of SNP Genotypes and SNP Loci
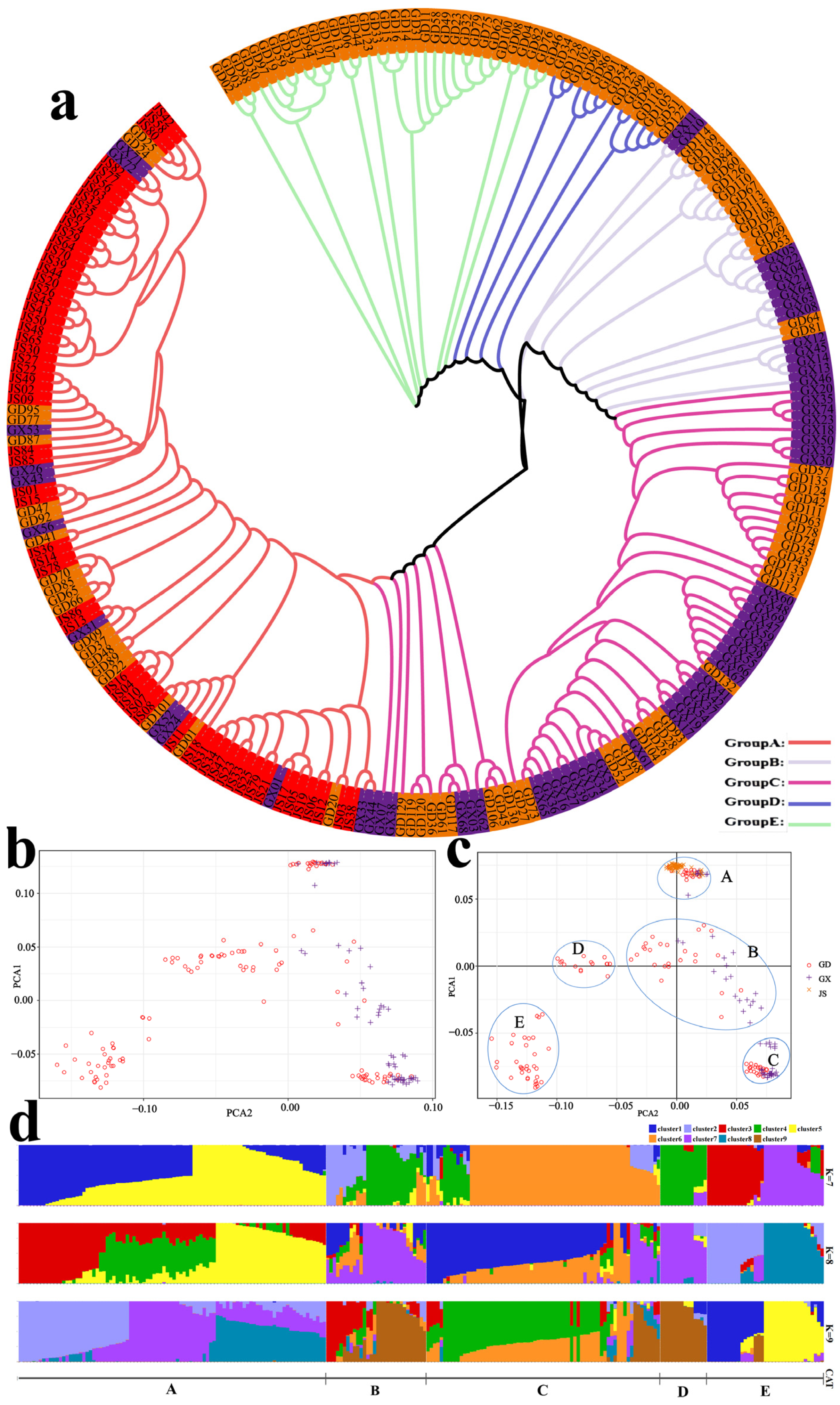
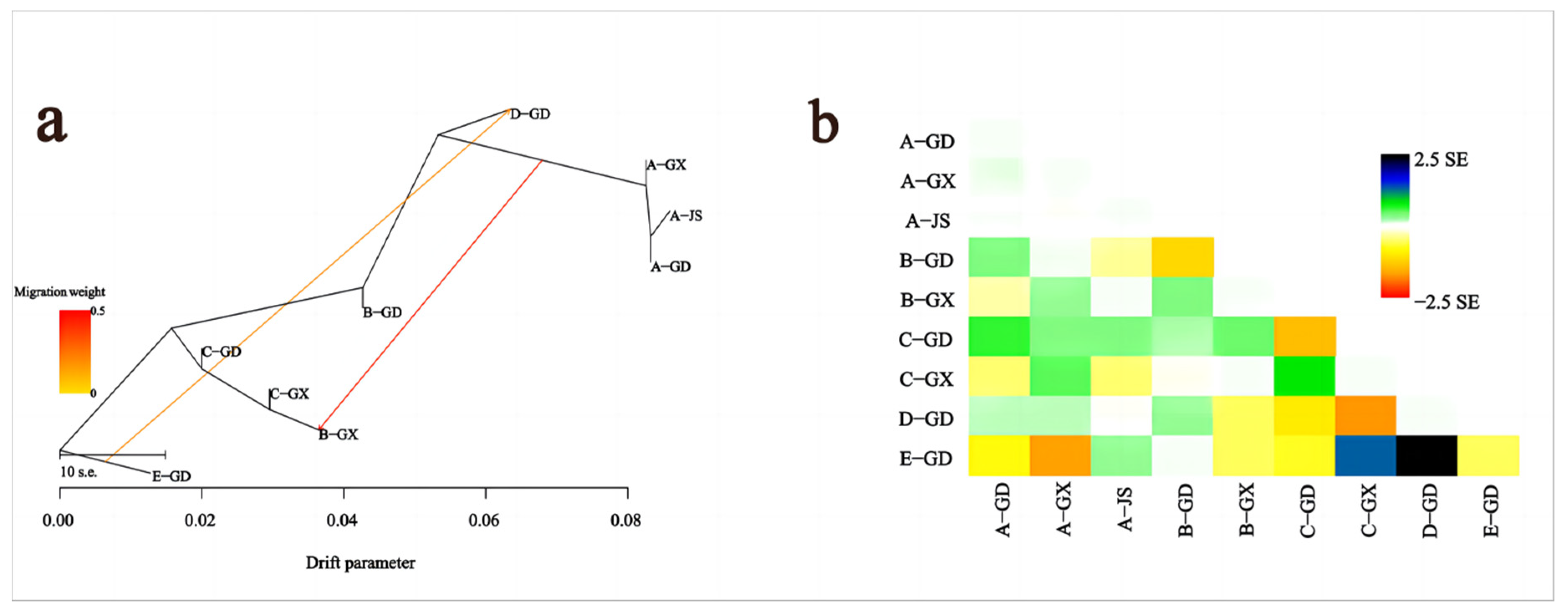
3.3. Analysis of Genetic Structure and Genetic Diversity
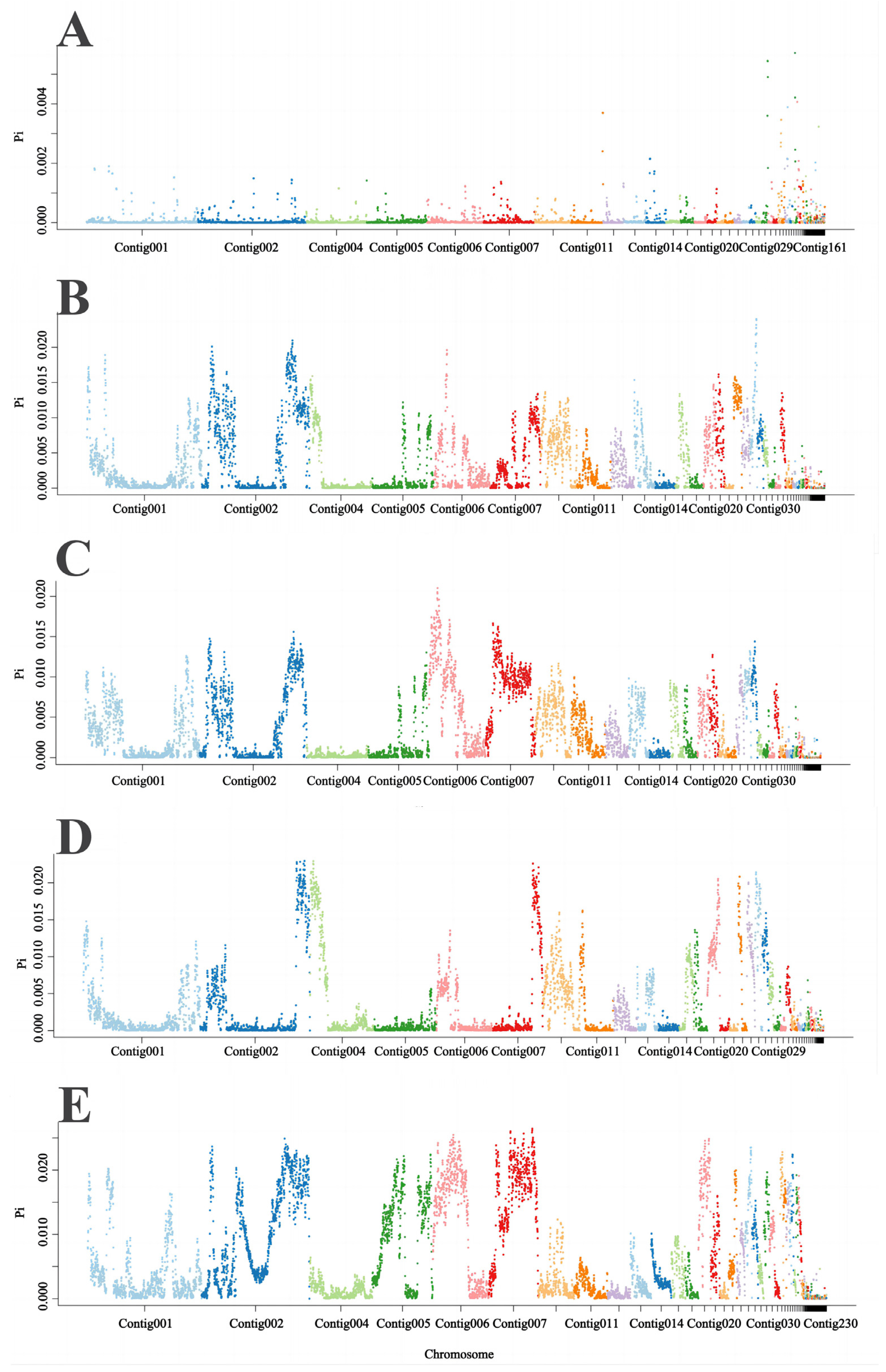
3.4. Selective Sweep Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Strain No. | Origin | Host | Sampling Date |
|---|---|---|---|
| GD01 | Fengkai County, Zhaoqing City, Guangdong Province | Pinus. massoniana | January 2015 |
| GD02 | Qingcheng District, Qingyuan City, Guangdong Province | P. massoniana | January 2015 |
| GD03 | Zengcheng City, Guangzhou City, Guangdong Province | P. massoniana | January 2015 |
| GD04 | Huiyang District, Huizhou City, Guangdong Province | P. massoniana | January 2015 |
| GD06 | Huidong County, Huizhou City, Guangdong Province | P. massoniana | January 2015 |
| GD08 | Boluo County, Huizhou City, Guangdong Province | P. massoniana | January 2015 |
| GD09 | Wujiang District, Shaoguan City, Guangdong Province | P. massoniana | January 2015 |
| GD11 | Zijin County, Heyuan City, Guangdong Province | P. massoniana | January 2015 |
| GD13 | Huicheng District, Huizhou City, Guangdong Province | P. massoniana | January 2015 |
| GD14 | Zhangmutou Town, Dongguan City, Guangdong Province | P. massoniana | January 2015 |
| GD15 | Zhangmutou Town, Dongguan City, Guangdong Province | P. massoniana | January 2015 |
| GD16 | Zhangmutou Town, Dongguan City, Guangdong Province | P. massoniana | January 2015 |
| GD17 | Tianhe District, Guangzhou City, Guangdong Province | P. massoniana | January 2015 |
| GD19 | Tianhe District, Guangzhou City, Guangdong Province | Pinus. yunnanensis | January 2015 |
| GD20 | Meixian District, Meizhou City, Guangdong Province | P. massoniana | January 2015 |
| GD22 | Qujiang District, Shaoguan City, Guangdong Province | P. massoniana | January 2015 |
| GD23 | Meijiang District, Meizhou City, Guangdong Province | P. massoniana | January 2015 |
| GD24 | Guangning County, Zhaoqing City, Guangdong Province | P. massoniana | August 2017 |
| GD25 | Guangning County, Zhaoqing City, Guangdong Province | P. massoniana | August 2017 |
| GD26 | Fengshun County, Meizhou City, Guangdong Province | P. massoniana | August 2017 |
| GD27 | Jiaoling County, Meizhou city, Guangdong Province | P. massoniana | August 2017 |
| GD28 | Jiaoling County, Meizhou city, Guangdong Province | P. massoniana | August 2017 |
| GD30 | Haifeng County, Shanwei City, Guangdong Province | P. massoniana | August 2017 |
| GD31 | Dongyuan County, Heyuan City, Guangdong Province | P. massoniana | August 2017 |
| GD32 | Dongyuan County, Heyuan City, Guangdong Province | P. massoniana | August 2017 |
| GD33 | Dongguan City, Guangdong Province | Unknown | Unknown |
| GD34 | Meizhou City, Guangdong province | Unknown | August 2022 |
| GD35 | Lianping County, Heyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD36 | Huaiji County, Zhaoqing City, Guangdong Province | P. massoniana | September 2022 |
| GD37 | Renhua County, Shaoguan City, Guangdong Province | Pinus. elliottii | September 2022 |
| GD39 | Guangzhou City, Guangdong Province | P. massoniana | September 2022 |
| GD41 | Longping Town, Lianzhou City, Guangdong Province | P. massoniana | September 2022 |
| GD42 | Wengyuan County, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD43 | Lianping County, Heyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD44 | Xinhui District, Jiangmen City, Guangdong Province | Unknown | September 2022 |
| GD45 | Nanlang Town, Zhongshan City, Guangdong Province | P. massoniana | September 2022 |
| GD46 | Yuancheng District, Meizhou City, Guangdong Province | Unknown | September 2022 |
| GD47 | Conghua District, Guangzhou City, Guangdong Province | P. massoniana | September 2022 |
| GD48 | Lianzhou city, Qingyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD49 | Luhe County, Shanwei City, Guangdong Province | P. massoniana | September 2022 |
