Study on Novel Modified Phenolic Foams with Added Pine Wood Sawdust
Abstract
1. Introduction
2. Materials and Methods
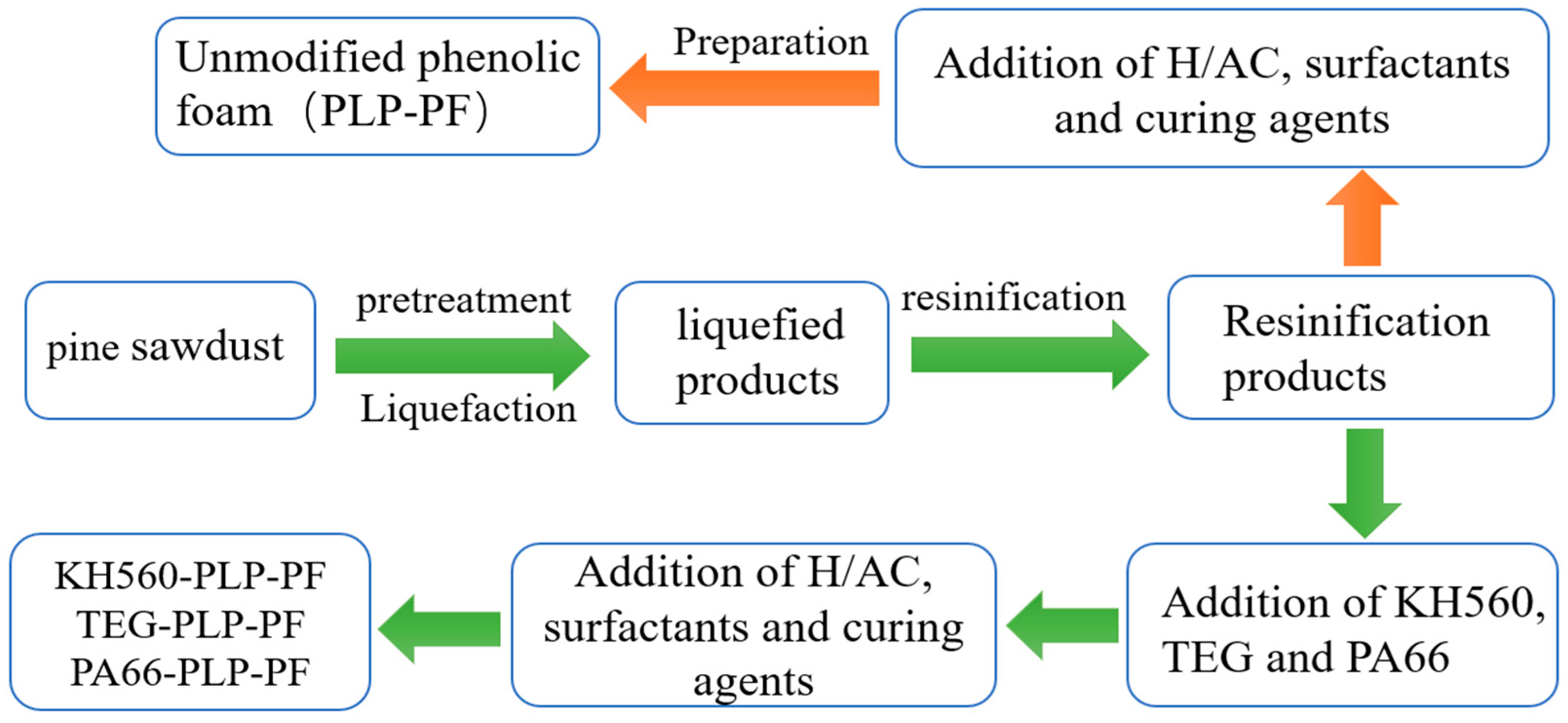
2.1. Preparation of MPFs
2.1.1. Materials
2.1.2. Pretreatment and Liquefaction of Pine Sawdust
2.1.3. Resinification of Liquefaction Products
2.1.4. Preparation of Resin Foam
2.1.5. Synthesis of Modified Liquefaction Products Resin
2.1.6. Preparation of Modified Resin Foam
2.2. Characterization Test
3. Results and Discussion
3.1. X-Ray Fluorescence Spectroscopy Analysis (XRF)
3.2. Infrared Analysis of Modified Resin Foam
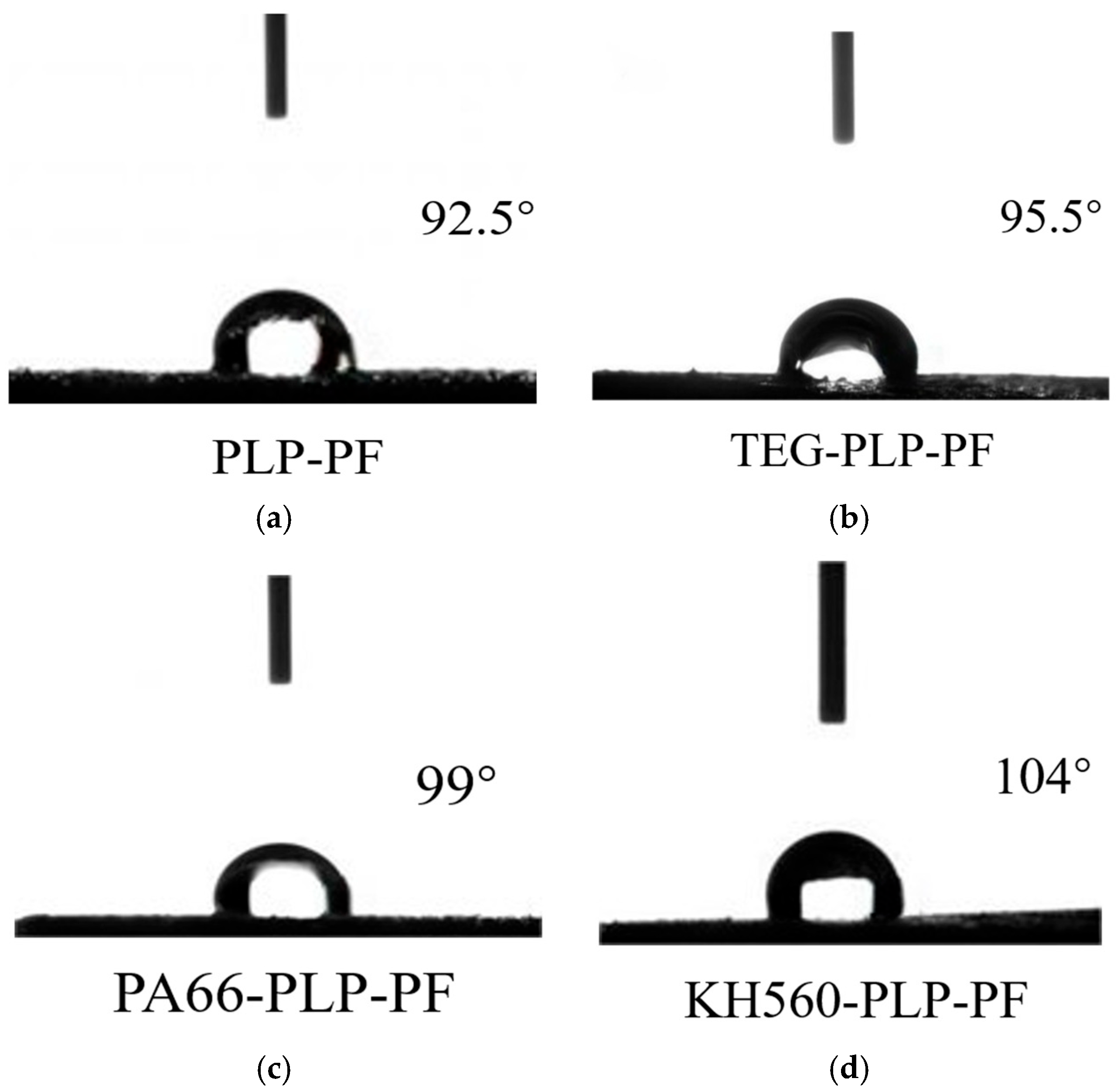
3.3. Contact Angle Analysis
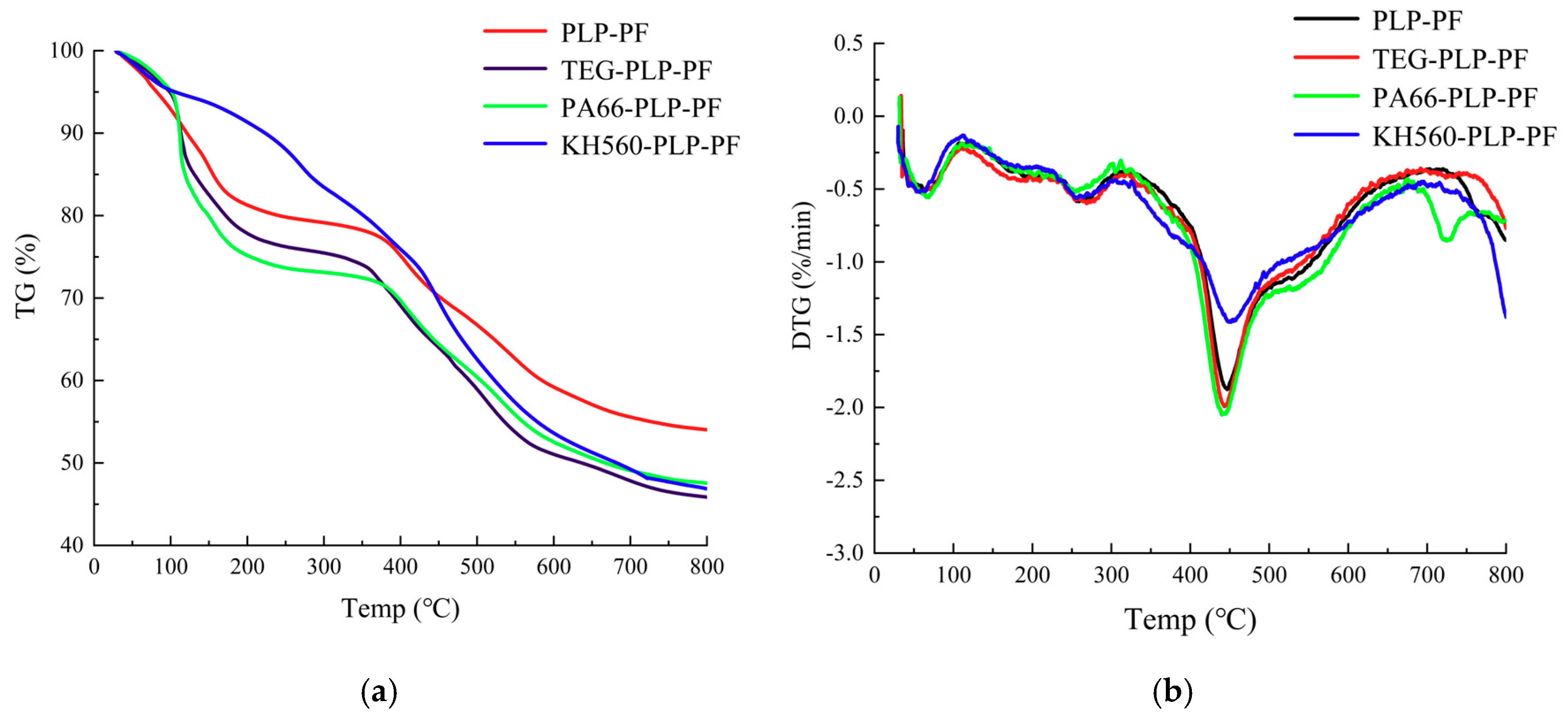
3.4. Thermogravimetric Analysis (TG-DTG)
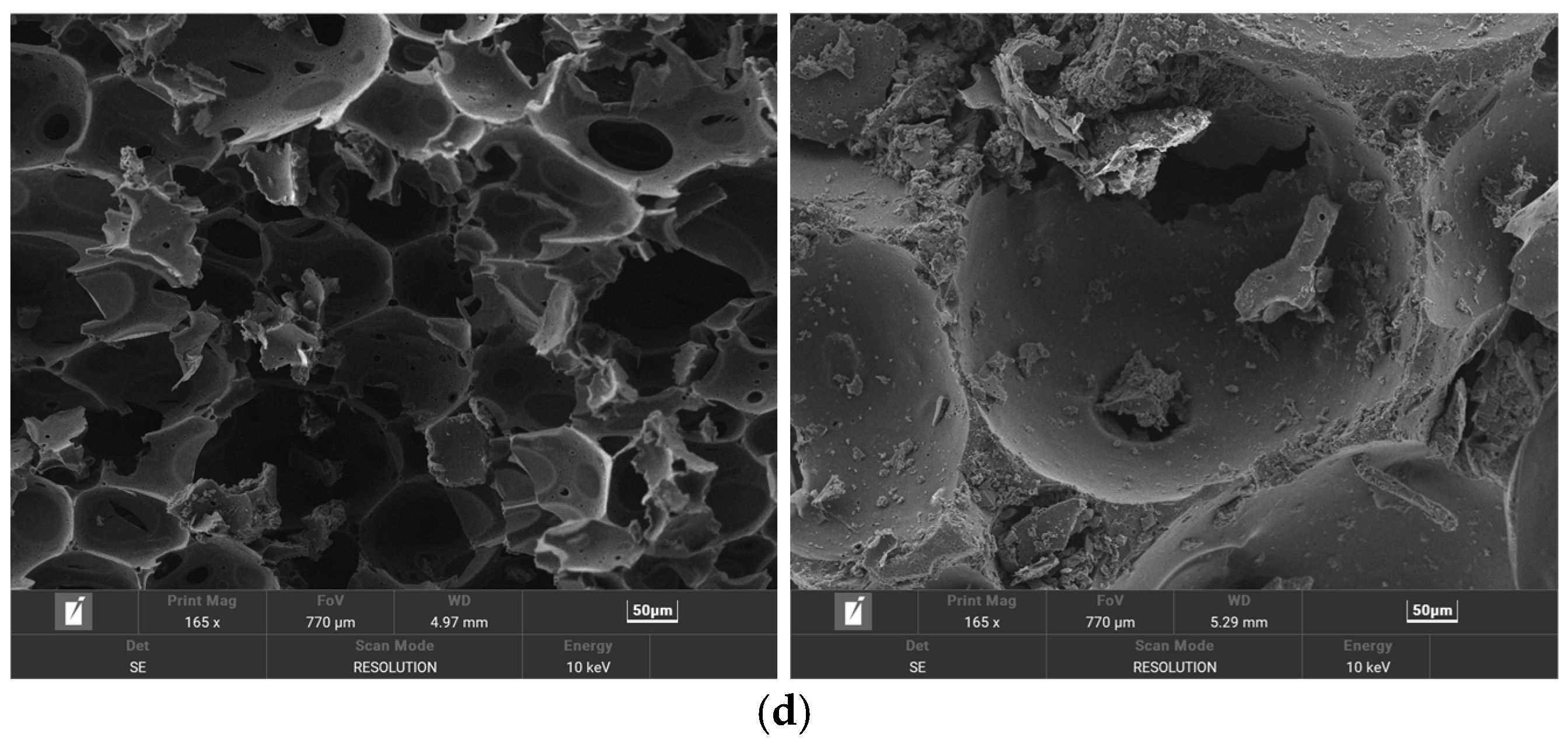
3.5. Scanning Electron Microscopy (SEM) Analysis
3.6. Open-Cell Ratio Analysis
3.7. Comparative Analysis of Foam Performance Indicators with Three Modifiers
3.7.1. Apparent Density and Thermal Conductivity
3.7.2. Compressive Strength Analysis
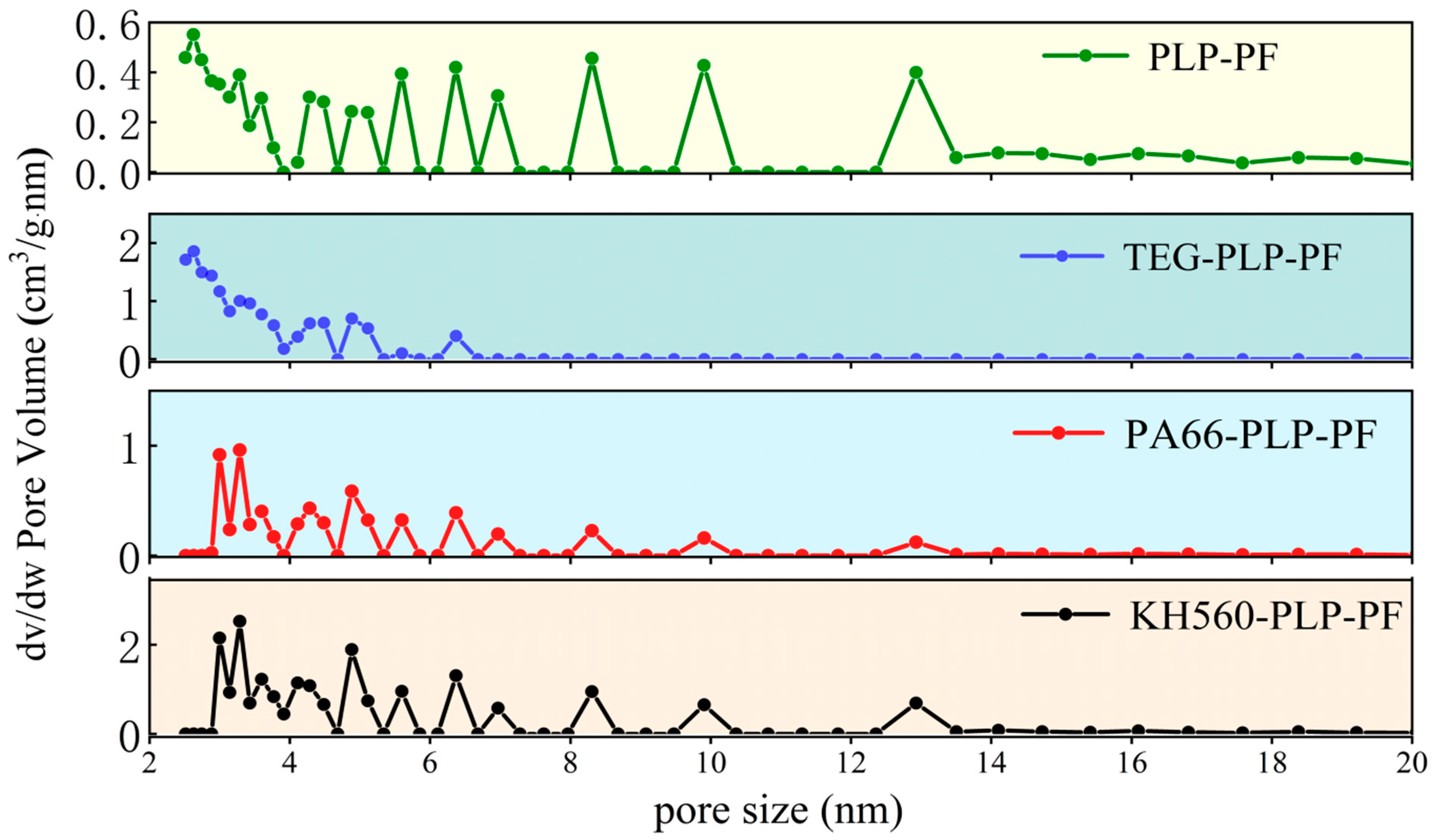
3.7.3. Microporous Structure Analysis
3.7.4. Analysis of Chalking Rate and Oxygen Index Results
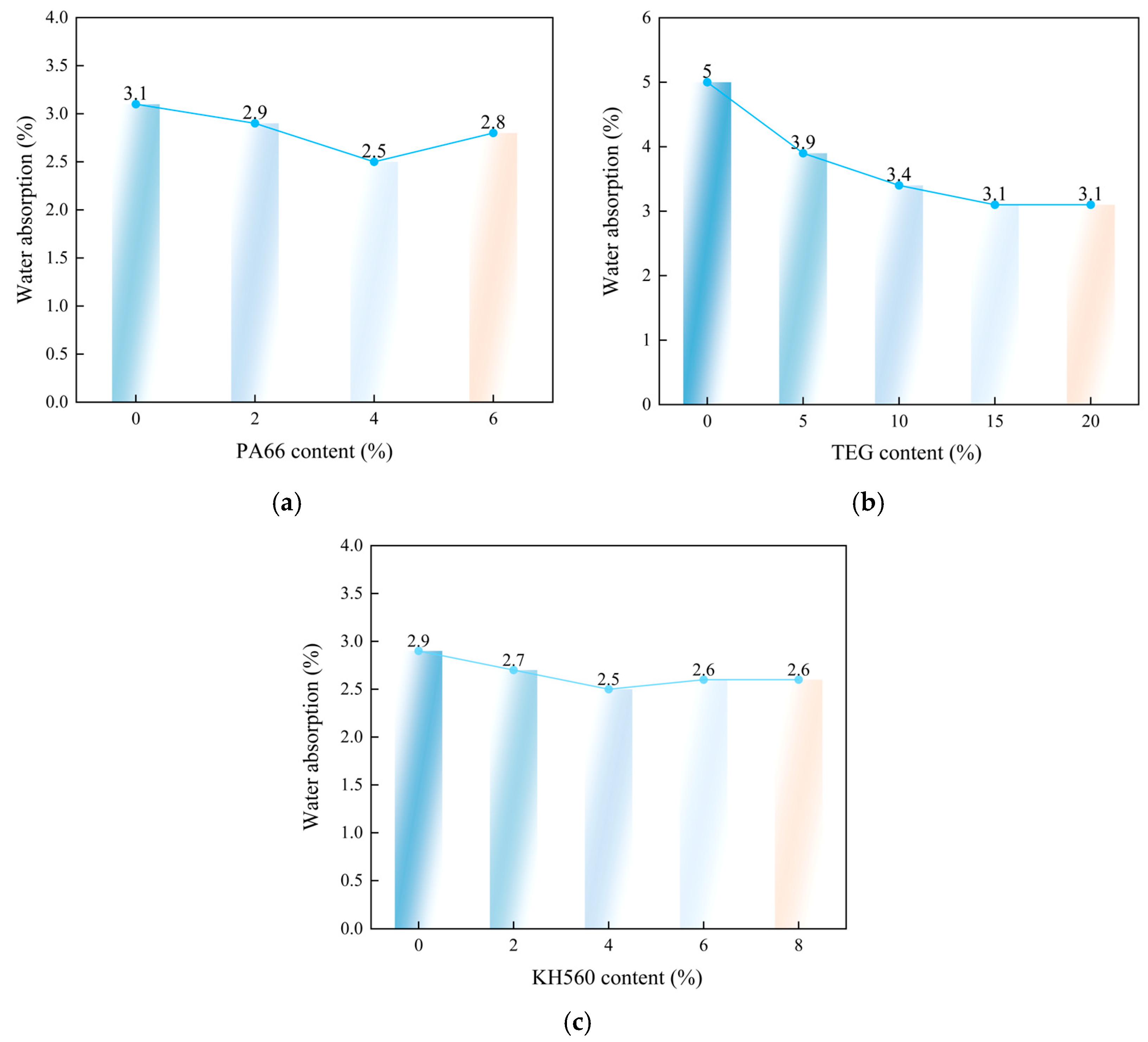
3.7.5. Water Absorption Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Zhang, Y.; Fang, J.; Ma, Z.; Li, C.; Yan, M.; Qiao, N.; Liu, Y.; Bian, M. Reduction and Reuse of Forestry and Agricultural Bio-Waste through Innovative Green Utilization Approaches: A Review. Forests 2024, 15, 1372. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, W.; Dong, X.; Wang, F.; Yang, W.; Liu, C.; Chen, D. A review on recent advances of biochar from agricultural and forestry wastes: Preparation, modification and applications in wastewater treatment. J. Environ. Chem. Eng. 2023, 12, 111638. [Google Scholar] [CrossRef]
