Wood-Decay Fungi Fructifying in Mediterranean Deciduous Oak Forests: A Community Composition, Richness and Productivity Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design
- Very Fine Woody Debris (VFWD): comprising branches and twigs with a diameter of ≤5 cm.
- Fine Woody Debris (FWD): encompassing logs with a diameter between 5 and 10 cm.
- Coarse Woody Debris (CWD): including logs or snags with a diameter of ≥10 cm.
- Stumps: for the wood-decay fungi preferences for species-specific woody debris sizes and decay description (surveyed during 2012, 2013 and 2014), stumps are categorized as CWD according to the classification by Albrecht et al. [39].
- Decay Stage 1 (DS1): Characterized by recently dead or cut trunks or pieces of wood, with firm wood, fresh bark and phloem. In this stage, the knife only penetrates a few millimeters into the wood.
- Decay Stage 2 (DS2): Represents an intermediate decomposition stage, where the wood is partially decayed and often accompanied by loosened pieces of bark. At this stage, the knife typically penetrates 2 ± 5 cm into the wood.
- Decay Stage 3 (DS3): Corresponds to the advanced stages of decomposition, where most of the wood is soft throughout. In this stage, the entire blade of the knife easily penetrates the wood.
2.3. Plot-Specific Woody Debris Variables
2.4. Data Analysis
3. Results
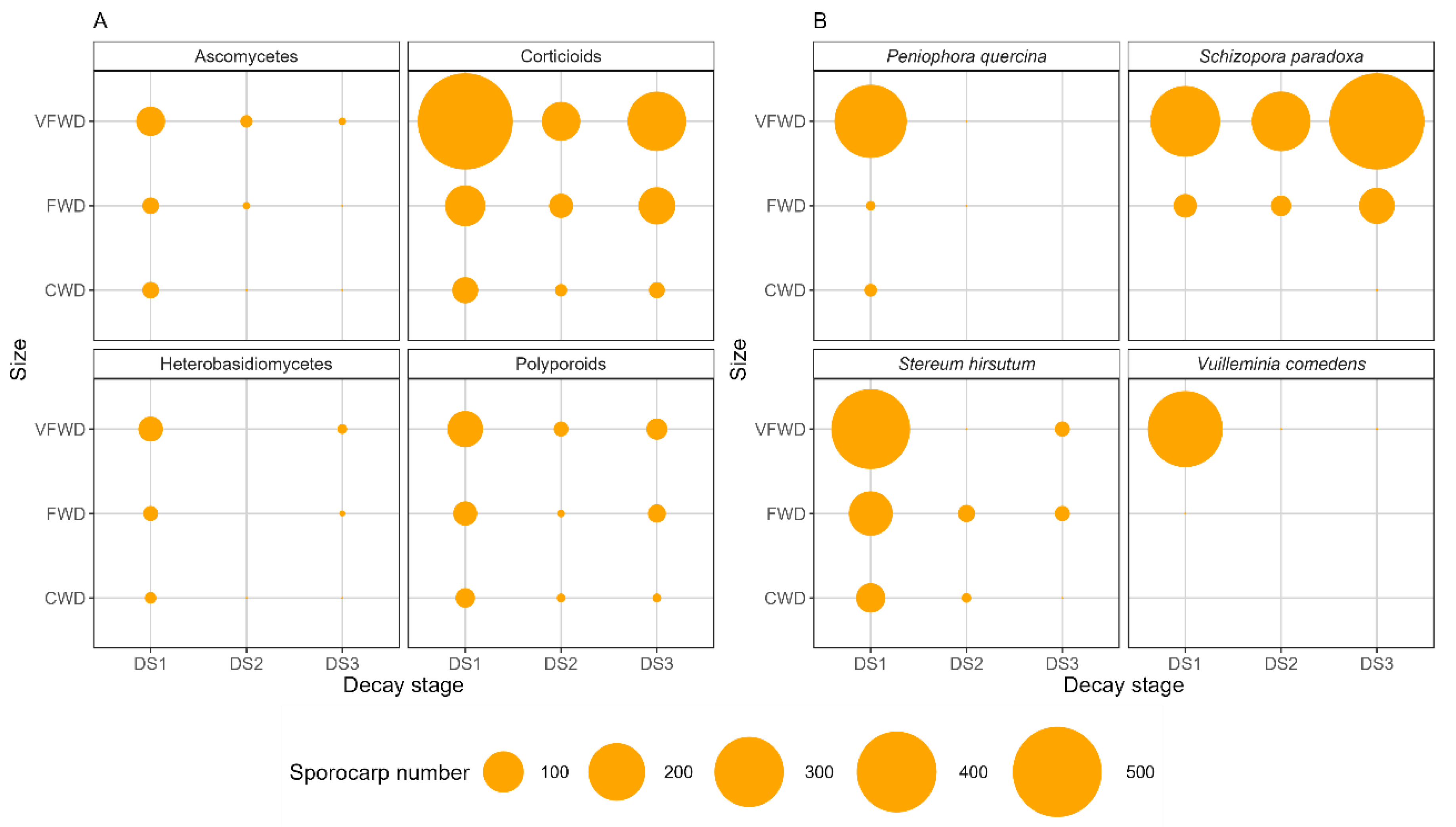
3.1. Wood-Decay Fungi Community Composition
3.2. Wood-Decay Fungi Productivity and Diversity and Plot-Specific Woody Size
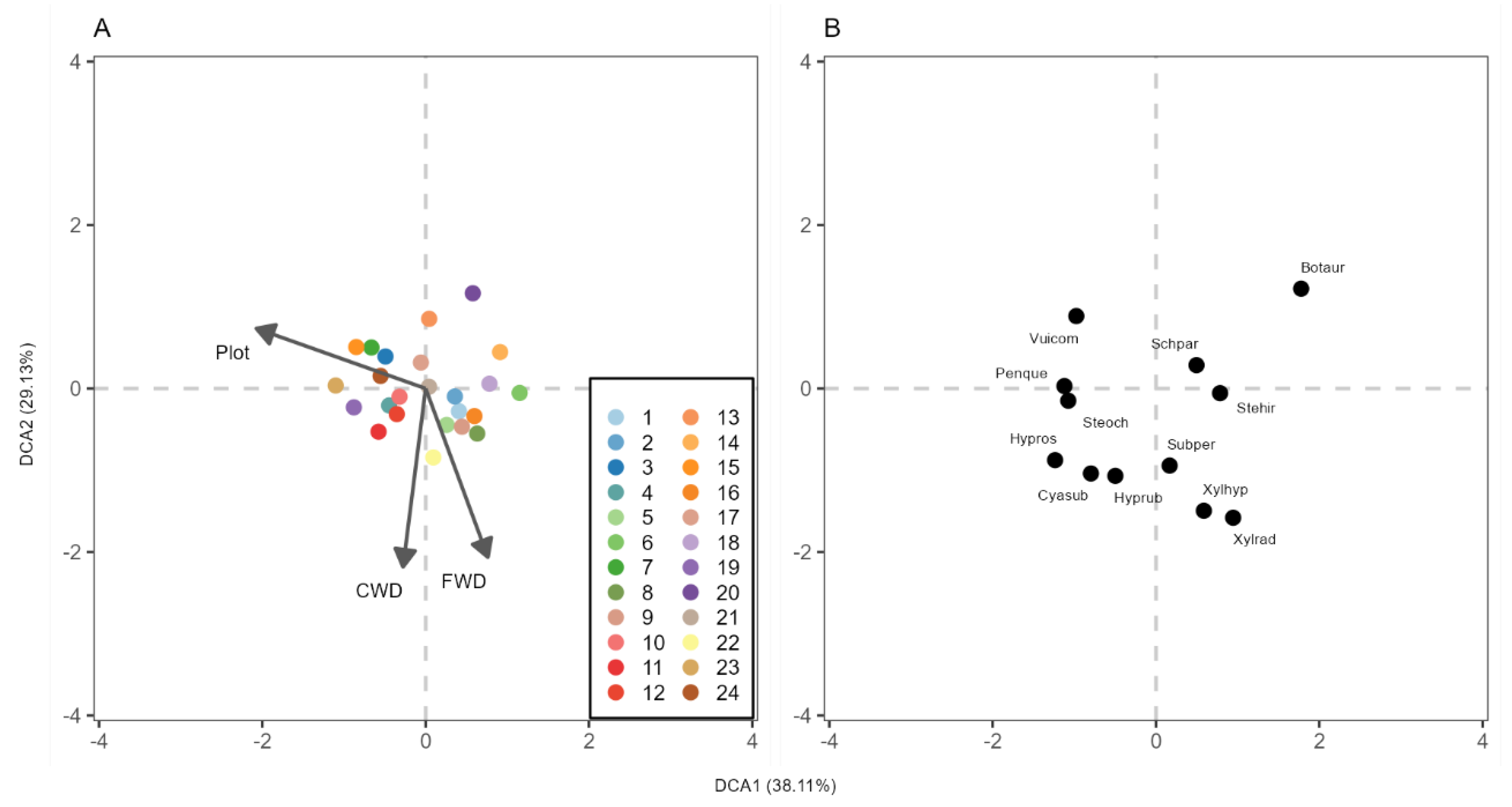
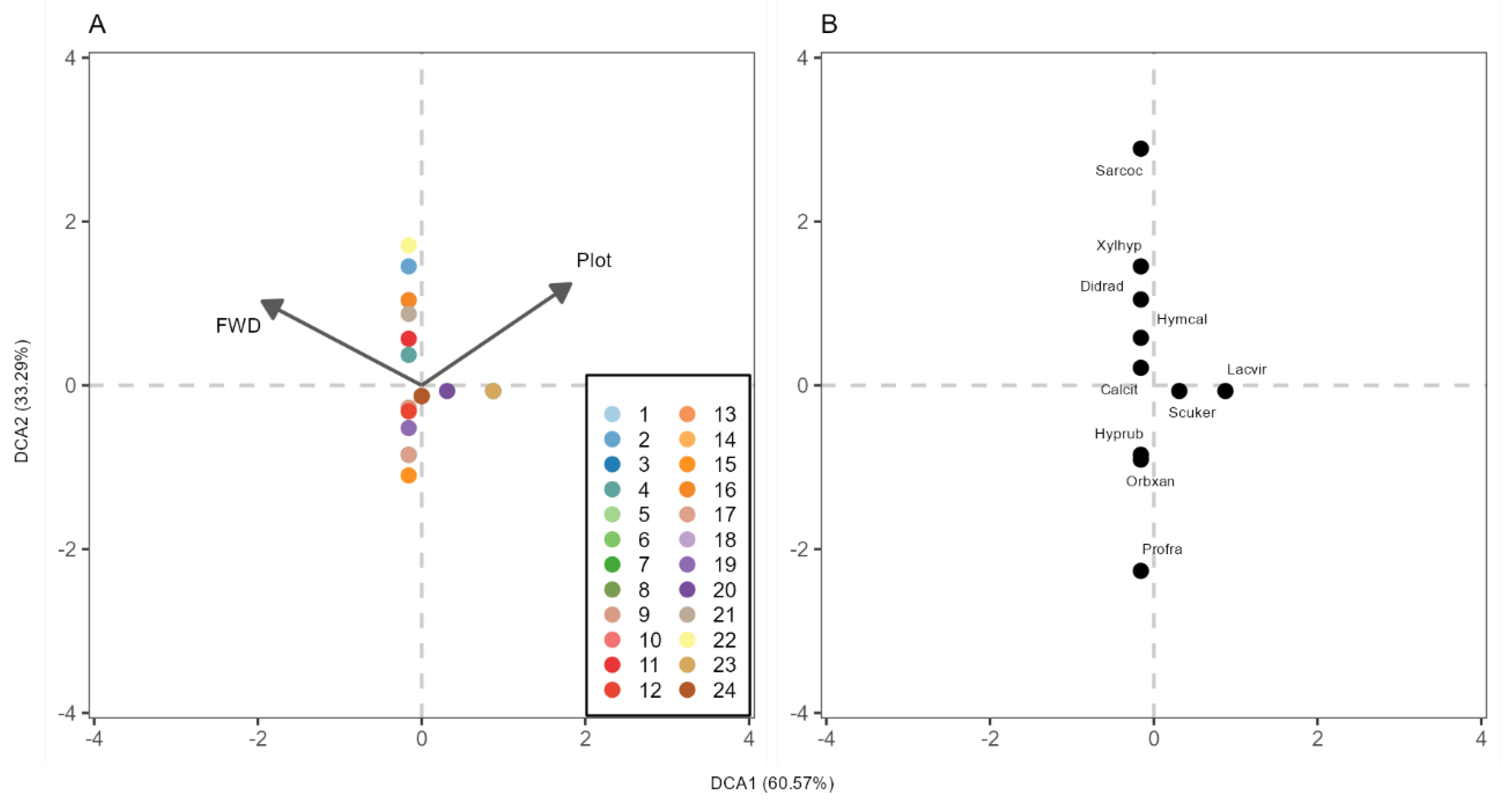
3.3. Wood-Decay Fungi Community Composition and Plot-Specific Woody Size
4. Discussion
4.1. Wood-Decay Fungi Community Composition
4.2. Wood-Decaying Fungi Productivity and Diversity and Plot-Specific Woody Size
4.3. Wood-Decaying Fungi Community Composition and Plot-Specific Woody Size
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Study Area | Season | Decay | Size | Group |
|---|---|---|---|---|---|
| Abortiporus biennis (Bull.) Singer | PAL | Autumn | 1 | C | P |
| Amylostereum laevigatum (Fr.) Boidin | COR/PAL | Spring/Autumn | 1, 2 | V | C |
| Antrodia albida (Fr.) Donk | PAL | Autumn | 1 | V | P |
| Antrodia ramentacea (Berk. & Broome) Donk | COR/PAL | Spring/Autumn | 1, 3 | V, F | P |
| Antrodiella genistae (Bourdot & Galzin) A. David | PAL | Autumn | 1 | V | P |
| Antrodiella romellii (Donk) Niemelä | COR/PAL | Spring/Autumn | 1, 3 | V, F | P |
| Antrodiella semisupina (Berk. & M.A. Curtis) Ryvarden | COR/PAL | Autumn | 1 | V, C | P |
| Athelia decipiens (Höhn. & Litsch.) J. Erikss. | COR/PAL | Autumn | 1 | V | C |
| Athelia epiphylla Pers. | COR/PAL | Spring/Autumn | 1, 3 | V, F | C |
| Athelia sp. | COR | Autumn | 5 | F | C |
| Athelia sp. 2 | PAL | Autumn | 1 | V | C |
| Athelia tenuispora Jülich | PAL | Autumn | 3 | F | C |
| Atheliachaete galactites (Bourdot & Galzin) Ţura, Zmitr., Wasser & Spirin | COR/PAL | Autumn | 1, 3 | V | C |
| Atheliachaete sanguinea (Fr.) Spirin & Zmitr. | PAL | Autumn | 1 | V | C |
| Auricularia auricula-judae (Bull.) Quél. | COR/PAL | Autumn | 1 | V, F, C | H |
