Abstract
The moisture, ash, and silicon content, as well as the phytolith morphotype and concentration in the tissue-cultured, seed-cultured, and grafted seedling leaves of Dendrocalamus brandisii were determined to investigate the differences in silicon uptake and phytolith morphology in the leaves from different rearing methods. The results showed that ash, silicon content, and phytolith concentration were higher in the mature leaves. Tissue-cultured seedlings had a significantly higher moisture content than grafted seedlings. Ash and silicon demonstrated the same order of grafted seedlings > tissue-cultured seedlings > seed-cultured seedlings. The highest phytolith concentration was found in tissue-cultured seedlings. The phytolith morphotypes in D. brandisii seedling leaves raised by different methods were identical and grouped into eight morphotypes. The phytolith assemblage was characterized by a high frequency of bilobate and saddle, accounting for more than 60%, whereas the morphotypes of elongate, blocky, flabellate, and circular phytoliths accounted for the smallest proportion, normally all below 4.5%. The phytolith size demonstrated an increasing trend in the maturing leaves. The sizes of bilobate, saddle, and acute phytoliths expanded the fastest in tissue-cultured seedling leaves, implying rapid growth of the cell in tissue-cultured seedlings. Accordingly, the tissue-cultured seedlings contained more silicon and phytoliths of larger sizes, which could be a better choice of stock supply for establishing large-scale plantations. If the stock of the seed-cultured and grafted seedlings is to be used, silicon fertilizer application is an optimal option to boost seedling growth.
1. Introduction
Silicon is a mineral element essential for the growth of most plants [1]. It is absorbed from soil by plant roots as soluble silica (Si (OH)4) and transported to different tissues of the plant system via the vascular system during evapotranspiration [2]. Ultimately, it is present in the form of hydrated silica (SiO2·nH2O) as precipitation in the plant cell walls, intracellular cavities, and between cell walls as phytoliths [3]. Phytolith is the main form of silicon in higher plants, accounting for more than 90% of total silicon in plants [3,4], and is perceived as a structure to support the mechanical properties of plant tissue [5,6]. The abrasive nature of phytoliths also deters herbivorous animals and phytophagous insects [7]. Leaf phytoliths facilitate the transmittance of light to the mesophyll, thereby improving the photosynthetic efficiency [8]. Moreover, silicon and phytolith can alleviate the adverse effects of different abiotic and biotic stresses via different mechanisms, including morphological, physiological, biochemical and genetic changes [9]. Despite these benefits, the mechanism driving the evolution of silica phytolith formation is still unclear [10]. The phytoliths “replicate” the original morphology of the plant cell and record the characteristics of the plant cell from which it originated. Different plants produce phytoliths with significantly different morphologies, sizes, numbers, and textures in specific cells and tissues [11,12,13].
Bamboo is an efficient silicon accumulator plant with high phytolith concentration. The silicon content and phytolith morphology varied among different tissues and organs of the bamboo [14]. Bamboo leaves had significantly higher silicon content and a greater diversity of phytolith morphotypes than any other organs [14,15]. Silicon deposition and phytolith concentration on the leaf surface increased with leaf aging [16,17]. Additionally, phytolith morphology in plants was influenced by factors such as nursery methods and environmental conditions [18,19,20].
Dendrocalamus brandisii is a sympodial bamboo species distributed primarily in the tropical and subtropical regions of China, and in Myanmar, Laos, Vietnam, and Thailand [21]. In China, D. brandisii acreage is among the largest forest plantations in southern and southwestern Yunnan. D. brandisi features a short growth cycle, strong regeneration capacity, and tall and straight culm, which is a fine substitute for wood. Increasing efforts are being made to utilize D. brandisii resources. High-quality seedlings are essential to establishing large bamboo plantations. Silicon is an essential element in the growth of bamboo. Zhan et al. [14] analyzed the silicon variation and phytolith morphology in different organs of D. brandisii plants in the wild and found that bamboo leaf contained significantly higher silicon content than any other organs, and its phytolith morphotypes were highly diverse. No other research has been conducted to assess the differences in silicon absorption and utilization by bamboo seedlings from different rearing methods. This study established three treatments of different nursery methods, viz. tissue-cultured, seed-cultured, and grafted, to explore the differences in silicon absorption in bamboo seedlings from different rearing methods. This provides a basis for the application of exogenous silicon fertilizers and helps select the most suitable seedlings as a stock supply for establishing large plantations.
2. Materials and Methods
2.1. Sample Collection and Preparation
Leaf samples were obtained from a nursery in the Bamboo Garden of Southwest Forestry University, Kunming, P. R. China (25°03′42″ N,102°45′40″ E; Altitude:1850 m) in January 2022. D. brandisii seeds were collected from a bamboo forest in the nursery center of the Xinping Forestry Bureau in Yunnan Province in 2019. All seedlings were raised in a seedling bed with a similar soil matrix (50% fermented soil, 50% peat mixture, water content 50%, pH 5, and thickness 30 cm) under natural climate conditions with an annual average temperature of 16.5 °C and average rainfall of 1450 mm for one year [22]. A total of 30 g (10 seedlings for different treatments, 3 g for each seedling) of fresh young and mature leaves were sampled from tissue-cultured, seed-cultured, and grafted seedlings at the same time. The young leaves were greenish, curled, or not fully stretched at the top of the twigs. The mature leaves were dark green in the middle part of the twigs growing about two months after the leaves were fully stretched.