- Zou, J.; Liu, X.; Xu, S.; Chen, M.; Yu, Q.; Xie, J. Combined hydrothermal pretreatment of agricultural and forestry wastes to enhance anaerobic digestion for methane production. Chem. Eng. J. 2024, 486, 150313. [Google Scholar] [CrossRef]
- Bilgen, S.; Sarıkaya, İ. Utilization of forestry and agricultural wastes. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 3484–3490. [Google Scholar] [CrossRef]
- Melro, E.; Duarte, H.; Antunes, F.E.; Valente, A.J.; Romano, A.; Norgren, M.; Medronho, B. Engineering novel phenolic foams with lignin extracted from pine wood residues via a new levulinic-acid assisted process. Int. J. Biol. Macromol. 2023, 248, 125947. [Google Scholar] [CrossRef]
- DSouza, G.C.; Chio, C.; Ray, M.B.; Prakash, A.; Qin, W.; Xu, C. Synthesis and characterization of biodegradable Kraft lignin-based hydrophilic phenol formaldehyde foams. Sustain. Mater. Technol. 2024, 41, e01064. [Google Scholar] [CrossRef]
- Chen, G.; Liu, J.; Zhang, W.; Han, Y.; Zhang, D.; Li, J.; Zhang, S. Lignin-based phenolic foam reinforced by poplar fiber and isocyanate-terminated polyurethane prepolymer. Polymers 2021, 13, 1068. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, M.; Lv, Y.; Guo, H.; Liu, Y.; Ye, X.; Shi, Y. Research on fire retardant lignin phenolic carbon foam with preferable smoke suppression performance. Chem. Eng. Sci. 2023, 282, 119305. [Google Scholar] [CrossRef]