| Auricularia mesenterica (Dicks.) Pers. | COR/PAL | Spring/Autumn | 1 | V, F, C | H |
| Basidiodendron caesiocinereum (Höhn. & Litsch.) Luck-Allen | COR/PAL | Autumn | 1, 3 | V, C | H |
| Biscogniauxia mediterranea (De Not.) Kuntze | PAL | Autumn | 1 | F | A |
| Bjerkandera adusta (Willd.) P. Karst. | PAL | Autumn | 2 | V | P |
| Botryobasidium aureum Parmasto | PAL | Spring/Autumn | 1, 2, 3 | V, F, C | C |
| Botryobasidium laeve (J. Erikss.) Parmasto | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | C |
| Botryohypochnus isabellinus (Fr.) J. Erikss. | COR/PAL | Autumn | 1, 2 | V | C |
| Byssomerulius corium (Pers.) Parmasto | COR/PAL | Autumn | 1, 3 | V, F | C |
| Byssomerulius hirtellus (Burt) Parmasto | COR | Spring/Autumn | 2, 3 | V, F | C |
| Calocera cornea (Batsch) Fr | COR/PAL | Autumn | 1, 3 | V | H |
| Calycina citrina (Hedw.) Gray | COR/PAL | Autumn | 1, 2 | V | A |
| Capitotricha bicolor (Bull.) Baral | PAL | Spring | 1 | V | A |
| Ceriporia excelsa S. Lundell ex Parmasto | COR | Autumn | 3 | V | P |
| Ceriporia purpurea (Fr.) Donk | COR/PAL | Spring | 2, 3 | V | P |
| Ceriporiopsis mucida (Pers.) Gilb. & Ryvarden | COR/PAL | Spring/Autumn | 1, 2, 3 | V, C | P |
| Cyanosporus subcaesius (A. David) B.K. Cui, L.L. Shen & Y.C. Dai | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F | P |
| Cylindrobasidium laeve (Pers.) Chamuris | COR/PAL | Spring/Autumn | 1, 2 | V, F, C | C |
| Dacrymyces stillatus Nees | COR/PAL | Autumn | 1 | V | H |
| Daedaleopsis nitida (Durieu & Mont.) Zmitr. & Malysheva | COR/PAL | Spring/Autumn | 1, 3 | V, F, C | P |
| Dasyscyphella nivea (R. Hedw.) Raitv. | COR/PAL | Spring/Autumn | 1, 3 | V, F | A |
| Dendrothele acerina (Pers.) P.A. Lemke | PAL | Autumn | 1, 3 | V | C |
| Diatrype stigma (Hoffm.) Fr. | PAL | Spring/Autumn | 1 | V | A |
| Diderma radiatum (L.) Morgan | COR/PAL | Spring | 1 | V | A |
| Ditiola peziziformis (Lév.) D.A. Reid | PAL | Autumn | 2 | V | A |
| Efibula tuberculata (P. Karst.) Zmitr. & Spirin | COR/PAL | Autumn | 1, 3 | V | C |
| Eutypa scabrosa (Bull.) Auersw. | COR | Autumn | 1 | V | A |
| Exidia glandulosa (Bull.) Fr. | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | H |
| Exidia recisa (Ditmar) Fr. | PAL | Autumn | 1 | F | H |
| Exidia thuretiana (Lév.) Fr. | COR/PAL | Autumn | 1 | V | H |
| Exidiopsis calcea (Pers.) K. Wells | COR/PAL | Spring/Autumn | 1, 3 | V, F | H |
| Exidiopsis effusa Bref. | PAL | Autumn | 1 | V | H |
| Fasciodontia bugellensis (Ces.) Yurchenko, Riebesehl & Langer | PAL | Autumn | 1 | V | C |
| Fibrodontia gossypina Parmasto | COR/PAL | Autumn | 3 | V | C |
| Fomitiporia robusta (P. Karst.) Fiasson & Niemelä | COR | Autumn | 1 | V, F | P |
| Fuscoporia contigua (Pers.) G. Cunn. | COR | Autumn | 1 | F | P |
| Fuscoporia ferruginosa (Schrad.) Murrill | COR/PAL | Spring/Autumn | 3 | V, F | P |
| Fuscoporia torulosa (Pers.) T. Wagner & M. Fisch. | COR/PAL | Autumn | 1 | V, F | P |
| Fuscopostia leucomallella (Murrill) B.K. Cui, L.L. Shen & Y.C. Dai | PAL | Autumn | 1, 3 | V, C | P |
| Ganoderma australe (Fr.) Pat. | PAL | Autumn | 1 | C | P |
| Hapalopilus rutilans (Pers.) Murrill | COR/PAL | Spring/Autumn | 1, 3 | V, F, C | P |
