Genetic Diversity and Structure of Higher-Resin Trees of Pinus oocarpa Schiede in Mexico: Implications for Genetic Improvement
Abstract
1. Introduction
2. Materials and Methods
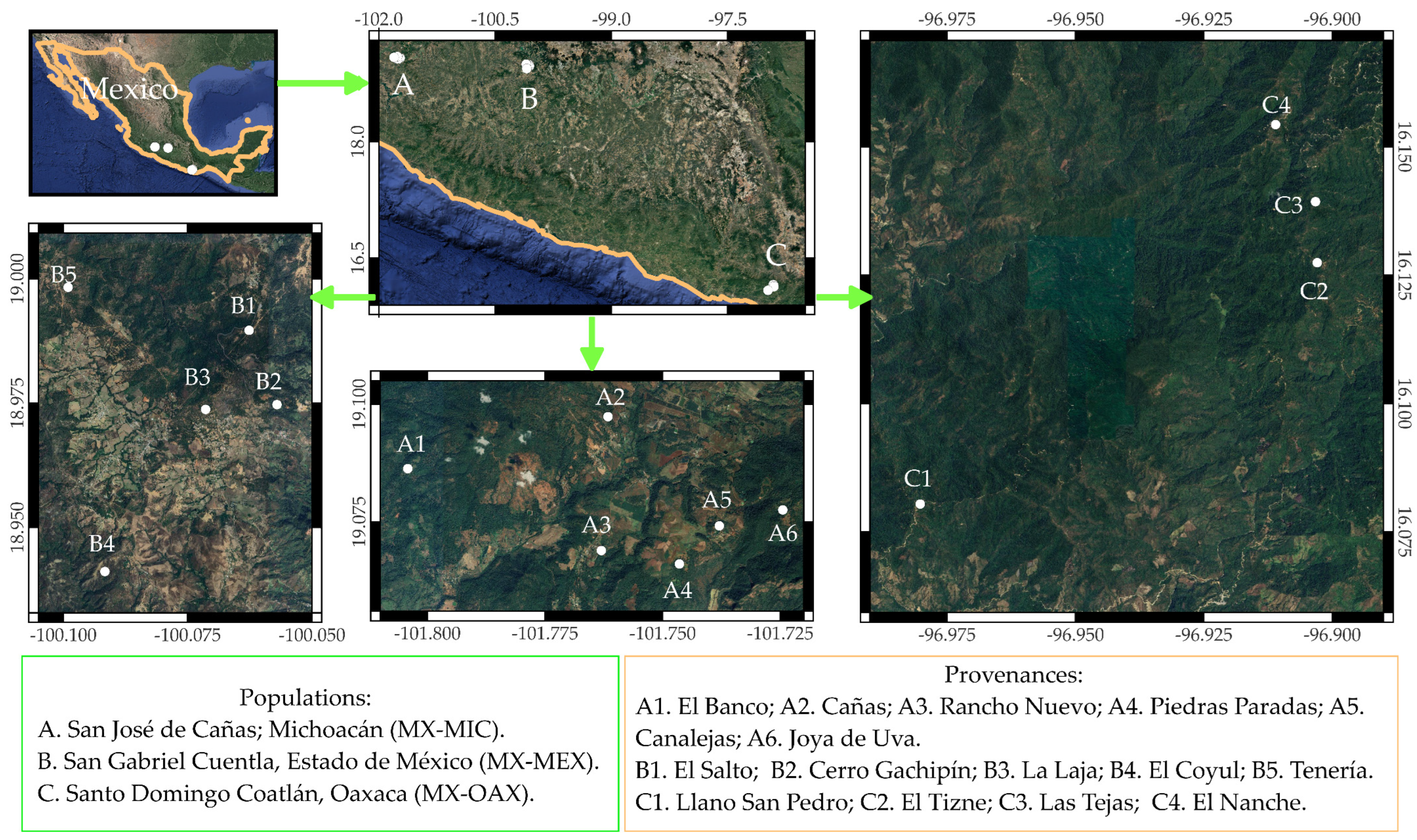
2.1. Selection of Superior Trees
2.2. Collection of Biological Material
2.3. DNA Extraction
2.4. Microsatellite Amplification and Fragment Analysis
2.5. Data Analysis
3. Results
3.1. Genetic Diversity and Allelic Variability
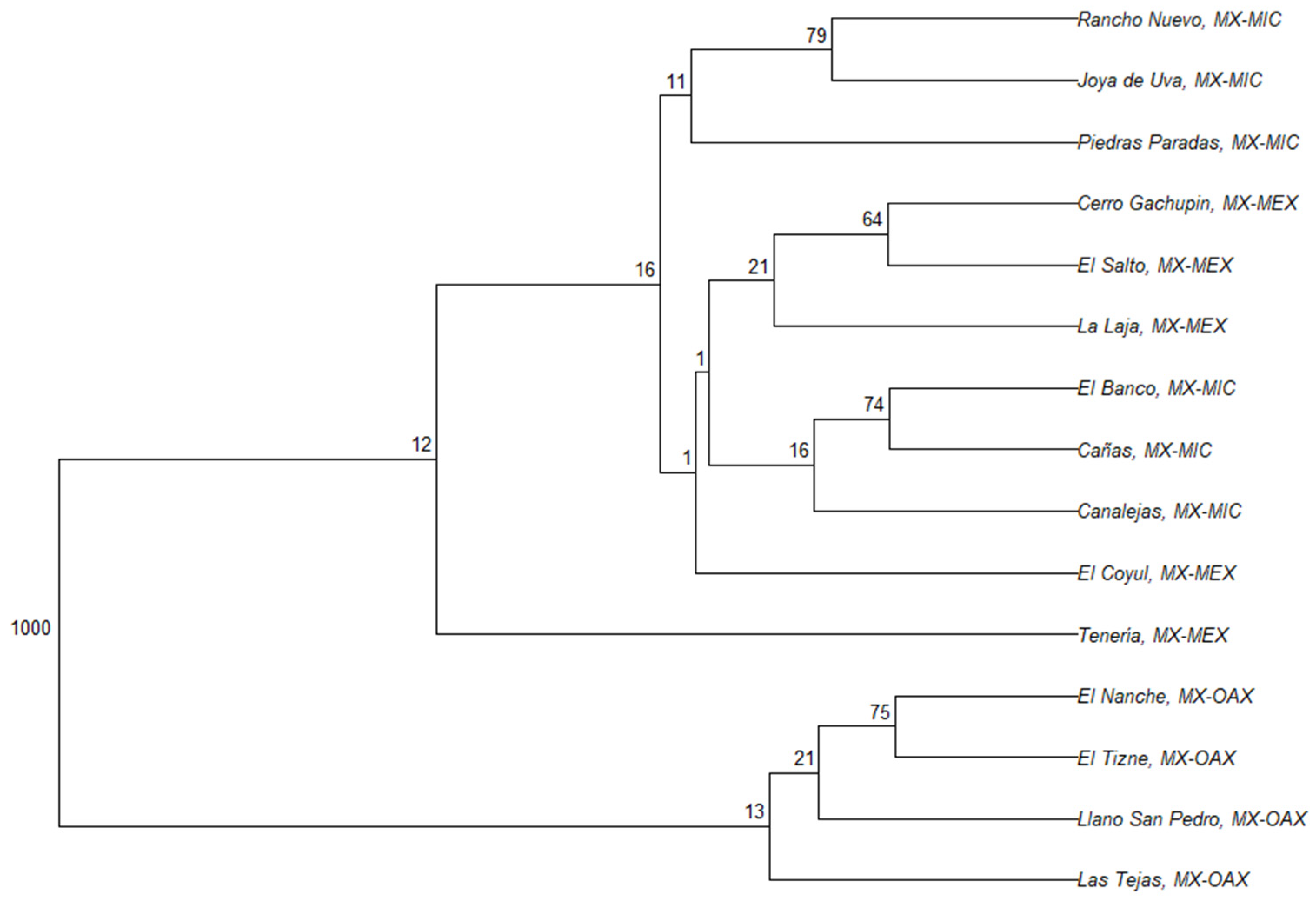
3.2. Genetic Structure and Differentiation
4. Discussion
4.1. Genetic Diversity
4.2. Genetic Structure
4.3. Implications for Genetic Improvement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, M.Y.; Son, S.; Herrando-Moraira, S.; Tang, C.Q.; Maki, M.; Kim, Y.-D.; López-Pujolj, J.; Hamrick, J.L.; Chung, M.G. Incorporating Differences Between Genetic Diversity of Trees and Herbaceous Plants in Conservation Strategies. Conserv. Biol. 2020, 34, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Kahilainen, A.; Puurtinen, M.; Kotiaho, J.S. Conservation Implications of Species-Genetic Diversity Correlations. Glob. Ecol. Conserv. 2014, 2, 315–323. [Google Scholar] [CrossRef]
- Kremer, A.; Ronce, O.; Robledo-Arnuncio, J.J.; Guillaume, F.; Bohrer, G.; Nathan, R.; Bridle, J.R.; Gomulkiewicz, R.; Klein, E.K.; Ritland, K.; et al. Long-Distance gene Flow and Adaptation of forest Trees to Rapid Climate Change. Ecol. Lett. 2012, 15, 378–392. [Google Scholar] [CrossRef]
- Wehenkel, C.; Mariscal-Lucero, S.R.; Jaramillo-Correa, J.P.; López-Sánchez, C.A.; Vargas-Hernández, J.J.; Sáenz-Romero, C. Genetic Diversity and Conservation of Mexican Forest Trees. In Biodiversity and Conservation of Woody Plants; Ahuja, M., Jain, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 17, pp. 37–67. [Google Scholar] [CrossRef]
