CfCHLM, from Cryptomeria fortunei, Promotes Chlorophyll Synthesis and Improves Tolerance to Abiotic Stresses in Transgenic Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation and Condition Setting of Plant Materials
2.2. Cloning of a CHLM cDNA Sequence
2.3. Phylogenetic and Protein Property Analysis of CfCHLM
2.4. Subcellular Localization and Transcriptional Activity Assay of CfCHLM
2.5. A. thaliana Transformation
2.6. Setting of Abiotic Stress Conditions in A. thaliana
2.7. Detection of Plant Phenotypic and Physiological Indicators
2.8. The Expression Pattern of CfCHLM
2.9. Data Statistics and Analysis
3. Results
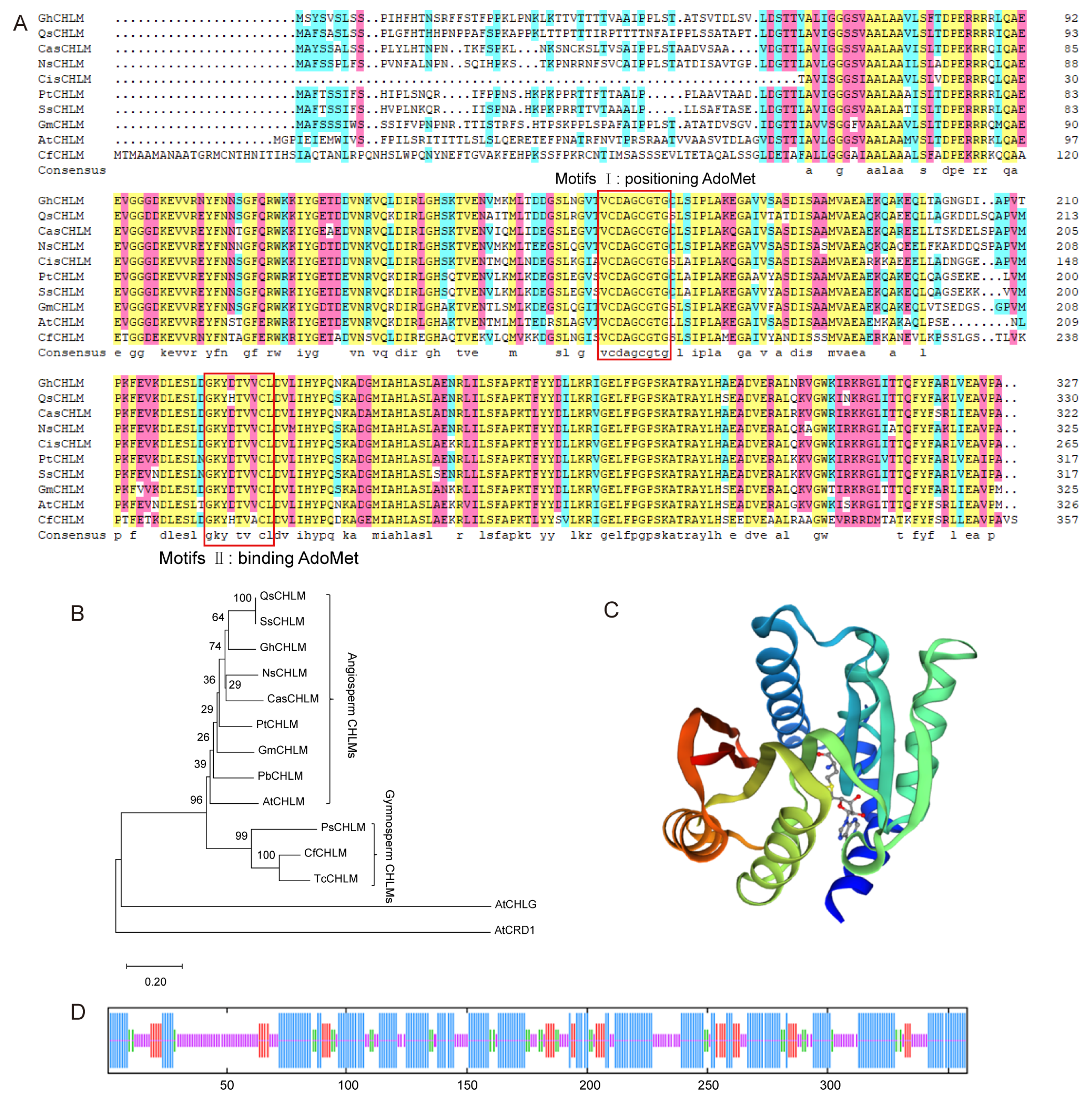
3.1. Full-Length and Phylogenetic Analysis of the CfCHLM Gene
3.2. Physicochemical Properties Analysis of CfCHLM Protein
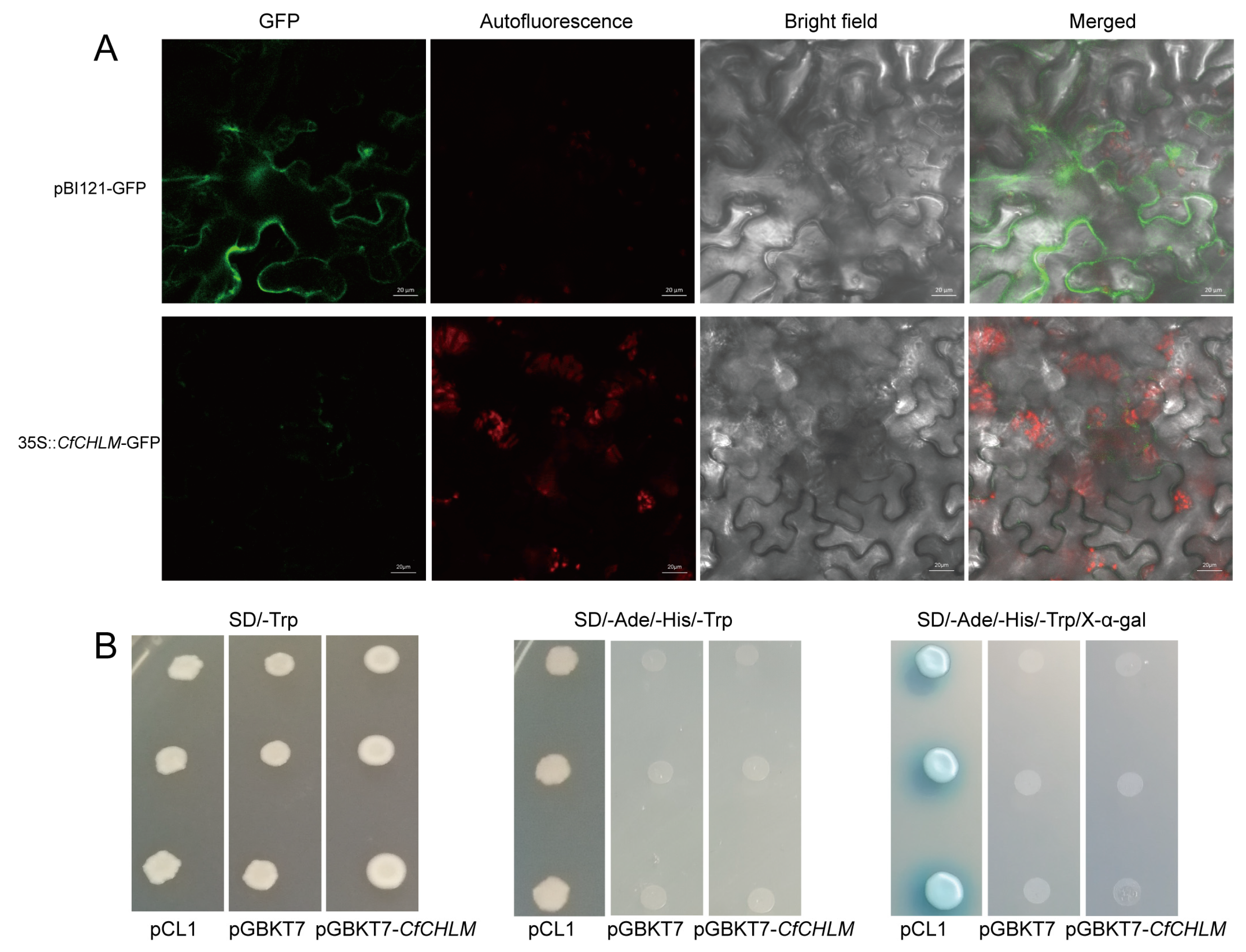
3.3. Subcellular Localization and Transcriptional Activity of CfCHLM
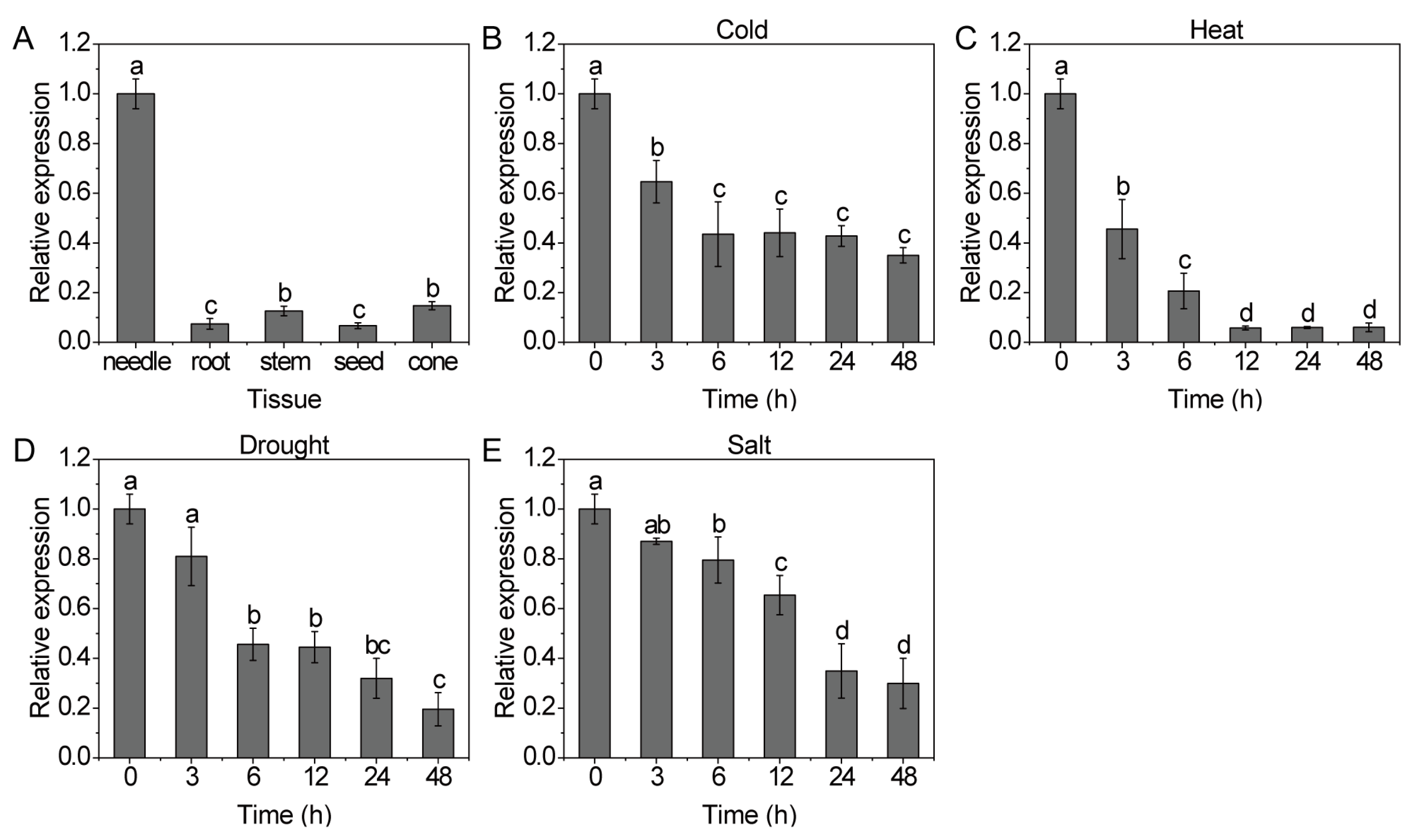
3.4. Expression Pattern of CfCHLM
3.5. Expression Level Assay of CfCHLM in Transgenic A. thaliana Lines
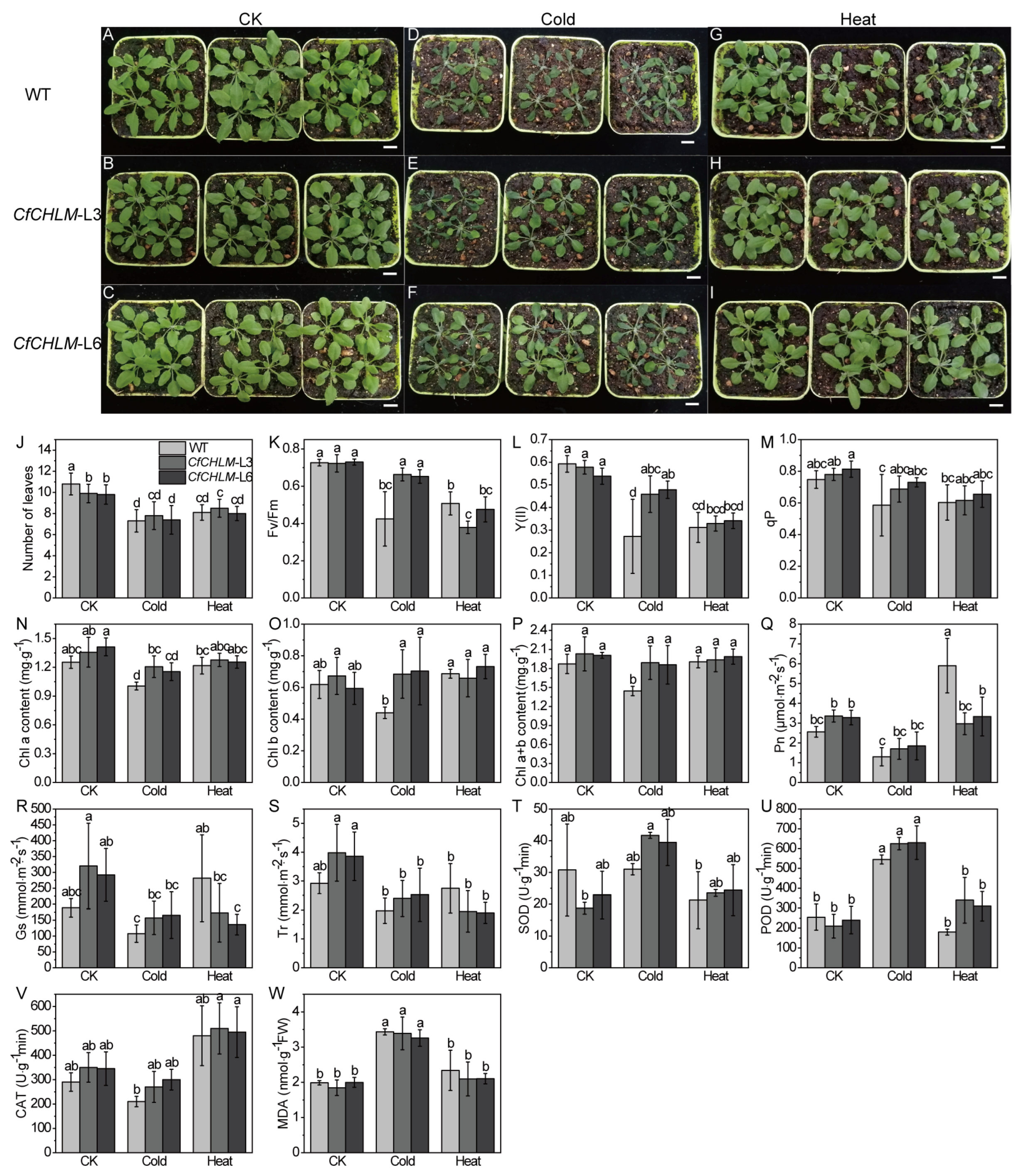
3.6. Response of CfCHLM Gene to Cold or Heat Stress
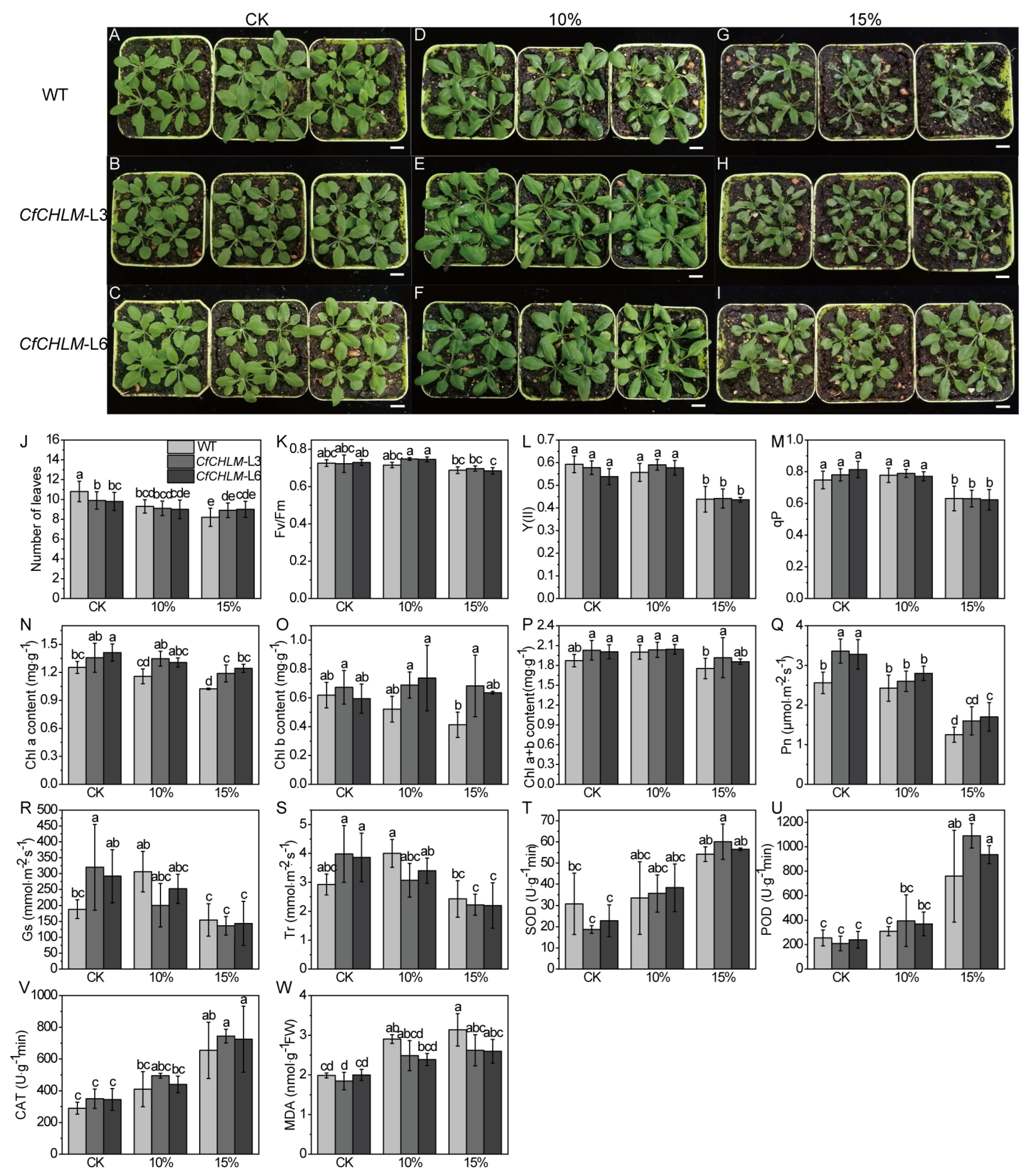
3.7. Response of CfCHLM Gene to Drought Stress
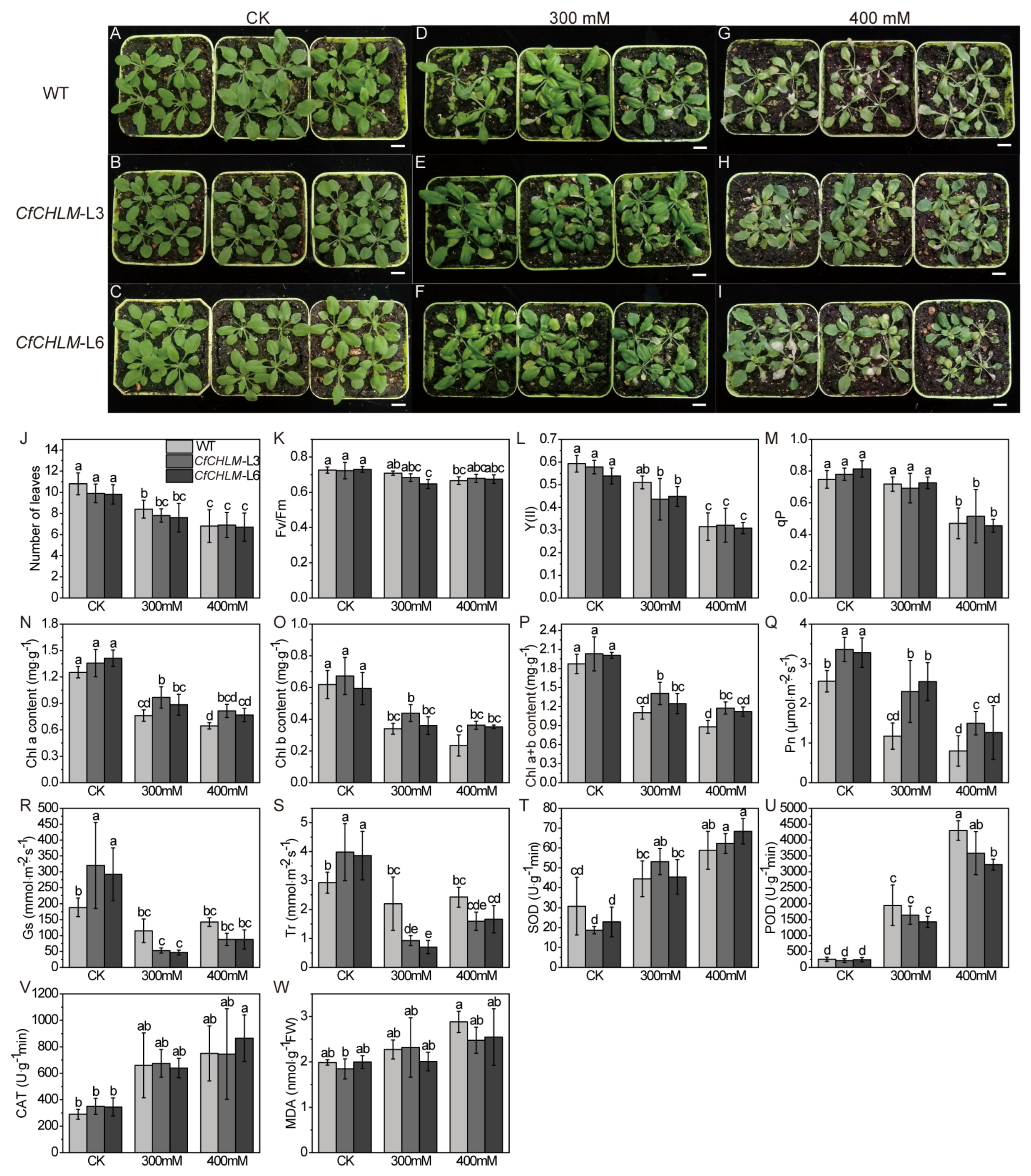
3.8. Response of CfCHLM Gene to Salt Stress
4. Discussion
4.1. Functional Analysis and Expression Pattern of CfCHLM
4.2. The Function of the CfCHLM Gene in Photosynthesis
4.3. The Function of the CfCHLM Gene in the Response to Abiotic Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Li, R.; He, Y.; Chen, J.; Zheng, S.; Zhuang, C. Research Progress in Improving Photosynthetic Efficiency. Int. J. Mol. Sci. 2023, 24, 9286. [Google Scholar] [CrossRef]