| Heteroradulum deglubens (Berk. & Broome) Spirin & Malysheva | COR | Spring/Autumn | 1 | V | H |
| Hymenochaete cinnamomea (Pers.) Bres | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | C |
| Hymenochaete rubiginosa (Dicks.) Lév. | COR/PAL | Spring/Autumn | 1 | V, F, C | C |
| Hymenoscyphus calyculus (Fr.) W. Phillips | PAL | Spring | 1 | V | A |
| Hyphoderma crustulinum (Bres.) Nakasone | PAL | Spring | 3 | F | C |
| Hyphoderma litschaueri (Burt) J. Erikss. & Å. Strid | COR | Autumn | 1, 2 | V | C |
| Hyphoderma medioburiense (Burt) Donk | PAL | Autumn | 1 | V | C |
| Hyphoderma nemorale K.H. Larss. | COR/PAL | Spring/Autumn | 1, 3 | V, F, C | C |
| Hyphoderma occidentale (D.P. Rogers) Boidin & Gilles | COR/PAL | Spring/Autumn | 1, 3 | V, F | C |
| Hyphoderma orphanellum (Bourdot & Galzin) Donk | COR | Autumn | 1 | V | C |
| Hyphoderma roseocremeum (Bres.) Donk | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | C |
| Hyphoderma setigerum (Fr.) Donk | COR/PAL | Spring/Autumn | 1, 2 | V, F | C |
| Hyphoderma sp. | PAL | Autumn | 3 | V | C |
| Hyphoderma transiens (Bres.) Parmasto | COR | Spring | 3 | F | C |
| Hyphodontia alutaria (Burt) J. Erikss. | COR | Autumn | 1 | V | C |
| Hyphodontia arguta (Fr.) J. Erikss. | COR | Autumn | 1 | F | C |
| Hyphodontia quercina (Pers.) J. Erikss. | COR/PAL | Spring/Autumn | 1, 3 | V, F | C |
| Hypochnicium cremicolor (Bres.) H. Nilsson & Hallenb. | COR | Spring | 3 | C | C |
| Hypoxylon fuscum (Pers.) Fr. | COR/PAL | Spring/Autumn | 1 | V | A |
| Hypoxylon rubiginosum (Pers.) Fr. | COR/PAL | Spring/Autumn | 1, 2 | V, F, C | A |
| Incrustoporia chrysella (Niemelä) Zmitr. | PAL | Autumn | 3 | F | P |
| Irpex lacteus (Fr.) Fr. | COR | Autumn | 1 | C | P |
| Junghuhnia nitida (Pers.) Ryvarden | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | P |
| Lachnum virgineum (Batsch) P. Karst. | COR/PAL | Spring | 1 | V | A |
| Laxitextum bicolor (Pers.) Lentz | COR | Autumn | 1 | V | P |
| Lindtneria chordulata (D.P. Rogers) Hjortstam | COR/PAL | Autumn | 1 | V | C |
| Lyomyces crustosus (Pers.) P. Karst. | COR/PAL | Spring/Autumn | 1, 3 | V, F | C |
| Lyomyces juniperi (Bourdot & Galzin) Riebesehl & Langer | COR/PAL | Spring/Autumn | 1, 3 | V | C |
| Lyomyces pruni (Lasch) Riebesehl & Langer | COR/PAL | Spring/Autumn | 1, 2 | V, F | C |
| Lyomyces sambuci (Pers.) P. Karst. | PAL | Autumn | 3 | V | C |
| Mycoacia aurea (Fr.) J. Erikss. & Ryvarden | COR | Spring | 3 | F | C |
| Mycoacia fuscoatra (Fr.) Donk | PAL | Spring | 1 | V | C |
| Mycoacia livida (Pers.) Zmitr. | COR | Spring/Autumn | 2, 3 | V, F | C |
| Mycoacia nothofagi (G. Cunn.) Ryvarden | COR | Spring | 3 | V | C |
| Mycoacia uda (Fr.) Donk | COR | Autumn | 1 | V | C |
| Mycoaciella bispora (Stalpers) J. Erikss. & Ryvarden | COR | Autumn | 1 | V | C |
| Neoantrodia serialis (Fr.) Audet | COR/PAL | Spring/Autumn | 2, 3 | V, C | P |
| Oligoporus sp. | COR | Autumn | 3 | F | P |
| Orbilia coccinella Fr. | COR | Autumn | 3 | V | A |
| Orbilia xanthostigma (Fr.) Fr. | COR/PAL | Autumn | 1, 2 | V, F | A |
| Peniophora boidinii D.A. Reid | PAL | Spring | 1 | V | C |