| GD51 | Jiedong District, Jieyang City, Guangdong Province | P. massoniana | September 2022 |
| GD52 | Luoding city, Yunfu city, Guangdong Province | P. massoniana | September 2022 |
| GD53 | Chenghai District, Shantou City, Guangdong Province | P. massoniana | September 2022 |
| GD54 | Deqing County, Zhaoqing City, Guangdong Province | P. massoniana | September 2022 |
| GD57 | Wengyuan County, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD58 | Yuancheng District, Heyuan City, Guangdong Province | Unknown | September 2022 |
| GD59 | Huadu District, Guangzhou City, Guangdong Province | P. massoniana | September 2022 |
| GD60 | Qingyuan City, Guangdong province | P. massoniana | September 2022 |
| GD61 | Qujiang District, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD62 | Yuancheng District, Heyuan City, Guangdong Province | Unknown | September 2022 |
| GD63 | Lianping County, Heyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD64 | Lechang City, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD65 | Qujiang District, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD66 | Yingde City, Qingyuan City, Guangdong Province | Unknown | September 2022 |
| GD67 | Xiangqiao District, Chaozhou City, Guangdong Province | Unknown | September 2022 |
| GD68 | Xingning City, Meizhou City, Guangdong Province | P. massoniana | September 2022 |
| GD69 | Chenghai District, Shantou City, Guangdong Province | P. massoniana | September 2022 |
| GD70 | Nanxiong City, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD71 | Chenghai District, Shantou City, Guangdong Province | P. massoniana | September 2022 |
| GD72 | Chenghai District, Shantou City, Guangdong Province | P. massoniana | September 2022 |
| GD73 | Lechang City, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD74 | Lianping County, Heyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD75 | Jieyang County, Jieyang City, Guangdong Province | P. massoniana | September 2022 |
| GD76 | Meizhou City, Guangdong Province | Unknown | September 2022 |
| GD77 | Longping Town, Qingyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD78 | Jiangxiong Village, Heyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD79 | Jieyang County, Jieyang City, Guangdong Province | P. massoniana | September 2022 |
| GD81 | Lechang city, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD82 | Meizhou City, Guangdong Province | Unknown | September 2022 |
| GD84 | Qingyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD85 | Nanao County, Shantou City, Guangdong Province | P. massoniana | September 2022 |
| GD86 | Qiufeng Town, Zhaoqing City, Guangdong Province | P. massoniana | September 2022 |
| GD87 | Fengwei Town, Zhaoqing City, Guangdong Province | Unknown | September 2022 |
| GD89 | Nanxiong City, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD90 | Deqing County, Zhaoqing City, Guangdong Province | P. massoniana | September 2022 |
| GD91 | Deqing County, Zhaoqing City, Guangdong Province | P. massoniana | September 2022 |
| GD92 | Shixing County, Shaoguan City, Guangdong Province | Unknown | September 2022 |
| GD93 | Xinbu, Meizhou City, Guangdong Province | Unknown | September 2022 |
| GD94 | Baiyun District, Guangzhou City, Guangdong Province | P. massoniana | September 2022 |
| GD95 | Shuikou Town, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD96 | Jiedong District, Jieyang City, Guangdong Province | P. massoniana | September 2022 |
| GD97 | Yangshan County, Qingyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD98 | Sanshui District, Foshan, Guangdong Province | P. massoniana | September 2022 |
| GD99 | Jiedong District, Jieyang City, Guangdong Province | P. massoniana | September 2022 |
| GD100 | Yuancheng District, Heyuan City, Guangdong Province | Unknown | September 2022 |
| GD101 | Fengwei Town, Zhaoqing City, Guangdong Province | P. massoniana | September 2022 |
| GD102 | Meizhou City, Guangdong Province | Unknown | September 2022 |