- Mougel, C.; Garnier, T.; Cassagnau, P.; Sintes-Zydowicz, N. Phenolic foams: A review of mechanical properties, fire resistance and new trends in phenol substitution. Polymer 2019, 164, 86–117. [Google Scholar] [CrossRef]
- Del Saz-Orozco, B.; Alonso, M.V.; Oliet, M.; Domínguez, J.C.; Rodriguez, F. Effects of formulation variables on density, compressive mechanical properties and morphology of wood flour-reinforced phenolic foams. Compos. Part B Eng. 2014, 56, 546–552. [Google Scholar] [CrossRef]
- Cheng, J.-Y.; Li, Z.-K.; Yan, H.-L.; Lei, Z.-P.; Yan, J.-C.; Ren, S.-B.; Wang, Z.-C.; Kang, S.-G.; Shui, H.-F. Preparation and characterization of low-temperature coal tar toughened phenolic foams. J. Fuel Chem. Technol. 2023, 51, 748–756. [Google Scholar] [CrossRef]
- Sarika, P.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Progress in Bio-Based Phenolic Foams: Synthesis, Properties, and Applications. ChemBioEng Rev. 2021, 8, 612–632. [Google Scholar] [CrossRef]
- Song, S.A.; Chung, Y.S.; Kim, S.S. The mechanical and thermal characteristics of phenolic foams reinforced with carbon nanoparticles. Compos. Sci. Technol. 2014, 103, 85–93. [Google Scholar] [CrossRef]
- Ziarati, H.B.; Fasihi, M. Phenolic foams: Foaming processes and applications. In Handbook of Thermosetting Foams, Aerogels, and Hydrogels; Elsevier: Amsterdam, The Netherlands, 2024; pp. 421–441. [Google Scholar]