All collected samples were washed and weighed after draining the water, dried at 105 °C for 30 min, followed by drying in an oven at 60 °C to a constant weight for storage.
2.2. Experimental Methods
2.2.1. Determination of Moisture Content
The moisture content was determined by the methods described by Viegas et al. [23]: the samples were weighed after wiping or washing to obtain fresh weight, dried in an oven at 105 °C for 30 m, followed by drying in an oven at 60 °C until the weight did not change, which was the dry weight.
All mean values were obtained by measuring ten samples, and each test was performed three times.
2.2.2. Determination of Ash and Silicon Content
Different samples were oven-dried at 60 °C for 24 h and then ground in a Wiley mill. Ground materials were passed through a no.40 mesh sieve shaker, but retained on no.60 mesh and were used for the determination of ash and silicon content. The ash content was determined using the Chinese national standard GB/T 2677.3-93 [24]; the samples were carbonized in a porcelain crucible on an electric stove, ignited at 575 ± 25 °C for 2 h in a muffle furnace, and then weighed. Ash samples were removed from the muffle furnace and cooled to room temperature. Then, following the standard for silicon GB/T7978 [25], 5 mL of 6 mol/liter HCl was added and evaporated in a steam bath. After three repetitions, the residue was rinsed in distilled water, filtered, and moved to a muffle furnace for heating at 575 ± 25 °C for 2 h, and then weighed for silicon content. Each test was performed in triplicate.
2.2.3. Phytolith Extraction and Observation
Phytoliths in the samples were extracted via chemical oxidation and slide-mounted in Canada balsam following the procedures outlined in Pearsall [26]. At least 3 slides were made for each sample. Identification and counting of at least 400 phytolith grains per slide were carried out, and the occurrence (%) of each morphotype present was calculated following the morphological classification proposed by the International Code for Nomenclature of Phytoliths (ICPN) 1.0 [27] and ICPN 2.0 [28]. Each phytolith morphotype was photographed, and the morphological parameters were measured under a microscope (Leica DM 1000, Tokyo, Japan) to obtain data for horizontal width (W, width) and vertical length (L, length) with objective lenses at 40×, following the measurements by Wang and Lu [3] for phytolith size.
The data obtained were analyzed and compared via one-way ANOVA using the least significant difference method (LSD) to assess the significance at p < 0.05.
3. Results
3.1. Variation in Moisture, Ash, Silicon, and Phytolith Concentration
The moisture content in D. brandisii seedling leaf from different rearing methods varied in the order of 55.41% (grafted seedlings), 59.27% (seed-cultured seedlings), and 60.42% (tissue-cultured seedlings) (Figure 1). Moisture in the young leaf of tissue-cultured seedlings was significantly higher than that in the seed-cultured seedlings. The moisture difference in the mature leaves among the three methods was insignificant. The young leaf of the seedlings from different rearing methods showed higher moisture than the mature leaf, but the difference was insignificant according to the t-test (Table 1).
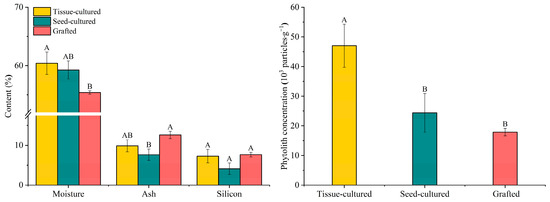
Figure 1.
Comparison of the average moisture, ash, silicon content, and phytolith concentration in D. brandisii seedling leaves raised by different methods. Different letters above bars denote the statistical difference among seedlings raised by different methods at p < 0.05 according to LSD.

Table 1.
Moisture, ash, Si content, and phytolith concentration in D. brandisii seedling leaves raised using different methods.
The ash content in D. brandisii leaf from different cultivation methods ranged from 4.56% to 14.61% (Figure 1). The ash content in the grafted seedling leaf was the highest (12.62%), followed by tissue-cultured seedlings (9.89%) and seed-cultured seedlings (7.65%). The ash content in grafted seedling leaf was significantly higher than that of the seed-cultured seedlings (Table 1; Figure 1). As the leaf matured, the ash content demonstrated an increasing trend. The ash content in the mature leaf from different rearing methods was significantly higher than that of the young leaf (Table 1).
Silicon in D. brandisii seedling leaves varied with different cultivation methods, which was consistent with that of ash, ranging from 3.57% to 10.99%. The grafted seedling leaves (7.66%) had the highest silicon, followed by tissue-cultured seedlings (7.28%) and seed-cultured seedlings (4.13%) (Figure 1). The young leaves of grafted seedlings had the highest silicon content (6.49%), which was significantly higher than that of tissue-cultured seedlings (3.57%) and seed-cultured seedlings (0.95%) (Table 1). With leaf maturation, the silicon content in different D. brandisii seedling leaves showed an increasing tendency. Specifically, the silicon content in the mature leaves of tissue-cultured seedlings increased dramatically and showed a significantly higher value (Table 1).
The phytolith concentration in D. brandisii seedling leaves from different rearing methods demonstrated the order of tissue-cultured seedlings (47.03 × 103 particles·g−1) > grafted seedlings (24.38 × 103 particles·g−1) > seed-cultured seedlings (17.88 × 103 particles·g−1). The tissue-cultured seedling leaves had significantly higher phytolith concentration than grafted and seed-cultured seedlings (Figure 1). Similarly to the trend observed for silicon, the phytolith concentration in the mature leaves of D. brandisii seedlings from the different cultivation methods increased sharply. The phytolith concentration in the mature leaves of tissue-cultured seedlings was far higher than that in the other two types of seedlings (Table 1).
