Nitrogen Addition Promotes the Accumulation of Soil Particulate Organic Carbon in a Subtropical Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Soil Physicochemical Characteristics
2.4. SOC Fractions
2.5. Exchangeable Cations and Soil Enzyme Activities
2.6. Aboveground Litter Product and Fine Root Biomass
2.7. Statistical Analyses
3. Results
3.1. Responses of SOC Fractions to Short-Term N Addition
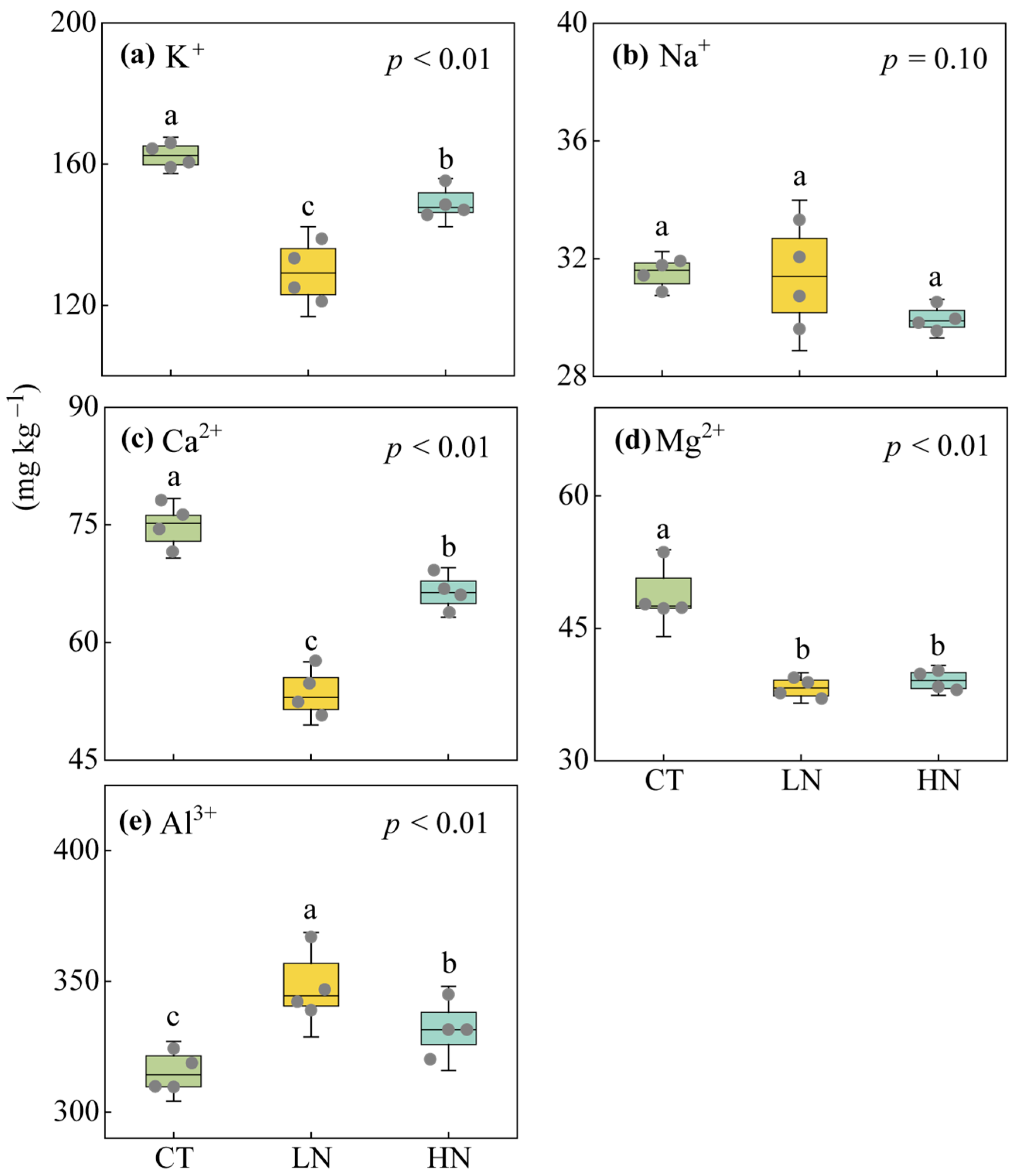
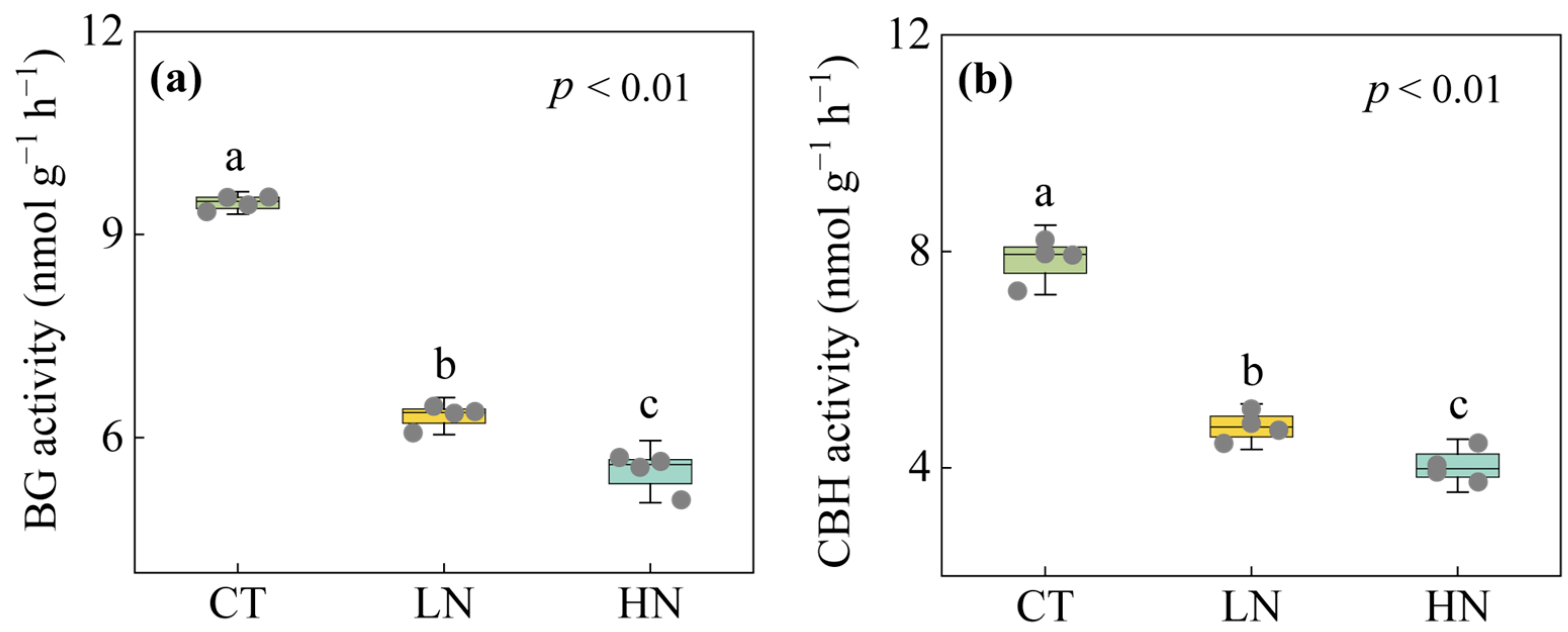
3.2. Responses of Soil Exchangeable Cations and Enzyme Activities to Short-Term N Addition
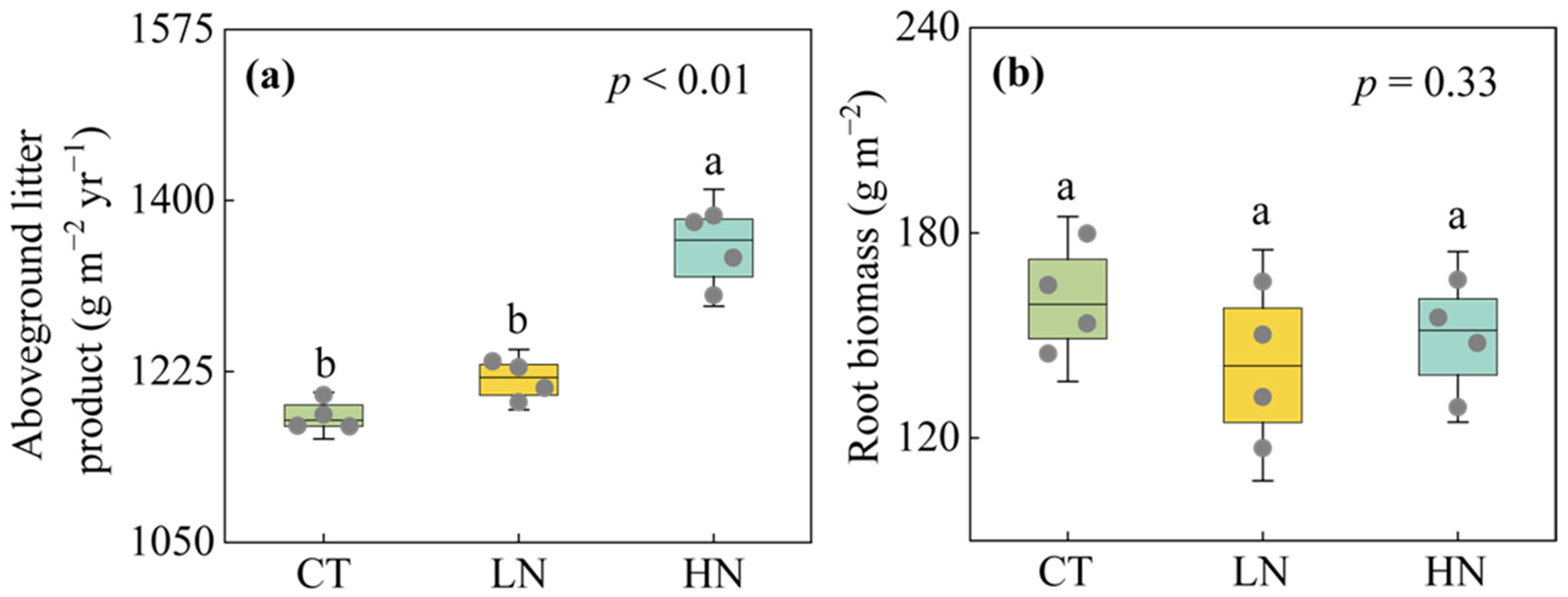
3.3. Responses of Aboveground Litter Product and Fine Root Biomass to Short-Term N Addition
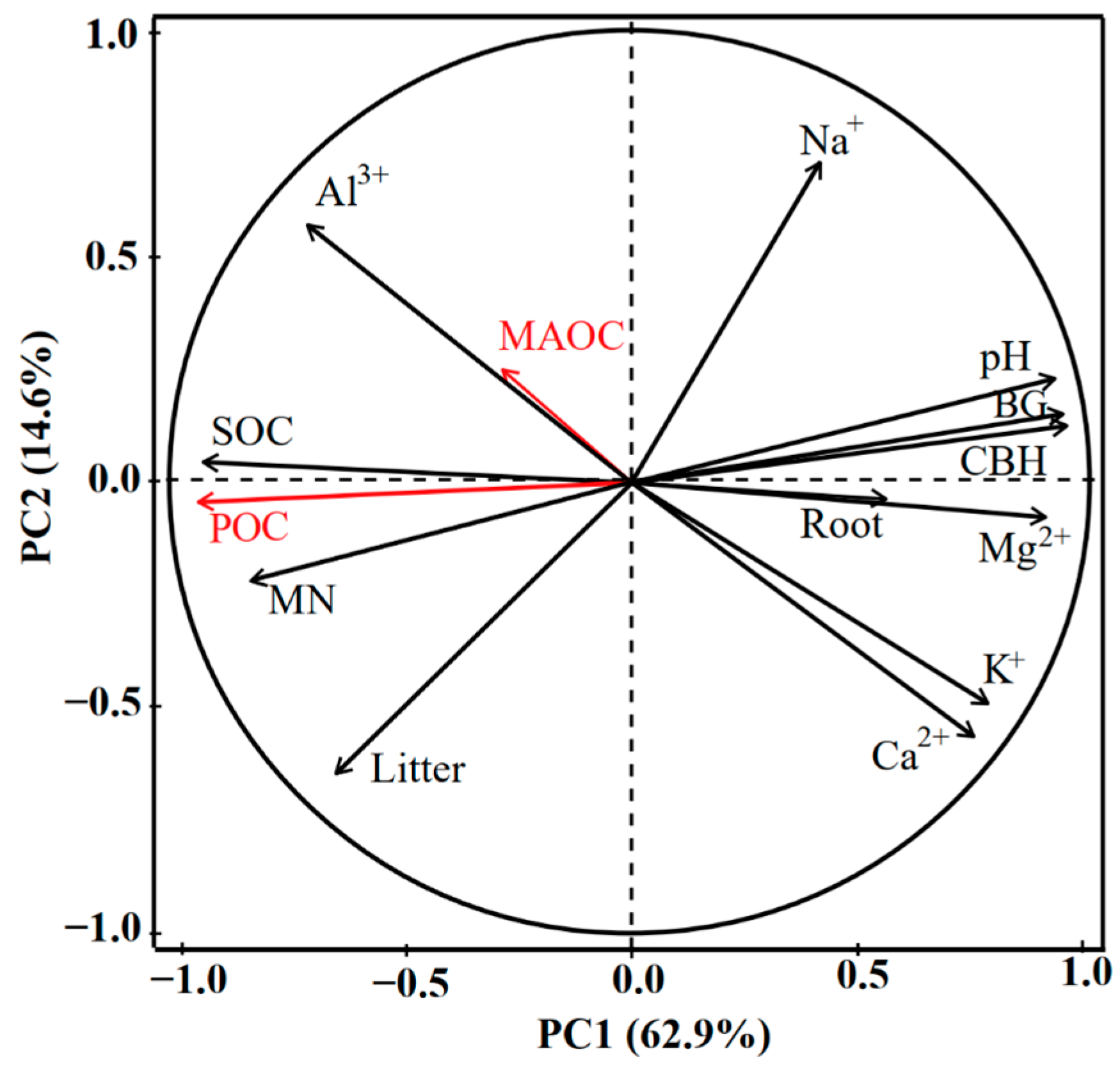
3.4. Analysis of Key Factors Influencing SOC Fraction Changes
4. Discussion
4.1. Positive Response of POC to Short-Term N Addition
4.2. Non-Significant Response of MAOC to Short-Term N Addition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradford, M.A.; Wieder, W.R.; Bonan, G.B.; Fierer, N.; Raymond, P.A.; Crowther, T.W. Managing uncertainty in soil carbon feedbacks to climate change. Nat. Clim. Change 2016, 6, 751–758. [Google Scholar] [CrossRef]
- Clark, C.M.; Tilman, D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 2008, 451, 712–715. [Google Scholar] [CrossRef]
- Ackerman, D.; Millet, D.B.; Chen, X. Global Estimates of Inorganic Nitrogen Deposition Across Four Decades. Glob. Biogeochem. Cycles 2019, 33, 100–107. [Google Scholar] [CrossRef]
- Yu, G.R.; Jia, Y.L.; He, N.P.; Zhu, J.X.; Chen, Z.; Wang, Q.F.; Piao, S.L.; Liu, X.J.; He, H.L.; Gou, X.B.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Greaver, T.L.; Clark, C.M.; Compton, J.E.; Vallano, D.; Talhelm, A.F.; Weaver, C.P.; Band, L.E.; Baron, J.S.; Davidson, E.A.; Tague, C.L.; et al. Key ecological responses to nitrogen are altered by climate change. Nat. Clim. Chang. 2016, 6, 836–843. [Google Scholar] [CrossRef]
