Abstract
Sonneratia apetala Buch., an alien species with strong growth and adaptability, has been introduced and cultivated in Southeastern China. Meanwhile, Derris trifoliata Lour., native to coastal and riparian areas in Guangdong, Guangxi, and Fujian provinces, has experienced a rapid surge in population, impacting the health of mangrove ecosystems. Our research focuses on understanding the interactions between Oriental mangroves and D. trifoliata, particularly their proliferation and long-term symbiotic relationships. We investigated how Oriental mangrove proliferation promotes excessive D. trifoliata growth and explored the underlying mechanisms. In Leizhou Bay, Guangxi, the annual growth rate surged from 12.03% (2005–2015) to 55.36% (2015–2019), indicating a significant acceleration post-2015 and a concerning trend towards overgrowth. D. trifoliata failed to produce seeds on sea rockets or bulrushes, instead yielding 10.5 and 97.43 seeds/m2 on native red mangroves and Oriental mangroves, respectively. Along riverbanks, 68% of Oriental mangroves hosted D. trifoliata, and the suitable regions for these species overlapped significantly. Oriental mangroves reach 15 m tall with 10 × 10 m crown diameters, providing ample vine space, optimal photosynthesis conditions, sturdy support, and convenient dispersal routes. This study offers insights into introduced–native species interactions in mangrove ecosystems, with significance for management and preservation.
1. Introduction
Mangrove ecosystems are essential and invaluable components of coastal regions and play a critical role in maintaining ecological balance and biodiversity. The introduction and spread of alien species have significantly affected these mangrove ecosystems [1,2]. Sonneratia apetala Buch., native to the coastal regions along the Indian Ocean [3], has found extensive introduction into the southeastern coastal areas of China, encompassing Hainan, Guangdong, Guangxi, and Fujian. This widespread introduction is attributed to its remarkable growth rate and adaptability [4]. This species covers an area of 2968 ha, representing 11.0% of the national mangrove area [5]. Plants thrive in optimal climatic conditions, maximizing their growth and reproduction. Exotic species can swiftly capitalize on additional resources, exhibiting rapid responses [6]. Sonneratia apetala has a high growth rate, tall trunks, and abundant sturdy seeds [7]. One distinctive feature is the absence of deadly herbivores in the regions where it has been introduced, leading to its extensive proliferation, and gradually making it a notable invasive species [8,9]. Following the formation of S. apetala forests, local mangrove species encounter challenges penetrating its dense canopy, while seedlings of native mangroves struggle to survive beneath it [10]. Additionally, S. apetala exerts allelopathic effects and displays strong interspecific competitiveness, further impeding the growth of native mangrove plants [11].
Derris trifoliata Lour., the fish vine, is another species of concern that has experienced significant spread and excessive growth in mangrove areas [12,13,14]. The harmful effects of D. trifoliata were predominantly noted in communities dominated by Aegiceras corniculatum (L.) Blanco. This invasive species ascends to the top of A. corniculatum, gradually forming dark green patches that spread from the contact points, leading to the withering, death, and collapse of the affected plant. The natural recovery of the disrupted mangrove ecosystem presents significant challenges [15]. D. trifoliata typically forms low-growing communities with heights not exceeding 1.2 m in open areas. Its branches, leaves, and flowers are frequently encrusted with mud, hindering seed formation and reproduction. While D. trifoliata inhabits open areas and coexists with native herbaceous plants like reeds and sedges, its spread is relatively gradual. Moreover, it typically undergoes leaf shedding between December and February, constraining its growth cycle.
This study aimed to investigate how the introduction and spread of S. apetala, a non-native species, have contributed to the overgrowth of D. trifoliata, a vine species, while uncovering the underlying ecological mechanisms. To achieve this objective, we conducted a detailed analysis of this specific interaction to elucidate general principles regarding the impact of invasive species on mangrove ecosystems. This research provides a scientific foundation for managing and conserving these ecosystems. Our primary hypothesis posits that the introduction and spread of Sonneratia apetala indirectly facilitate the overgrowth of D. trifoliata by altering the structure and function of the ecosystem. To test this hypothesis, we employed ecological methods and techniques, including field surveys, laboratory analyses, and model simulations, to thoroughly investigate the interactions between these two species and their impacts on the mangrove ecosystem. The contribution of this study is its novel insights into species interactions within mangrove ecosystems and theoretical and practical guidance for the formulation of targeted management strategies. Additionally, by examining the mutual influences of Sonneratia apetala and D. trifoliata, we provide new perspectives and approaches for protecting and restoring threatened mangrove ecosystems.
2. Materials and Methods
2.1. Distribution Survey of S. apetala and D. trifoliata
Multiple mangrove sites were selected in river estuaries, including the Lianzhou (109°6′3.59″, 21°33′2.52″), Qinzhou (108°34′12.00″, 21°44′19.32″), and Dandouhai Bays (109°39′7.20″, 21°34′22.44″), and the Dafeng (108°51′36.00″, 21°45′40.32″), Maoling (108°28′4.81″, 21°50′53.88″), and Beilun Rivers (108°5′9.59″, 21°31′52.68″), to include locations with varying degrees of S. apetala spread. The distribution of D. trifoliata and S. apetala in the study area was established through field surveys, which integrated ground reconnaissance with the use of drone aerial images and high-resolution satellite imagery. This approach enabled identifying the locations and extent of S. apetala and D. trifoliata distributions.