According to the correlation analysis, no significant correlation was found between the moisture and ash, silicon, and phytolith concentrations in D. brandisii leaves from different seedling raising methods (Figure 2). However, there was a highly significant positive correlation (p < 0.01) between the ash and silicon, as well as phytolith concentrations in leaves from seed-cultured and grafted seedlings (Figure 2). Moreover, a significant positive correlation (p < 0.05) was noted between the ash and silicon content, as well as the phytolith concentration for the tissue-cultured seedlings. Additionally, there was a highly significant positive correlation (p < 0.01) between the silicon and phytolith concentrations in D. brandisii leaves from different seedling raising methods.
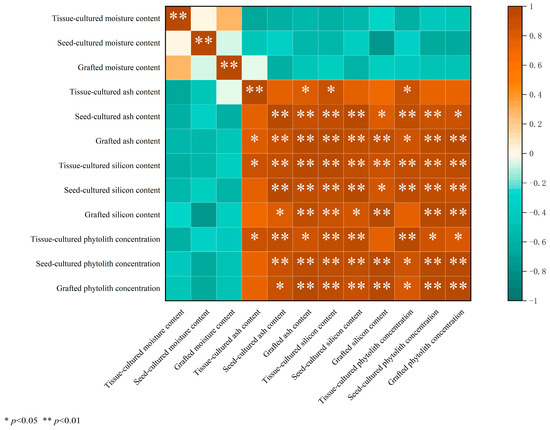
Figure 2.
Correlation between moisture, ash, and Si content, and phytolith concentration in D. brandisii seedling leaves raised by different methods.
3.2. Variation in Phytolith Morphology
Phytolith morphotypes in D. brandisii seedling leaves raised by different methods were identical and grouped into eight morphotypes, including bilobate, saddle, acute, rondel, elongate, blocky, flabellate, and circular based on the classification systems ICPN 1.0 [27] and ICPN 2.0 [28] (Figure 3).
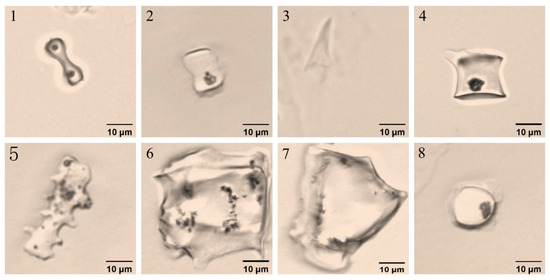
Figure 3.
Phytolith morphotypes in D. brandisii seedling leaves raised by different methods. (1) Bilobate. (2) Saddle. (3) Acute. (4) Rondel. (5) Elongate. (6) Blocky. (7) Flabellate. (8) Circular.
Phytolith assemblages in D. brandisii seedling leaves raised by different methods were characterized by a high frequency of bilobate and saddle, accounting for more than 60%, whereas the morphotypes of elongate, blocky, flabellate, and circular phytolith accounted for the smallest proportion, normally below 4.5% (Table 2; Figure 4 and Figure 5).

Table 2.
Percentages of different phytolith morphotypes in D. brandisii seedling leaves raised by different methods.
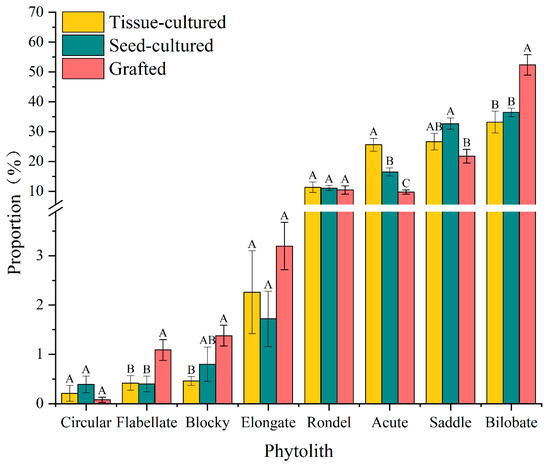
Figure 4.
Average proportion of phytolith morphotypes in D. brandisii seedling leaves raised by different methods. Note: Different capital letters represent the differences in the proportion of the same phytolith morphotype in different seedlings.
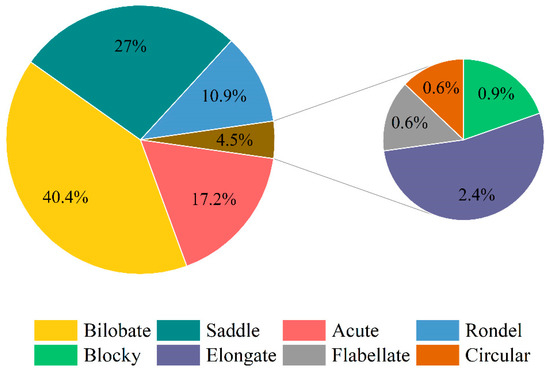
Figure 5.
Average proportion of phytolith morphotypes in D. brandisii seedling leaves.
The bilobate phytolith had the highest frequency (40.4%) and varied in the order of grafted seedlings > seed-cultured seedlings > tissue-cultured seedlings (Figure 4 and Figure 5). The grafted seedling leaves had a significantly higher bilobate phytolith percentage than the other two seedling types. With the leaf maturing, bilobate phytoliths demonstrated an increasing trend, and their proportion in mature leaves was higher than that in young leaves. Specifically, bilobate phytoliths increased sharply in tissue-cultured seedling leaves and their proportion became greater than that in seed-cultured seedlings (Table 2).