- Cheng, S.J.; Hess, P.G.; Wieder, W.R.; Thomas, R.Q.; Nadelhoffer, K.J.; Vira, J.; Lombardozzi, D.L.; Gundersen, P.; Fernandez, I.J.; Schleppi, P.; et al. Decadal fates and impacts of nitrogen additions on temperate forest carbon storage: A data-model comparison. Biogeosciences 2019, 16, 2771–2793. [Google Scholar] [CrossRef]
- Ullah, S.; Raza, M.M.; Abbas, T.; Guan, X.; Zhou, W.; He, P. Responses of soil microbial communities and enzyme activities under nitrogen addition in fluvo-aquic and black soil of North China. Front. Microbiol. 2023, 14, 1249471. [Google Scholar] [CrossRef]
- Wu, Z.F.; Tang, Z.H.; Yu, T.Y.; Zhang, J.C.; Zheng, Y.M.; Yang, J.S.; Wu, Y. Nitrogen fertilization rates mediate rhizosphere soil carbon emissions of continuous peanut monoculture by altering cellulose-specific microbes. Front. Plant Sci. 2023, 14, 1109860. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Li, F.C.; Dong, L.L.; Wang, X.; Han, L.B.; Olesen, J.E. Effects of exogenous organic/inorganic nitrogen addition on carbon pool distribution and transformation in grassland soil. Sci. Total Environ. 2023, 858, 159919. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Qi, P.; Chen, J.; Wang, X.J.; Zhang, G.Z.; Cai, L.Q.; Jiao, Y.P.; Li, Z.Q.; Han, G.J. Changes in soil particulate and mineral-associated organic carbon concentrations under nitrogen addition in China—A meta-analysis. Plant Soil 2023, 489, 439–452. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-Associated forms to address global change in the 21st century. Glob. Chang. Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef] [PubMed]
- Poeplau, C.; Don, A.; Six, J.; Kaiser, M.; Benbi, D.; Chenu, C.; Cotrufo, M.F.; Derrien, D.; Gioacchini, P.; Grand, S.; et al. Isolating organic carbon fractions with varying turnover rates in temperate agricultural soils-A comprehensive method comparison. Soil Biol. Biochem. 2018, 125, 10–26. [Google Scholar]