- Koji, K.; Toshiyuki, S.; Ryo, N.; Seiji, A.; Takehiro, S.; Naoshi, D.; Min, C.; Suleyman, I.A.; Jian, R.S.; Fusamichi, A.; et al. Structural basis for the adaptation and function of chlorophyll f in photosystem I. Nat. Commun. 2020, 11, 238. [Google Scholar] [CrossRef]
- Hu, X.; Gu, T.; Khan, I.; Zada, A.; Jia, T. Research Progress in the Interconversion, Turnover and Degradation of Chlorophyll. Cells 2021, 10, 3134. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, O.B. Studies of chlorophyll biosynthesis in Russia. Photosynth. Res. 2003, 76, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Bollivar, D.W. Recent advances in chlorophyll biosynthesis. Photosynth. Res. 2006, 89, 1–22. [Google Scholar] [CrossRef]
- Masuda, T. Recent overview of the Mg branch of the tetrapyrrole biosynthesis leading to chlorophylls. Photosynth. Res. 2008, 96, 121–143. [Google Scholar] [CrossRef]
- Bae, C.H.; Abe, T.; Matsuyama, T.; Fukunishi, N.; Nagata, N.; Nakano, T.; Kaneko, Y.; Miyoshi, K.; Matsushima, H.; Yoshida, S. Regulation of Chloroplast Gene Expression is Affected in ali, a Novel Tobacco Albino Mutant. Ann. Bot. 2001, 88, 545–553. [Google Scholar] [CrossRef]
- Jung, K.H.; Hur, J.; Ryu, C.H.; Choi, Y.; Chung, Y.Y.; Miyao, A.; Hirochika, H.; An, G.H. Characterization of a rice chlorophyll-deficient mutant using the T-DNA gene-trap system. Plant Cell Physiol. 2003, 44, 463–472. [Google Scholar] [CrossRef]
- Shmakov, N.A.; Vasiliev, G.V.; Shatskaya, N.V.; Doroshkov, A.V.; Khlestkina, E.K. Identification of nuclear genes controlling chlorophyll synthesis in barley by RNA-seq. BMC Plant Biol. 2016, 16, 3. [Google Scholar] [CrossRef]