Saddle phytoliths ranked in the second highest proportion (27%) and varied in the order of seed-cultured seedlings > tissue-cultured seedlings > grafted seedlings (Figure 4 and Figure 5). However, contrary to the trend of bilobate phytoliths, the saddle phytoliths demonstrated a decreasing trend with leaf maturation (Table 2).
The average proportion of acute phytoliths was also relatively high (17.2%), which varied in the order of tissue-cultured seedlings > seed-cultured seedlings > grafted seedlings. Tissue-cultured seedling leaves had the highest percentage of acute phytoliths, which were significantly higher than tissue- and seed-cultured seedlings (Figure 4 and Figure 5). With the growth of the leaves, the proportion of acute phytoliths all showed an increasing trend in different seedlings, and a significant increase was observed in tissue-cultured seedling leaves (Table 2).
The proportion of rondel phytoliths was highest in tissue-cultured young leaves, followed by grafted and seed-cultured seedlings (Figure 4). With the growth of leaves, their proportion in different seedlings showed a decreasing trend, and a dramatic decrease was observed in tissue-cultured seedling leaves (Table 2).
The proportion of elongate phytoliths was the highest in grafted seedling leaves, followed by seed-cultured and tissue-cultured seedling leaves (Figure 4). With leaf maturation, the elongate phytoliths in tissue-cultured and seed-cultured seedling leaves showed an increasing trend, while showing a slightly decreasing trend in the grafted seedling leaves (Table 2).
Grafted seedling leaves had the highest blocky phytolith proportion, but with leaf maturation, blocky phytoliths showed an increasing trend in tissue- and seed-cultured seedling leaves (Figure 4; Table 2). In mature leaves, it was observed that the proportion was higher in seed-cultured seedlings than in grafted and tissue-cultured seedlings (Table 2).
The proportion of flabellate phytoliths was the highest in grafted seedling leaves (Figure 4). With leaf maturation, the proportion increased in the tissue- and seed-cultured seedlings, whereas grafted seedlings showed a decreasing trend, which was similar to that of elongate and blocky phytoliths (Table 2).
Circular phytoliths showed the highest content in seed-cultured seedling leaves (Figure 4). However, when the leaf matured, its proportion decreased in tissue-cultured seedling leaves, whereas a significant increase was observed in seed-cultured seedling leaves.
3.3. Variation in Phytolith Size
The phytolith assemblage in D. brandisii seedling leaves was characterized by a high frequency of bilobate, saddle, and acute, the sizes of which varied in seedling leaves raised by different methods (Table 3).

Table 3.
The phytolith size in D. brandisii seedling leaves raised by different methods.
The mean length of the bilobate phytoliths varied in the order of seed-cultured seedlings > tissue-cultured seedlings > grafted seedlings, whereas the width varied in the order of tissue-cultured seedlings > seed-cultured seedlings > grafted seedlings (Figure 6). Both the length and width of the bilobate phytoliths in seed- and tissue-cultured seedling leaves were significantly greater than those in grafted seedling leaves (Figure 6). With leaf maturation, the bilobate phytolith size demonstrated an increasing trend, and the mature leaves of tissue-cultured seedlings had significantly longer bilobate phytoliths than those of young leaves (Table 3).
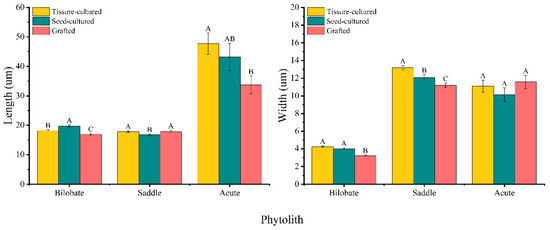
Figure 6.
Average size of phytolith morphotypes in D. brandisii seedling leaves raised by different methods. Different letters above bars denote the statistical differences among seedlings raised by different methods at p < 0.05 according to LSD.
The saddle phytolith was much longer in grafted and tissue-cultured seedling leaves than in seed-cultured seedling leaves, and the saddle phytolith in tissue-cultured seedling leaves was wider (Figure 6). With leaf maturation, both the length and width of saddle phytoliths in D. brandisii tissue-cultured seedling leaves demonstrated an increasing trend. The largest saddle phytolith size was found in the mature leaves of tissue-cultured seedlings. However, the saddle phytolith in seed-cultured seedling leaves showed an opposite trend: their length and width in mature leaves were significantly smaller than those in young leaves (Table 3).
The acute phytolith was significantly longer in tissue-cultured seedling leaves than in grafted seedling leaves. The width of acute phytoliths differed insignificantly among the three seedling leaves (Figure 6). Young leaves of tissue-cultured seedlings had an obviously greater length, whereas the greatest width was found in the grafted seedlings. The size of acute phytoliths in the young leaves of seed-cultured seedlings was the smallest. With leaf maturation, both the length and width of acute phytoliths increased in the seed-cultured seedlings, which decreased in the grafted seedling leaves. It could be noted that the size of the acute phytolith was the largest in the mature leaves of tissue-cultured seedlings (Table 3).