- Gao, M.; Wu, W.; Wang, Y.; Wang, Y.; Wang, H. Phenolic foam modified with dicyandiamide as toughening agent. J. Therm. Anal. Calorim. 2016, 124, 189–195. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, F.; Luo, L.; Wang, L.; Liu, Y.; Leng, J. Design and preparation of shape memory phenol–formaldehyde foam composites with excellent thermal stability and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2023, 174, 107738. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Yuan, H.; Song, L.; Hu, Y.; Yuen, R.K. Fire performance and mechanical properties of phenolic foams modified by phosphorus-containing polyethers. J. Polym. Res. 2012, 19, 1–10. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhou, C. Mechanical and thermal properties of modified red mud-reinforced phenolic foams. Polym. Int. 2018, 67, 528–534. [Google Scholar] [CrossRef]
- Song, S.A.; Lee, Y.; Kim, Y.S.; Kim, S.S. Mechanical and thermal properties of carbon foam derived from phenolic foam reinforced with composite particles. Compos. Struct. 2017, 173, 1–8. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Xu, P.; Chang, J. Preparation and characterization of phenolic foam modified with bio-oil. Materials 2018, 11, 2228. [Google Scholar] [CrossRef]
- Del Saz-Orozco, B.; Alonso, M.V.; Oliet, M.; Domínguez, J.C.; Rojo, E.; Rodriguez, F. Lignin particle-and wood flour-reinforced phenolic foams: Friability, thermal stability and effect of hygrothermal aging on mechanical properties and morphology. Compos. Part B Eng. 2015, 80, 154–161. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, X.; Fan, Z.; Ni, R.; Liu, X.; Tian, Y.; Zhou, B. Using lignin degraded to synthesize phenolic foams with excellent flame retardant property. Eng. Asp. 2023, 666, 131373. [Google Scholar] [CrossRef]