2.2. Comparison of Seed Quantity of D. trifoliata on Different Plants
In October 2023, several D. trifoliata communities within Lianzhou Bay were chosen for study. These included those found climbing on Cyperus malaccensis subsp., Phragmites australis (Cav.) Trin., A. corniculatum, Acanthus ilicifolius L., Kandelia obovata Sheue et al., Excoecaria agallocha L., as well as S. apetala and D. trifoliata growing on a sandy shore. Eight types of D. trifoliata communities were selected for the study, each with five 1 × 1 m plots. Representative D. trifoliata plants entwined in the crown were selected. The maximum stem diameter, height, and D. trifoliata fruit yield per unit area were recorded.
2.3. Survey of D. trifoliata Damage on S. apetala
The primary tributaries of the Nanliu River (109°4′26.39″, 21°36′3.24″) in Lianzhou Bay were selected for this survey. A combination of on-site observations and drone imagery was used to assess the extent of D. trifoliata damage to S. apetala along the banks of the river and record the coordinates of S. apetala and the extent of damage to S. apetala by D. trifoliata. The degree of damage to S. apetala was divided into three levels: serious damage (II) when D. trifoliata was distributed on the crown of S. apetala; slight damage (I) when D. trifoliata was distributed on the branches of S. apetala; and no D. trifoliata damage when D. trifoliata was not observed on the trunk.
2.4. MaxEnt Model
The MaxEnt model 3.4.1 (Columbia University in the United States, New York, NY, USA) with the Kuenm package optimization parameters [16] was used to predict the spatial distributions of D. trifoliata and S. apetala. The sources of environmental data (Table 1) and the selection of environmental factors (Table 2) in this study refer to the research of Li et al. [13]. This study obtained the parameters for the MaxEnt model using the Kuenm package. D. trifoliata had a feature class of threshold features (T), product features (T), and linear features (L). S. apetala had a feature class of quadratic (Q) and threshold features (T). D. trifoliata and S. apetala had regulation multipliers of 0.3 and 1.5, respectively.

Table 1.
Sources of environmental data.

Table 2.
Environmental variables used to predict the distributions of D. trifoliata and S. apetala in the Beibu Gulf, Guangxi, China.
The MaxEnt model was utilized to compute the receiver operating characteristic (ROC) curve, with the area under the curve (AUC) serving as the diagnostic test value. AUC values, ranging from 0 to 1, were indicative of prediction accuracy, with higher values indicating greater accuracy. Subsequently, the grid output results were visually converted and analyzed using ArcGIS 10.4 (Environmental Systems Research Institute, Redlands, CA, USA). Each grid’s pixel value denoted the probability distribution of mangroves within the grid, varying from 0 to 1. Larger pixel values indicated higher potential distributions and higher habitat suitability of D. trifoliata and S. apetala. In this study, we used the natural breakpoint method to grade the fitness results as follows: 0–0.2, no fitness; 0.2–0.5, low fitness; 0.5–0.7, medium fitness; and >0.7, high fitness [13,17].
2.5. Data Analysis
The ecological niche overlap between the two species was analyzed using ENMTools 1.3 (University of California, Los Angeles, CA, USA) [18], and the formula was calculated as follows:
where and are the geographical ecological niche overlap of species X and Y; and are the number of individuals utilizing resource i (i = 1, 2, …, n) [19]. The D and I values range from 0 to 1, with values closer to 1 indicating a higher ecological niche overlap between the two species.
3. Results and Analysis
3.1. Overgrowth of D. trifoliata and Spread of S. apetala after Introduction
The area covered by D. trifoliata in the mangroves of Leizhou Bay, Guangxi, showed significant variation over different years (Figure 1). In 2005, it covered 11.4 ha, increasing to 35.5 ha by 2015, with an annual growth rate of 12.03%. By 2019, the coverage had expanded rapidly to 206.8 ha, and since 2015, there has been a notable acceleration in the spread of D. trifoliata in Leizhou Bay, Guangxi, indicating an overgrowth trend.
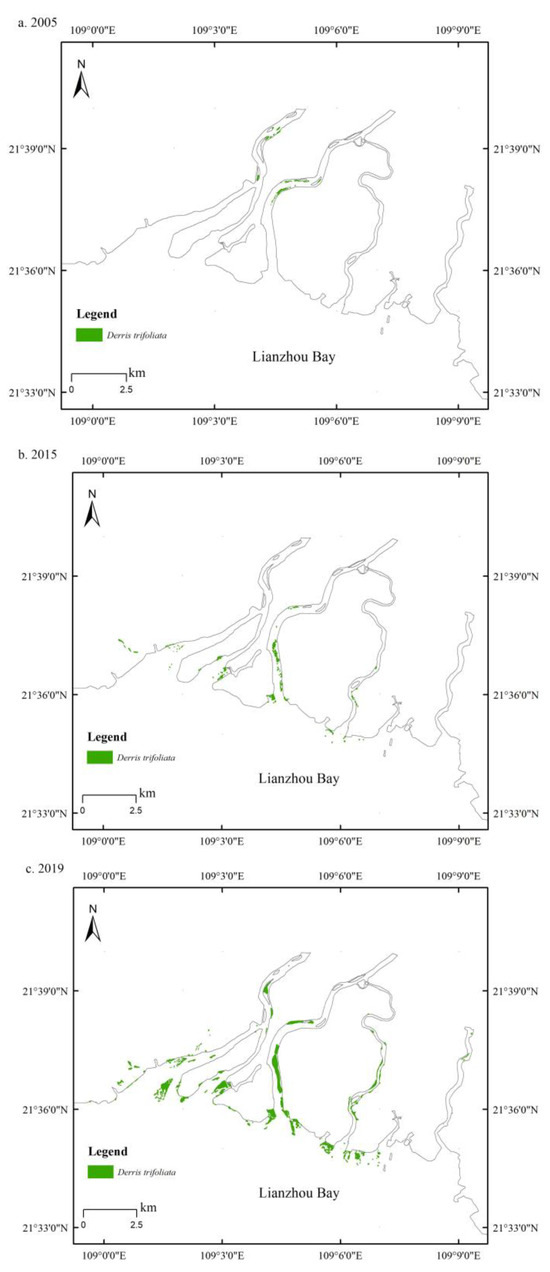
Figure 1.