4. Discussion
4.1. Comparison of Moisture, Ash, Si, and Phytolith Concentration in Different D. brandisii Seedling Leaves
In D. brandisii seedlings raised by different methods, the mature leaves had higher ash, silicon, and phytolith concentrations than the young leaves (Table 1). This was consistent with the findings of Zhan [29] that silicon was higher in mature tissues than in young tissues of the bamboo in the wild, and that the number of phytoliths increased with the leaf age. This was also in agreement with the results of Zhu et al. [18] that the ash and silicon in bamboo leaves under different phenological stages varied in the order of old leaf > mature leaf > young leaf. The silicon content in young tissues was significantly lower than that in mature tissues, as the growth and elongation of young tissues had not been completed; therefore, the silicon deposition was insufficient, resulting in significantly lower phytolith concentration in young leaves. Motomura et al. [16] reported that Sasa veitchii Rehder (Poaceae: Bambusoideae) leaves continuously accumulated silica throughout their life and phytoliths were continuously produced with the aging of the tissues. Therefore, the silicon and phytolith content in mature leaves of D. brandisii seedlings raised by different methods showed an increasing trend.
In the present study, the moisture content in D. brandisii seedling leaves raised by different rearing methods increased in the order of tissue-cultured seedlings > seed-cultured seedlings > grafted seedlings. The moisture content was significantly lower in grafted seedling leaves than in tissue-cultured seedlings, whereas the silicon in grafted seedling leaves was higher than that in tissue- and seed-cultured seedlings (Figure 1). This was because silicon deposition occurred passively due to plant water loss, and the low moisture in grafted seedling leaves, resulting in excessive water loss, may have caused an increase in silicon. In addition, the silicon in grafted seedling leaves was higher than that in tissue- and seed-cultured seedlings, which could be attributed to the relatively well-developed root system of the grafted stock, leading to a stronger ability to absorb soluble monosilicic acid in the soil. Silicon promoted the growth of rice roots, increased rooting sprouting (by 20%–30%), enhanced root vitality (by 1.12–1.13 times), and prolonged the functional period of roots to avoid premature senescence [30,31]. Therefore, grafted seedlings may be more conducive to growth performance in later stages.
The phytolith concentration followed the order of tissue-cultured seedlings > seed-cultured seedlings > grafted seedlings, which was consistent with the variation in moisture (Figure 1). Silicon absorbed in the plant changed the permeability of plant tissues and affected the transpiration and water evaporation on the leaf surface [23,32,33]. The cell wall of phytoliths in plants also prevented plant cells from swelling and cracking due to excessive water absorption and stabilized the osmotic pressure of plant cells to a certain extent. Meanwhile, the water in phytoliths played a role in regulating water in plants [34]. In addition, phytoliths straightened the leaves, improved their photosynthetic efficiency [8], and helped sustain the normal photosynthesis and transpiration of plants under changing environments to alleviate plant stress [35]. Thus, silicon and phytoliths promoted water retention and regulated water use efficiency, photosynthesis, and transpiration, thereby improving the drought and high-temperature resistance of plants to some extent. The tissue-cultured seedling leaves had the highest phytolith concentration, possibly contributing to stronger drought and high-temperature resistance, whereas with the lowest phytolith concentration, the grafted seedlings might demonstrate weaker resistance to drought and high temperature.
The variations in ash and silicon in D. brandisii seedling leaves with different rearing methods were positively correlated with the concentration of phytoliths, with the highest concentration observed in the tissue culture seedlings, followed by the seed-cultured seedlings and grafted seedlings (Figure 2). This trend was consistent with previous research showing a transfer of phytolith content to biogenic silica content [36], and a significant positive correlation between the silicon and phytolith content in leaves of three bamboo species grown in soils of different parent materials [37]. The present study found that the ash content in the leaves of the D. brandisii seedlings raised by different rearing methods was higher than that of silicon because the ash content also contained calcium and potassium in addition to silicon [38]. Wang et al. [39] reported that more than 90% of silicon in plant bodies existed in the form of phytoliths, indicating that the variation in phytolith concentration reflected the variation in silicon in bamboo to a certain extent.
4.2. Morphotypes and Proportions of Phytoliths in Different D. brandisii Seedling Leaves
Phytolith composition and abundance in plants were primarily controlled by species [3,40]. Different morphotypes of phytoliths originated from different cells and had different functions. Li et al. [41] found that the morphotypes of phytoliths in bamboo leaves were diverse, including long saddle, fan, rectangular, elongate, hairs, and silicified stomata-shaped phytoliths. Dumb-bell and saddle phytoliths originated from short cells [3], which made a great contribution to the total percentage of phytoliths in bamboo [14,15,42,43], and the high morphological diversity of saddles inside the subfamily Bambusoideae might be related to the earliest origin of the Poaceae family [42]. In the present study, we observed that the phytolith assemblage in D. brandisii seedling leaves raised by different methods was characterized by a high frequency of bilobates and saddles, accounting for more than 60% of the total (Figure 5), which was consistent with previous studies.
The proportion of acute phytoliths was 17.2% (Figure 5), whereas it was only 1.58%-3.05% in D. brandisii reported by Zhan et al. [14], which might be because we classified acute, acute bulbous, and extended acute phytoliths together as acute, resulting in a larger proportion difference. Acute phytoliths were mainly formed in trichome cells and acted as a defense against vertebrate and invertebrate herbivores [44]. The proportion of acute phytoliths was the highest in tissue-cultured seedlings, followed by seed-cultured and grafted seedlings (Figure 4), which may imply a stronger ability to resist biotic and abiotic stress and better adaptability.
