Abstract
Truffles represent one of the most expensive edible fungi. About two-thirds of all known truffle records originate from Europe. Historically, the Mediterranean region in Southern Europe is associated with truffle cultivation and it is widely regarded as a center of truffle distribution in Europe. On the contrary, little is known about truffles in the central and northern regions of Europe. Here, native truffle species in Poland, their symbiotic ectomycorrhizal (ECM) tree partners, and their continental-scale distribution were studied. Altogether, 16 truffle species were identified based on the sequences of the Internal Transcribed Spacer (ITS) rDNA, a barcode region for fungi. Truffle species were associated with deciduous tree species of Fagales (Fagaceae, Betulaceae, Juglandaceae), Malvales (Malvaceae), Malpighiales (Salicaceae), and coniferous trees of Pinales (Pinaceae). Fagales trees constituted 70% of ECM associations formed by truffle species. Three genera of the order Fagales, Quercus, Fagus, and Corylus formed 50% of all ECM associations. Coniferous trees of Pinales formed 16% of associations. Two other orders of deciduous trees, Malvales and Malpighiales, together formed 14% of ECM associations. All but three identified truffle species exhibited low specificity to their ECM tree partners. Two-thirds of truffle species formed ECM symbiosis with both coniferous and deciduous tree species, but the share of coniferous tree partners was considerably lower than that of deciduous trees, reaching up to 30% for T. anniae, T. puberulum, and T. borchii (clade/puberulum). All the identified truffles were noted in both Central Europe and the Mediterranean region. Among them, about 80% of truffle species were widely distributed across the continent and represented by similar or higher numbers of records in Northern Europe, i.e., above the 48th parallel north, than in Southern Europe. This study showed higher taxa richness of native truffle species in Poland, but low specificity to their ECM tree partners. However, further studies on the regional-scale distribution of truffles in Poland are needed to improve the knowledge of the patterns of truffle distribution in forest ecosystems and the potential productivity of edible truffle species with high economic value.
1. Introduction
The genus Tuber (Pezizales, Ascomycota) is represented by numerous edible fungal species that are considered delicious foods. Due to their high economic value, truffles are widely cultivated in Europe, North America, and Asia [1,2,3]. The distribution of truffle species varies among the world’s regions. The highest number of ~20,000 records (about 60% of all available records) for the genus Tuber was noted in Europe. Numerous records were also found in Asia (~8000 records) and North America (~5000 records), but less than 1% of all records of Tuber species were found in the Southern Hemisphere [4].
The European production of edible truffles comes almost exclusively from truffle orchards located in southern France and northern Spain and Italy. In these countries, the history of truffle cultivation goes back to the 18th century. The long-lasting tradition of truffle harvesting and farming is considered a cultural heritage and a key component of the local history of the Mediterranean countries, which makes edible European truffles are considered Mediterranean species. However, edible truffle species such as T. aestivum and T. melanosporum also occur in central and northern regions of Europe, among others, in England, Germany, Poland, Estonia, and the Scandinavian Peninsula [5,6,7,8,9]. Truffle cultivation and their harvest from wild stands are well developed in Southern Europe, but are still developing in Northern Europe. For instance, the first crop of the burgundy truffle in an experimental truffle orchard in Poland was harvested in 2017, followed by abundant truffle harvests from the wild stands [10].
In Poland, the principles of truffle harvest from wild stands have not yet been determined by legal regulations. Only one truffle species, Tuber mesentericum, has been given legal protection in Poland, and five other truffle species appear on the Red List of Plants and Fungi in Poland (T. aestivum, T. dryophilum, T. maculatum, T. nitidum, T. rapaeodorum). Unfortunately, the abovementioned Red List does not involve legal regulations. The large-scale distribution of native truffle species and the threats to their occurrence in wild stands have not yet been studied in Poland. However, it can be expected that climate change and human pressure, such as deforestation, environmental pollution, habitat fragmentation, biological invasions of alien species, and other disturbances, threaten native truffle species in Poland. The data available on social network sites such as Facebook provide numerous examples of uncontrolled truffle harvest from wild stands in Poland despite the fact that digging in the forests, managed by the State Forests of Poland (~80% of forest area in Poland), is prohibited by legal regulations. Nevertheless, illegal truffle harvest, together with the damage to forest habitats, is increasingly observed in Poland.