Distribution of Derris trifoliata in Lianzhou Bay, Guangxi Province, in 2005, 2015, and 2019.
In 2004, S. apetala was first introduced in Leizhou Bay, Guangxi, China, with an initial area of approximately 0.7 ha. Despite the absence of large-scale artificial planting activities, S. apetala has rapidly spread through natural propagation in the estuarine areas of Leizhou Bay. The natural expansion of S. apetala plants was observed along all rivers entering the sea in Leizhou Bay. Preliminary statistics indicate that by 2023, the number of mature S. apetala plants will have reached no less than 2000, which is expected to continue increasing. This suggests rapid natural spread of S. apetala in the Leizhou Bay area of Guangxi following its introduction.
3.2. Long-Term Seed Production of D. trifoliata on S. apetala
Figure 1 shows that the area covered by D. trifoliata in the Leizhou Bay mangrove forest exhibited significant variation in different years. In 2005, the D. trifoliata area was 11.4 ha, which increased to 35.5 ha by 2015, with an average annual growth rate of 12.03%. Subsequently, in 2019, the D. trifoliata area sharply increased to 206.8 ha, with an extraordinarily high average annual growth rate of 55.36%. Thus, since 2015, the expansion of D. trifoliata in Leizhou Bay has accelerated significantly, manifesting an overgrowth trend.
Derris trifoliata exhibited variations in seed production and stem diameter in different plant species, including herbaceous, shrub, and tree species in the Lianzhou Bay mangrove forest (Table 3). From the herbaceous species to the shrubs and then the tree species, both the fruit production and stem diameter of D. trifoliata gradually increased (Table 1).

Table 3.
Comparative analysis of the damage and seed output of Derris trifoliata on different plants.
When D. trifoliata adhered to herbaceous plants such as C. malaccensis and P. australis, it produced no fruits and 1.6 fruits/m2, respectively. These herbaceous plants often wither and collapse during winter, and considering their limited structural support, they cannot withstand the extensive growth of D. trifoliata. This phenomenon frequently results in the collapse of both the herbaceous plants and D. trifoliata. Consequently, the growth and propagation of D. trifoliata on C. malaccensis and P. australis was limited.
On the other hand, when D. trifoliata adheres to shrubs such as A. corniculatum and Sonneratia caseolaris, it produces 1.50 and 10.5 seed/m2, respectively. This suggests that D. trifoliata can generate seeds for propagation when grown on supportive plants like A. corniculatum and S. caseolaris. However, shrubs, especially A. corniculatum, often die relatively quickly, causing D. trifoliata to lose its support structure and subsequently die or form low shrub-like patches. In such cases, D. trifoliata does not produce or generate few seeds. Thus, D. trifoliata experienced self-limitation when growing on native shrub mangrove plants.
When D. trifoliata clung to trees, such as K. obovata and E. agallocha, it produced 21.55 and 53.17 seed/m2, respectively. D. trifoliata has more space to grow and reproduce sexually when climbing trees than herbaceous and shrub vegetation do. K. obovata and E. agallocha are native plants with relatively few natural herbivores. This reduces the likelihood of D. trifoliata clinging to these plants, decreasing the opportunities for further expansion.
The fruit production of D. trifoliata on S. apetala (97.43 seed/m2) exceeded that of native plants (Table 1). D. trifoliata readily attaches to S. apetala, capitalizing on ample sunlight that fosters efficient photosynthesis, thereby boosting both the quantity and quality of seeds (see Figure 2). Consequently, the introduction and propagation of S. apetala promoted sexual reproduction in D. trifoliata, thereby aiding its external expansion. S. apetala, which bears fruit twice a year, yields a notable number of seeds and exhibits remarkable adaptability to the environment, thereby enhancing its dispersal capabilities [20]. These features enabled the rapid propagation of S. apetala in the estuarine areas of Leizhou Bay, providing favorable conditions for the growth of D. trifoliata. D. trifoliata has clung to S. apetala for over five years (Table 1), allowing for their long-term symbiosis and providing D. trifoliata with opportunities to produce seeds and expand through creeping stems.

Figure 2.
Seeds of Derris trifoliata growing on Sonneratia apetala.
In summary, the introduced species S. apetala was more conducive to D. trifoliata overgrowth than native mangrove plants.
3.3. Active Propagation of S. apetala and Its Rapid Growth Facilitate the Expansion of D. trifoliata
The introduction and propagation of S. apetala has led to its extensive distribution in estuarine areas, allowing it to preferentially occupy the growth zones of native plants, such as C. malaccensis and S. grossus (Figure 3). This provides an opportunity for D. trifoliata to thrive and expand beyond its original habitats. Additionally, the natural spread of S. apetala in estuarine regions has established pathways and corridors that facilitate the spread of D. trifoliata to new areas, promoting its overgrowth. With the extensive propagation of S. apetala, D. trifoliata plants can now climb into the crowns of S. apetala trees, providing them with additional growing space and reproductive opportunities, further facilitating their expansion.