Generally, silicon deposition in the long cells of plants formed elongate phytoliths, which functioned as a mechanical support to protect the vascular tissue of the leaves to enhance photosynthetic capacity [3,42]. In this study, there were no significant differences in the proportion of elongate phytoliths in D. brandisii seedling leaves raised by different methods, indicating relatively balanced photosynthetic activity in response to the sunshine.
Additionally, the phytolith morphotypes in D. brandisii seedling leaves raised by different methods were identical, and the proportion of the eight phytolith morphotypes varied in the same order (Figure 4), implying that different rearing methods did not affect the phytolith morphotypes and their proportions.
4.3. Phytolith Sizes in Different D. brandisii Seedling Leaves
In this study, the phytolith assemblage in D. brandisii seedlings raised by different methods was characterized by a high frequency of bilobate, saddle, and acute phytoliths (Figure 4). It was found that both the length and width of the bilobate phytolith in seed- and tissue-cultured seedling leaves were significantly greater than those in grafted seedling leaves. The length and width of the saddle phytoliths in D. brandisii tissue-cultured seedling leaves also demonstrated an increasing trend, similar to that of bilobate phytoliths. The largest average size of the saddle phytolith was found in the mature leaves of the tissue-cultured seedlings. Additionally, it was also observed that the acute phytolith was the largest in the mature leaves of tissue-cultured seedlings (Table 3). The phytoliths could “replicate” the original morphology of the cell and record the characteristics of the plant cell they originated from [11]. The size of the phytoliths of bilobate, saddle, and acute developed the fastest in tissue-cultured seedling leaves (Table 3), implying rapid cell growth in tissue-cultured seedlings. Tissue-cultured seedlings would have better and faster growth than the seed-cultured and grafted seedlings in the later stage.
Li et al. [41] conducted a study on 64 species from 19 genera of subfamily Bambusoideae in the Poaceae family and found that the length and width of saddles ranged from 15.0 μm to 21.0 μm and from 9.0 μm to 15.0 μm, respectively. Our study showed that the length and width of saddle phytoliths in D. brandisii seedlings raised by different methods were 16.12 μm to 19.05 μm and 11.00 μm to 13.05 μm, respectively, which was in or close to the size range of bamboo species. Li et al. [41] classified types of bamboo species based on the average size of saddle phytoliths, with the monopodial bamboo (length 20.6 ± 0.2 μm, width 12.8 ± 0.5 μm) and the sympodial bamboo length 18.0 ± 1.8 μm, width 9.7 ± 0.5 μm). In this study, the mean length and width of saddle phytoliths in D. brandisii seedling leaves were 17.47 ± 0.19 μm and 12.15 ± 0.17 μm, respectively, which was close to the range of sympodial bamboo (Table 3). Tao et al. [45] conducted morphometric and clustering analyses of phytoliths in 17 woody bamboo species in southwestern China and found that the size of phytoliths could be used to classify bamboo species at the genus level. Future research on phytolith size could help to analyze the value of phytolith size in classifying bamboo at the genus or species level.
5. Conclusions
In D. brandisii seedlings raised by different methods, the ash, silicon, and phytolith concentrations were higher in mature leaves than in young leaves. Tissue-cultured seedlings had a significantly higher moisture content than that of grafted seedlings. The ash and silicon demonstrated the same order of grafted seedlings > tissue-cultured seedlings > seed-cultured seedlings. The highest phytolith concentration was found in tissue-cultured seedlings. There was a significant positive correlation between ash, silicon, and phytolith concentrations in the leaves of D. brandisii raised by different methods.
The phytolith morphotypes in D. brandisii seedling leaves raised by different methods were identical and grouped into eight morphotypes as follows: bilobate, saddle, acute, rondel, elongate, blocky, flabellate, and circular. The phytolith assemblage was characterized by a high frequency of bilobate and saddle, accounting for more than 60%, while the morphotypes of elongate, blocky, flabellate, and circular phytoliths accounted for the smallest proportion, normally below 4.5%. The phytolith size demonstrated an increasing trend in maturing leaves. The sizes of bilobate, saddle, and acute phytoliths expanded the fastest in tissue-cultured seedling leaves, implying rapid cell growth in tissue-cultured seedlings. Accordingly, the tissue-cultured seedlings contained more silicon and phytoliths with larger sizes, which could be a better choice of stock supply for establishing large-scale plantations. If the stock of the seed-cultured and grafted seedlings is to be used, silicon fertilizer application is an optimal option to boost seedling growth.
Author Contributions
C.D. and R.X. wrote the main manuscript text. L.Y. and F.Z. collected the data. J.L. and S.W. prepared the figures. M.L. and H.Z. made important revisions to the paper. C.W. and H.Z. approved the final manuscript to be submitted. All authors have read and agreed to the published version of the manuscript.
Funding
This study was jointly funded by the Natural Science Foundation of Yunnan Province (NO. 202001AT070108), National Natural Science Foundation of China (NO. 32160415); Yunnan Provincial Joint Special Project for Basic Research in Agriculture (202101BD070001-114); and Xingdian Talent Support Plan Project.