- Li, C.; Ma, F.; Jiao, R.; Chen, C.; Wang, Q.; Xiao, F.; Sun, C.; Deng, X.; Dong, C.; Wang, P. Mutation in Mg-Protoporphyrin IX Monomethyl ester cyclase causes yellow and spotted leaf phenotype in rice. Plant Mol. Biol. Rep. 2019, 37, 253–264. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Guo, R.; Fan, J.; Liu, S.; Liao, J.; Huang, Y.; Wang, Z. Physiological, Cytological, and Transcriptomic Analysis of Magnesium Protoporphyrin IX Methyltransferase Mutant Reveal Complex Genetic Regulatory Network Linking Chlorophyll Synthesis and Chloroplast Development in Rice. Plants 2023, 12, 3785. [Google Scholar] [CrossRef] [PubMed]
- Kagan, R.M.; Clarke, S. Widespread occurrence of three sequence motifs in diverse S-adenosylmethionine-dependent methyltransferases suggests a common structure for these enzymes. Arch. Biochem. Biophys. 1994, 310, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Pontier, D.; Albrieux, C.; Joyard, J.; Lagrange, T.; Block, M.A. Knock-out of the Mg protoporphyrin IX methyltransferase gene in Arabidopsis: Effects on chloroplast development and on chloroplast-to-nucleus signaling. J. Biol. Chem. 2007, 282, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Block, M.A.; Tewari, A.K.; Albrieux, C.; Maréchal, E.; Joyard, J. The plant S-adenosyl-l-methionine: Mg-protoporphyrin IX methyltransferase is located in both envelope and thylakoid chloroplast membranes. Eur. J. Biochem. 