Truffles are closely associated with their tree partners through ectomycorrhizal symbiosis, a mutualistic relationship between fungal hyphae and the roots of tree species. As a result, the distribution of truffle species, both as ectomycorrhizas and ascomata, is determined by the presence of appropriate tree partners for ectomycorrhizal symbiosis, in addition to the climate and soil variables. The symbiotic specificity between truffle species and their symbiotic tree partners remains poorly known for the majority of ECM fungal species, including native truffle species in Poland. Edible truffle species were reported as symbiotic partners of deciduous trees, among others numerous oak species (e.g., Quercus robur, Q. petraea, Q. ilex, Q. cerris, and Q. pubescens), common hornbeam (Carpinus betulus), common hazel (Corylus avellana), and European beech (Fagus sylvatica) [1,11,12,13]. Coniferous trees are less frequently reported as symbiotic partners of edible truffles; however, T. aestivum has established ectomycorrhizas on the roots of Picea abies in Germany [14], and T. melanosporum on the roots of Pinus nigra in Spain [15].
Climatic conditions are among the most important factors that explain the large-scale distribution of species [16,17], including truffles [11,18,19] and ectomycorrhizal tree species [20,21]. Climate change shifts the distribution of ecological niches for tree species [20,21] and ECM fungal symbionts associated with them [19,22,23,24]. Importantly, the impact of climate change on tree species is more severe in Southern than in Northern Europe [25,26]. Considerable climate changes, including climate warming and seasonal droughts, have already been observed in the Mediterranean region [27], and are expected to accelerate rapidly in the near future [28,29]. Concurrently, truffles considered Mediterranean species, such as T. melanosporum, can be cultivated further north [6,7]. Truffle orchards in Central and Northern Europe can support European truffle production in the future when suitable niches for economically important truffles decrease in the area in the south.
In view of the above, this study expands the knowledge of the diversity and distribution of native truffle species in Poland and their symbiotic ECM associations with forest tree species. The obtained results present a valuable insight into the ecology of truffle species in Poland, which is crucial to establishing current recommendations for species protection and habitat protection in relation to the potential risks and benefits of future truffle cultivation and harvest in Poland. In addition, this study outlines future perspectives for the cultivation and conservation of and further research on truffle species in Poland.
2. Methods
To identify the ectomycorrhizal symbiotic associations formed by truffles (Tuber spp.) on the roots of their ectomycorrhizal tree partners in Poland, the sequences of the Internal Transcribed Spacer (ITS) barcode region of fungal rDNA, noted in various studies conducted in Poland, were used. The DNA sequences, which represent the molecular identification of truffle species from studies conducted on root-associated ECM fungal communities during the years 2010–2022 in Poland, were gathered and analyzed. The studies were conducted at more than 160 sites (>800 samples) across the country and gathered into 12 regions (Figure S1). Studies on ECM fungi associated with roots of native tree species in managed forests and forest reserves [30,31], native young trees in forest nurseries [32], native tree species in nutrient-poor coniferous forest ecosystems of sand dunes [33], lowland and mountain forest ecosystems [34], and exotic alien tree species in botanical garden and under forest conditions [34,35,36,37] were included. In addition, molecular data on truffle species identified as ECM roots in ongoing studies were included. Studies on the old-growth oak–hornbeam, spruce, and oak forests in Białowieża National Park (since 2017; unpublished except for conference materials and research report; a few Tuber species were involved in the list of ECM fungi in Białowieża Forest [38]), old-growth beech and oak–pine forests in Woliński National Park (since 2020; unpublished except for conference materials and research report), old-growth oak–hornbeam and mixed oak–spruce forests in Wigierski National Park (since 2021; unpublished except for conference materials and research report), and pine and oak forests distributed in 6 regions in 4 provinces across Central and Western Poland, with Wielkopolski National Park as one of the regions (since 2021, unpublished except for conference materials) were taken into account in the consideration of truffle distribution in Poland. Studies in Białowieża, Woliński, and Wigierski National Parks were conducted on behalf of the State Forests in Poland. Research reports with partially complete data on the distribution of ECM fungi were submitted to the State Forests, but have not yet been published, except for Białowieża [38]. Due to the high number of conservation-relevant species among native truffles, the rapid growth of truffle hunting in Poland, threats to truffles in the wild, and many records in strictly protected forest areas, the coordinates of truffle species have not been published. Records could be made available upon reasonable request for scientific and legal purposes only.
