Long-Term Successional Subculture Dynamics and Their Effects on the Proliferation Efficiency, Embryogenic Potential, and Genetic Stability of Embryogenic Tissues in Larix principis-rupprechtii Mayr
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Embryogenic Tissue Proliferation
2.3. Pre-Treatment and Maturation
2.4. Staining and Examination of Embryogenic Tissue
2.5. Genomic DNA Extraction
2.6. SSR Identification
2.7. Data Analysis
| ATW (g) = Weight of tissue and culture medium − Weight of culture medium | |
| Proliferation culture PA (g) = 14 d ATW − 0 d ATW | |
| Proliferation culture PR = (Proliferation culture PA/0 d ATW) × 100% | |
| Pre-treatment culture PA (g) = 10 d ATW − 0 d ATW | |
| Pre-treatment culture PR = (Pre-treatment culture PA/0 d ATW) × 100% |
3. Results
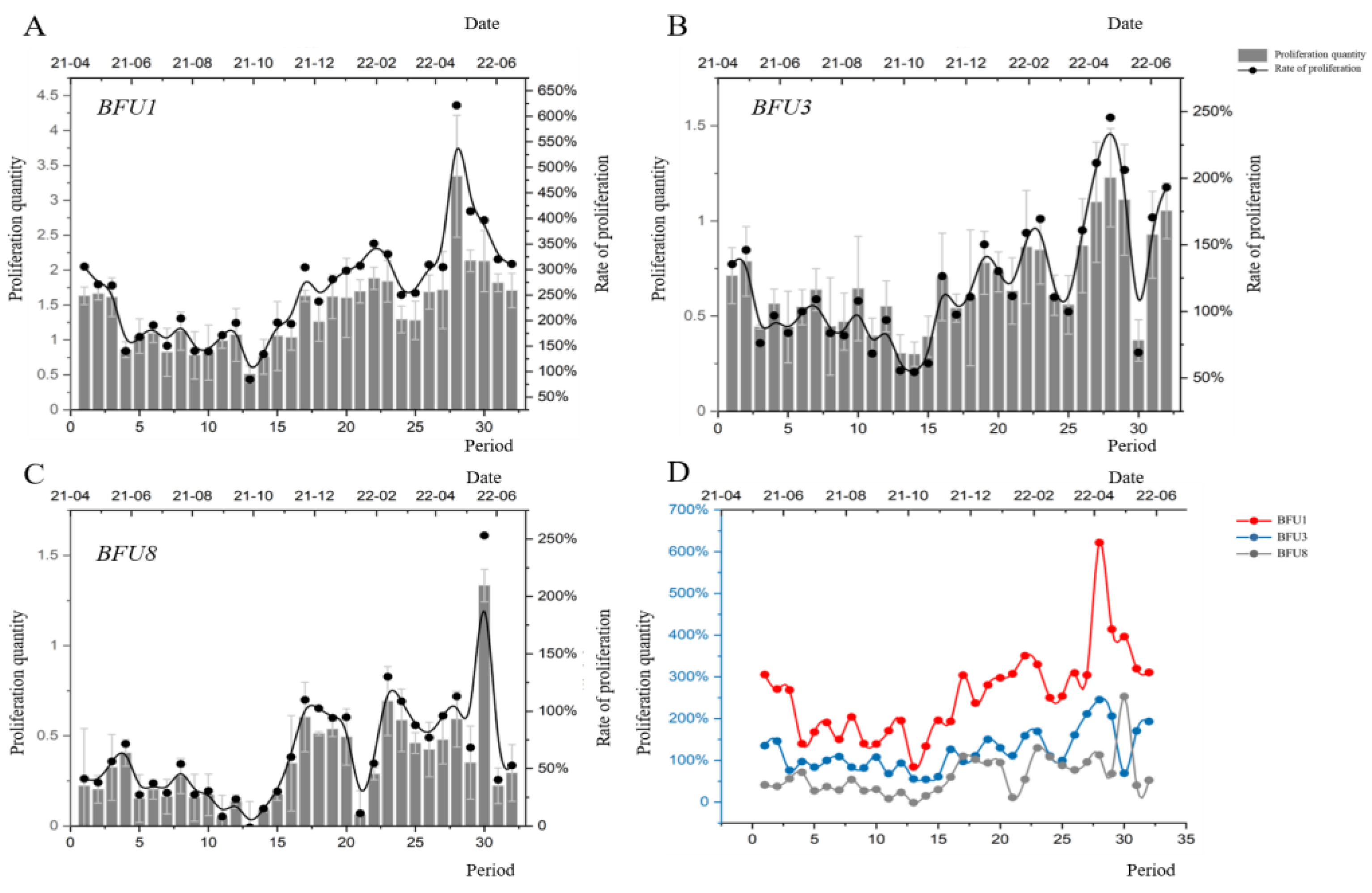
3.1. Proliferation Efficiency of Embryogenic Tissues during Long-Term Successional Subculture
3.2. Proliferation Efficiency of Embryogenic Tissues on Pre-Treatment Medium for Long-Term Successional Subculture
3.3. Maturation Capacity of Long-Term Successional Subculture
3.4. Correlation Analysis of the Number of Successive Generations, Proliferation Efficiency during Proliferative Culture, Proliferation Efficiency of Pre-Treatment, and Maturation Capacity
3.5. SSR Genetic Stability Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Li, H.; Fu, S.; Chen, B.; Sun, W.; Zhang, J.; Zhang, J. An iTRAQ-Based Proteomics Approach to Clarify the Molecular Physiology of Somatic Embryo Development in Prince Rupprecht’s Larch (Larix principis-rupprechtii Mayr). PLoS ONE 2015, 10, e0119987. [Google Scholar] [CrossRef] [PubMed]
- Diao, S.; Hou, Y.; Xie, Y.; Sun, X. Age Trends of Genetic Parameters, Early Selection and Family by Site Interactions for Growth Traits in Larix kaempferi Open-Pollinated Families. BMC Genet. 2016, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.D.; Long, S.M.; Sniezko, R.A. Sexual Reproduction and Crossing Barriers in White Pines: The Case between Pinus lambertiana (Sugar Pine) and P. monticola (Western White Pine). Tree Genet. Genomes 2005, 1, 143–150. [Google Scholar] [CrossRef]
- Rampart, M. Seed Germination Variation among Crop Years from a Pinus sylvestris Clonal Seed Orchard. Asian J. Res. Agric. For. 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Wang, D. Assessment of Tree-Ring Mercury Radial Translocation and Age Effect in Masson Pine: Implications for Historical Atmospheric Mercury Reconstruction. J. Environ. Sci. 2024, 138, 266–276. [Google Scholar] [CrossRef]
- Rubilar, R.; Bozo, D.; Albaugh, T.; Cook, R.; Campoe, O.; Carter, D.; Allen, H.L.; Álvarez, J.; Pincheira, M.; Zapata, Á. Rotation-Age Effects of Subsoiling, Fertilization, and Weed Control on Radiata Pine Growth at Sites with Contrasting Soil Physical, Nutrient, and Water Limitations. For. Ecol. Manag. 2023, 544, 121213. [Google Scholar] [CrossRef]