- Chen, J.G.; Wen, X.; Zheng, C.Y.; Zhu, B. Nitrogen addition has contrasting effects on particulate and mineral-associated soil organic carbon in a subtropical forest. Soil Biol. Biochem. 2020, 142, 107708. [Google Scholar] [CrossRef]
- Feng, H.L.; Guo, J.H.; Peng, C.H.; Kneeshaw, D.; Roberge, G.; Pan, C.; Ma, X.H.; Zhou, D.; Wang, W.F. Nitrogen addition promotes terrestrial plants to allocate more biomass to aboveground organs: A global meta-analysis. Glob. Chang. Biol. 2023, 29, 3970–3989. [Google Scholar]
- Sun, T.; Mao, X.L.; Han, K.F.; Wang, X.J.; Cheng, Q.; Liu, X.; Zhou, J.J.; Ma, Q.X.; Ni, Z.H.; Wu, L.H. Nitrogen addition increased soil particulate organic carbon via plant carbon input whereas reduced mineral−associated organic carbon through attenuating mineral protection in agroecosystem. Sci. Total Environ. 2023, 899, 165705. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Nierop, K.G.J.; Simpson, M.J. Plant or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biol. Biochem. 2021, 156, 108–189. [Google Scholar]
- Craig, M.E.; Geyer, K.M.; Beidler, K.V.; Brzostek, E.R.; Frey, S.D.; Stuart, G.A.; Liang, C.; Phillips, R.P. Fast-decaying plant litter enhances soil carbon in temperate forests but not through microbial physiological traits. Nat. Commun. 2022, 13, 1229. [Google Scholar] [CrossRef]
- Tian, D.S.; Niu, S.L. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Rocci, K.S.; Lavallee, J.M.; Stewart, C.E.; Cotrufo, M.F. Soil organic carbon response to global environmental change depends on its distribution between mineral-associated and particulate organic matter: A meta-analysis. Sci. Total Environ. 2021, 793, 148569. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E. Mechanisms driving the soil organic matter decomposition response to nitrogen enrichment in grassland soils. Soil Biol. Biochem. 2016, 99, 54–65. [Google Scholar] [CrossRef]