| GD103 | Yangchun City, Yangjiang City, Guangdong Province | P. massoniana | September 2022 |
| GD104 | Lianzhou, Qingyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD105 | Chaoan District, Chaozhou City, Guangdong Province | P. massoniana | September 2022 |
| GD106 | Puning City, Jieyang City, Guangdong Province | P. massoniana | September 2022 |
| GD107 | Huadu District, Guangzhou City, Guangdong Province | P. massoniana | September 2022 |
| GD108 | Chaonan District, Shantou City, Guangdong Province | P. massoniana | September 2022 |
| GD109 | Yangchun City, Yangjiang City, Guangdong Province | P. massoniana | September 2022 |
| GD110 | Xiangqiao District, Chaozhou City, Guangdong Province | Unknown | September 2022 |
| GD111 | Longchuan County, Heyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD112 | Yangchun City, Yangjiang City, Guangdong Province | P. massoniana | September 2022 |
| GD113 | Chaoan District, Chaozhou City, Guangdong Province | Unknown | September 2022 |
| GD114 | Yangchun City, Yangjiang City, Guangdong Province | P. massoniana | September 2022 |
| GD115 | Chaonan District, Shantou City, Guangdong Province | P. massoniana | September 2022 |
| GD116 | Jiedong District, Jieyang City, Guangdong Province | P. massoniana | September 2022 |
| GD117 | Shixing County, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD119 | Qingxin District, Qingyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD120 | Qingxin District, Qingyuan City, Guangdong Province | P. massoniana | September 2022 |
| GD121 | Huadu District, Guangzhou City, Guangdong Province | P. massoniana | September 2022 |
| GD123 | Luhe county, Shanwei City, Guangdong Province | P. massoniana | September 2022 |
| GD124 | Heping County, Heyuan City, Guangdong Province | Unknown | September 2022 |
| GD125 | Haojiang District, Shantou City, Guangdong Province | Unknown | September 2022 |
| GD126 | Xiangqiao District, Chaozhou City, Guangdong Province | Unknown | September 2022 |
| GD127 | Chaoan District, Chaozhou City, Guangdong Province | P. massoniana | September 2022 |
| GD128 | Meizhou City, Guangdong Province | Unknown | September 2022 |
| GD129 | Park of Meizhou city, Guangdong Province | Unknown | September 2022 |
| GD130 | Nanlang Town, Zhongshan City, Guangdong Province | P. massoniana | September 2022 |
| GD131 | Xingning City, Meizhou City, Guangdong Province | P. massoniana | September 2022 |
| GD132 | Lechang city, Shaoguan City, Guangdong Province | P. massoniana | September 2022 |
| GD133 | Xinfeng County, Shaoguan City, Guangdong Province | P. massoniana | October 2022 |
| GD135 | Xinfeng County, Shaoguan City, Guangdong Province | P. massoniana | October 2022 |
| GX01 | Yulin City, Guangxi Zhuang Autonomous Region | P. massoniana | January 2015 |
| GX03 | Yulin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2016 |
| GX04 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | April 2019 |
| GX05 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | April 2019 |
| GX07 | Chongzuo City, Guangxi Zhuang Autonomous Region | P. massoniana | April 2019 |
| GX08 | Guigang City, Guangxi Zhuang Autonomous Region | P. massoniana | April 2019 |
| GX10 | Guigang City, Guangxi Zhuang Autonomous Region | Unknown | April 2019 |
| GX11 | Wuzhou City, Guangxi Zhuang Autonomous Region | Unknown | April 2019 |
| GX12 | Liuzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX13 | Liuzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX14 | Liuzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX15 | Liuzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX16 | Liuzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX17 | Liuzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX18 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX19 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX20 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX21 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX22 