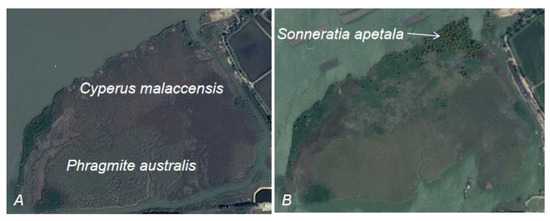
Figure 3.
Sonneratia apetala rapidly invades habitats of Cyperus malaccensis and Phragmites australis. ((A) native salt algae plants (B) Sonneratia apetala diffuses into native salt algae plants).
Sonneratia apetala, as an introduced fast-growing tree species, can grow up to 0.8–3.4 m annually [21]. In the Leizhou Bay area, the average height of S. apetala stands at 7.86 m, surpassing that of other native mangrove plants (see Table 1). Additionally, the maximum stem diameter of D. trifoliata clinging to S. apetala measures 28.4 mm, significantly larger than that of D. trifoliata on native plants (Table 1). The distribution of S. apetala and D. trifoliata along the Nanliu River is shown in Figure 4 and Table 4, where mature S. apetala with D. trifoliata displaying mild damage (I) reached 32.97%, and those with moderate damage (II) accounted for 27.33%. The percentage of young S. apetala with mild damage (I) was 14.43%, and no young S. apetala with moderate damage was observed. Surveys of S. apetala and D. trifoliata on both banks of the Nanliu River revealed that the rate of D. trifoliata attachment to mature S. apetala was 60.30%. The attachment rate for young S. apetala was 14.43%, resulting in a combined attachment rate of 68% for S. apetala on both banks of the Nanliu River.

Figure 4.
Sonneratia apetala covered with Derris trifoliata on the outer edge of a beach at Nanliu River in mid-October 2023.

Table 4.
Damage percentage of Sonneratia apetala.
Derris trifoliata effectively utilizes support structures for climbing, expanding its growth space alongside tall support trees (see Figure 5). As it ascends, D. trifoliata distances itself from periodically inundated and highly saline environments, favoring optimal growth conditions. This indicates that S. apetala provides a more conducive environment for D. trifoliata growth compared to other native mangrove plants. Simultaneously, the extensive propagation of S. apetala in the estuarine regions provided substantial support for the growth of D. trifoliata (Figure 4). D. trifoliata uses S. apetala as climbing structures to grow and reproduce abundantly, resulting in a rapid increase in the number of D. trifoliata plants and, consequently, overgrowth. This phenomenon strongly supports the hypothesis that the introduction and propagation of S. apetala promotes the overgrowth of D. trifoliata.
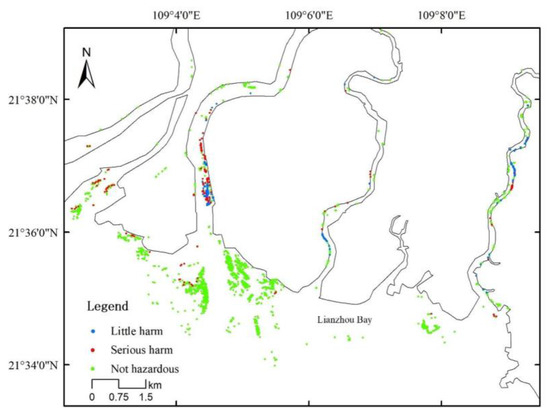
Figure 5.
Sonneratia apetala damaged by Derris trifoliata in Lianzhou Bay in mid-October 2023.
Sonneratia apetala relies on hydrodynamics for dispersal [22], typically spreading to locations along estuaries, coastlines, and water edges where its expansion is more susceptible to the influence of tides and water currents (Figure 4). Moreover, these aquatic edges allow the waterborne dispersal of D. trifoliata seeds due to the transportation of seeds to distant areas by tides and water currents, facilitating their rapid spread to other locations.
Synchronized growth between S. apetala and D. trifoliata also contributes to their cooperative dissemination. The fruit maturation period of S. apetala aligns with that of D. trifoliata owing to their shared tidal cycle. This synchrony facilitates their joint spread, fostering interactions.
In summary, the introduction and propagation of S. apetala have enhanced the growth of D. trifoliata. This includes increasing the likelihood of D. trifoliata attachment, boosting seed quantity per unit area, and resulting in significant variations in D. trifoliata stem diameters among different tree species. This improvement allows D. trifoliata to grow and reproduce under improved conditions while promoting interactions and ecological balance among species within the mangrove ecosystem. This is of considerable significance for understanding the complex interactions between introduced and native species and for the conservation and management of mangrove ecosystems.
3.4. High Overlap in the Suitable Areas for D. trifoliata and S. apetala
This study unveiled a correlation between a higher abundance of D. trifoliata and an increased number of S. apetala individuals, with D. trifoliata commonly entwining around S. apetala trees. In regions like Guangxi Lianzhou and Qinzhou Bays, as well as the Dandouhai, Maoling, and Fengjia Rivers, severe infestations of D. trifoliata were observed, indicating its detrimental impact on the stability of these ecosystems. However, in these areas, we observed the natural expansion of S. apetala, providing strong support for the excessive growth of D. trifoliata. In these regions, S. apetala naturally expands over a wide range, and the habitats suitable for S. apetala and D. trifoliata exhibit significant overlap. For example, S. apetala has spread naturally over a wide area in Lianzhou Bay. S. apetala both heavily and mildly infested with D. trifoliata were observed in this region (Figure 5).