- Ledig, F.T. Human Impacts on Genetic Diversity in Forest Ecosystems. Oikos 1992, 63, 87–108. [Google Scholar] [CrossRef]
- Fabbio, G.; Merlo, M.; Tosi, V. Silvicultural Management in Maintaining Biodiversity and Resistance of Forests in Europe—The Mediterranean Region. J. Environ. Manag. 2003, 67, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Finkeldey, R.; Ziehe, M. Genetic Implications of Silvicultural Regimes. For. Ecol. Manag. 2004, 197, 231–244. [Google Scholar] [CrossRef]
- Buiteveld, J.; Vendramin, G.G.; Leonardi, S.; Kamer, K.; Geburek, T. Genetic Diversity and Differentiation in European Beech (Fagus sylvatica L.) Stands Varying in Management History. For. Ecol. Manag. 2007, 247, 98–106. [Google Scholar] [CrossRef]
- Ratnam, W.; Rajora, O.P.; Finkeldey, R.; Aravanopoulos, F.; Bouvet, J.M.; Vaillancourt, R.E.; Kanashiro, M.; Fady, B.; Tomita, M.; Vinson, C. Genetic Effects of Forest Management Practices: Global Synthesis and Perspectives. For. Ecol. Manag. 2014, 333, 52–65. [Google Scholar] [CrossRef]
- Hubert, J.; Lee, S. A review of the Relative Roles of Silviculture and Tree Breeding in Tree Improvement: The Example of Sitka Spruce in Britain and Possible Lessons for Hardwood Breeding. Forestry 2005, 78, 109–120. [Google Scholar] [CrossRef][Green Version]
- White, T.L.; Adams, W.T.; Neale, D.B. Forest Genetics; CABI Publishing: Cambrige, MA, USA, 2007; p. 682. Available online: https://www.cabidigitallibrary.org/doi/book/10.1079/9781845932855.0000 (accessed on 15 November 2024).