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX23 | Hezhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX24 | Yulin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX26 | Yulin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX27 | Guigang City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX28 | Guigang City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX29 | Guigang City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX30 | Laibin city, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX31 | Hezhou, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX32 | Laibin city, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX33 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX34 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX35 | Hezhou City, Guangxi Zhuang Autonomous Region | P. massoniana | October2021 |
| GX36 | Liuzhou city, Guangxi Zhuang Autonomous Region | P. massoniana | October2021 |
| GX37 | Liuzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | October2021 |
| GX38 | Hezhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX39 | Hezhou city, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX41 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX42 | Guilin city, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX43 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX44 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX45 | Liuzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX46 | Liuzhou city, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX47 | Qinzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX48 | Qinzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX50 | Qinzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX51 | Hezhou City, Guangxi Zhuang Autonomous Region | P. massoniana | September 2021 |
| GX52 | Nanning city, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX53 | Nanning city, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX54 | Rongxian, Yulin, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX55 | Rongxian, Yulin, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX56 | Yulin city, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX59 | Hezhou City, Guangxi Zhuang Autonomous Region | P. massoniana | September 2021 |
| GX60 | Hezhou city, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX61 | Hezhou city, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX62 | Guigang City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX63 | Guigang City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX64 | Guigang City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX65 | Wuzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX66 | Wuzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX67 | Wuzhou City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX68 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX69 | Guilin City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX71 | Nanning City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX72 | Nanning City, Guangxi Zhuang Autonomous Region | P. massoniana | August 2021 |
| GX74 | Hezhou City, Guangxi Zhuang Autonomous Region | P. massoniana | October 2021 |
| JS01 | Lishui District, Nanjing City, Jiangsu Province | P. massoniana | December 2014 |
| JS02 | Runzhou District, Zhenjiang City, Jiangsu Province | P. massoniana | December 2014 |
| JS03 | Dantu District, Zhenjiang City, Jiangsu Province | P. massoniana | December 2014 |
| JS04 | Jurong city, Zhenjiang City, Jiangsu Province | P. massoniana | December 2014 |
| JS05 | Mausoleum of Sun Yat-sen in Nanjing, Jiangsu Province | P. massoniana | December 2014 |
| JS06 | Binhu District, Wuxi City, Jiangsu Province | P. massoniana | December 2014 |
| JS07 | Huishan District, Wuxi City, Jiangsu Province | P. massoniana | December 2014 |
| JS08 | Yixing city, Wuxi City, Jiangsu Province | P. massoniana | December 2014 |