2002, 269, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Alawady, A.; Reski, R.; Yaronskaya, E.; Grimm, B. Cloning and expression of the tobacco CHLM sequence encoding Mg protoporphyrin IX methyltransferase and its interaction with Mg chelatase. Plant Mol. Biol. 2005, 57, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Hong, X.; Hu, K.K.; Wang, Y.; Wang, X.X.; Du, S.Y.; Li, Y.; Hu, D.D.; Cheng, K.X.; An, B.G.; et al. Impaired Magnesium Protoporphyrin IX Methyltransferase (ChlM) Impedes Chlorophyll Synthesis and Plant Growth in Rice. Front. Recent. Dev. Plant Sci. 2017, 8, 1694. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; David, M.P.; Cao, Z.L.; Xiao, L.T. Mutation of chloroplast CHLM contributes to down-regulation of multiple stress response genes in Arabidopsis. Plant Growth Regul. 2020, 91, 209–219. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Yang, L.W.; Zhang, M.; Yang, J.J.; Cui, J.B.; Hu, H.L.; Xu, J. CfAPX, a cytosolic ascorbate peroxidase gene from Cryptomeria fortunei, confers tolerance to abiotic stress in transgenic Arabidopsis. Plant Physiol. Biochem. 2022, 172, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Hyodo, H.; Takemura, M.; Yokota, A.; Ohyama, K.; Kohchi, T. Systematic Isolation of Highly Transcribed Genes in Inflorescence Apices in Arabidopsis thaliana from an Equalized cDNA Library. Biosci. Biotechnol. Biochem. 2000, 64, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Zhu, L.J.; Xue, J.Y.; Yang, J.J.; Hu, H.L.; Cui, J.B.; Xu, J. Selection and verification of appropriate reference genes for expression normalization in Cryptomeria fortunei under abiotic stress and hormone treatments. Genes 2021, 12, 791. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, Z.; Chang, X.; Zhang, Z.; Shi, W.; Zhang, Z.; Zhao, B.; Sun, J. Analysis of the Alternative Splicing Events of Exogenous δ-Aminolevulinic Acid under NaCl Stress in Wild Jujube Seedlings. Forests 2022, 13, 2076. [Google Scholar] [CrossRef]