| Peniophora cinerea (Pers.) Cooke | COR/PAL | Autumn | 1, 3 | V, C | C |
| Peniophora incarnata (Pers.) P. Karst. | COR | Autumn | 1 | V | C |
| Peniophora lycii (Pers.) Höhn. & Litsch. | COR/PAL | Spring/Autumn | 1 | V | C |
| Peniophora meridionalis Boidin | COR/PAL | Autumn | 1 | V | C |
| Peniophora quercina (Pers.) Cooke | COR/PAL | Spring/Autumn | 1, 2 | V, F, C | C |
| Peniophorella praetermissa (P. Karst.) K.H. Larss. | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | C |
| Phaeophlebiopsis ravenelii (Cooke) Zmitr. | COR/PAL | Autumn | 1, 2, 3 | V, F | C |
| Phanerochaete calotricha (P. Karst.) J. Erikss. & Ryvarden | PAL | Autumn | 1 | F | C |
| Phanerochaete laevis (Fr.) J. Erikss. & Ryvarden | COR | Spring | 1 | V | C |
| Phanerochaete sordida (P. Karst.) J. Erikss. & Ryvarden | PAL | Spring/Autumn | 1, 3 | V | C |
| Phanerochaete velutina (DC.) P. Karst. | COR/PAL | Spring/Autumn | 1, 3 | V, F, C | C |
| Phellinus pomaceus (Pers.) Maire | COR | Spring | 1 | V, F | P |
| Phlebia lilascens (Bourdot) J. Erikss. & Hjortstam | PAL | Autumn | 2 | V | C |
| Phlebia sp. | COR/PAL | Autumn | 1 | V, F | C |
| Phlebia subochracea (Alb. & Schwein.) J. Erikss. & Ryvarden | COR/PAL | Spring | 1, 3 | V, F | C |
| Postia simanii (Pilát ex Pilát) Jülich | PAL | Spring | 3 | F | P |
| Propolis farinosa (Pers.) Fr. | COR | Autumn | 1, 2 | V | A |
| Radulomyces confluens (Fr.) M.P. Christ. | PAL | Autumn | 1 | V | C |
| Radulomyces molaris (Chaillet ex Fr.) M.P. Christ. | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | C |
| Resiniporus resinascens (Romell) Zmitr. | COR | Autumn | 3 | F | P |
| Rutstroemia bolaris (Batsch) Rehm | PAL | Autumn | 1 | V | A |
| Rutstroemia firma (Pers.) P. Karst. | PAL | Autumn | 1 | V | A |
| Sarcoscypha coccinea (Gray) Boud. | COR/PAL | Autumn | 1, 3 | V | A |
| Schizopora paradoxa (Schrad.) Donk | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | C |
| Scutellinia kerguelensis (Berk.) Kuntze | COR | Spring | 1, 2, 3 | V, C | A |
| Sebacina sp. | COR | Autumn | 1 | V | H |
| Sidera vulgaris (Fr.) Miettinen | COR/PAL | Spring/Autumn | 2, 3 | F, C | P |
| Skeletocutis nivea (Jungh.) Jean Keller | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | P |
| Skeletocutis percandida (Malençon & Bertault) Jean Keller | COR | Autumn | 3 | F | P |
| Steccherinum fimbriatum (Pers.) J. Erikss. | COR/PAL | Spring/Autumn | 1, 3 | V, F, C | C |
| Steccherinum lacerum (P. Karst.) Kotir. & Saaren. | COR | Spring/Autumn | 1 | F | C |
| Steccherinum ochraceum (Pers.) Gray | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | C |
| Steccherinum semisupiniforme (Murrill) Miettinen | PAL | Autumn | 1 | V | P |
| Stereum gausapatum (Fr.) Fr. | COR/PAL | Spring/Autumn | 1 | V, F | C |
| Stereum hirsutum (Willd.) Pers. | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | C |
| Stereum ochraceoflavum (Schwein.) Sacc. | COR/PAL | Spring/Autumn | 1 | V, F | C |
| Stereum reflexulum Lloyd | COR | Autumn | 1 | V | C |
| Subulicystidium longisporum (Pat.) Parmasto, | PAL | Autumn | 1, 2 | V, C | C |
| Subulicystidium perlongisporum Boidin & Gilles | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | C |