Data Availability Statement
The datasets generated during and/or analyzed during the study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Manivannan, A.; Soundararajan, P.; Jeong, B.R. Silicon: A “Quasi-Essential” element’s role in plant physiology and development. Front. Plant Sci. 2023, 14, 1157185. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ohnishi, Y.; Kitagishi, K. Chemical forms, mobility and deposition of silicon in rice plant. Soil. Sci. Plant Nutr. 1962, 8, 15–21. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H. Phytolith Study and Its Application; China Ocean Press: Beijing, China, 1993. [Google Scholar]
- Twiss, P.C.; Suess, E.; Smith, R.M. Morphological classification of grass phytoliths. Soil. Sci. Soc. Am. J. 1969, 33, 109–114. [Google Scholar] [CrossRef]
- Currie, H.A.; Perry, C.C. Silica in plants: Biological biochemical and chemical studies. Ann. Bot. 2007, 100, 1383–1389. [Google Scholar] [CrossRef]
- Yamanaka, S.; Takeda, H.; Komatsubara, S.; Ito, F.; Usami, H.; Togawa, E.; Yoshino, k. Structures and physiological functions of silica bodies in the epidermis of rice plants. Appl. Phys. Lett. 2009, 95, 123703. [Google Scholar] [CrossRef]
- Massey, F.P.; Hartley, S.E. Physical defences wear you down: Progressive and irreversible impacts of silica on insect herbivores. J. Anim. Ecol. 2009, 78, 281–291. [Google Scholar] [CrossRef]
- Sato, K.; Yamauchi, A.; Ozaki, N.; Ishigure, T.; Oaki, Y.; Imai, H. Optical properties of biosilicas in rice plants. RSC Adv. 2016, 6, 109168–109173. [Google Scholar] [CrossRef]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of silicon uptake, transport, and deposition in plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef]
- Strömberg, C.A.E.; Di Stilio, V.S.; Song, Z. Functions of phytoliths in vascular plants: An evolutionary perspective. Funct. Ecol. 2016, 30, 1286–1297. [Google Scholar] [CrossRef]
- Bozarth, S.R. Classification of opal phytoliths formed in selected dicotyledons native to the Great Plains. In Phytolith Systematics: Emerging Issues; Springer: Berlin/Heidelberg, Germany, 1992; pp. 193–214. [Google Scholar] [CrossRef]
- Lu, Y.H. Observation of epidermal micromorphology of 23 bamboo leaves. J. Bamboo Res. 1996, 2, 42–44. [Google Scholar]
- Wang, R.H.; Xia, N.H.; Lin, R.S. Epidermal characteristics of the leaves of the genus Bamboo and the genus Peony chophyllum (subfamily Bamboo). J. Trop. Subtrop. Bot. 2002, 10, 22–26. [Google Scholar]
- Zhan, H.; Li, J.; Niu, Z.H.; Li, M.B.; Wang, C.M.; Wang, S.G. Silicon variation and phytolith morphology in different organs of Dendrocalamus brandisii (Munro) Kurz (Bambusoideae). Braz. J. Bot. 2019, 42, 529–541. [Google Scholar] [CrossRef]
- Xu, R.; He, H.; Guo, H.; Zhu, F.; Wang, S.; Dai, C.; Zheng, X.; Xie, D.; Li, H.; Wang, C.; et al. Characteristics of silicon and phytolith distribution in bamboo (Ferrocalamus strictus): Variations between different organs and ages. Rev. Palaeobot. Palynol. 2023, 311, 104817. [Google Scholar] [CrossRef]
- Motomura, H.; Fujii, T.; Suzuki, M. Silica deposition in relation to ageing of leaf tissues in Sasa veitchii (Carriere) Rehder (Poaceae: Bambusoideae). Ann. Bot. 2004, 93, 235–248. [Google Scholar] [CrossRef]
- Niu, Z.; He, W.; Wang, C.; Zhang, L.; Zhan, H.; Wang, S. The Changes in Phytolith Morphology in Culms of Dendrocalamus Giganteus. J. Bamboo Res. 2016, 3, 9–14+25. [Google Scholar] [CrossRef]
- Zhu, F.W.; Niu, Z.H.; Li, J.; Yu, L.X.; Wang, S.G.; Wang, C.M.; Zhan, H. Changes in silicon content and phytolith morphology in Dendrocalamus giganteus at different phenological stages. J. Southwest For. Univ. 2022, 42, 71–77. [Google Scholar] [CrossRef]
- Yi, X.X.; Ma, X.; Liu, M.L.; Wang, F.; Zhang, Q.P. Research progress on effect of silicon on plant stress tolerance and its mechanism. Crop Res. 2020, 34, 398–404. [Google Scholar]
- Li, R.; Fan, J.; Carter, J.; Jiang, N.; Gu, Y.S. Monthly variations of phytoliths in the leaves of the bamboo Dendrocalamus ronganensis (Poaceae: Bambusoideae). Rev. Palaeobot. Palynol. 2017, 246, 62–69. [Google Scholar] [CrossRef]
- Li, D.Z.; Wang, Z.P.; Zhu, Z.D.; Xia, N.H.; Jia, L.Z.; Guo, Z.H.; Yang, G.Y.; Stapleton, C.M.A. Bambuseae. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China, 2006. [Google Scholar]
- He, R.; Qiu, J.; Luo, B. Analysis of Ash and Silica Content of Six Bamboo Species. World Bamboo Ratt. 2016, 14, 4. [Google Scholar] [CrossRef]
- Viegas, D.X.; Viegas, T.P.; Ferreira, A.D. Moisture content of fine forest fuels and fire occurrencein central Portugal. Int. J. Wildland Fire 1992, 2, 69–85. [Google Scholar] [CrossRef]
- GB/T2677.3-1993; Fibrous Raw Material—Determination of Ash. Standards Press of China: Qinhuangdao, China, 1993.