- Chandrasekaran, U.; Byeon, S.; Kim, K.; Kim, S.H.; Park, C.O.; Han, A.R.; Lee, Y.S.; Kim, H.S. Short-term severe drought influences root volatile biosynthesis in eastern white pine (Pinus strobus L.). Front. Plant Sci. 2022, 13, 1030140. [Google Scholar] [CrossRef]
- Rossi, S.; Huang, B. Heat-induced Leaf Senescence in Creeping Bentgrass Suppressed by Aminoethoxyvinylglycine Involving Regulation of Chlorophyll Metabolism. J. Am. Soc. Hortic. Sci. 2023, 148, 126–133. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Yuan, L.; Zhou, H.; Hou, X.; Liu, T. Cold acclimation can specifically inhibit chlorophyll biosynthesis in young leaves of Pakchoi. BMC Plant Biol. 2021, 21, 172. [Google Scholar] [CrossRef]
- Dong, L.; Ravelombola, W.; Weng, Y.; Qin, J.; Zhou, W.; Bhattarai, G.; Zia, B.; Yang, W.; Shi, L.; Mou, B.; et al. Change in Chlorophyll Content Over Time Well-differentiated Salt-tolerant, Moderately Salt-tolerant, and Salt-susceptible Cowpea Genotypes. HortScience 2019, 54, 1477–1484. [Google Scholar] [CrossRef]
- Syamsia, S.; Idhan, A.; Noerfitryani, N.; Nadir, M.; Reta, R.; Kadir, M. Paddy chlorophyll concentrations in drought stress condition and endophytic fungi application. IOP Conf. Ser. Earth Environ. Sci. 2018, 156, 012040. [Google Scholar] [CrossRef]