| Szczepkamyces campestris (Quél.) Zmitr. | COR/PAL | Autumn | 1 | V, F | P |
| Tapesia fusca (Pers.) Fuckel | PAL | Autumn | 1 | V | A |
| Terana coerulea (Lam.) Kuntze | COR/PAL | Autumn | 1 | V | C |
| Tomentella asperula (P. Karst.) Höhn. & Litsch. | COR | Autumn | 1 | C | C |
| Tomentella ferruginea (Pers.) Pat. | PAL | Autumn | 3 | F | C |
| Trametes ochracea (Pers.) Gilb. & Ryvarden | PAL | Autumn | 1, 3 | F, C | P |
| Trametes versicolor (L.) Lloyd | COR | Autumn | 1 | V | P |
| Trechispora cohaerens (Schwein.) Jülich & Stalpers | PAL | Autumn | 1 | V | C |
| Trechispora farinacea (Pers.) Liberta | COR | Autumn | 1, 3 | V | C |
| Trechispora fastidiosa (Pers.) Liberta | COR | Autumn | 3 | F | C |
| Trechispora microspora (P. Karst.) Liberta | PAL | Autumn | 1 | V | C |
| Trechispora mollusca (Pers.) Liberta | COR/PAL | Spring/Autumn | 2, 3 | V, F, C | C |
| Trechispora nivea (Pers.) K.H. Larss. | COR | Spring | 3 | F | C |
| Tremella globispora D.A. Reid | PAL | Autumn | 1 | V | H |
| Tremella mesenterica Retz. | COR | Autumn | 1 | V | H |
| Trichaptum biforme (Fr.) Ryvarden | COR/PAL | Spring/Autumn | 1, 2, 3 | F, C | P |
| Tubulicrinis medius (Bourdot & Galzin) Oberw. | COR | Autumn | 1 | F | C |
| Tulasnella pallida Bres. | COR/PAL | Autumn | 1, 3 | V, F, C | H |
| Vitreoporus dichrous (Fr.) Zmitr. | COR | Autumn | 1 | V, C | P |
| Vuilleminia comedens (Nees) Maire | COR/PAL | Spring/Autumn | 1, 3, 5 | V, F | C |
| Xenasmatella ardosiaca (Bourdot & Galzin) Stalpers | COR | Autumn | 1, 3 | V, C | C |
| Xylaria hypoxylon (L.) Grev. | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F, C | A |
| Xylodon asper (Fr.) Hjortstam & Ryvarden | COR | Autumn | 1, 3 | V, F | C |
| Xylodon brevisetus (P. Karst.) Hjortstam & Ryvarden | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F | C |
| Xylodon flaviporus (Berk. & M.A. Curtis ex Cooke) Riebesehl & Langer | COR/PAL | Spring/Autumn | 1, 2, 3 | F, C | C |
| Xylodon nesporii (Bres.) Hjortstam & Ryvarden | COR/PAL | Spring/Autumn | 1, 2, 3 | V, F | C |
| Xylodon radula (Fr.) Ţura, Zmitr., Wasser & Spirin (Fr.) Nobles | COR/PAL | Autumn | 1, 2, 3 | V, F, C | C |
| Xylodon raduloides Riebesehl & Langer | COR/PAL | Spring/Autumn | 1, 3 | V, F | C |
References
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Christensen, M. Does size matter? On the importance of various dead wood fractions for fungal diversity in Danish beech forests. For. Ecol. Manag. 2004, 201, 105–117. [Google Scholar] [CrossRef]
- Lonsdale, D.; Pautasso, M.; Holdenrieder, O. Wood-decaying fungi in the forest: Conservation needs and management options. Eur. J. For. Res. 2008, 127, 1–22. [Google Scholar] [CrossRef]
- Fukasawa, Y. Ecological impacts of fungal wood decay types: A review of current knowledge and future research directions. Ecol. Res. 2021, 36, 910–931. [Google Scholar] [CrossRef]
- McComb, W. Dying, Dead, and Down Trees. Maintaining Biodiversity in Forest Ecosystems; Cambridge University Press: Cambridge, UK, 1999; pp. 335–372. [Google Scholar]
- Lindenmayer, D.B.; Noss, R.F. Salvage logging, ecosystem processes, and biodiversity conservation. Conserv. Biol. 2006, 20, 949–958. [Google Scholar] [CrossRef]