The Maximum Entropy Model was employed to predict the distribution of D. trifoliata and S. apetala. The AUC values for S. apetala in both the training and test sets were 0.973 and 0.968, while for D. trifoliata, the corresponding AUC values were 0.979 and 0.978, respectively. Thus, the model demonstrated high reliability. The optimal suitable habitat area for S. apetala was 1800 hm2 (Figure 6 and Table 5), and for D. trifoliata, it was 3000 hm2 (Figure 7 and Table 5). Regions with a high degree of suitability for both species were mainly located in Lianzhou Bay, the Guangxi Shankou Mangrove Nature Reserve, and the Guangxi Maowei Sea Provincial Mangrove Nature Reserve, showing similar spatial suitability. Using ENMTools 1.3 software to analyze the ecological niche overlap between D. trifoliata and S. apetala revealed that the ecological overlap value was 0.7559, the geographical distribution overlap (I) was 0.7988, and the D value was 0.5955. These findings indicate a significant habitat similarity between the two species. These results further confirm the observed interaction between D. trifoliata and S. apetala, where S. apetala provided a growth structure that promoted the growth of D. trifoliata.
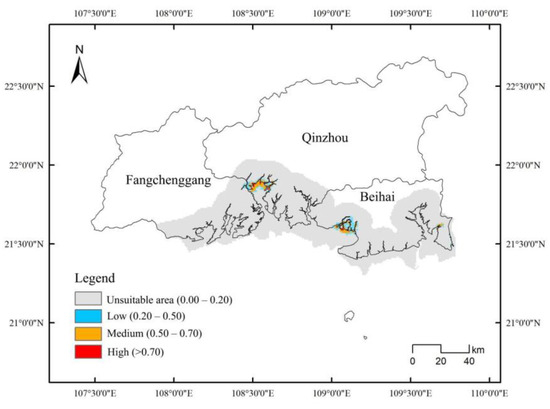
Figure 6.
Predicted distribution map of Sonneratia apetala.

Table 5.
Suitable area for D. trifoliata and S. apetala.
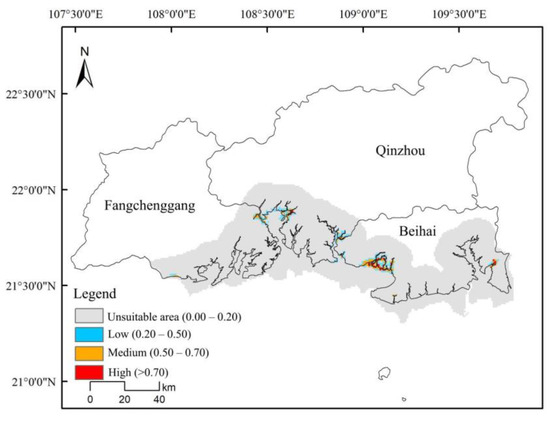
Figure 7.
Predicted distribution map of Derris trifoliata.
These spatial distribution results further support our hypothesis that a strong association exists between the introduction and spread of S. apetala and D. trifoliata overgrowth, emphasizing the importance of their mutually symbiotic relationship within the mangrove ecosystem.
Through observing and analyzing their spatial distribution, we have provided compelling evidence that enhances the understanding of the complex ecological interactions between these two species. These findings have significant implications for mangroves and other ecological conservation.
4. Discussion
This study explored the impact of the introduction of Sonneratia apetala on the overgrowth of D. trifoliata, revealing the profound effects of their interaction on mangrove ecosystems. Our results indicate a significant correlation between the introduction of Sonneratia apetala and the overgrowth of D. trifoliata. This finding aligns with ecological theories suggesting that introducing invasive species can alter species interactions and competitive dynamics within ecosystems, thereby influencing their structure and function [23,24].
While some scholars believe that S. apetala has increased the fixation rate of N2, which is significant for the sustainable management and restoration of mangrove ecosystems [24], the negative impact of S. apetala has not been fully considered. Our results provide insights into the potential adverse effects of invasive species introduction on mangrove ecosystems, contrasting with findings in the literature [25]. Existing research mainly focuses on the ecological impacts of a single invasive species [26], yet our study offers a comprehensive perspective on the interactions between two species, thereby expanding our understanding of ecosystem impacts.
These findings offer strong support for existing theories, particularly regarding the impact of invasive species introduction on ecosystem health [9]. The rapid spread of Sonneratia apetala provides additional space for the survival and reproduction of D. trifoliata, promoting its overgrowth. This interaction may exacerbate the degradation of native mangrove ecosystems, affecting the habitat and ecological services they provide to native species [27].
Our results not only deepen the understanding of species interactions within mangrove ecosystems but also offer practical guidance for managers in developing effective ecosystem management and conservation strategies. By understanding the relationship between the introduction of S. apetala and the overgrowth of D. trifoliata, targeted measures can be implemented to control or mitigate the spread of these species, protecting and restoring mangrove ecosystems.
While this study offers valuable insights, it also has limitations. For instance, it focuses on species interactions within a specific region, which may not fully represent situations in different areas. For example, in areas lacking rivers, certain dynamics may not be as apparent [28]. Additionally, owing to the limited duration of this study, we may not have captured changes in long-term ecological processes, such as mudflat elevation [29], soil nutrient accumulation [30,31,32,33], allelopathy [34], light impact [35,36], adaptability to salinity [37,38,39], natural enemy factors [40,41], gene differentiation [42], etc. Hence, further research should encompass a broader geographical scope and longer time scales to obtain a more comprehensive understanding.