- Ye, C.L.; Chen, D.M.; Hall, S.J.; Pan, S.; Yan, X.B.; Bai, T.S.; Guo, H.; Zhang, Y.; Bai, Y.F.; Hu, S.J. Reconciling multiple impacts of nitrogen enrichment on soil carbon: Plant, microbial and geochemical controls. Ecol. Lett. 2018, 21, 1162–1173. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.H.; Zhou, Z.Y.; Zhang, Y.Y. Inorganic nitrogen addition affects soil respiration and belowground organic carbon fraction for a Pinus tabuliformis forest. Forests 2019, 10, 369. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhang, H.; Pan, Y.T.; Cheng, X.L.; Zhang, K.R.; Liu, G.H. Particulate organic carbon is more sensitive to nitrogen addition than mineral-associated organic carbon: A meta-analysis. Soil Tillage Res. 2023, 232, 105770. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Lugato, E.; Lavallee, J.M.; Haddix, M.L.; Panagos, P.; Cotrufo, M.F. Different climate sensitivity of particulate and mineral-associated soil organic matter. Nat. Geosci. 2021, 14, 295–300. [Google Scholar] [CrossRef]
- Zeng, Q.X.; Fan, Y.X.; Zhang, Q.F.; Yuan, X.C.; Lin, K.M.; Zhou, J.C.; Lin, H.Y.; Xie, H.; Cui, J.Y.; Wu, Y.; et al. Differential factors determine the response of soil P fractions to N deposition in wet and dry seasons in a subtropical Moso bamboo forest. Plant Soil 2022. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Yan, J.H. The influences of regional atmospheric precipitation characteristics and its element inputs on the existence and development of Dinghushan forest ecosystems. Acta Ecol. Sin. 2001, 21, 2002–2012. [Google Scholar]
- Yuan, L.; Li, W.; Chen, W.; Zhang, J.; Cai, Z. Characteristics of nitrogen deposition in Daiyun Mountain National Nature Reserve. Environ. Sci. 2016, 37, 4142–4146. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for soil microbial biomass-C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Abhiram, G.; Grafton, M.; Jeyakumar, P.; Bishop, P.; Davies, C.E.; McCurdy, M. The Nitrogen Dynamics of Newly Developed Lignite-Based Controlled-Release Fertilisers in the Soil-Plant Cycle. Plants 2022, 11, 3288. [Google Scholar] [CrossRef]