- GB/T 7978-1987; Pulps—Determination of Alcohol–Silicon Dioxide. Standards Press of China: Qinhuangdao, China, 1987.
- Pearsall, D.M. Paleoethnobotany: A Handbook of Procedures, 3rd ed.; Routledge: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Madella, M.; Alexandre, A.; Ball, T. International Code for Phytolith Nomenclature 1.0. Ann. Bot. 2005, 96, 253–260. [Google Scholar] [CrossRef]
- Neumann, K.; Stromberg, C.; Ball, T.; Albert, R.; Vrydaghs, L.; Cummings, L.S. International Code for Phytolith Nomenclature (ICPN) 2.0. Ann. Bot. 2019, 124, 189–199. [Google Scholar] [CrossRef]
- Zhan, H. Morphological Characteristics of Phytolithic Body of 10 Kinds of Clumped Bamboo and the Effect of Exogenous Silicon on the Cold Tolerance of Bamboo Seedlings. Master’s Thesis, Southwest Forestry University, Kunming, China, 2017. [Google Scholar]
- Matichenkov, V.V.; Bochamikova, E.A. Chapter 13 The relationship between silicon and soil physical and chemical properties. Stud. Plant Sci. 2001, 8, 209–219. [Google Scholar] [CrossRef]
- Gong, J.L.; Zhang, H.C.; Long, H.Y.; Hu, Y.Q.; Dai, Q.G.; Huo, Z.Y.; Xu, K.; Wei, H.Y.; Gao, H. Progress in research of nutrition functions and physiological mechanisms of silicon in rice. J. Plant Physiol. 2012, 48, 1–10. [Google Scholar] [CrossRef]
- Yoshida, S.; Ohnishi, Y.; Kitagishi, K. Histochemistry of silicon in rice plant. Soil. Sci. Plant Nutr. 1962, 8, 36–41. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Interaction between calcium and silicon in water-cultured rice plants. Plant Soil. 1993, 148, 107–113. [Google Scholar] [CrossRef]
- Agarie, S.; Uchida, H.; Agata, W.; Kubota, F.; Kaufman, P.B. Effects of silicon on transpiration and leaf conductance in rice plants (Oryza sativa L.). Plant Prod. Sci. 1998, 1, 89–95. [Google Scholar] [CrossRef]
- Zhang, G.Q. Effect of Silicon on the Growth and Some Physiological Characteristics of Ginger. Master’s Thesis, Shandong Agricultural University, Taian, China, 2008. [Google Scholar] [CrossRef]
- Rastogi, A.; Yadav, S.; Hussain, S.; Kataria, S.; Hajihashemi, S.; Kumari, P.; Yang, X.; Brestic, M. Does silicon really matter for the photosynthetic machinery in plants…? Plant Physiol. Biochem. 2021, 169, 40–48. [Google Scholar] [CrossRef]
- Song, Z.; Liu, H.; Li, B.; Yang, X.M. The production of phytolith-occluded carbon in China’s forests: Implications to biogeochemical carbon sequestration. Glob. Chang. Biol. 2013, 19, 2907–2915. [Google Scholar] [CrossRef]
- Liu, J.X.; Huang, Z.T.; Jiang, P.K.; Huang, C.P.; Feng, S.F.; Chen, C.; Yin, S. Effects of parent rock and bamboo age on silicon and phytolith-occluded carbon in the leaves of Moso bamboo. Chin. J. Appl. Ecol. 2017, 28, 2917–2922. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, B.Z.; Li, X.J.; Kong, W.J.; Weng, C.H.; Hu, X.L. Carbon storage and vertical spatial distribution of Dendrocalamopsis vario-striata ecosystem. J. Trop. Subtrop. Bot. 2012, 20, 72–77. [Google Scholar] [CrossRef]
- Piperno, D.R.; Pearsall, D.M. The silica bodies of tropical American grasses: Morphology, taxonomy, and implications for grass systematics and fossil phytolith identification. Smithson. Contrib. Bot. 1998, 85, 1–46. [Google Scholar] [CrossRef]
- Li, Q.; Xu, D.K.; Lu, H.Y. Morphology of phytolith in Bambusoideae (Poaceae) and its ecological significance. Quat. Sci. 2005, 25, 777–784. [Google Scholar] [CrossRef]
- Gu, Y.S.; Liu, H.Y.; Wang, H.L.; Li, R.C.; Yu, J.X. Phytoliths as a method of identification for three genera of woody bamboos (Bambusoideae) in tropical southwest China. J. Archaeol. Sci. 2016, 68, 46–53. [Google Scholar] [CrossRef]
- Tao, X.Y. Study on the Taxonomic Significance of Bamboo Leaf Phytolithic Morphology and Micro-Element Composition. Master’s Thesis, Guilin University of Technology, Guilin, China, 2021. [Google Scholar] [CrossRef]
- Zhang, X.; Gélin, U.; Spicer, R.A.; Wu, F.; Farnsworth, A.; Chen, P.; Del, R.C.; Li, S.; Liu, J.; Jian, H.; et al. Rapid Eocene diversification of spiny plants in subtropical woodlands of central Tibet. Nat. Commun. 2022, 13, 3787. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.Y.; Wen, M.D.; Li, R.C.; Vachula, R.S.; Pang, L.N.; Li, C.; Yang, K.Q.; Jiang, N. Phytolith sizes and assemblages differentiate genera and ecotypes of woody bamboos in subtropical Southwest China. Rev. Palaeobot. Palynol. 2020, 272, 104129. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).