- Weng, S.; Li, Z.; Bo, C.; Song, F.; Xu, Y.; Hu, L.; Zhou, Y.; Jia, P. Design lignin doped with nitrogen and phosphorus for flame retardant phenolic foam materials. React. Funct. Polym. 2023, 185, 105535. [Google Scholar] [CrossRef]
- Wei, D.; Li, D.; Zhang, L.; Zhao, Z.; Ao, Y. Study on phenolic resin foam modified by montmorillonite and carbon fibers. Procedia Eng. 2012, 27, 374–383. [Google Scholar] [CrossRef]
- Patle, V.K.; Mehta, Y.; Kumar, R. Nickel and iron nanoparticles decorated carbon fibers reinforced phenolic resin-based carbon composites foam for excellent electromagnetic interference shielding. Diam. Relat. Mater. 2024, 145, 111069. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Patle, V.K.; Dhawan, R.; Abhash, A.; Dwivedi, N.; Mondal, D.; Srivastava, A. Phenol formaldehyde resin derived carbon-MCMB composite foams for electromagnetic interference shielding and thermal management applications. Compos. Commun. 2020, 22, 100433. [Google Scholar] [CrossRef]
- Radotić, K.; Mićić, M. Methods for extraction and purification of lignin and cellulose from plant tissues. In Sample Preparation Techniques for Soil, Plant, and Animal Samples; Univerzitet u Beogradu: Belgrade, Serbia, 2016; pp. 365–376. [Google Scholar]
- Lan, W.; Liu, C.-F.; Sun, R.-C. Fractionation of bagasse into cellulose, hemicelluloses, and lignin with ionic liquid treatment followed by alkaline extraction. J. Agric. Food Chem. 2011, 59, 8691–8701. [Google Scholar] [CrossRef]