- Pérez-Oliver, M.A.; González-Mas, M.D.C.; Renau-Morata, B.; Arrillaga, I.; Sales, E. Heat-Priming during Somatic Embryogenesis Increased Resilience to Drought Stress in the Generated Maritime Pine (Pinus pinaster) Plants. Int. J. Mol. Sci. 2023, 24, 9299. [Google Scholar] [CrossRef]
- Hassani, S.B.; Trontin, J.-F.; Raschke, J.; Zoglauer, K.; Rupps, A. Constitutive Overexpression of a Conifer WOX2 Homolog Affects Somatic Embryo Development in Pinus pinaster and Promotes Somatic Embryogenesis and Organogenesis in Arabidopsis Seedlings. Front. Plant Sci. 2022, 13, 838421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Fei, Q.; Xia, X.-R.; Ke, X.; Ye, J.-R.; Zhu, L.-H. Pinus massoniana Somatic Embryo Maturation, Mycorrhization of Regenerated Plantlets and Its Resistance to Bursaphelenchus xylophilus. Front. Plant Sci. 2023, 14, 1130471. [Google Scholar] [CrossRef]
- Sales, E.; Cañizares, E.; Pereira, C.; Pérez-Oliver, M.A.; Nebauer, S.G.; Pavlović, I.; Novák, O.; Segura, J.; Arrillaga, I. Changing Temperature Conditions during Somatic Embryo Maturation Result in Pinus pinaster Plants with Altered Response to Heat Stress. Int. J. Mol. Sci. 2022, 23, 1318. [Google Scholar] [CrossRef]
- Durzan, D.J.; Chalupa, V. Growth and Metabolism of Cells and Tissue of Jack Pine (Pinus banksiana). 3. Growth of Cells in Liquid Suspension Cultures in Light and Darkness. Can. J. Bot. 1976, 54, 456–467. [Google Scholar] [CrossRef]
- Arrillaga, I.; Morcillo, M.; Zanón, I.; Lario, F.; Segura, J.; Sales, E. New Approaches to Optimize Somatic Embryogenesis in Maritime Pine. Front. Plant Sci. 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Castander-Olarieta, A.; Montalbán, I.A.; Moncaleán, P. Multi-Strategy Approach towards Optimization of Maturation and Germination in Radiata Pine Somatic Embryogenesis. Plant Cell Tissue Organ Cult. 2023, 153, 173–190. [Google Scholar] [CrossRef]
- do Nascimento, A.M.M.; Polesi, L.G.; Back, F.P.; Steiner, N.; Guerra, M.P.; Castander-Olarieta, A.; Moncaleán, P.; Montalbán, I.A. The Chemical Environment at Maturation Stage in Pinus Spp. Somatic Embryogenesis: Implications in the Polyamine Profile of Somatic Embryos and Morphological Characteristics of the Developed Plantlets. Front. Plant Sci. 2021, 12, 771464. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, X.; Gao, Y.; Cui, Y.; Kong, L.; Zhao, J.; Zhang, J. Plant Regeneration via Somatic Embryogenesis in Larix principis-rupprechtii Mayr. Forests 2021, 12, 1335. [Google Scholar] [CrossRef]
- Li, F.; Yao, J.; Hu, L.; Chen, J.; Shi, J. Multiple Methods Synergistically Promote the Synchronization of Somatic Embryogenesis Through Suspension Culture in the New Hybrid Between Pinus elliottii and Pinus caribaea. Front. Plant Sci. 2022, 13, 857972. [Google Scholar] [CrossRef] [PubMed]
- Lineros, Y.; Rojas-Rioseco, M.; Hernández, M.; Ríos, D.; Muñoz, X.; Hasbún, R. Effects of Long-Term Subculture on Maturation Ability and Plant Conversion in Pinus Radiata: Using FT-IR Spectroscopy to Determine Biomarkers of Embryogenic Tissue Aging. Forests 2023, 14, 1446. [Google Scholar] [CrossRef]