- Banks, J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practitioners. Tree Physiol. 2017, 37, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Liu, C.; Li, K.; Li, X.X.; Xu, M.M.; Guo, Y.F. CLE14 functions as a “brake signal” to suppress age-dependent and stress-induced leaf senescence by promoting JUB1-mediated ROS scavenging in Arabidopsis. Mol. Plant 2022, 15, 179–188. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Torres, R.; Romero, J.M.; Lagorio, M.G. Effects of sub-optimal illumination in plants. Comprehensive chlorophyll fluorescence analysis. J. Photochem. Photobiol. B 2021, 218, 112182. [Google Scholar] [CrossRef] [PubMed]
- Arminian, A.; Bidgoli, R.D. Simultaneous responses of photosystem II and soluble proteins of rapeseed to cold acclimation. Cell. Mol. Biol. 2019, 65, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, Y.; Yu, J.; Zhang, C. The difference of photosynthetic characteristics and chlorophyll fluorescence parameters in two cucumber varieties with heat tolerance under high temperature stress. In Advances in Biomedical Photonics and Imaging, Proceedings of the 6th International Conference on Photonics and Imaging in Biology and Medicine, Wuhan, China, 4–6 November 2007; World Scientific Publishing: Singapore, 2008. [Google Scholar] [CrossRef]
- Hou, Q.; Sun, K.; Zhang, H.; Su, X.; Fan, B.; Feng, H. The responses of photosystem II and intracellular ATP production of Arabidopsis leaves to salt stress are affected by extracellular ATP. J. Plant Res. 2018, 131, 331–339. [Google Scholar] [CrossRef]
- Ohsumi, A.; Hamasaki, A.; Nakagawa, H.; Homma, K.; Horie, T.; Shiraiwa, T. Response of leaf photosynthesis to vapor pressure difference in rice (Oryza sativa L.) varieties in relation to stomatal and leaf internal conductance. Plant Prod. Sci. 2015, 11, 184–191. [Google Scholar] [CrossRef]