- Maser, C.; Trappe, J. The seen and unseen world of the fallen tree. USDA Forest Service, Pacific Northwest Forest and Range Experiment Station. In General Technical Report PNW-164; Pacific Northwest Research Station: Corvallis, OR, USA, 1984; 56p. [Google Scholar]
- Stevens, V. The Ecological Role of Coarse Woody Debris: An Overview of the Ecological Role of CWD in BC Forests; British Columbia Ministry of Forests: Victoria, BC, Canada, 1997. [Google Scholar]
- Bujoczek, L.; Bujoczek, M. Factors influencing the diversity of deadwood, a crucial microhabitat for many rare and endangered saproxylic organisms. Ecol. Indic. 2022, 142, 109197. [Google Scholar] [CrossRef]
- Rydin, H.; Diekmann, M.; Hallingbäck, T. Biological Characteristics, Habitat Associations, and Distribution of Macrofungi in Sweden. Conserv. Biol. 1997, 11, 628–640. [Google Scholar] [CrossRef]
- Sippola, A.L.; Renvall, P. Wood-decomposing fungi and seed-tree cutting: A 40-year perspective. For. Ecol. Manag. 1999, 115, 183–201. [Google Scholar] [CrossRef]
- Boddy, L. Fungal community ecology and wood decomposition processes in angiosperms: From standing tree to complete decay of coarse woody debris. Ecol. Bull. 2001, 49, 43–56. [Google Scholar]
- Nordén, B.; Ryberg, M.; Götmark, F.; Olausson, B. Relative importance of coarse and fine woody debris for the diversity of wood-inhabiting fungi in temperate broadleaf forests. Biol. Conserv. 2004, 117, 1–10. [Google Scholar] [CrossRef]
- Juutilainen, K.; Halme, P.; Kotiranta, H.; Mönkkönen, M. Size matters in studies of dead wood and wood-inhabiting fungi. Fungal Ecol. 2011, 4, 342–349. [Google Scholar] [CrossRef]
- Küffer, N.; Gillet, F.; Senn-Irlet, B.; Job, D.; Aragno, M. Ecological determinants of fungal diversity on dead wood in European forests. Fungal Divers. 2008, 30, 83–95. [Google Scholar]
- D'Aguanno, M.; Perini, C.; Cantini, D.; Salerni, E. Analysis of diversity of wood-inhabiting fungi retrieved from a Mediterranean forest dominated by Pinus pinaster Aiton. Ital. J. Mycol. 2016, 45, 1–12. [Google Scholar] [CrossRef]
- Persiani, A.M.; Lombardi, F.; Lunghini, D.; Granito, V.M.; Tognetti, R.; Maggi, O.; Pioli, S.; Marchetti, M. Stand structure and deadwood amount influences saproxylic fungal biodiversity in Mediterranean mountain unmanaged forests. Iforest-Biogeosciences For. 2015, 9, 115–124. [Google Scholar] [CrossRef]
- Abrego, N.; Salcedo, I. Response of wood-inhabiting fungal community to fragmentation in a beech forest landscape. Fungal Ecol. 2014, 8, 18–27. [Google Scholar] [CrossRef]
- Abrego, N.; Salcedo, I. Variety of woody debris as the factor influencing wood-inhabiting fungal richness and assemblages: Is it a question of quantity or quality? For. Ecol. Manag. 2013, 291, 377–385. [Google Scholar] [CrossRef]
- Abrego, N.; Salcedo, I. How does fungal diversity change based on woody debris type? A case study in Northern Spain. Ekologija 2011, 57, 109–119. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T. Ecosystems and biodiversity hotspots in the Mediterranean basin threats and conservation efforts. Sci. Adv. Environ. Toxicol. Ecotoxicol. 2011, 10, 1–24. [Google Scholar]