- Kang, K.S.; Lindgren, D.; Mullin, T.J. Prediction of Genetic Gain and Gene Diversity in Seed Orchard Crops Under Alternative Management Strategies. Theor. Appl. Genet. 2001, 103, 1099–1107. [Google Scholar] [CrossRef]
- Kang, K.S.; El-Kassaby, Y.A.; Choi, W.Y.; Han, S.U.; Kim, C.S. Genetic Gain and Diversity Caused by Genetic Thinning in a Clonal Seed Orchard of Pinus densiflora. Silvae Genet. 2003, 52, 220–223. Available online: https://www.thuenen.de/media/institute/fg/PDF/Silvae_Genetica/2003/Vol._52_Heft_5-6/52_5-6_220.pdf (accessed on 15 November 2024).
- Zobel, B.J.; Talbert, J.T. Applied Forest Tree Improvement; John Wiley & Sons, Inc.: New York, NY, USA, 1984; 505p. [Google Scholar]
- Méndez-Neri, M.; Ramírez-Herrera, C.; Vargas-Hernández, J.J.; Martínez-Trinidad, T.; López-Upton, J.; López, P.A. Diversidad Genética en Dos Huertos Semilleros de Pinus patula Schiede ex SchltdL. et Cham. Rev. Fitotec. Mex. 2020, 43, 113–119. [Google Scholar] [CrossRef]
- Mendoza-Hernández, N.B. Diversidad Genética de Un Huerto Semillero Sexual de Pinus patula. Master’s Thesis, Colegio de Postgraduados, Texcoco, Mexico, 2016. Available online: http://colposdigital.colpos.mx:8080/xmlui/handle/10521/3507 (accessed on 15 November 2024).
- Farjon, A.; Styles, B.T. Pinus (Pinaceae). Flora Neotropical Monograph 75; The New York Botanical Gardens: New York, NY, USA, 1997. [Google Scholar]
- Dvorak, W.S.; Potter, K.M.; Hipkins, V.D.; Hodge, G.R. Genetic Diversity and Gene Exchange in Pinus oocarpa, a Mesoamerican Pine with Resistance to the Pitch Canker Fungus (Fusarium circinatum). Int. J. Plant Sci. 2009, 170, 609–626. [Google Scholar] [CrossRef]
- SEMARNAT. Anuario Estadístico de la Producción Forestal 2018; SEMARNAT: Ciudad de México, Mexico, 2021; pp. 139–146. Available online: https://www.gob.mx/semarnat/documentos/anuarios-estadisticos-forestales (accessed on 19 December 2024).
- Quiroz Carranza, J.A.; Magaña Alejandro, M.A. Natural resins of Mexican Plant Species: Current and Potential End–Uses. Madera Bosques 2015, 21, 171–183. [Google Scholar] [CrossRef][Green Version]
- Gallo Corredor, J.A.; Sarria Villa, R.A.; Palta Angulo, J.C. Comparación de La Producción Resinera de Dos Especies de Pino Cultivadas en el Municipio de Cajibla, Departamento del Cauca, Colombia. J. Cienc. Ing. 2012, 4, 37–42. Available online: https://jci.uniautonoma.edu.co/2012/2012-6.pdf (accessed on 15 November 2024).
- CONAFOR. La Producción de Resina de Pino en México, 1st ed.; CONAFOR: Guadalajara, Mexico, 2013; p. 93. Available online: http://www.conafor.gob.mx:8080/documentos/docs/43/6046La%20producci%C3%B3n%20de%20resina%20de%20pino%20en%20M%C3%A9xico.pdf (accessed on 19 December 2024).
- Fabián-Plesníková, I. Estudio de Caracteres que Intervienen en la Producción de Resina y su Posible Control Genético en Pinus oocarpa. Ph.D. Thesis, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mexico, 2020. Available online: http://bibliotecavirtual.dgb.umich.mx:8083/xmlui/handle/DGB_UMICH/3741 (accessed on 15 December 2023).