- Maruyama, T.E.; Hosoi, Y. Progress in Somatic Embryogenesis of Japanese Pines. Front. Plant Sci. 2019, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Gao, F.; Wang, H.; Nikolaevna Tretyakova, I.; Mikhaylovich Nosov, A.; Shen, H.; Yang, L. Suspension Culture and Somatic Embryogenesis of Korean Pine. Phyton 2022, 91, 223–238. [Google Scholar] [CrossRef]
- Salaj, T.; Klubicová, K.; Matusova, R.; Salaj, J. Somatic Embryogenesis in Selected Conifer Trees Pinus nigra Arn. and Abies Hybrids. Front. Plant Sci. 2019, 10, 13. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Bueno, N.; Cañas, R.A.; Avila, C.; Cánovas, F.M.; Ordás, R.J. Analysis of the WUSCHEL-RELATED HOMEOBOX Gene Family in Pinus pinaster: New Insights into the Gene Family Evolution. Plant Physiol. Biochem. 2018, 123, 304–318. [Google Scholar] [CrossRef]
- Kang, Y.; Li, W.; Zhang, L.; Qi, L. Over-Expression of the Cell-Cycle Gene LaCDKB1;2 Promotes Cell Proliferation and the Formation of Normal Cotyledonary Embryos during Larix kaempferi Somatic Embryogenesis. Genes 2021, 12, 1435. [Google Scholar] [CrossRef]
- Peng, C.; Gao, F.; Tretyakova, I.N.; Nosov, A.M.; Shen, H.; Yang, L. Transcriptomic and Metabolomic Analysis of Korean Pine Cell Lines with Different Somatic Embryogenic Potential. Int. J. Mol. Sci. 2022, 23, 13301. [Google Scholar] [CrossRef]
- Breton, D.; Harvengt, L.; Trontin, J.F.; Bouvet, A.; Favre, J.M. Long-Term Subculture Randomly Affects Morphology and Subsequent Maturation of Early Somatic Embryos in Maritime Pine. Plant Cell Tissue Organ Cult. 2006, 87, 95–108. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Noceda, C.; Pelletier, G.; Label, P.; Rodriguez, R.; Lelu-Walter, M.A. Biological Characterization of Young and Aged Embryogenic Cultures of Pinus pinaster (Ait.). Vitr. Cell. Dev. Biol. Plant 2009, 45, 20–33. [Google Scholar] [CrossRef]
- Neelakandan, A.K.; Wang, K. Recent Progress in the Understanding of Tissue Culture-Induced Genome Level Changes in Plants and Potential Applications. Plant Cell Rep. 2012, 31, 597–620. [Google Scholar] [CrossRef]
- Zayova, E.; Vassilevska-Ivanova, R.; Stoeva, D.; Kraptchev, B. Somaclonal Variation through Indirect Organogenesis in Eggplant (Solanum melongena L.). Biol. Divers. Conserv. 2010, 3, 1–5. [Google Scholar]
- Hume, S.; Bo, D.; Simon, S.A.; Suhua, F.; Maria, B.; Matteo, P.; Guo-Liang, W.; Meyers, B.C.; Jacobsen, S.E. Plants Regenerated from Tissue Culture Contain Stable Epigenome Changes in Rice. eLife 2013, 2, e00354. [Google Scholar]
- George, E.F. Plant Propagation by Tissue Culture—Part 1: The Technology; Exegetics Limited: Westbury, UK, 1993. [Google Scholar]
- Kaeppler, S.M.; Kaeppler, H.F.; Rhee, Y.; Kaeppler, S.M.; Kaeppler, H.F.; Rhee, Y. Epigenetic Aspects of Somaclonal Variation in Plants. Plant Mol. Biol. 2000, 43, 179–188. [Google Scholar] [CrossRef]
- Lee, M.; Phillips, R.L. The Chromosomal Basis of Somaclonal Variation. Plant Mol. Biol. 1988, 39, 413–437. [Google Scholar] [CrossRef]