- Marriott, E.E.; Wander, M.M. Total and labile soil organic matter in organic and conventional farming systems. Soil Sci. Soc. Am. J. 2006, 70, 950–959. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E.; Bach, E.M.; Hofmockel, K.S.; Kazanski, C.E. Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry 2015, 125, 203–219. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Feng, W.; Niu, S.; Plante, A.; Luo, Y.; Wang, K. Different responses of soil organic carbon fractions to additions of nitrogen. Eur. J. Soil Sci. 2018, 69, 1098–1104. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Lavallee, J.M. Chapter One-Soil organic matter formation, persistence, and functioning: A synthesis of current understanding to inform its conservation and regeneration. Adv. Agron. 2022, 172, 1–66. [Google Scholar]
- Yang, X.; Wang, Y.; Xu, Q.; Liu, W.; Liu, L.; Wu, Y.; Jiang, L.; Lu, J. Soil fertility underlies the positive relationship between island area and litter decomposition in a fragmented subtropical forest landscape. CATENA 2021, 204, 105414. [Google Scholar] [CrossRef]
- Sokol, N.W.; Kuebbing, S.E.; Karlsen-Ayala, E.; Bradford, M.A. Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. New Phytol. 2019, 221, 233–246. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Averill, C.; Waring, B. Nitrogen limitation of decomposition and decay: How can it occur. Glob. Chang. Biol. 2018, 24, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.G.; Sun, Y.; He, G.X.; He, R.L.; Zhang, M.Y.; Fang, H.D.; Shi, L.T. Nitrogen enrichment affects soil enzymatic stoichiometry via soil acidification in arid and hot land. Pedobiologia 2020, 81–82, 150663. [Google Scholar] [CrossRef]