- López-Álvarez, Ó.; Zas, R.; Marey-Perez, M. Resin tapping: A review of the main factors modulating pine resin yield. Ind. Crops Prod. 2023, 202, 117105. [Google Scholar] [CrossRef]
- Reyes-Ramos, A.; Cruz de León, J.; Martínez-Palacios, A.; Lobit, P.C.M.; Ambríz-Parra, J.E.; Sánchez-Vargas, N.M. Ecological and Dendrometric Characters in Which Influence Resin Production of Pinus oocarpa of Michoacán, Mexico. Madera Bosques 2019, 25, e2511414. [Google Scholar] [CrossRef]
- Fabián-Plesníková, I.; Sáenz-Romero, C.; de León, J.C.; Martínez-Trujillo, M.; Sánchez-Vargas, N.M. Growth Trait Genetic Parameters in a Progeny Trial of Pinus oocarpa. Madera Bosques 2020, 26, e2632014. [Google Scholar] [CrossRef]
- Fabián-Plesníková, I.; Sáenz-Romero, C.; Cruz-De-León, J.; Martínez-Trujillo, M.; Sánchez-Vargas, N.M.; Terrazas, T. Heritability and Characteristics of Resin Ducts in Pinus oocarpa Stems in Michoacán, Mexico. IAWA J. 2021, 42, 258–278. [Google Scholar] [CrossRef]
- Fabián-Plesníková, I.; Sáenz-Romero, C.; Terrazas, T.; Reyes-Ramos, A.; Martínez-Trujillo, M.; Cruz-De-León, J.; Sánchez-Vargas, N.M. Traumatic Ducts Size Varies Genetically and is Positively Associated to Resin Yield of Open–Pollinated Progenies. Silvae Genet. 2022, 71, 10–19. [Google Scholar] [CrossRef]
- Romero-Sanchez, M.E.; Velasco-Garcia, M.V.; Perez-Miranda, R.; Velasco-Bautista, E.; Gonzalez-Hernandez, A. Different Modelling Approaches to Determine Suitable Areas for Conserving Egg–Cone Pine (Pinus oocarpa Schiede) Plus Trees in the Central Part of Mexico. Forests 2022, 13, 2112. [Google Scholar] [CrossRef]
- Velasco-García, M.V.; Hernandez-Hernandez, A. Altitudinal Genetic Variation of Pinus oocarpa Seedling Emergence in the Southern Mountains, Oaxaca, Mexico. Seeds 2024, 3, 1–15. [Google Scholar] [CrossRef]
- Velasco-García, M.V.; Hernández-Hernández, A. Geographic and Climatic Variation in Resin Components and Quality of Pinus oocarpa in Southern Mexico Provenances. Plants 2024, 13, 1755. [Google Scholar] [CrossRef]
- Velasco-García, M.V.; Muñoz-Gutiérrez, L.; Martínez-Cantera, G. Seedling Emergence Capacity and Morphological Traits are Under Strong Genetic Control in the Resin Tree Pinus oocarpa. iForest 2024, 17, 245–251. [Google Scholar] [CrossRef]
- Rajora, O.P.; Rahman, M.H.; Buchert, G.P.; Dancik, B.P. Microsatellite DNA Analysis of Genetic Effects of Harvesting in Old-Growth Eastern White Pine (Pinus strobus) in Ontario, Canada. Mol. Ecol. 2000, 9, 339–348. [Google Scholar] [CrossRef]
- Chaisurisri, K.; El-Kassaby, Y.A. Genetic Diversity in a Seed Production Population vs. Natural Populations of Sitka Spruce. Biodivers. Conserv. 1994, 3, 512–523. [Google Scholar] [CrossRef]
- El-Kassaby, Y.A.; Ritland, K. Impact of selection and breeding on the genetic diversity in Douglas-fir. Biodivers. Conserv. 1996, 5, 795–813. [Google Scholar] [CrossRef]
- Telfer, E.; Graham, N.; Stanbra, L.; Manley, T.; Wilcox, P. Extraction of High Purity Genomic DNA from Pone for Use in a High-Throughput Genotyping Platform. N. Z. J. For. Sci. 2013, 43, 3. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. Genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 117693430500100. [Google Scholar] [CrossRef]
- Goudet, J.; Jombart, T. Hierfstat: Estimation and Tests of Hierarchical F-Statistics, R package version 0.5–11; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Karhu, A.; Vogl, C.; Moran, G.F.; Bell, J.C.; Savolainen, O. Analysis of Microsatellite Variation in Pinus radiata Reveals Effects of Genetic Drift but no Recent Bottlenecks. J. Evol. Biol. 2006, 19, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Arámbula, A.R.; de la Rosa, J.P.; Arias, A.; Rajora, O.P. Cross–Species Transferability of Eastern White Pine (Pinus strobus) Nuclear Microsatellite Markers to Five Mexican White Pines. Genet. Mol. Res. 2014, 13, 7571–7576. [Google Scholar] [CrossRef] [PubMed]
- Delgado Valerio, P.; Núñez Medrano, J.; Rocha Granados, M.; Muñoz Flores, H.J. Variación Genética de Dos Áreas Semilleras de Pino Establecidas en el Estado de Michoacán. Rev. Mex. Cienc. For. 2013, 4, 104–115. Available online: https://www.scielo.org.mx/scielo.php?pid=S2007-11322013000400008&script=sci_arttext (accessed on 15 November 2024).