- Etienne, H.; Bertrand, B. Somaclonal Variation in Coffea arabica: Effects of Genotype and Embryogenic Cell Suspension Age on Frequency and Phenotype of Variants. Tree Physiol. 2003, 23, 419–426. [Google Scholar] [CrossRef]
- Pijut, P.M.; Beasley, R.R.; Lawson, S.S.; Palla, K.J.; Stevens, M.E.; Wang, Y. In Vitro Propagation of Tropical Hardwood Tree Species—A Review (2001–2011). Propag. Ornam. Plants 2012, 12, 25–51. [Google Scholar]
- Sharma, S.K.; Bryan, G.J.; Millam, W.S. Stability of Potato (Solanum tuberosum L.) Plants Regenerated via Somatic Embryos, Axillary Bud Proliferated Shoots, Microtubers and True Potato Seeds: A Comparative Phenotypic, Cytogenetic and Molecular Assessment. Planta 2007, 226, 1449–1458. [Google Scholar] [CrossRef]
- Nwauzoma, A.B.; Jaja, E.T. A Review of Somaclonal Variation in Plantain (Musa Spp): Mechanisms and Applications. J. Appl. Biosci. 2013, 67, 5252–5260. [Google Scholar] [CrossRef]
- Bairu, M.W.; Fennell, C.W.; van Staden, J. The Effect of Plant Growth Regulators on Somaclonal Variation in Cavendish banana (Musa AAA Cv. ’Zelig’). Sci. Hort. Amst. 2006, 108, 347–351. [Google Scholar] [CrossRef]
- Rival, A.; Ilbert, P.; Labeyrie, A.; Torres, E.; Doulbeau, S.; Personne, A.; Dussert, S.; Beulé, T.; Durand-Gasselin, T.; Tregear, J.W. Variations in Genomic DNA Methylation during the Long-Term In Vitro Proliferation of Oil Palm Embryogenic Suspension Cultures. Plant Cell Rep. 2013, 32, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Egertsdotter, U. Plant Physiological and Genetical Aspects of the Somatic Embryogenesis Process in Conifers. Scand. J. For. Res. 2019, 34, 360–369. [Google Scholar] [CrossRef]
- Peng, C.; Gao, F.; Wang, H.; Shen, H.; Yang, L. Optimization of Maturation Process for Somatic Embryo Production and Cryopreservation of Embryogenic Tissue in Pinus koraiensis. Plant Cell Tissue Organ Cult. 2021, 144, 185–194. [Google Scholar] [CrossRef]
- Ree, J.F.; Polesi, L.G.; Back, F.; Bertolazi, A.A.; Guerra, M.P. Aging Peach Palm (Bactris gasipaes Kunth) Cultures Lose Embryogenic Potential and Metabolic Cellular Function Due to Continuous Culture in Hypoxic Environments. Plant Cell Tissue Organ Cult. 2019, 140, 49–67. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Zhu, L.; Liu, X.; Wang, Q.; Ye, J. Evaluation of Somatic Embryo Production during Embryogenic Tissue Proliferation Stage Using Morphology, Maternal Genotype, Proliferation Rate and Tissue Age of Pinus thunbergii Parl. J. For. Res. 2022, 33, 445–454. [Google Scholar] [CrossRef]
- Dong, M.; Gao, J.; Sun, W.; Fan, Y.; Yuan, D.; Zhang, J. Genetic Diversity and Population Structure of Elite Larix principis-rupprechtii Mayr in Beijing. J. Plant Genet. Resour. 2016, 17, 616–624. [Google Scholar] [CrossRef]
- Fan, Y. Genetic Diversity of Larix principis rupprechtii Revealed by SSR Marker; Beijing Forestry University: Beijing, China, 2014. [Google Scholar]