- Tang, G.L.; Li, X.Y.; Lin, L.S.; Zeng, F.J. Different causes of photosynthetic decline and water status in different stages of girdling in Alhagi sparsifolia Shap. (Fabaceae). Rev. Bras. Bot. 2016, 39, 519–529. [Google Scholar] [CrossRef]
- Yang, H.M.; Zhang, X.Y.; Zhao, L. Stomatal control partly explains different photosynthetic characteristics in Helianthus laetiflora and H-annuus. N. Z. J. Crop Hortic. Sci. 2009, 37, 33–39. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.C.; Zhang, Y.P. Effects of drought stress on the photosynthesis of wild apricot. Acta Hortic. 2008, 772, 287–290. [Google Scholar] [CrossRef]
- Ying, Y.Q.; Song, L.L.; Jacobs, D.F.; Mei, L.; Liu, P.; Jin, S.H.; Wu, J.S. Physiological response to drought stress in Camptotheca acuminata seedlings from two provenances. Front. Recent Dev. Plant Sci. 2015, 6, 361. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Wang, L.L.; Wei, Y.X.; Zhang, S.W.; Zhang, Y.L. Cross adaptation tolerance in rice seedlings exposed to peg induced salinity and drought stress. Int. J. Agric. Biol. 2015, 18, 535–541. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar] [CrossRef]
- Suda, I.; Furuta, S.; Nishiba, Y. Fluorometric determination of a 1,3-Diethyl-2-thiobarbituric Acid-Malondialdehyde adduct as an index of lipid peroxidation in plant materials. Biosci. Biotechnol. Biochem. 1994, 58, 14–17. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, H.B.; Chen, S.H.; He, J.; Guo, S.L. Changes in antioxidant enzyme activity and transcript levels of related genes in limonium sinense kuntze seedlings under nacl stress. J. Chem. 2014, 2014, 749047. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, T.; Hu, J.; Han, R.; Zhu, Y.; Guan, Y.; Zhu, S. Relationship between endogenous salicylic acid and antioxidant enzyme activities in maize seedlings under chilling stress. Exp. Agric. 2013, 49, 295–308. [Google Scholar] [CrossRef]
- Khanna-Chopra, R.; Chauhan, S. Wheat cultivars differing in heat tolerance show a differential response to oxidative stress during monocarpic senescence under high temperature stress. Protoplasma 2015, 252, 1241–1251. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, G.; Zhang, Y.; Yang, Y.; Yang, J.; Xu, J. CfCHLM, from Cryptomeria fortunei, Promotes Chlorophyll Synthesis and Improves Tolerance to Abiotic Stresses in Transgenic Arabidopsis thaliana. Forests 2024, 15, 628. https://doi.org/10.3390/f15040628
Wei G, Zhang Y, Yang Y, Yang J, Xu J. CfCHLM, from Cryptomeria fortunei, Promotes Chlorophyll Synthesis and Improves Tolerance to Abiotic Stresses in Transgenic Arabidopsis thaliana. Forests. 2024; 15(4):628. https://doi.org/10.3390/f15040628
Chicago/Turabian StyleWei, Guangqian, Yingting Zhang, Ye Yang, Junjie Yang, and Jin Xu. 2024. "CfCHLM, from Cryptomeria fortunei, Promotes Chlorophyll Synthesis and Improves Tolerance to Abiotic Stresses in Transgenic Arabidopsis thaliana" Forests 15, no. 4: 628. https://doi.org/10.3390/f15040628
APA StyleWei, G., Zhang, Y., Yang, Y., Yang, J., & Xu, J. (2024). CfCHLM, from Cryptomeria fortunei, Promotes Chlorophyll Synthesis and Improves Tolerance to Abiotic Stresses in Transgenic Arabidopsis thaliana. Forests, 15(4), 628. https://doi.org/10.3390/f15040628