| JS09 | Guiwu Town, Xuyi County, Huai’an City, Jiangsu Province | P. massoniana | January 2015 |
| JS10 | Jintan City, Changzhou City, Jiangsu Province | P. massoniana | January 2015 |
| JS11 | Haizhou District, Lianyungang City, Jiangsu Province | Pinus. densiflora | January 2015 |
| JS12 | Yizheng City, Yangzhou City, Jiangsu Province | P. massoniana | January 2015 |
| JS13 | Lianyun District, Lianyungang City, Jiangsu Province | P. massoniana | January 2015 |
| JS14 | Pukou District, Nanjing City, Jiangsu Province | P. massoniana | February 2015 |
| JS15 | Changshu City, Suzhou City, Jiangsu Province | P. massoniana | February 2015 |
| JS16 | Baima Town, Lishui County, Nanjing city, Jiangsu Province | P. massoniana | February 2015 |
| JS17 | Gaochun District, Nanjing City, Jiangsu Province | P. massoniana | February 2015 |
| JS18 | Tianmuhu Town, Changzhou City, Jiangsu Province | P. massoniana | February 2015 |
| JS19 | Changshu City, Suzhou City, Jiangsu Province | P. massoniana | October 2017 |
| JS20 | Changshu City, Suzhou City, Jiangsu Province | P. massoniana | October 2017 |
| JS21 | Changshu City, Suzhou City, Jiangsu Province | P. massoniana | October 2017 |
| JS22 | Pukou District, Nanjing City, Jiangsu Province | P. massoniana | October 2017 |
| JS23 | Pukou District, Nanjing City, Jiangsu Province | P. massoniana | October 2017 |
| JS24 | Pukou District, Nanjing City, Jiangsu Province | P. massoniana | October 2017 |
| JS25 | Dingshu Town, Yixing City, Wuxi City, Jiangsu Province | P. massoniana | October 2017 |
| JS26 | Dingshu Town, Yixing City, Wuxi City, Jiangsu Province | P. massoniana | October 2017 |
| JS27 | Hufu Town, Yixing City, Wuxi City, Jiangsu Province | P. massoniana | October 2017 |
| JS29 | Yizheng City, Yangzhou City, Jiangsu Province | P. massoniana | October 2017 |
| JS30 | Yizheng City, Yangzhou City, Jiangsu Province | P. massoniana | October 2017 |
| JS31 | Lishui District, Nanjing City, Jiangsu Province | P. massoniana | October 2017 |
| JS32 | Lishui District, Nanjing City, Jiangsu Province | P. massoniana | October 2017 |
| JS33 | Lishui District, Nanjing City, Jiangsu Province | P. massoniana | October 2017 |
| JS34 | Liyang City, Changzhou City, Jiangsu Province | P. massoniana | October 2017 |
| JS35 | Liyang City, Changzhou City, Jiangsu Province | P. massoniana | October 2017 |
| JS36 | Liyang City, Changzhou City, Jiangsu Province | P. massoniana | October 2017 |
| JS38 | Lianyun District, Lianyungang City, Jiangsu Province | Pinus. thunbergii | November 2017 |
| JS39 | Lianyun District, Lianyungang City, Jiangsu Province | P. thunbergii | November 2017 |
| JS41 | Runzhou District, Zhenjiang City, Jiangsu Province | P. massoniana | November 2017 |
| JS42 | Runzhou District, Zhenjiang City, Jiangsu Province | P. massoniana | November 2017 |
| JS44 | Jurong city, Zhenjiang City, Jiangsu Province | P. massoniana | October 2017 |
| JS45 | Jurong city, Zhenjiang City, Jiangsu Province | P. massoniana | October 2017 |
| JS47 | Jiangning District, Nanjing City, Jiangsu Province | P. massoniana | October 2017 |
| JS48 | Tea Hill, Jiangning District, Nanjing City, Jiangsu Province | P. massoniana | October 2017 |
| JS49 | Jintan District, Changzhou City, Jiangsu Province | P. massoniana | October 2017 |
| JS50 | Jintan District, Changzhou City, Jiangsu Province | P. massoniana | October 2017 |
| JS56 | Binhu District, Wuxi City, Jiangsu Province | P. massoniana | October 2017 |
| JS58 | Xuanwu District, Nanjing city, Jiangsu Province | P. massoniana | November 2017 |
| JS63 | Huai’an City, Jiangsu Province | P. massoniana | November 2017 |
| JS64 | Qixia District, Nanjing City, Jiangsu Province | P. massoniana | November 2017 |
| JS65 | Qixia District, Nanjing City, Jiangsu Province | P. massoniana | November 2017 |
| JS67 | Jianshan, Yuhuatai District, Nanjing City, Jiangsu Province | P. massoniana | October 2017 |
| JS70 | Liuhe District, Nanjing City, Jiangsu Province | P. massoniana | October 2017 |