Moreover, future research should incorporate additional ecological factors, such as pollution [43], biological characteristics of species [32], and external disturbances, such as climate change [39] and human activities [44], to assess their combined effects on ecosystems. Furthermore, long-term monitoring and interdisciplinary approaches are crucial for comprehending and managing the interactions between invasive and native species in mangrove ecosystems.
In summary, our study underscores the importance of understanding and managing species interactions in mangrove ecosystems, especially in the context of invasive species introduction. By examining the interactions between S. apetala and D. trifoliata, we enhanced the understanding of the function and health of this specific ecosystem and provided practical insights and strategies for ecosystem management to overcome the challenges posed by introducing invasive species.
5. Conclusions
Based on our findings, we examined the relationship and underlying mechanisms between the introduction and spread of S. apetala and the proliferation of D. trifoliata. In the mangrove forests of Liangzhou Bay, Guangxi, the area covered by D. trifoliata experienced a notable annual average growth rate of 12.03% from 2005 to 2015. Subsequently, from 2015 to 2019, this rate surged to 55.36%, indicating a pronounced phase of overgrowth. This suggests a significant acceleration in the spread of D. trifoliata after 2015.
We also observed that D. trifoliata rarely produces seeds when climbing on native plants, such as C. malaccensis and P. australis. By contrast, on native mangrove plants, such as S. apetala, the seed density of D. trifoliata reached 97.43 seeds/m2. This study further revealed that 68% of the S. apetala trees were affected by D. trifoliata, and the natural spread of S. apetala was observed in several regions, including Liangzhou Bay, Guangxi. The analysis indicated a high ecological and geographic distribution overlap between D. trifoliata and S. apetala, highlighting the substantial spatial similarity in their habitats.
This study also revealed that the tall S. apetala provides more climbing space for D. trifoliata, photosynthesis conditions, a stable support structure, and more convenient pathways for dispersion, increasing the duration for which D. trifoliata grow on them, increasing seed production, and promoting dispersion. A unique finding of this study is the identification of a neglected phenomenon, where D. trifoliata wraps around S. apetala and stably produces many seeds over a long period, the dispersion of which promotes the excessive growth of D. trifoliata. However, because S. apetala is relatively tall, D. trifoliata generally does not cause its death; thus, the control of D. trifoliata on S. apetala is often overlooked. Additionally, a synergistic dispersion phenomenon between the seeds of D. trifoliata and S. apetala was discovered, indicating that the seeds of both often disperse together to new areas. This study provides important insights into the complex interactions between introduced and local species in mangrove ecosystems, offering scientific value for the management and conservation of mangrove ecosystems.
Author Contributions
Conceptualization, W.L. and L.L.; methodology, W.L.; software, L.L.; validation, H.W., W.J. and Q.L.; formal analysis, H.W. and Y.X.; investigation, W.L., Y.X. and Y.T.; resources, W.L.; data curation, L.L.; writing—original draft preparation, W.L.; writing—review and editing, L.L.; visualization, Q.L.; supervision, L.L. and W.J.; project administration, W.L.; funding acquisition, W.L. and W.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China [grant number U21A2022 and 32060282], the Open Research Fund Program of Guangxi Key Lab of Mangrove Conservation and Utilization [grant number GKLMC-20A06], and Guangxi Science and Technology Program (GuiKeAD22080040).
Data Availability Statement
No data were used for the research described in the article.
Acknowledgments
We gratefully acknowledge the support of Guangxi Key Lab of Mangrove Conservation. We gratefully acknowledge associate researcher Lianghao Pan for identifying the name of Phragmites australis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Biswas, S.R.; Biswas, P.L.; Limon, S.H.; Yan, E.R.; Xu, M.S.; Khan, M.S.I. Plant invasion in mangrove forests worldwide. For. Ecol. Manag. 2018, 429, 480–492. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, K.M. Overview and trends in research on biological invasion in mangrove ecosystems. Acta Pharmacol. Sin. 2015, 39, 283–299. [Google Scholar] [CrossRef]
- Pan, L.H.; Shi, X.F.; Zeng, C.; Chen, Y.S. Plant types in mangroves of Guangxi. Guangxi Sci. 2018, 25, 352–362. [Google Scholar] [CrossRef]
- Wu, D.Q. Research on the spread status of Sonneratia apetala in the Zhangjiangkou national mangrove nature Reserve. For. Prot. Sci. Technol. 2016, 33–35. [Google Scholar] [CrossRef]