- Dong, M. High-Density Genetic Linkage Map Construction and QTLs Analysis of Phenotypic Traits in Larix principis-rupprechtii Mayr; Beijing Forestry University: Beijing, China, 2020. [Google Scholar]
- Businge, E.; Brackmann, K.; Moritz, T.; Egertsdotter, U. Metabolite Profiling Reveals Clear Metabolic Changes during Somatic Embryo Development of Norway Spruce (Picea abies). Tree Physiol. 2012, 32, 232–244. [Google Scholar] [CrossRef]
- Leonardo, J.; Dos, S.A.L.W.; Bueno, C.A.; Barbosa, H.R.; Floh, E.I.S. Proteomic Analysis and Polyamines, Ethylene and Reactive Oxygen Species Levels of Araucaria angustifolia (Brazilian pine) Embryogenic Cultures with Different Embryogenic Potential. Tree Physiol. 2014, 34, 94–104. [Google Scholar]
- Pullman, G.S.; Namjoshi, K.; Zhang, Y. Somatic Embryogenesis in Loblolly Pine (Pinus taeda L.): Improving Culture Initiation with Abscisic Acid and Silver Nitrate. Plant Cell Rep. 2003, 22, 85–95. [Google Scholar] [CrossRef]
- Miguel, C.; Gonçalves, S.; Tereso, S.; Marum, L.; Maroco, J.; Oliveira, M.M. Somatic Embryogenesis from 20 Open-Pollinated Families of Portuguese Plus Trees of Maritime Pine. Plant Cell Tissue Organ Cult. 2004, 76, 121–130. [Google Scholar] [CrossRef]
- Quiroz-Figueroa, F.; Méndez-Zeel, M.; Sánchez-Teyer, F.; Rojas-Herrera, R.; Loyola-Vargas, V.M. Differential Gene Expression in Embryogenic and Non-Embryogenic Clusters from Cell Suspension Cultures of Coffea arabica. J. Plant Physiol. 2002, 159, 1267–1270. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Han, S.; Yang, W.; Li, W.; Wei, H.; Li, X.; Qi, L. Four Abiotic Stress-Induced miRNA Families Differentially Regulated in the Embryogenic and Non-Embryogenic Callus Tissues of Larix leptolepis. Biochem. Biophys. Res. Commun. 2010, 398, 355–360. [Google Scholar] [CrossRef]
- Breton, D.; Harvengt, L.; Trontin, J.F.; Bouvet, A.; Favre, J.M. High Subculture Frequency, Maltose-Based and Hormone-Free Medium Sustained Early Development of Somatic Embryos in Maritime Pine. Vitr. Cell. Dev. Biol. Plant 2005, 41, 494–504. [Google Scholar] [CrossRef]
- Khosroushahi, A.Y.; Naderi-Manesh, H.; Simonsen, H.T. Effect of Antioxidants and Carbohydrates in Callus Cultures of Taxus brevifolia: Evaluation of Browning, Callus Growth, Total Phenolics and Paclitaxel Production. Bioimpacts Bi 2011, 1, 37. [Google Scholar]
- Li, W.F.; Zhang, S.G.; Han, S.Y.; Wu, T.; Zhang, J.H.; Qi, L.W. Regulation of LaMYB33 by miR159 during Maintenance of Embryogenic Potential and Somatic Embryo Maturation in Larix kaempferi (Lamb.) Carr. Plant Cell Tissue Organ Cult. 2013, 113, 131–136. [Google Scholar] [CrossRef]
- Xia, X.-R.; Yang, F.; Ke, X.; Chen, Y.-M.; Ye, J.-R.; Zhu, L.-H. Somatic Embryogenesis of Masson Pine (Pinus massoniana): Initiation, Maturation and Genetic Stability Analysis at SSR Loci. Plant Cell Tissue Organ Cult. 2021, 145, 667–677. [Google Scholar] [CrossRef]
- Lelu-Walter, M.A.; Paques, L.E. Simplified and Improved Somatic Embryogenesis of Hybrid Larches (Larix × eurolepis and Larix × marschlinsii). Perspectives for Breeding. Ann. For. Sci. 2009, 66, 104. [Google Scholar] [CrossRef]