| JS77 | Jurong Forest Farm, Zhenjiang City, Jiangsu Province | P. massoniana | September 2021 |
| JS78 | Jurong Forest Farm, Zhenjiang City, Jiangsu Province | P. massoniana | September 2021 |
| JS79 | Jurong Forest Farm, Zhenjiang City, Jiangsu Province | P. massoniana | September 2021 |
| JS80 | Jurong Forest Farm, Zhenjiang City, Jiangsu Province | P. massoniana | September 2021 |
| JS82 | Jurong Forest Farm, Zhenjiang City, Jiangsu Province | P. massoniana | September 2021 |
| JS84 | Gaochun District, Nanjing City, Jiangsu Province | P. massoniana | October 2022 |
| JS85 | Gaochun District, Nanjing City, Jiangsu Province | P. massoniana | October 2022 |
| JS86 | Gaochun District, Nanjing City, Jiangsu Province | P. massoniana | October 2022 |
References
- Wang, Z.; Wang, C.Y.; Fang, Z.M.; Zhang, D.L.; Liu, L.; Lee, M.R.; Li, Z.; Li, J.J.; Sung, C.K. Advances in research of pathogenic mechanism of pine wilt disease. Afr. J. Microbiol. Res. 2010, 4, 437–442. [Google Scholar]
- Li, Y.L.; Fan, C.J.; Jiang, X.H.; Tian, X.Y.; Han, Z.M. Bursaphelenchus xylophilus: An Important Pathogenic Factor of Pine Wilt Disease and Its Relationship with Bursaphelenchus mucronatus. Plant Dis. 2021, 105, 3055–3062. [Google Scholar] [CrossRef]
- Diao, J.; Hao, X.; Ma, W.; Ma, L. Bioinformatics analysis of structure and function in the MRP gene family and its expression in response to various drugs in Bursaphelenchus xylophilus. J. For. Res. 2020, 32, 779–787. [Google Scholar] [CrossRef]
- Cao, J.X.; Hao, X.; Li, Y.; Tan, R.A.; Cui, Z.X.; Li, L.; Zhang, Y.; Cao, J.Y.; Min, M.R.; Liang, L.W.; et al. Exploring the role of detoxification genes in the resistance of Bursaphelenchus xylophilus to different exogenous nematicidal substances using transcriptomic analyses. Pestic. Biochem. Physiol. 2023, 194, 105527. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T.; Matsunaga, K.; Shirasawa, K. Quantitative Trait Loci Analysis Based on High-Density Mapping of Single-Nucleotide Polymorphisms by Genotyping-by-Sequencing Against Pine Wilt Disease in Japanese Black Pine (Pinus thunbergii). Front. Plant Sci. 2022, 13, 850660. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.Y.; Cheng, X.Y.; Shi, J.; Zhang, Q.W.; Dai, S.M.; Cheng, F.X.; Luo, Y.Q. Mechanisms of invasive population establishment and spread of pinewood nematodes in China. Sci. China Ser. C-Life Sci. 2009, 52, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.G.; Yuan, Y.D.; Li, X.M.; Zhang, J.C. Maximum Entropy Modeling to Predict the Impact of Climate Change on Pine Wilt Disease in China. Front. Plant Sci. 2021, 12, 652500. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, Y. History of pine wilt disease in Japan. J. Nematol. 1988, 20, 219–226. [Google Scholar] [PubMed]
- Kishi, Y. The Pine Wood Nematode and the Japanese Pine Sawyer; Thomas Company Ltd.: Tokyo, Japan, 1995; p. 302. [Google Scholar]
- Jung, J.K.; Kim, M.; Nam, Y.; Koh, S.H. Changes in spatial and temporal distributions of Monochamus beetles along the fire severity in burned Pinus densiflora forests. J. Asia-Pac. Entomol. 2020, 23, 404–410. [Google Scholar] [CrossRef]
- Mota, M.M.; Bonifácio, L.; Bravo, M.A.; Naves, P.; Penas, A.C.; Pires, J.; Sousa, E.; Vieira, P. Discovery of Pine Wood Nematode in Portugal and in Europe. In Proceedings of the International Workshop on Pinewood Nematode, Bursaphelenchus Xylophilus, Univ Evora, Evora, Portugal, 20–22 August 2001; University Evora: Evora, Portugal, 2001; pp. 1–5. [Google Scholar]
- Zamora, P.; Rodríguez, V.; Renedo, F.; Sanz, A.V.; Domínguez, J.C.; Pérez-Escolar, G.; Miranda, J.; Alvarez, B.; González-Casas, A.; Mayor, E.; et al. First Report of Bursaphelenchus xylophilus Causing Pine Wilt Disease on Pinus radiata in Spain. Plant Dis. 2015, 99, 1449. [Google Scholar] [CrossRef]