- Bowman, W.D.; Cleveland, C.C.; Lubos, H.; Hresko, J.; Baron, J.S. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci. 2008, 1, 767–770. [Google Scholar] [CrossRef]
- Lu, X.K.; Vitousek, P.M.; Mao, Q.G.; Gilliam, F.S.; Luo, Y.Q.; Turner, B.L.; Zhou, G.Y.; Mo, J.M. Nitrogen deposition accelerates soil carbon sequestration in tropical forests. Proc. Natl. Acad. Sci. USA 2021, 118, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Hou, Y.H.; Zhou, S.R.; Zhu, B. Particulate organic carbon is more vulnerable to nitrogen addition than mineral-associated organic carbon in soil of an alpine meadow. Plant Soil 2021, 458, 93–103. [Google Scholar]
- Feng, J.G.; He, K.Y.; Zhang, Q.F.; Han, M.G.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Chang. Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar]
- Tang, B.; Rocci, K.S.; Lehmann, A.; Rillig, M.C. Nitrogen increases soil organic carbon accrual and alters its functionality. Glob. Chang. Biol. 2023, 29, 1971–1983. [Google Scholar] [CrossRef]
- Yu, G.H.; Xiao, J.; Hu, S.J.; Polizzotto, M.L.; Zhao, F.J.; Mcgrath, S.P.; Li, H.; Ran, W.; Shen, Q.R. Mineral availability as a key regulator of soil carbon storage. Environ. Sci. Technol. 2017, 51, 4960–4969. [Google Scholar] [CrossRef]
- Waychunas, G.A.; Kim, C.S.; Banfield, J.F. Nanoparticulate iron oxide minerals in soils and sediments: Unique properties and contaminant scavenging mechanisms. J. Nanopart. Res. 2005, 7, 409–433. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhang, D.D.; Chen, Q.; Feng, J.; Li, Q.X.; Yang, F.; Zhang, Q.; Cheng, X.L. Shifts in soil organic carbon dynamics under detritus input manipulations in a coniferous forest ecosystem in subtropical China. Soil Biol. Biochem. 2018, 126, 1–10. [Google Scholar] [CrossRef]
- Whalen, E.D.; Lounsbury, N.; Geyer, K.; Anthony, M.; Morrison, E.; van Diepen, L.T.A.; Le Moine, J.; Nadelhoffer, K.; vanden Enden, L.; Simpson, M.J.; et al. Root control of fungal communities and soil carbon stocks in a temperate forest. Soil Biol. Biochem. 2021, 161, 108390. [Google Scholar] [CrossRef]