- Klubicová, K.; Uvácková, L.; Danchenko, M.; Nemecek, P.; Skultéty, L.; Salaj, J.; Salaj, T. Insights into the Early Stage of Pinus nigra Arn. Somatic Embryogenesis Using Discovery Proteomics. J. Proteom. 2017, 169, 99–111. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Overton, C.; Stewart, D.; Rutledge, R.G. Initiation of Somatic Embryos and Regeneration of Plants from Primordial Shoots of 10-Year-Old Somatic White Spruce and Expression Profiles of 11 Genes Followed during the Tissue Culture Process. Planta 2011, 233, 635–647. [Google Scholar] [CrossRef]
- Santos, A.L.W.D.; Steiner, N.; Guerra, M.P.; Zoglauer, K.; Moerschbacher, B.M. Somatic Embryogenesis in Araucaria angustifolia. Biol. Plant. 2008, 52, 195–199. [Google Scholar] [CrossRef]
- Santos, A.L.W.D.; Jo, L.; Santa-Catarina, C.; Guerra, M.P.; Floh, E.I.S. Somatic Embryogenesis in Brazilian Pine: Establishment of Biochemical Markers for Selection of Cell Lines with High Embryogenic Potential. In Proceedings of the IUFRO Working Party 2.09.02 Conference “Integrating Vegetative Propagation, Biotechnologies and Genetic Improvement for Tree Production and Sustainable Forest Management”, Brno, Czech Republic, 25–28 June 2012. [Google Scholar]
- Bozhkov, P.V.; Filonova, L.H.; von Arnold, S. A Key Developmental Switch during Norway Spruce Somatic Embryogenesis Is Induced by Withdrawal of Growth Regulators and Is Associated with Cell Death and Extracellular Acidification. Biotechnol. Bioeng. 2002, 77, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska, K.; Hargreaves, C.; Lelu-Walter, M.A.; Trontin, J.F. Advances in Conifer Somatic Embryogenesis Since Year 2000. In In Vitro Embryogenesis in Higher Plants, Methods in Molecular Biology; Springer Science + Business Media: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Ahn, Y.E. Propagation and Cryopreservation of Ulleungdo Hemlock (Tsuga ulleungensis) via Somatic Embryogenesis. Trees. Struct. Funct. 2018, 32, 1801–1808. [Google Scholar] [CrossRef]
- Varis, S.; Ahola, S.; Jaakola, L.; Aronen, T. Reliable and Practical Methods for Cryopreservation of Embryogenic Cultures and Cold Storage of Somatic Embryos of Norway Spruce. Cryobiology 2017, 76, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Brar, D.S.; Jain, S.M. Somaclonal Variation: Mechanism and Applications in Crop Improvement. In Somaclonal Variation and Induced Mutations in Crop Improvement; Springer: Dordrecht, Netherlands, 1998. [Google Scholar] [CrossRef]
- Marum, L.; Rocheta, M.; Maroco, J.; Oliveira, M.M.; Miguel, C. Analysis of Genetic Stability at SSR Loci during Somatic Embryogenesis in Maritime Pine (Pinus pinaster). Plant Cell Rep. 2009, 28, 673–682. [Google Scholar] [CrossRef]
- Bozhkov, K.B.A.H.P.; Arnold, S. Developmental and Genetic Variation in Nuclear Microsatellite Stability during Somatic Embryogenesis in Pine. J. Exp. Bot. 2007, 58, 687–698. [Google Scholar]