- Soliman, T.; Mourits, M.C.; van der Werf, W.; Hengeveld, G.M.; Robinet, C.; Lansink, A.G. Framework for Modelling Economic Impacts of Invasive Species, Applied to Pine Wood Nematode in Europe. PLoS ONE 2012, 7, e45505. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, Y. Pathology of the Pine Wilt Disease Caused by Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.R. Epidemic Status of Pine Wilt Disease in China and Its Prevention and Control Techniques and Counter Measures. Sci. Silvae Sin. 2019, 55, 1–10. [Google Scholar]
- Futai, K. Pine Wood Nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.N.; Liu, P.X.; Shi, Y.; Wu, H.; Yu, H.Y.; Jiang, S.W. Difference analysis on pine wilt disease between liaoning province of northeastern China and other epidemic areas in China. Beijing For. Univ. 2021, 43, 155–160. [Google Scholar]
- Li, Y.X.; Zhang, X.Y. Analysis on the trend of invasion and expansion of Bursaphelenchus xylophilus. For. Pest Dis. 2018, 37, 1–4. [Google Scholar]
- Gao, R.H.; Liu, L.; Li, R.J.; Fan, S.M.; Dong, J.H.; Zhao, L.J. Predicting potential distributions of Monochamus saltuarius, a novel insect vector of pine wilt disease in China. Front. For. 2023, 6, 1243996. [Google Scholar] [CrossRef]
- Rutherford, T.A.; Mamiya, Y.; Webster, J.M. Nematode-induced pine wilt disease: Factors influencing its occurrence and distribution. For. Sci. 1990, 36, 145–155. [Google Scholar] [CrossRef]
- Rutherford, T.A.; Webster, J.M. Distribution of pine wilt disease with respect to temperature in North America, Japan, and Europe. Can. J. For. Res. 1987, 17, 1050–1059. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Estoup, A.; Guillemaud, T. Reconstructing routes of invasion using genetic data: Why, how and so what? Mol. Ecol. 2010, 19, 4113–4130. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, T.; Kanzaki, N.; Maehara, N. ITS-RFLP pattern of Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) does not reflect nematode virulence. J. For. Res. 2012, 18, 384–388. [Google Scholar] [CrossRef]
- Vieira, P.; Burgermeister, W.; Mota, M.; Metge, K.; Silva, G. Lack of genetic variation of Bursaphelenchus xylophilus in Portugal revealed by RAPD-PCR analyses. J. Nematol. 2007, 39, 118–126. [Google Scholar] [PubMed]
- Valadas, V.; Laranjo, M.; Barbosa, P.; Espada, M.; Mota, M.; Oliveira, S. The pine wood nematode, Bursaphelenchus xylophilus, in Portugal: Possible introductions and spread routes of a serious biological invasion revealed by molecular methods. Nematology 2012, 14, 899–911. [Google Scholar] [CrossRef]
- Mallez, S.; Castagnone, C.; Espada, M.; Vieira, P.; Eisenback, J.D.; Harrell, M.; Mota, M.; Aikawa, T.; Akiba, M.; Kosaka, H. Worldwide invasion routes of the pinewood nematode: What can we infer from population genetics analyses? Biol. Invasions 2015, 17, 1199–1213. [Google Scholar] [CrossRef]
- Fengmao, C.; Jianren, Y.; Xiaoqin, W.; Lin, H.; Jiajin, T. SCAR Marker and Detection Technique of Bursaphelenchus xylophilus. Sci. Silvae Sin. 2012, 48, 88–94. [Google Scholar]
- Jung, J.; Han, H.; Ryu, S.; Kim, W. Amplified fragment length polymorphism analysis and genetic variation of the pinewood nematode Bursaphelenchus xylophilus in South Korea. Anim. Cells Syst. 2010, 14, 31–36. [Google Scholar] [CrossRef]
- Shinya, R.; Takeuchi, Y.; Ichimura, K.; Takemoto, S.; Futai, K. Establishment of a set of inbred strains of the pine wood nematode, Bursaphelenchus xylophilus (Aphelenchida: Aphelenchoididae), and evidence of their varying levels of virulence. Appl. Entomol. Zool. 2012, 47, 341–350. [Google Scholar] [CrossRef]
- Ding, X.L.; Guo, Y.F.; Ye, J.R.; Wu, X.Q.; Lin, S.X.; Chen, F.M.; Zhu, L.H.; Huang, L.; Song, X.F.; Zhang, Y.; et al. Population differentiation and epidemic tracking of Bursaphelenchus xylophilus in China based on chromosome-level assembly and whole-genome sequencing data. Pest Manag. Sci. 2022, 78, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.X.; Ding, X.L.; Feng, Y.; Chen, T.T.; Ye, J.R. Genetic Diversity and Population Structure of Bursaphelenchus xylophilus in Central China Based on SNP Markers. Forests 2023, 14, 1443. [Google Scholar] [CrossRef]