- Wang, X.; Jang, J.; Su, Y.; Liu, J.; Zhang, H.; He, Z.; Ni, Y. Starting materials, processes and characteristics of bio-based foams: A review. J. Bioresour. Bioprod. 2024, 9, 160–173. [Google Scholar] [CrossRef]
- Kashyap, P.; Brzezińska, M.; Keller, N.; Ruppert, A.M. Influence of Impurities in the Chemical Processing Chain of Biomass on the Catalytic Valorisation of Cellulose towards γ-Valerolactone. Catalysts 2024, 14, 141. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Behin, J. Detrimental effect of industrial toluene organic impurities on the density of rigid polyurethane foam and their removal. Korean J. Chem. Eng. 2021, 38, 204–214. [Google Scholar] [CrossRef]
- GB/T 2406.1-2008; Plastics—Determination of Burning Behaviour by Oxygen Index. Standardization Administration of China: Beijing, China, 2008.
- GB/T 10297-2015; Test Method for Thermal Conductivity of Nonmetal Solid Materials—Hot - Wire Method. State General Administration of the People’s Republic of China for Quality Supervision, Inspection and Quarantine. Standardization Administration of the People’s Republic of China: Beijing, China, 2015.
- GB/T 10799-2008; Rigid Cellular Plastics—Determination of the Volume Percentage of Open Cells and of Closed Cells. State General Administration of the People’s Republic of China for Quality Supervision, Inspection and Quarantine. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- GB/T 8810-2005; Determination of Water Absorption of Rigid Cellular Plastics. State General Administration of the People’s Republic of China for Quality Supervision, Inspection and Quarantine. Standardization Administration of the People’s Republic of China: Beijing, China, 2005.