- Kristensen, J.A.; Svenning, J.; Georgiou, K.; Malhi, Y. Can large herbivores enhance ecosystem carbon persistence. Trends Ecol. Evol. 2022, 37, 117–128. [Google Scholar]

| Treatment | CT | LN | HN | p Value |
|---|---|---|---|---|
| pH | 4.94 ± 0.04 a | 4.58 ± 0.07 b | 4.46 ± 0.12 b | <0.01 |
| SOC (g kg−1) | 51.0 ± 3.1 b | 57.5 ± 5.1 a | 58.0 ± 3.2 a | <0.01 |
| TN (g kg−1) | 3.85 ± 0.27 b | 4.44 ± 0.18 a | 4.65 ± 0.26 a | <0.01 |
| SOC:TN | 13.3 ± 0.8 a | 13.0 ± 1.3 a | 12.5 ± 0.5 a | 0.58 |
| Mineral N (mg kg−1) | 56.2 ± 5.6 b | 70.8 ± 8.4 a | 76.8 ± 7.2 a | <0.01 |
| MBC (mg kg−1) | 1790 ± 67 a | 1429 ± 146 b | 1314 ± 75 b | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, Q.; Dai, H.; Feng, J.; Zeng, Q.; Sun, X.; Peng, Y.; Chen, W.; Zhu, B.; Chen, Y. Nitrogen Addition Promotes the Accumulation of Soil Particulate Organic Carbon in a Subtropical Forest. Forests 2024, 15, 619. https://doi.org/10.3390/f15040619
Chen J, Zhang Q, Dai H, Feng J, Zeng Q, Sun X, Peng Y, Chen W, Zhu B, Chen Y. Nitrogen Addition Promotes the Accumulation of Soil Particulate Organic Carbon in a Subtropical Forest. Forests. 2024; 15(4):619. https://doi.org/10.3390/f15040619
Chicago/Turabian StyleChen, Jingqi, Qiufang Zhang, Hui Dai, Jiguang Feng, Quanxin Zeng, Xueqi Sun, Yuanzhen Peng, Wenwei Chen, Biao Zhu, and Yuehmin Chen. 2024. "Nitrogen Addition Promotes the Accumulation of Soil Particulate Organic Carbon in a Subtropical Forest" Forests 15, no. 4: 619. https://doi.org/10.3390/f15040619
APA StyleChen, J., Zhang, Q., Dai, H., Feng, J., Zeng, Q., Sun, X., Peng, Y., Chen, W., Zhu, B., & Chen, Y. (2024). Nitrogen Addition Promotes the Accumulation of Soil Particulate Organic Carbon in a Subtropical Forest. Forests, 15(4), 619. https://doi.org/10.3390/f15040619