- Viglierchio, D.R.; Schmitt, R.V. On the methodology of nematode extraction from field samples: Baermann funnel modifications. J. Nematol. 1983, 15, 438–444. [Google Scholar] [PubMed]
- Son, J.A.; Moon, Y.S. Migrations and Multiplications of Bursaphelenchus xylophilus and B. mucronatus in Pinus thumbergii in Relation to Their Pathogenicity. Plant Pathol. J. 2013, 29, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Gish, W.; States, D.J. Identification of protein coding regions by database similarity search. Nat. Genet. 1993, 3, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.; Simoes, M.J.; Gomes, P.; Barroso, C.; Pinho, D.; Conceiçao, L.; Fonseca, L.; Abrantes, I.; Pinheiro, M.; Egas, C. Assessment of the Geographic Origins of Pinewood Nematode Isolates via Single Nucleotide Polymorphism in Effector Genes. PLoS ONE 2013, 8, e83542. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Xing, L.; Liu, X.L.; Pu, Y.L.; Yang, Y.Q.; Fu, Y.Y. Potential Impact of Climate Change on the Distribution of the Pinewood Nematode Bursaphelenchus xylophilus in Chongqing, China. Pak. J. Zool. 2022, 54, 809–816. [Google Scholar] [CrossRef]
- Wang, B.W.; Ma, L.; Wang, F.; Wang, B.Y.; Hao, X.; Xu, J.Y.; Ma, Y. Low Temperature Extends the Lifespan of Bursaphelenchus xylophilus through the cGMP Pathway. Int. J. Mol. Sci. 2017, 18, 2320. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.X.; Ding, X.L.; Feng, Y.; Zhao, R.W.; Ye, J.R. Genetic diversity and genome-wide association analysis of pine wood nematode populations in different regions of China. Front. Plant Sci. 2023, 14, 1183772. [Google Scholar]
- Rui, L.; Liu, H.B.; Liang, R.; Wu, X.Q. Resistance genes mediate differential resistance to pine defensive substances α-Pinene and H2O2 in Bursaphelenchus xylophilus with different levels of virulence. J. For. Res 2021, 32, 1753–1762. [Google Scholar] [CrossRef]
- Zhou, J.P.; Dong, Y.Y.; Gao, Y.J.; Tang, X.H.; Li, J.J.; Yang, Y.J.; Xu, B.; Xie, Z.R.; Huang, Z.X. Characterization of a family 3 polysaccharide lyase with broad temperature adaptability, thermo-alkali stability, and ethanol tolerance. Biotechnol. Bioprocess Eng. 2012, 17, 729–738. [Google Scholar] [CrossRef]
- Huang, D.M.; Song, Y.Y.; Liu, Y.L.; Qin, Y. A new strain of Aspergillus tubingensis for high-activity pectinase production. Braz. J. Microbiol. 2019, 50, 53–65. [Google Scholar] [CrossRef] [PubMed]
- He, L.X.; Wu, X.Q.; Xue, Q.; Qiu, X.W. Effects of Endobacterium (Stenotrophomonas maltophilia) on Pathogenesis-Related Gene Expression of Pine Wood Nematode (Bursaphelenchus xylophilus) and Pine Wilt Disease. Int. J. Mol. Sci. 2016, 17, 778. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Shibuya, H.; Aikawa, T.; Jones, J.T. Cloning and characterization of pectate lyases expressed in the esophageal gland of the pine wood nematode Bursaphelenchus xylophilus. Mol. Plant-Microbe Interact. 2006, 19, 280–287. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Jian, W.; Ding, X.; Ye, J. Genetic Diversity and Population Structure of Bursaphelenchus xylophilus in Guangdong, Guangxi, and Jiangsu Provinces in China. Forests 2024, 15, 934. https://doi.org/10.3390/f15060934
Feng Y, Jian W, Ding X, Ye J. Genetic Diversity and Population Structure of Bursaphelenchus xylophilus in Guangdong, Guangxi, and Jiangsu Provinces in China. Forests. 2024; 15(6):934. https://doi.org/10.3390/f15060934
Chicago/Turabian StyleFeng, Yuan, Wenjing Jian, Xiaolei Ding, and Jianren Ye. 2024. "Genetic Diversity and Population Structure of Bursaphelenchus xylophilus in Guangdong, Guangxi, and Jiangsu Provinces in China" Forests 15, no. 6: 934. https://doi.org/10.3390/f15060934
APA StyleFeng, Y., Jian, W., Ding, X., & Ye, J. (2024). Genetic Diversity and Population Structure of Bursaphelenchus xylophilus in Guangdong, Guangxi, and Jiangsu Provinces in China. Forests, 15(6), 934. https://doi.org/10.3390/f15060934