- Andreas, H.; Sara, V.A.; Kornel, B.; Bozhkov, P.V. High Stability of Nuclear Microsatellite Loci during the Early Stages of Somatic Embryogenesis in Norway Spruce. Tree Physiol. 2004, 24, 1181–1186. [Google Scholar]
- Harvengt, L.; Trontin, J.F.; Reymond, I.; Canlet, F.; Paques, M. Molecular Evidence of True-to-Type Propagation of a 3-Year-Old Norway Spruce through Somatic Embryogenesis. Planta 2001, 213, 828–832. [Google Scholar] [CrossRef]
- Valledor, L.; Hasbún, R.; Meijón, M.; Rodríguez, J.L.; Santamaría, E.; Viejo, M.; Berdasco, M.; Feito, I.; Fraga, M.F.; Rodríguez, R.; et al. Involvement of DNA methylation in tree development and development micropropagation. Plant Cell Tissue Organ Cult. 2007, 91, 75–86. [Google Scholar] [CrossRef]
- Corredoira, E.; Cano, V.; Bárány, I.; Solís, M.-T.; Rodríguez, H.; Vieitez, A.-M.; Risueño, M.C.; Testillano, P.S. Initiation of Leaf Somatic Embryogenesis Involves High Pectin Esterification, Auxin Accumulation and DNA Demethylation in Quercus alba. J. Plant Physiol. 2017, 213, 42. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Yuan, D.; Jin, F.; Zhang, Y.; Xu, J. Transcript Profiling Reveals Complex Auxin Signalling Pathway and Transcription Regulation Involved in Dedifferentiation and Redifferentiation during Somatic Embryogenesis in Cotton. BMC Plant Biol. 2012, 12, 110. [Google Scholar] [CrossRef]
- Tretyakova, I.N.; Kudoyarova, G.R.; Park, M.E.; Kazachenko, A.S.; Shuklina, A.S.; Akhiyarova, G.R.; Korobova, A.V.; Veselov, S.U. Content and Immunohistochemical Localization of Hormones during in Vitro Somatic Embryogenesis in Long-Term Proliferating Larix sibirica Cultures. Plant Cell Tissue Organ Cult. 2019, 136, 511–522. [Google Scholar] [CrossRef]
- Li, Q.; Deng, C.; Zhu, T.; Ling, J.; Zhang, H.; Kong, L.; Zhang, S.; Wang, J.; Chen, X. Dynamics of Physiological and miRNA Changes after Long-Term Proliferation in Somatic Embryogenesis of Picea balfouriana. Trees 2019, 33, 469–480. [Google Scholar] [CrossRef]
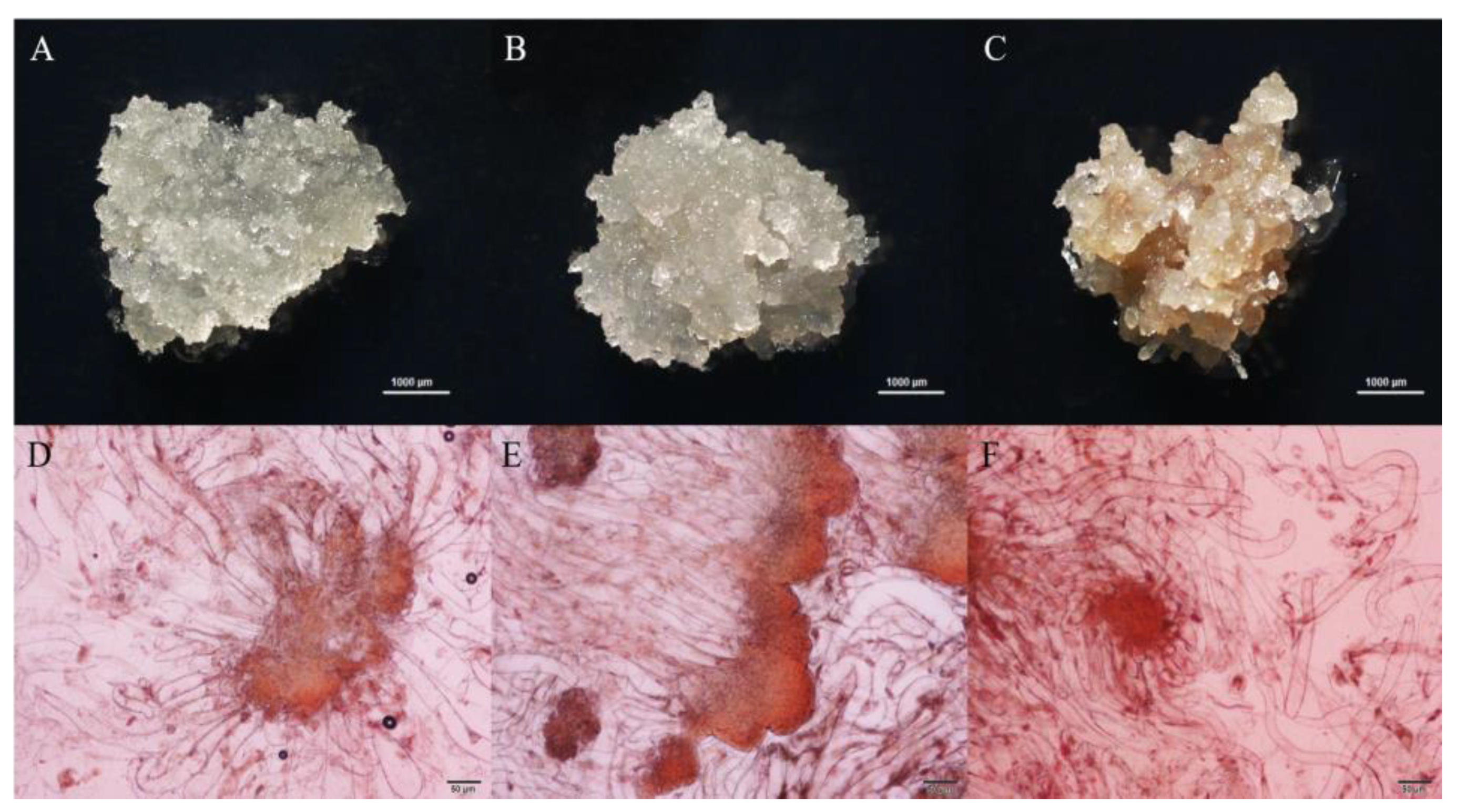



| Number | Primer Name | Sequence | Repetitive Unit | Tm (°C) | Fragment Size (bp) | References |
|---|---|---|---|---|---|---|
| 1 | F42 | F:AACACTTTAATCCCCTCCC | (CAGGAA)5 | 51 | 190–220 | [43] |
| R:CTGGACCTGATACTCCTCTTC | ||||||
| 2 | F94 | F:GCCGTTGACAACAATTACAT | (ACTGG)5 | 55 | 150–180 | [43] |
| R:AAAGAATAGCAACCCGCAGT | ||||||
| 3 | F1 | F:AAGGAGGAGGGTCAGGGAA | (TCAGGC)5 | 55 | 160–190 | [43] |
| R:CATGCGGAGGTTGAGTGTG | ||||||
| 4 | SSR11 | F:ATGGGTTTGACAGCGGATAA | (GCA)6 | 55 | 240–260 | [42] |
| R:CGTGTTCTTGTTTTGGGTGG | ||||||
| 5 | Lar_eSSR111 | F:GATATCAACTCCCTGCGGAA | (CCTGAA)4 | 55 | 230 | [44] |
| R:AGCTGTGAGCGAGAGAGAGG | ||||||
| 6 | Lar_eSSR115 | F:TTGTGATGCTTCTTTGACCG | (TTGTCT)4 | 55 | 239 | [44] |
| R:GAGGCAGATAGAGGGCTTCC | ||||||
| 7 | Lar_eSSR78 | F:CAATCCGATAAAACGCCATC | (GTGTCG)4 | 55 | 262 | [44] |
| R:CAGTAACACTCCCGCCTAGC | ||||||
| 8 | Lar_eSSR54 | F:GCGCGCTCTTCTTTTCTCT | (ACCCGC)6 | 55 | 162 | [44] |