- Alfonso-Corrado, C.; Campos-Contreras, J.; Sánchez-García, G.; Monsalvo-Reyes, A.; Clark-Tapia, R. Manejo Forestal y Diversidad Genética de Pinus patula Schiede ex Schltdl, & Cham, en Sierra Juárez, Oaxaca. Madera Bosques 2014, 20, 11–22. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Tapia-Olivares, B.L. Pinus oocarpa Isoenzymatic Variation Along an Altitudinal Gradient in Michoacán, México. Silvae Genet. 2003, 52, 237–240. Available online: https://www.thuenen.de/media/institute/fg/PDF/Silvae_Genetica/2003/Vol._52_Heft_5-6/52_5-6_237.pdf (accessed on 15 November 2024).
- Lise, Y.; Kaya, Z.; Isik, F.; Sabuncu, R.; Kandemir, I.; Onde, S. The Impact of Over-Exploitation on the Genetic Structure of Turkish Red Pine (Pinus brutia Ten.) Populations Determined by RAPD Markers. Silva Fenn. 2007, 41, 211–220. [Google Scholar] [CrossRef]
- Lowe, A.; Boshier, D.; Ward, M.; Bacles, C.; Navarro, C. Genetic Resource Impacts of Habitat Loss and Degradation; Reconciling Empirical Evidence and Predicted Theory for Neotropical Trees. Heredity 2005, 95, 255–273. [Google Scholar] [CrossRef]
- Aravanopoulos, F.A. Do Silviculture and Forest Management Affect the Genetic Diversity and Structure of Long–Impacted Forest Tree Populations? Forests 2018, 9, 355. [Google Scholar] [CrossRef]
- Parraguirre-Lezama, C.; Vargas-Hernández, J.; Ramírez-Vallejo, P.; Ramírez-Herrera, C. Sistema de Cruzamiento en Cuatro Poblaciones Naturales de Pinus greggii Engelm. Agrociencia 2004, 38, 107–119. Available online: https://www.redalyc.org/pdf/302/30238111.pdf (accessed on 15 November 2024).
- Velasco-García, M.V.; Ramírez-Herrera, C.; López-Upton, J.; Valdez-Hernández, J.I.; López-Sánchez, H.; López-Mata, L. Diversity and Genetic Structure of Dioon holmgrenii (Cycadales: Zamiaceae) in the Mexican Pacific Coast Biogeographic Province: Implications for Conservation. Plants 2021, 10, 2250. [Google Scholar] [CrossRef]
- Williams, C.G. Long-Distance Pine Pollen Still Germinates After Meso-Scale Dispersal. Am. J. Bot. 2010, 97, 846–855. Available online: https://www.jstor.org/stable/20700415 (accessed on 15 November 2024). [CrossRef] [PubMed]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. Introduction to Conservation Genetics, 2nd ed.; Cambridge University Press: New York, NY, USA, 2009; p. 644. [Google Scholar]
- Allendorf, F.W.; Luikart, G.H.; Aitken, S.N. Conservation and The Genetics of Populations, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 602. [Google Scholar]
- Díaz, V.; Ferrer, E. Genetic Variation of Populations of Pinus oocarpa Revealed by Resistance Gene Analog Polymorphism (RGAP). Genome 2003, 46, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Nepamuceno-Martínez, F.; Sánchez-Alpizar, V. Variaciones Morfológicas en 10 Poblaciones de Pinus oocarpa Schiede. Rev. Cienc. For. 1987, 12, 3–17. [Google Scholar]
- Gutiérrez-Vázquez, B.N.; Flores-Montaño, A. Patrón y Magnitud de la Variación de la Densidad de la Madera en Rodales Semilleros de Pinus oocarpa. Madera Bosques 2019, 25, e2531615. [Google Scholar] [CrossRef]
- Wang, B.; Mao, J.F.; Zhao, W.; Wang, X.R. Impact of geography and climate on the genetic differentiation of the subtropical pine Pinus yunnanensis. PLoS ONE 2013, 8, e67345. [Google Scholar] [CrossRef]
- Tóth, E.G.; Tremblay, F.; Housset, J.M.; Bergeron, Y.; Carcaillet, C. Geographic Isolation and Climatic Variability Contribute to Genetic Differentiation in Fragmented Populations of the Long-Lived Subalpine Conifer Pinus cembra L. in the Western Alps. BMC Evol. Biol. 2019, 19, 190. [Google Scholar] [CrossRef]
- Mosca, E.; González-Martínez, S.C.; Neale, D.B. Environmental versus geographical determinants of genetic structure in two subalpine conifers. New Phytol. 2014, 201, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Peñaloza-Ramírez, J.M.; Rodríguez-Correa, H.; González-Rodríguez, A.; Rocha-Ramírez, V.; Oyama, K. High genetic diversity and stable Pleistocene distributional ranges in the widespread Mexican red oak Quercus castanea Née (1801) (Fagaceae). Ecol. Evol. 2020, 10, 4204–4219. [Google Scholar] [CrossRef]
- Anguiano-Constante, M.A.; Zamora-Tavares, P.; Ruiz-Sanchez, E.; Dean, E.; Rodríguez, A.; Munguía-Lino, G. Population differentiation and phylogeography in Lycianthes moziniana (Solanaceae: Capsiceae), a perennial herb endemic to the Mexican Transition Zone. Biol. J. Linn. Soc. 2021, 132, 359–373. [Google Scholar] [CrossRef]
- Anducho-Reyes, M.A.; Cognato, A.I.; Hayes, J.L.; Zúñiga, G. Phylogeography of the bark beetle Dendroctonus mexicanus Hopkins (Coleoptera: Curculionidae: Scolytinae). Mol. Phylogenetics Evol. 2008, 49, 930–940. [Google Scholar] [CrossRef]
- Mastretta-Yanes, A.; Moreno-Letelier, A.; Piñero, D.; Jorgensen, T.H.; Emerson, B.C. Biodiversity in the Mexican highlands and the interaction of geology, geography and climate within the Trans- Mexican Volcanic Belt. J. Biogeogr. 2015, 42, 1586–1600. [Google Scholar] [CrossRef]
- Rzedowski, J. Vegetación de México, 1st ed.; CONABIO: Ciudad de México, Mexico, 2005; p. 504. Available online: https://www.biodiversidad.gob.mx/publicaciones/librosDig/pdf/VegetacionMx_Cont.pdf (accessed on 15 November 2024).