- Venturella, G. Fungal Diversity in the Mediterranean Area. Diversity 2020, 12, 253. [Google Scholar] [CrossRef]
- Krah, F.S.; Seibold, S.; Brandl, R.; Baldrian, P.; Müller, J.; Bässler, C. Independent effects of host and environment on the diversity of wood-inhabiting fungi. J. Ecol. 2018, 106, 1428–1442. [Google Scholar] [CrossRef]
- Purhonen, J.; Huhtinen, S.; Kotiranta, H.; Kotiaho, J.S.; Halme, P. Detailed information on fruiting phenology provides new insights on wood-inhabiting fungal detection. Fungal Ecol. 2017, 27, 175–177. [Google Scholar] [CrossRef]
- Baber, K.; Otto, P.; Kahl, T.; Gossner, M.M.; Wirth, C.; Gminder, A.; Bässler, C. Disentangling the effects of forest-stand type and dead-wood origin of the early successional stage on the diversity of wood-inhabiting fungi. For. Ecol. Manag. 2016, 377, 161–169. [Google Scholar] [CrossRef]
- Hoppe, B.; Purahong, W.; Wubet, T.; Kahl, T.; Bauhus, J.; Arnstadt, T.; Hofrichter, M.; Buscot, F.; Krüger, D. Linking molecular deadwood-inhabiting fungal diversity and community dynamics to ecosystem functions and processes in Central European forests. Fungal Divers. 2016, 77, 367–379. [Google Scholar] [CrossRef]
- Landi, M.; Salerni, E.; Ambrosio, E.; D’Aguanno, M.; Nucci, A.; Saveri, C.; Perini, C.; Angiolini, C. Concordance between vascular plant and macrofungal community composition in broadleaf deciduous forests in central Italy. Iforest-Biogeosciences For. 2015, 8, 279. [Google Scholar] [CrossRef]
- Angiolini, C.; Landi, M.; Salerni, E.; Perini, C.; Piazzini, S.; Frignani, F.; Amici, V.; Geri, F.; Leonardi, P.; Pecoraro, L.; et al. Piano di Gestione Naturalistico della Riserva Naturale di Palazzo; Ufficio Territoriale per la Biodiversità di Siena—Tiporafia “Il Lecio”: Siena, Italy, 2011. [Google Scholar]
- Angiolini, C.; Landi, M.; Salerni, E.; Perini, C.; Piazzini, S.; Frignani, F.; Amici, V.; Geri, F.; Leonardi, P.; Pecoraro, L.; et al. Piano di Gestione Naturalistico della Riserva Naturale di Cornocchia; Ufficio Territoriale per la Biodiversità di Siena—Tiporafia “Il Lecio”: Siena, Italy, 2011. [Google Scholar]
- Eriksson, J.; Ryvarden, L. The Corticiaceae of North Europe; Fungiflora: Oslo, Norway, 1973–1988; pp. 2–8. [Google Scholar]
- Breitenbach, J.; Kränzlin, J. Fungi of Switzerland; Mykologica: Lucerne, Switzerland, 1981, 1986; Volume 1,2. [Google Scholar]
- Ryvarden, L.; Gilbertson, R.L. European Polypores; Fungiflora: Oslo, Norway, 1993–1994; Volume 1–2, 344p. [Google Scholar]
- Kotiranta, H. The Corticiaceae of Finland; Publications in Botany from the University of Helsinki: Helsinki, Finland, 2001; 278p. [Google Scholar]
- Bernicchia, A. Polyporaceae s.l. Fungi Europaei; Edizioni Candusso: Alassio, Italy, 2005; Volume 10, 808p. [Google Scholar]
- Medardi, G. Atlante Fotografico Degli Ascomiceti d’Italia; AMB: Trento, Italy, 2006; 403p. [Google Scholar]
- Bernicchia, A.; Gorjón, S.P. Corticiaceae s.l. Fungi Europaei; Edizioni Candusso: Alassio, Italy, 2010; Volume 12, 1008p. [Google Scholar]
- CABI-Bioscience Database of Fungal Names. Available online: www.speciesfungorum.org (accessed on 2 April 2023).