| R:CGCCGTCGACTGTATAACCT | ||||||
| 9 | Lar_eSSR11 | F:AATCCAAATTTCTGGACCCC | (CAAGGG)4 | 56 | 239 | [44] |
| R:CCTGCAAAAAGAGGATAGCG | ||||||
| 10 | SSR12 | F:GCCTTCGCTGATCTGTTT | (AC)6(AT)6 | 55 | 170–190 | [42] |
| R:TTTCCTCTTGACGGACTCTT |
| Name | Variate | Correlation | Proliferation Rate | Pre-Treated Proliferation Rate | Maturation Capacity |
|---|---|---|---|---|---|
| BFU1 | Culture period | pertinence | 0.283 | 0.622 ** | −0.409 * |
| significance | 0.117 | <0.001 | 0.020 | ||
| BFU3 | pertinence | 0.277 | 0.562 ** | −0.427 * | |
| significance | 0.125 | 0.001 | 0.015 | ||
| BFU8 | pertinence | −0.333 | 0.511 ** | −0.621 ** | |
| significance | 0.063 | 0.003 | <0.001 |
| Name | Variate | Correlation | Proliferation Rate | Pre-Treated Proliferation Rate | Maturation Capacity |
|---|---|---|---|---|---|
| BFU1 | Proliferation rate | pertinence | 1 | 0.411 * | 0.025 |
| significance | -- | 0.019 | 0.890 | ||
| Pre-treated proliferation rate | pertinence | 0.411 * | 1 | −0.513 ** | |
| significance | 0.019 | -- | 0.003 | ||
| BFU3 | Proliferation rate | pertinence | 1 | 0.178 | 0.056 |
| significance | -- | 0.330 | 0.763 | ||
| Pre-treated proliferation rate | pertinence | 0.178 | 1 | −0.340 | |
| significance | 0.330 | -- | 0.057 | ||
| BFU8 | Proliferation rate | pertinence | 1 | −0.106 | 0.404 * |
| significance | -- | 0.562 | 0.022 | ||
| Pre-treated proliferation rate | pertinence | −0.106 | 1 | −0.264 | |
| significance | 0.562 | -- | 0.145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, C.; Yuan, D.; Wang, X.; Zhao, H.; Zhang, L.; Kong, L.; Zhang, J.; Zhao, J. Long-Term Successional Subculture Dynamics and Their Effects on the Proliferation Efficiency, Embryogenic Potential, and Genetic Stability of Embryogenic Tissues in Larix principis-rupprechtii Mayr. Forests 2024, 15, 627. https://doi.org/10.3390/f15040627
Chen X, Liu C, Yuan D, Wang X, Zhao H, Zhang L, Kong L, Zhang J, Zhao J. Long-Term Successional Subculture Dynamics and Their Effects on the Proliferation Efficiency, Embryogenic Potential, and Genetic Stability of Embryogenic Tissues in Larix principis-rupprechtii Mayr. Forests. 2024; 15(4):627. https://doi.org/10.3390/f15040627
Chicago/Turabian StyleChen, Xiaoyi, Chengbi Liu, Deshui Yuan, Xiuqi Wang, Huanhuan Zhao, Luyao Zhang, Lisheng Kong, Jinfeng Zhang, and Jian Zhao. 2024. "Long-Term Successional Subculture Dynamics and Their Effects on the Proliferation Efficiency, Embryogenic Potential, and Genetic Stability of Embryogenic Tissues in Larix principis-rupprechtii Mayr" Forests 15, no. 4: 627. https://doi.org/10.3390/f15040627
APA StyleChen, X., Liu, C., Yuan, D., Wang, X., Zhao, H., Zhang, L., Kong, L., Zhang, J., & Zhao, J. (2024). Long-Term Successional Subculture Dynamics and Their Effects on the Proliferation Efficiency, Embryogenic Potential, and Genetic Stability of Embryogenic Tissues in Larix principis-rupprechtii Mayr. Forests, 15(4), 627. https://doi.org/10.3390/f15040627