- Gautam, S.; Timilsina, S.; Shrestha, M. The effects of forest management activities on genetic diversity of forest trees. Indones. J. Soc. Environ. Issues (IJSEI) 2021, 2, 110–118. [Google Scholar] [CrossRef]
- Vallejos, J.; Badilla, Y.; Picado, F.; Murillo, O. Metodología Para la Selección e Incorporación de Árboles Plus en Programas de Mejoramiento Genético Forestal. Agron. Costarric. 2010, 34, 105–119. Available online: https://www.scielo.sa.cr/scielo.php?pid=S0377-94242010000100011&script=sci_arttext&tlng=en (accessed on 15 November 2024). [CrossRef]
- Djerrad, Z.; Kadika, L.; Djouahri, A. Chemical Variability and Antioxidant activities Among Pinus halepensis Mill. Essential Oils Provenances, Depending on Geographic Variation and Environmental Conditions. Ind. Crops Prod. 2015, 74, 440–449. [Google Scholar] [CrossRef]
- Sukarno, A.; Hardiyanto, E.B.; Marsoem, S.N.; Na’iem, M. Oleoresin Production, Turpentine Yield and Components of “Pinus merkusii” from Various Indonesian Provenances. J. Trop. For. Sci. 2015, 27, 136–141. Available online: https://www.jstor.org/stable/43150981 (accessed on 15 November 2024).
- Rajčević, N.; Nikolić, B.; Marin, P.D. Different Responses to Environmental Factors in Terpene Composition of Pinus heldreichii and P. peuce: Ecological and Chemotaxonomic Considerations. Arch. Biol. Sci. 2019, 71, 629–637. [Google Scholar] [CrossRef]
- Zas, R.; Touza, R.; Sampedro, L.; Lario, F.J.; Bustingorri, G.; Lema, M. Variation in Resin Flow Among Maritime Pine Populations: Relationship with Growth Potential and Climatic Responses. For. Ecol. Manag. 2020, 474, 118351. [Google Scholar] [CrossRef]
- Machado, J.A.R.; Freitas, M.L.M.; Paiva, D.I.; De Souza, B.M.; De Sousa, V.A.; Martins, K.; Oliveira, E.B.; De Aguiar, A.V. Economic Evaluation of Conservation Through Use of an Araucaria angustifolia Provenance and Progeny Test. Plants 2024, 13, 2580. [Google Scholar] [CrossRef]
- Degen, B.; Müller, N.A. A simulation study comparing advanced marker-assisted selection with genomic selection in tree breeding programs. G3 Genes Genomes Genet. 2023, 13, jkad164. [Google Scholar] [CrossRef]
- Lee, K.; Kim, I.S.; Choi, W.Y. Enhancing Breeding Potential and Genetic Conservation: A Comprehensive Approach to Plus-Tree Selection for Tilia amurensis Improvement. Forests 2023, 14, 1972. [Google Scholar] [CrossRef]
- Li, Y.; Klápště, J.; Telfer, E.; Wilcox, P.; Graham, N.; Macdonald, L.; Dungey, H.S. Genomic selection for non-key traits in radiata pine when the documented pedigree is corrected using DNA marker information. BMC Genom. 2019, 20, 1026. [Google Scholar] [CrossRef]
- Barrera-Ramírez, R.; Vargas-Hernández, J.J.; López-Aguillón, R.; Muñoz-Flores, H.J.; Treviño-Garza, E.J.; Aguirre-Calderón, O.A. Impact of External and Internal Factors on Successful Grafting of Pinus pseudostrobus var. oaxacana (Mirov) Harrison. Rev. Chapingo Ser. Cienc. For. Ambiente 2021, 27, 243–256. [Google Scholar] [CrossRef]
- Castro-Garibay, S.L.; Villegas-Monter, A.; López-Upton, J.; SandovalVilla, M.; Arévalo-Galarza, L. Effective Protocol to Increase the Percentage of Grafting Success of Pinus greggii Engelm. var. australis Donahue et López. Rev. Chapingo Ser. Cienc. For. Ambiente 2022, 28, 225–240. [Google Scholar] [CrossRef]
- Pérez Luna, A.; López Upton, J.; Prieto Ruíz, J.Á.; Barrera Ramírez, R. Cloning of Pinus patula Schiede ex Schltdl. & Cham. by Grafting: Effect of Phenology and Type of Scion. Rev. Mex. Cienc. For. 2024, 15, 29–49. [Google Scholar] [CrossRef]
- Lindgren, D.; Mullin, T.J. Balancing Gain and Relatedness in Selection. Silvae Genet. 1997, 46, 124–129. Available online: https://www.thuenen.de/media/institute/fg/PDF/Silvae_Genetica/1997/Vol._46_Heft_2-3/46_2-3_124.pdf (accessed on 15 November 2024).