- Zhao, C.; Qin, C.Z.; Wang, Z.; Mao, D.; Wang, Y.; Jia, M. Decision surface optimization in mapping exotic mangrove species (Sonneratia apetala) across latitudinal coastal areas of China. ISPRS J. Photogramm. 2022, 193, 269–283. [Google Scholar] [CrossRef]
- Knauf, A.E.; Litton, C.M.; Cole, R.J.; Sparks, J.P.; Giardina, C.P.; Gerow, K.G.; Quiones-Santiago, M. Nutrient use strategy and not competition determines native and invasive species response to changes in soil nutrient availability. Restor. Ecol. 2021, 29, e13374. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liao, B.W.; Zheng, S.F.; Li, M.; Song, X.Y. Dynamic Changes and Species Diversity of the Mangrove Community with Excoecaria agallocha, Kandelia candel, and Avicennia marina. Chin. J. Appl. Ecol. 2004, 15, 924–928. [Google Scholar]
- Huang, Z.J.; Li, Z.; Wang, M.H.; Zhu, D.H.; Yang, Q.; Yu, S.X. Coastal nutrient enrichments facilitated reproductive output in exotic mangrove species over two decades. Front. For. Glob. Chang. 2023, 6, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Q.; Peng, Y.; Pan, L.; Chen, Y.; Zhang, Y.; Chen, L. Distributions of the non-native mangrove Sonneratia apetala in China: Based on google earth imagery and field survey. Wetlands 2022, 42, 35. [Google Scholar] [CrossRef]
- Hu, Z.W.; Wu, J.J.; Wang, J.Z.; Zhang, Y.H.; Zhou, H.C.; Gao, C.J.; Wang, J.J.; Wu, G.F. How exotic Sonneratia species affect the spatiotemporal dynamics of mangroves in Shenzhen Bay, China: A remote sensing perspective. Ecol. Indic. 2023, 153, 110479. [Google Scholar] [CrossRef]
- Chen, G.G.; Li, Y.Y.; Cai, L.Q.; Xu, J.J.; Lin, P.R.; Chen, W.C.; Huang, S.L. Influence of the exotic mangrove species Excoecaria agallocha on the morphological characteristics and biomass of the indigenous species Avicennia marina. Oceanol. Limnol. Sin. 2017, 41, 26–33. [Google Scholar]
- Cao, H.; Liu, S.H.; Liu, S.Y. Ecological health assessment and protection strategies of mangrove ecosystem in Huachang Bay, Hainan. S. China For. Investig. Plan. 2020, 39, 16–19+28. [Google Scholar] [CrossRef]
- Li, L.F.; Liu, W.I.; Ai, J.W.; Cai, S.J.; Dong, J.W. Predicting Mangrove Distributions in the Beibu Gulf, Guangxi, China, Using the MaxEnt Model: Determining Tree Species Selection. Forests 2023, 14, 149. [Google Scholar] [CrossRef]
- Li, L.F.; Lin, J. Distribution and control suggestions of Excoecaria agallocha in Dongzhaigang national nature Reserve, Hainan. Trop. For. 2019, 47, 36–38. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhong, C.; Chen, S.Y.; Liu, Y.; Liang, S.C. Harm and management of Fishvine (Kandelia candel) on Mangrove Plants. Wetl. Sci. Manag. 2015, 11, 26–29. [Google Scholar]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Chao, B.X.; Hu, W.J.; Chen, B.; Zhang, D.; Chen, G.C.; Yu, W.W.; Ma, Z.Y.; Lei, G.C.; Wang, Y.Y. Potential suitable habitat of mangroves and conservation gap analysis in Guangdong Province with MaxEnt Modeling. Chin. J. Ecol. 2020, 39, 3785–3794. [Google Scholar] [CrossRef]
- Wang, W.T.; Gao, S.Y.; Wang, S.F. Predictive Study on Potential Invasion Areas of Four Toxic Weeds in Gansu Grasslands. Acta Ecol. Sin. 2019, 39, 5301–5307. [Google Scholar] [CrossRef]
- Peng, Y.G.; Xu, Z.C.; Liu, M.C. Introduction and Ecological Impact of the Exotic Mangrove Plant Sonneratia apetala. Acta Ecol. Sin. 2012, 32, 2259–2270. [Google Scholar] [CrossRef]
- Wu, Z.H.; Cai, J.X.; Ye, Q.H. Analysis of the introduction and promotion effect of Sonneratia apetala. Guangdong For. Sci. Technol. 2000, 16, 6–10. [Google Scholar]
- Hong, P.; Wen, Y.; Xiong, Y.; Diao, L.; Gu, X.; Feng, H.; Yang, C.; Chen, L. Latitudinal gradients and climatic controls on reproduction and dispersal of the non-native mangrove Sonneratia apetala in China. Estuar. Coast. Shelf Sci. 2021, 248, 106749. [Google Scholar] [CrossRef]
- Powell, K.I.; Chase, J.M.; Knight, T.M. Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science 2013, 339, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Kumar, D.; Singh, R.K.; Gupta, A.K.; Premkumar, K.; Kewat, A.K. Investigating the phenology and interactions of competitive plant species co-occurring with invasive Lantana camara in Indian Himalayan Region. Sci. Rep. 2024, 14, 400. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Yang, Q.S.; Feng, J.X.; Yang, Z.H.; Yu, C.X.; Zhang, J.; Ling, J.; Dong, J.D. Introduction of exotic species Sonneratia apetala alters diazotrophic community and stimulates nitrogen fixation in mangrove sediments. Ecol. Indic. 2022, 142, 109179. [Google Scholar] [CrossRef]
- Ren, H.; Lu, H.F.; Shen, W.J.; Huang, C.; Guo, Q.F.; Li, Z.A.; Jian, S.G. Sonneratia apetala Buch. Ham in the mangrove ecosystems of China: An invasive species or restoration species? Ecol. Eng. 2009, 35, 1243–1248. [Google Scholar] [CrossRef]
- Eysink, G.G.J.; Hatamura, E.; Schaffer-Novelli, Y. First occurrence in mangroves of South America of the exotic species Sonneratia apetala Buch.-Ham. from the Indo-Malayan region. Biota Neotrop. 2023, 23, e20231575. [Google Scholar] [CrossRef]