- Küffer, N.; Senn-Irlet, B. Influence of forest management on the species richness and composition of wood-inhabiting basidiomycetes in Swiss forests. Biodivers. Conserv. 2005, 14, 2419–2435. [Google Scholar] [CrossRef]
- Albrecht, L. Grundlagen, Ziele und Methodik der waldökologischen Forschung in Naturwaldreservaten. In Schriften-Reihenaturwaldreservate in Bayern; Bayerisches Staatsministerium für Ernährung, Landwirtschaft und Forsten: München, Germany, 1990; Volume 1, pp. 1–221. [Google Scholar]
- Lüdecke, D.; Makowski, D.; Waggoner, P.; Patil, I. Performance: Assessment of Regression Models Performance, R package version 0.4; 2020; Volume 5. [Google Scholar]
- R Development Core Tea. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R package version 2.5-7; 2020. [Google Scholar]
- Bernicchia, A.; Gorjón, S.P. Polypores of the Mediterranean Region; Romar: Segrate, Italy, 2020; p. 904. [Google Scholar]
- Mammarella, B.; D’Aguanno, M.; Cantini, D.; Salerni, E.; Perini, C. Macromiceti lignicoli in ambiente mediterraneo: Il caso studio del Parco Regionale della Maremma. Micol. Veget. Medit. 2014, 29, 65–74. [Google Scholar]
- Saitta, A.; Bernicchia, A.; Gorjón, S.P.; Altobelli, E.; Granito, V.M.; Losi, C.; Lunghini, D.; Maggi, O.; Medardi, G.; Padovan, F.; et al. Biodiversity of wood-decay fungi in Italy. Plant Biosyst. 2011, 145, 958–968. [Google Scholar] [CrossRef]
- Moore, D.; Gange, A.C.; Gange, E.G.; Boddy, L. Fruit bodies: Their production and development in relation to environment. Br. Mycol. Soc. Symp. Ser. 2008, 28, 79–103. [Google Scholar] [CrossRef]
- Salerni, E.; Laganà, A.; Perini, C.; Loppi, S.; De Dominicis, V. Effects of temperature and rainfall on fruiting of macrofungi in oak forests of the Mediterranean area. Isr. J. Plant Sci. 2002, 50, 189–198. [Google Scholar] [CrossRef]
- Ryvarden, L. Studies in Neotropical polypore. Some new and interesting species from tropical America. Synop. Fungorum 2014, 32, 58–67. [Google Scholar]
- Granito, V.M.; Lunghini, D.; Maggi, O.; Persiani, A.M. Wood-inhabiting fungi in southern Italy forest stands: Morphogroups, vegetation types and decay classes. Mycologia 2015, 107, 1074–1088. [Google Scholar] [CrossRef]
- Cantini, D.; D’Aguanno, M.; Mammarella, B.; Salerni, E.; Perini, C. Le Riserve Naturali Statali di Cornocchia e Palazzo: Macromiceti lignicoli in querceti decidui. Micol. Ital. 2014, 43, 1-2-3. [Google Scholar]
- Klockow, P.A.; D’Amato, A.W.; Bradford, J.B.; Fraver, S. Nutrient concentrations in coarse and fine woody debris of Populus tremuloides Michx. -dominated forests, northern Minnesota, USA. Silva Fenn. 2014, 48, 962. [Google Scholar] [CrossRef]
- Lasota, J.; Piaszczyk, W.; Błońska, E. Fine woody debris as a biogen reservoir in forest ecosystems. Acta Oecol. 2022, 115, 103822. [Google Scholar] [CrossRef]
- Pioli, S.; Antonucci, S.; Giovannelli, A.; Traversi, M.L.; Borruso, L.; Bani, A.; Brusetti, L.; Tognetti, R. Community fingerprinting reveals increasing wood-inhabiting fungal diversity in unmanaged Mediterranean forests. For. Ecol. Manag. 2018, 408, 202–210. [Google Scholar] [CrossRef]
- Kubartová, A.; Ottosson, E.; Dahlberg, A.; Stenlid, J. Patterns of fungal communities among and within decaying logs, revealed by 454 sequencing. Mol. Ecol. 2012, 21, 4514–4532. [Google Scholar] [CrossRef]


| Min. | Mean | Median | Max. | |
|---|---|---|---|---|
| Total sporocarp productivity | 8 | 26 ± 9.43 | 25 | 42 |
| Ascomycota sporocarp productivity | 0 | 1 ± 1.47 | 1 | 5 |
| Corticioids sporocarp productivity | 4 | 20 ± 7.93 | 17 | 35 |
| Polyporoids sporocarp productivity | 0 | 3 ± 2.73 | 3 | 10 |
| Heterobasidiomycetes sporocarp productivity | 0 | 1 ± 1.40 | 1 | 5 |
| Total sporocarp richness | 8 | 14 ± 4.37 | 15 | 23 |
| Ascomycota sporocarp richness | 0 | 1 ± 1.14 | 1 | 4 |
| Corticioids sporocarp richness | 4 | 9 ± 3.07 | 9 | 16 |
| Polyporoids sporocarp richness | 0 | 3 ± 1.97 | 2 | 7 |
| Heterobasidiomycetes sporocarp richness | 0 | 1 ± 0.86 | 1 | 3 |
| VFWD abundance | 15 | 35 ± 13.64 | 32 | 72 |
| FWD abundance | 0 | 6 ± 4.68 | 6 | 19 |
| CWD abundance | 0 | 2 ± 1.39 | 2 | 5 |
| Stump abundance | 0 | 3 ± 2.00 | 2 | 8 |
| Total woody debris | 23 | 46 ± 16.64 | 41 | 85 |
| Variety | 0 | 0 ± 0.13 | 0 | 1 |
| Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Explanatory Variables | Sporocarp Productivity | Sporocarp Richness | ||||||||
| Total | Asco | Corti | Polyp | Heterob | Total | Asco | Corti | Polyp | Heterob | |
| Intercept | +2.60 *** | −0.75 | +2.41 *** | +0.54 * | - | +2.01 *** | −0.88 | +1.68 *** | −0.09 | - |
| VFWD | - | +0.03 ** | - | - | - | - | +0.03 * | - | - | - |
| FWD | - | - | - | +0.06 ** | - | - | - | - | - | - |
| CWD | - | - | - | +0.28 ** | - | - | - | - | - | - |
| STUMP | - | - | - | - | - | - | - | - | - | - |
| Total_WD | - | - | - | - | - | - | - | - | - | - |
| Variety | +1.70 *** | - | +1.43 *** | - | - | +1.66 *** | - | +1.32 * | +2.74 ** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce, Á.; Salerni, E.; D’Aguanno, M.N.; Perini, C. Wood-Decay Fungi Fructifying in Mediterranean Deciduous Oak Forests: A Community Composition, Richness and Productivity Study. Forests 2023, 14, 1326. https://doi.org/10.3390/f14071326
Ponce Á, Salerni E, D’Aguanno MN, Perini C. Wood-Decay Fungi Fructifying in Mediterranean Deciduous Oak Forests: A Community Composition, Richness and Productivity Study. Forests. 2023; 14(7):1326. https://doi.org/10.3390/f14071326
Chicago/Turabian StylePonce, Ángel, Elena Salerni, Maria Nives D’Aguanno, and Claudia Perini. 2023. "Wood-Decay Fungi Fructifying in Mediterranean Deciduous Oak Forests: A Community Composition, Richness and Productivity Study" Forests 14, no. 7: 1326. https://doi.org/10.3390/f14071326
APA StylePonce, Á., Salerni, E., D’Aguanno, M. N., & Perini, C. (2023). Wood-Decay Fungi Fructifying in Mediterranean Deciduous Oak Forests: A Community Composition, Richness and Productivity Study. Forests, 14(7), 1326. https://doi.org/10.3390/f14071326