- Williams, C.G.; Savolainen, O. Inbreeding Depression in Conifers: Implications for Breeding Strategy. For. Sci. 1996, 42, 102–117. [Google Scholar] [CrossRef]
- Alberto, F.J.; Aitken, S.N.; Alía, R.; González-Martínez, S.C.; Hänninen, H.; Kremer, A.; Lefèvre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for Evolutionary Responses to Climate Change–Evidence from Tree Populations. Glob. Change Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef] [PubMed]
| Population | Locus | N | Na ± SE | Ne ± SE | Ii ± SE | Ho ± SE | He ± SE | F ± SE |
|---|---|---|---|---|---|---|---|---|
| MX-MIC | PtTX3013 | 50 | 12 ± 0.57 | 3.01 ± 0.77 | 1.56 ± 0.11 | 0.50 ± 0.05 | 0.67 ± 0.04 | 0.25 ± 0.03 |
| NZPR1078 | 50 | 11 ± 0.33 | 3.11 ± 0.81 | 1.60 ± 0.10 | 0.48 ± 0.02 | 0.68 ± 0.04 | 0.29 ± 0.03 | |
| PtTX2146 | 50 | 9 ± 1.20 | 3.36 ± 0.19 | 1.50 ± 0.04 | 0.68 ± 0.06 | 0.70 ± 0.01 | 0.03 ± 0.07 | |
| PtTX3107 | 50 | 5 ± 0.57 | 2.60 ± 0.29 | 1.17 ± 0.05 | 0.58 ± 0.10 | 0.61 ± 0.05 | 0.06 ± 0.11 | |
| PtTX3034 | 50 | 13 ± 0.33 | 7.63 ± 1.24 | 2.25 ± 0.15 | 0.82 ± 0.07 | 0.87 ± 0.05 | 0.06 ± 0.04 | |
| Average | 10 ± 1.41 | 3.94 ± 0.93 | 1.62 ± 0.17 | 0.61 ± 0.06 | 0.71 ± 0.04 | 0.14 ± 0.05 | ||
| MX-MEX | PtTX3013 | 50 | 11 ± 0.57 | 4.48 ± 0.77 | 1.84 ± 0.11 | 0.58 ± 0.05 | 0.78 ± 0.04 | 0.25 ± 0.03 |
| NZPR1078 | 50 | 10 ± 0.33 | 4.40 ± 0.81 | 1.79 ± 0.10 | 0.54 ± 0.02 | 0.77 ± 0.04 | 0.30 ± 0.03 | |
| PtTX2146 | 50 | 5 ± 1.20 | 3.43 ± 0.19 | 1.36 ± 0.04 | 0.56 ± 0.06 | 0.71 ± 0.01 | 0.21 ± 0.07 | |
| PtTX3107 | 50 | 4 ± 0.57 | 2.97 ± 0.29 | 1.19 ± 0.05 | 0.58 ± 0.10 | 0.66 ± 0.05 | 0.12 ± 0.11 | |
| PtTX3034 | 50 | 13 ± 0.33 | 6.82 ± 1.24 | 2.17 ± 0.15 | 0.84 ± 0.07 | 0.85 ± 0.05 | 0.02 ± 0.04 | |
| Average | 8.6 ± 1.75 | 4.42 ± 0.66 | 1.67 ± 0.17 | 0.62 ± 0.05 | 0.75 ± 0.03 | 0.18 ± 0.05 | ||
| MX-OAX | PtTX3013 | 46 | 10 ± 0.57 | 5.68 ± 0.77 | 1.92 ± 0.11 | 0.70 ± 0.05 | 0.82 ± 0.04 | 0.16 ± 0.03 |
| NZPR1078 | 46 | 10 ± 0.33 | 5.91 ± 0.81 | 1.97 ± 0.10 | 0.50 ± 0.02 | 0.83 ± 0.04 | 0.40 ± 0.03 | |
| PtTX2146 | 46 | 6 ± 1.20 | 3.98 ± 0.19 | 1.48 ± 0.04 | 0.78 ± 0.06 | 0.75 ± 0.01 | −0.05 ± 0.07 | |
| PtTX3107 | 46 | 6 ± 0.57 | 1.96 ± 0.29 | 1.03 ± 0.05 | 0.28 ± 0.10 | 0.49 ± 0.05 | 0.42 ± 0.11 | |
| PtTX3034 | 46 | 12 ± 0.33 | 3.56 ± 1.24 | 1.75 ± 0.15 | 0.61 ± 0.07 | 0.72 ± 0.05 | 0.15 ± 0.04 | |
| Average | 46 | 8.8 ± 1.2 | 4.22 ± 0.73 | 1.63 ± 0.17 | 0.57 ± 0.09 | 0.72 ± 0.06 | 0.22 ± 0.09 |
| Source Variation | Sum of Squares | Variance Components | Percentage of Variation | F-Statistics (p-Value) |
|---|---|---|---|---|
| Between populations | 46.84 | 0.21 | 10.24 | FCT = 0.10238 (0.00000) |
| Between provenances within populations | 32.07 | 0.05 | 2.34 | FSC = 0.02607 (0.00684) |
| Within provenances | 496.27 | 1.80 | 87.42 | FST = 0.12578 (0.00000) |
| Total | 575.18 | 2.06 | 100.00 |