- Lu, W.Z.; Yang, S.C.; Chen, L.Z.; Wang, W.Q.; Du, X.N.; Wang, C.M.; Ma, Y.; Lin, G.X.; Lin, G.H. Changes in carbon pool and stand structure of a native subtropical mangrove forest after inter-planting with exotic species Sonneratia apetala. PLoS ONE 2014, 9, e91238. [Google Scholar] [CrossRef]
- Xin, K.; Zhou, Q.; Arndt, S.K.; Yang, X. Invasive Capacity of the Mangrove Sonneratia apetala in Hainan Island, China. J. Trop. For. Sci. 2013, 25, 70–78. [Google Scholar]
- Huang, X.F.; Feng, J.X.; Yang, Q.S.; Chen, L.X.; Zhang, J.; Yang, B.; Tang, X.Y.; Yu, C.X.; Ling, J.; Dong, J.D. High site elevation enhanced nitrogen fixation and the stability of diazotrophic community in planted Sonneratia apetala mangrove sediments. Appl. Soil Ecol. 2023, 191, 105059. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, K.; Liao, B.W.; Sheng, N.; Ai, X.H. The characteristics of pods and seeds of liana species Derris trifoliata and their relationship with environmental factors in Guangdong, China. Ecol. Indic. 2021, 129, 107930. [Google Scholar] [CrossRef]
- Xu, Y.W.; Liao, B.W.; Jiang, Z.M.; Xin, K.; Xiong, Y.M.; Zhang, Y. Examining the Differences between Invasive Sonneratia apetala and Native Kandelia obovata for Mangrove Restoration: Soil Organic Carbon, Nitrogen, and Phosphorus Content and Pools. J. Coast. Res. 2021, 37, 708–715. [Google Scholar] [CrossRef]
- Teng, Z.Z.; Lin, X.B. Sediment nitrates reduction processes affected by non-native Sonneratia apetala plantation in South China. Sci. Total Environ. 2024, 906, 167523. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.X.; Feng, J.X.; Liu, K.; Wang, G.; Zhu, Y.H.; Cheng, H.; Guan, D.S. Changes of ecosystem carbon stock following the plantation of exotic mangrove Sonneratia apetala in Qi’ao Island, China. Sci. Total Environ. 2020, 717, 137142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, F.P.; Yang, Y.; Li, J.W.; Zhang, J.W.; Zhang, S.J.; Bai, H.; Liu, Q.; Zhong, C.R.; Li, L. Allelopathic effects of leachates from two alien mangrove species, Sonneratia apetala and laguncularia racemosa on seed germination, seedling growth and antioxidative activity of native mangrove species sonneratia caseolaris. Allelopath. J. 2018, 44, 119–129. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Guan, W.; Xiong, Y.M.; Li, M.; Chen, Y.J.; Liao, B.W. Interactive Effects of Intertidal Elevation and Light Level on Early Growth of Five Mangrove Species under Sonneratia apetala Buch. Hamplantation Canopy: Turning Monocultures to Mixed Forests. Forests 2019, 10, 83. [Google Scholar] [CrossRef]
- Zhu, D.H.; Hui, D.F.; Huang, Z.J.; Qiao, X.T.; Tong, S.; Wang, M.Q.; Yang, Q.; Yu, S.X. Comparative impact of light and neighbor effect on the growth of introduced species Sonneratia apetala and native mangrove species in China: Implications for restoration. Restor. Ecol. 2022, 30, e13522. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, T.T.; Yang, Z.Y.; Yang, S.X.; Chen, J.H. PacBio Full-Length Transcriptome Sequencing Reveals the Mechanism of Salt Stress Response in Sonneratia apetala. Plants 2023, 12, 3849. [Google Scholar] [CrossRef]
- Nasrin, S.; Hossain, M.; Rahman, M.M. Adaptive responses to salinity: Nutrient resorption efficiency of Sonneratia apetala (Buch.-Ham.) along the salinity gradient in the Sundarbans of Bangladesh. Wetl. Ecol. Manag. 2019, 27, 343–351. [Google Scholar] [CrossRef]
- Rahman, M.S.; Sass-Klaassen, U.; Zuidema, P.A.; Chowdhury, M.Q.; Beeckman, H. Salinity drives growth dynamics of the mangrove tree Sonneratia apetala Buch. -Ham. in the Sundarbans, Bangladesh. Dendrochronologia 2020, 62, 125711. [Google Scholar] [CrossRef]
- Simlai, A.; Gangwar, A.; Sarthaki, G.; Roy, A. Antimicrobial and Antioxidative Activities in the Stem Extracts of Derris trifoliata, a Mangrove Shrub. J. Pharm. Res. Int. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Hardy, G.E.S.; Le, T.V.; Nguyen, H.Q.; Le, D.H.; Nguyen, T.V.; Dell, B. Mangrove Dieback and Leaf Disease in Sonneratia apetala and Sonneratia caseolaris in Vietnam. Forests 2021, 12, 1273. [Google Scholar] [CrossRef]
- Wu, B.; Geng, S.L.; Shu, B. Genetic variation and the conservation of isolated populations of Derris trifoliata (Leguminosae), a mangrove-associated vine, in southern China. Biochem. Syst. Ecol. 2012, 40, 118–125. [Google Scholar] [CrossRef]
- Li, R.L.; Chai, M.W.; Li, R.Y.; Xu, H.L.; He, B.; Qiu, G.Y. Influence of introduced Sonneratia apetala on nutrients and heavy metals in intertidal sediments, South China. Environ. Sci. Pollut. Res. 2017, 24, 2914–2927. [Google Scholar] [CrossRef]
- Yang, X.L.; Hu, C.Y.; Wang, B.; Lin, H.; Xu, Y.P.; Guo, H.; Liu, G.Z.; Ye, J.Q.; Gao, D.Z. Sediment nitrogen mineralization and immobilization affected by non-native Sonneratia apetala plantation in an intertidal wetland of South China. Environ. Pollut. 2022, 305, 119289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).