- Xu, S.-C.; Zhang, N.-L.; Yang, J.-F.; Wang, B.; Kim, C.-Y. Silicon carbide-based foams derived from foamed SiC-filled phenolic resin by reactive infiltration of silicon. Ceram. Int. 2016, 42, 14760–14764. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Hu, Y. Synthesis of a novel flame retardant containing phosphorus, nitrogen and boron and its application in flame-retardant epoxy resin. Polym. Degrad. Stab. 2016, 133, 358–366. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Z.; Xiao, Y.; Pan, Z.; Jia, P.; Feng, G.; Bao, C.; Zhou, Y.; Hua, L. Lignin-based phenolic resin modified with whisker silicon and its application. J. Bioresour. Bioprod. 2020, 5, 67–77. [Google Scholar] [CrossRef]
- Tang, K.; He, X.; Xu, G.; Tang, X.; Ge, T.; Zhang, A. Effect of formaldehyde to phenol molar ratio on combustion behavior of phenolic foam. Polym. Test. 2022, 111, 107626. [Google Scholar] [CrossRef]
- Suttaphakdee, P.; Neramittagapong, S.; Theerakulpisut, S.; Neramittagapong, A.; Kumsaen, T.; Jina, P.; Saengkhamsuk, N. Comparison of dehydration methods for untreated lignin resole by hot air oven and vacuum rotary evaporator to synthesize lignin-based phenolic foam. Heliyon 2022, 8, e08769. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Y.; Liu, S.; Zhang, A.; Liu, W.; Huang, S. A tannic acid-based intumescent flame retardant for improving flame retardancy of epoxy composites. Adv. Ind. Eng. Polym. Res. 2024, in press. [Google Scholar] [CrossRef]
- Saifaldeen, Z.S.; Khedir, K.R.; Camci, M.T.; Ucar, A.; Suzer, S.; Karabacak, T. The effect of polar end of long-chain fluorocarbon oligomers in promoting the superamphiphobic property over multi-scale rough Al alloy surfaces. Appl. Surf. Sci. 2016, 379, 55–65. [Google Scholar] [CrossRef]
- Song, F.; Li, Z.; Jia, P.; Bo, C.; Zhang, M.; Hu, L.; Zhou, Y. Phosphorus-containing tung oil-based siloxane toughened phenolic foam with good mechanical properties, fire performance and low thermal conductivity. Mater. Des. 2020, 192, 108668. [Google Scholar] [CrossRef]
- Song, F.; Jia, P. Flame Retardant Modification of Phenolic Foam. In Phenolic Based Foams: Preparation, Characterization, and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 195–207. [Google Scholar]
- Wang, L.; Hu, A.; Fan, L.; Yang, S. Structures and properties of closed-cell polyimide rigid foams. J. Appl. Polym. Sci. 2013, 130, 3282–3291. [Google Scholar] [CrossRef]
- Xiong, W.; Wu, J.; Tian, H.; Xiang, A.; Wang, C.; Wu, Q. Enhanced mechanical and thermal properties of polyurethane-imide foams with the addition of expended vermiculite. Polym. Compos. 2020, 41, 886–892. [Google Scholar] [CrossRef]
- Xu, Q.; Gong, R.; Cui, M.-Y.; Liu, C.; Li, R.-H. Preparation of high-strength microporous phenolic open-cell foams with physical foaming method. High Perform. Polym. 2015, 27, 852–867. [Google Scholar] [CrossRef]
- Gao, J.; Wu, Y.; Li, J.; Peng, X.; Yin, D.; Jin, H.; Wang, S.; Wang, J.; Wang, X.; Jin, M. A review of the recent developments in flame-retardant nylon composites. Compos. Part C Open Access 2022, 9, 100297. [Google Scholar] [CrossRef]
- Tang, K.; Tang, X.; Liu, X.; Zhang, A.; Ge, T.; Li, Y. Phenolic Foams Toughened with Triethylene Glycol by In Situ Polymerization and Prepolymerization Processes. ACS Appl. Polym. Mater. 2022, 4, 8303–8314. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, W.; Wang, S. PVA fiber/cement-based interface in silane coupler KH560 reinforced high performance concrete–Experimental and molecular dynamics study. Constr. Build. Mater. 2023, 395, 132184. [Google Scholar] [CrossRef]
- Simonini, L.; Sorze, A.; Maddalena, L.; Carosio, F.; Dorigato, A. Mechanical reprocessing of polyurethane and phenolic foams to increase the sustainability of thermal insulation materials. Polym. Test. 2024, 138, 108539. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Zhang, W.; Han, Y. Phenolic resin foam composites reinforced by acetylated poplar fiber with high mechanical properties, low pulverization ratio, and good thermal insulation and flame retardant performance. Materials 2019, 13, 148. [Google Scholar] [CrossRef]