| MX-OAX | MX-MEX | MX-MIC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| El Nanche | Las Tejas | El Tizne | Llano San Pedro | El Salto | Cerro Gachupín | La Laja | Tenería | El Coyul | Joya de Uva | Canalejas | Piedras Paradas | Rancho Nuevo | Cañas | El Banco | ||
| MX-OAX | El Nanche | 0.000 | ||||||||||||||
| Las Tejas | −0.004 | 0.000 | ||||||||||||||
| El Tizne | −0.020 | −0.020 | 0.000 | |||||||||||||
| Llano San Pedro | 0.061 | 0.074 | 0.078 | 0.000 | ||||||||||||
| MX-MEX | El Salto | 0.108 | 0.092 | 0.098 | 0.136 | 0.000 | ||||||||||
| Cerro Gachupín | 0.205 | 0.184 | 0.178 | 0.250 | 0.021 | 0.000 | ||||||||||
| La Laja | 0.098 | 0.096 | 0.097 | 0.150 | 0.000 | 0.035 | 0.000 | |||||||||
| Tenería | 0.190 | 0.167 | 0.200 | 0.237 | −0.001 | 0.014 | 0.012 | 0.000 | ||||||||
| El Coyul | 0.181 | 0.138 | 0.153 | 0.192 | 0.019 | 0.019 | 0.045 | 0.016 | 0.000 | |||||||
| MX-MIC | Joya de Uva | 0.161 | 0.117 | 0.145 | 0.177 | 0.011 | 0.071 | 0.036 | 0.042 | 0.020 | 0.000 | |||||
| Canalejas | 0.215 | 0.160 | 0.197 | 0.222 | 0.038 | 0.096 | 0.071 | 0.103 | 0.036 | −0.002 | 0.000 | |||||
| Piedras Paradas | 0.261 | 0.212 | 0.267 | 0.279 | 0.064 | 0.142 | 0.073 | 0.161 | 0.086 | 0.026 | −0.038 | 0.000 | ||||
| Rancho Nuevo | 0.256 | 0.208 | 0.236 | 0.270 | 0.045 | 0.028 | 0.076 | 0.027 | 0.037 | 0.022 | −0.002 | 0.035 | 0.000 | |||
| Cañas | 0.166 | 0.132 | 0.160 | 0.194 | 0.022 | 0.083 | 0.048 | 0.028 | 0.029 | 0.024 | 0.032 | 0.061 | 0.020 | 0.000 | ||
| El Banco | 0.188 | 0.169 | 0.199 | 0.220 | 0.025 | 0.112 | 0.032 | 0.067 | 0.077 | 0.037 | 0.036 | 0.006 | 0.036 | 0.002 | 0.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallejo-Reyna, M.Á.; Velasco-García, M.V.; Aguilera-Martínez, V.; Méndez-Sánchez, H.; Muñoz-Gutiérrez, L.; Gómez-Cárdenas, M.; Hernández-Hernández, A. Genetic Diversity and Structure of Higher-Resin Trees of Pinus oocarpa Schiede in Mexico: Implications for Genetic Improvement. Forests 2024, 15, 2250. https://doi.org/10.3390/f15122250
Vallejo-Reyna MÁ, Velasco-García MV, Aguilera-Martínez V, Méndez-Sánchez H, Muñoz-Gutiérrez L, Gómez-Cárdenas M, Hernández-Hernández A. Genetic Diversity and Structure of Higher-Resin Trees of Pinus oocarpa Schiede in Mexico: Implications for Genetic Improvement. Forests. 2024; 15(12):2250. https://doi.org/10.3390/f15122250
Chicago/Turabian StyleVallejo-Reyna, Miguel Ángel, Mario Valerio Velasco-García, Viridiana Aguilera-Martínez, Hilda Méndez-Sánchez, Liliana Muñoz-Gutiérrez, Martín Gómez-Cárdenas, and Adán Hernández-Hernández. 2024. "Genetic Diversity and Structure of Higher-Resin Trees of Pinus oocarpa Schiede in Mexico: Implications for Genetic Improvement" Forests 15, no. 12: 2250. https://doi.org/10.3390/f15122250
APA StyleVallejo-Reyna, M. Á., Velasco-García, M. V., Aguilera-Martínez, V., Méndez-Sánchez, H., Muñoz-Gutiérrez, L., Gómez-Cárdenas, M., & Hernández-Hernández, A. (2024). Genetic Diversity and Structure of Higher-Resin Trees of Pinus oocarpa Schiede in Mexico: Implications for Genetic Improvement. Forests, 15(12), 2250. https://doi.org/10.3390/f15122250