- Schinazi, G.; Price, E.J.; Schiraldi, D.A. Fire testing methods of bio-based flame-retardant polymeric materials. In Bio-Based Flame-Retardant Technology for Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 61–95. [Google Scholar]
- Bo, C.; Shi, Z.; Hu, L.; Pan, Z.; Hu, Y.; Yang, X.; Jia, P.; Ren, X.; Zhang, M.; Zhou, Y. Cardanol derived P, Si and N based precursors to develop flame retardant phenolic foam. Sci. Rep. 2020, 10, 12082. [Google Scholar] [CrossRef]
- Zhi, M.; Liu, Q.; Chen, H.; Chen, X.; Feng, S.; He, Y. Thermal stability and flame retardancy properties of epoxy resin modified with functionalized graphene oxide containing phosphorus and silicon elements. ACS Omega 2019, 4, 10975–10984. [Google Scholar] [CrossRef]
- Ge, T.; Hu, X.; Tang, K.; Wang, D. The preparation and properties of terephthalyl-alcohol-modified phenolic foam with high heat aging resistance. Polymers 2019, 11, 1267. [Google Scholar] [CrossRef]
- Sarika, P.R.; Nancarrow, P.; Makkawi, Y.; Ibrahim, T.H. Preparation and Characterization of Date Palm Bio-Oil Modified Phenolic Foam. Polymers 2024, 16, 955. [Google Scholar] [CrossRef]
- Ge, T.; Tang, K.; Tang, X. Preparation and properties of acetoacetic ester-terminated polyether pre-synthesis modified phenolic foam. Materials 2019, 12, 334. [Google Scholar] [CrossRef]



| Materials | KH560 | PA66 | TEG | Activated Carbon | n-Hexane | Aqueous Formaldehyde |
|---|---|---|---|---|---|---|
| Dosage | 4.1% | 6% | 15% | 2.5 g | 1 g | 37% |
| Mass (%) | Na | Mg | Si | P | S | Cl | K | Ca | Fe | Zn | Al | Cr | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLP-PF | 19.4 | 0.39 | 1.9 | 2.8 | 49.8 | 15.3 | 2.45 | 3.98 | 2.1 | 1.02 | 0.43 | 0.10 | 0.19 |
| TEG-PLP-PF | 14.6 | 0.27 | 1.65 | 2.73 | 46.2 | 22.9 | 2.98 | 4.65 | 2.1 | 0.57 | 0.47 | 0.15 | 0.25 |
| KH560-PLP-PF | 10.7 | 0.15 | 11 | 2.99 | 49.7 | 14.2 | 4.0 | 2.85 | 1.8 | 0.89 | / | 0.12 | 0.22 |
| PA66-PLP-PF | 15.8 | 0.22 | 1.46 | 2.37 | 38.7 | 33.4 | 2.36 | 2.9 | 1.3 | 0.49 | 0.39 | / | 0.12 |
| Skeleton Volume (cm3) | True Density (g/cm3) | Sampling Volume (cm3) | Open Porosity (%) | |
|---|---|---|---|---|
| PLP-PF | 0.3549 | 1.5522 | 2.3969 | 85.19 |
| TEG-PLP-PF | 0.3080 | 1.4889 | 2.3780 | 87.05 |
| PA66-PLP-PF | 0.1264 | 1.3054 | 2.1631 | 94.16 |
| KH560-PLP-PF | 0.1209 | 2.1541 | 2.7982 | 95.68 |
| Foam Type | Apparent Density (kg/m3) | Pore Volume (cm3/g) | Compressive Strength (MPa) | Specific Surface Area (m2/g) | Thermal Conductivity (W/m·K) | Average Pore Diameter (μm) |
|---|---|---|---|---|---|---|
| PLP-PF | 0.38 ± 0.037 | 0.175 ± 0.014 | 4.56 ± 0.21 | 1.921 ± 0.12 | 0.023 ± 0.002 | 115 |
| TEG-PLP-PF | 0.42 ± 0.055 | 0.098 ± 0.019 | 5.93 ± 0.35 | 2.164 ± 0.18 | 0.018 ± 0.001 | 104 |
| KH560-PLP-PF | 0. 52 ± 0.045 | 0.088 ± 0.018 | 12.22 ± 0.56 | 2.252 ± 0.18 | 0.011 ± 0.001 | 111 |
| PA66-PLP-PF | 0.46 ± 0.062 | 0.045 ± 0.01 | 7.02 ± 0.34 | 1.882 ± 0.2 | 0.017 ± 0.001 | 117 |
| Compressive Strength (MPa) | Oxygen Index (%) | Chalking Rate (%) | Water Absorption (%) | Thermal Conductivity (W/m·K) | Literatures |
|---|---|---|---|---|---|
| 0.125 | 31.5 | 5.2 | / | 0.048 | [7] |
| 0.28 | / | 8.9 ± 0.2 | / | / | [20] |
| 0.245 | 41.7 | / | / | 0.0366 | [43] |
| 0.18 | 33.9 | 6.5 | / | 0.056 | [52] |
| ≈0.25 | / | ≈3.5 | ≈4.3 | / | [56] |
| 0.294 | / | / | / | 0.038 | [57] |
| 0.203 | / | 1.3 | ≈6.5 | / | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, J.; Lu, S.; Liu, S.; Li, X.; Liu, J. Study on Novel Modified Phenolic Foams with Added Pine Wood Sawdust. Forests 2024, 15, 2249. https://doi.org/10.3390/f15122249
Ling J, Lu S, Liu S, Li X, Liu J. Study on Novel Modified Phenolic Foams with Added Pine Wood Sawdust. Forests. 2024; 15(12):2249. https://doi.org/10.3390/f15122249
Chicago/Turabian StyleLing, Jianwei, Shiyu Lu, Shouqing Liu, Xuemei Li, and Jianxiang Liu. 2024. "Study on Novel Modified Phenolic Foams with Added Pine Wood Sawdust" Forests 15, no. 12: 2249. https://doi.org/10.3390/f15122249
APA StyleLing, J., Lu, S., Liu, S., Li, X., & Liu, J. (2024). Study on Novel Modified Phenolic Foams with Added Pine Wood Sawdust. Forests, 15(12), 2249. https://doi.org/10.3390/f15122249