The species identity of ectomycorrhizal fungi, which formed ECM tips on the roots of tree species, was determined using the ITS (Internal Transcribed Spacer) region of fungal rDNA, widely accepted as the barcode region for fungi [39]. The DNA was extracted from individual ECM root tips, and the ITS region was amplified using ITS4 and ITS1-F primers and sequenced with the ITS4 primer. A detailed description of the DNA isolation, amplification, sequencing, reagents, and equipment used to perform the DNA analyses is given in previous studies [30,31,32,33,40], based on the modified methodological approach to DNA isolation from ECM roots formed by Ascomycota and Basidiomycota [41].
To visualize the continental-scale distribution of identified truffle species, molecular data on ECM roots were supplemented by European records of truffle species from the Global Biodiversity Information Facility (GBIF) database ([4]; modified). GBIF contains both DNA sequences obtained from ECM roots, ascomata, and soil, and human observations of ascomata. However, the ECM symbiotic associations between truffles and their tree partners were determined using the DNA sequences of the ITS rDNA region only.
Using UNITE v. 9.0 (https://unite.ut.ee/ (accessed on 1 December 2023), the DNA sequences of identified truffle species were assigned to Species Hypotheses (threshold 1.5), according to the UNITE data, accurate as of December 2022. The DNA sequences assigned to the Species Hypotheses (SH) by the members and associates of the UNITE Community were used, if available, and were supplemented by the Species Hypotheses assigned automatically by the UNITE program. In the next step, tree species reported as symbiotic partners of ectomycorrhizal fungal species were extracted from the UNITE database and analyzed, using the phylogenetic identity of truffle and tree species available in the UNITE databases and the literature [42,43,44,45,46].
Symbiotic tree partners of truffle species in Poland were identified based on the morphological features of the trees being sampled. In the studies on tree seedlings, entire root systems of distinct seedlings were analyzed. In the studies on mature trees, monoculture stands (pure stands) were used if available. In the monoculture stands, used, among others, for Carya, Fagus, Quercus, Picea, and Pinus species, all tree roots belonged to a single tree species. In the mixed forests composed of one coniferous tree and one deciduous tree species (e.g., oak–pine forests, spruce forests with common hazel), the roots of deciduous and coniferous trees were distinguished based on the morphological features of the roots. The fine roots of coniferous trees are considerably thicker than the roots of deciduous trees and are characterized by regular dichotomous branching of ectomycorrhizas [47,48]. In the mixed stands composed of many tree species, such as oak–hornbeam forests with lime or the artificial ecosystems of botanical gardens, roots were traced from the trunk of the tree species to the exposed root system to confirm the plant species identity of the roots and ensure that sampled roots were attached to the tree species being sampled [35,49,50,51].
3. Results
Altogether, 16 truffle species were identified as ECM symbionts of tree species in forest ecosystems in Poland. The 156 ectomycorrhizal associations formed by truffle species were identified. Among them, 131 ECM associations (84% of all associations) were formed by deciduous tree species of the orders Fagales (Fagaceae, Betulaceae, Juglandaceae), Malpighiales (Salicaceae), and Malvales (Malvaceae), and 25 symbiotic ECM associations (16% of all associations) were formed by coniferous tree species of the Pinales order (Pinaceae) (Figure 1). Deciduous trees from Fagales formed 69.8% of symbiotic the ECM associations of truffle species. Among them, Fagaceae (Fagus and Quercus) and Betulaceae (Corylus, Carpinus, Betula) formed the most associations (36.6% and 28.2% of all associations, respectively), but Juglandaceae (Carya) formed 5.1% of all ECM associations. Other orders of deciduous trees, Malvales (Tilia) and Malpighiales (Salix, Populus), formed 7.7% and 6.4% of all ECM associations, respectively. Coniferous trees of the order Pinales (Pinus, Picea, Abies, Larix) formed 16% of all ECM associations of truffle species (Figure 1).
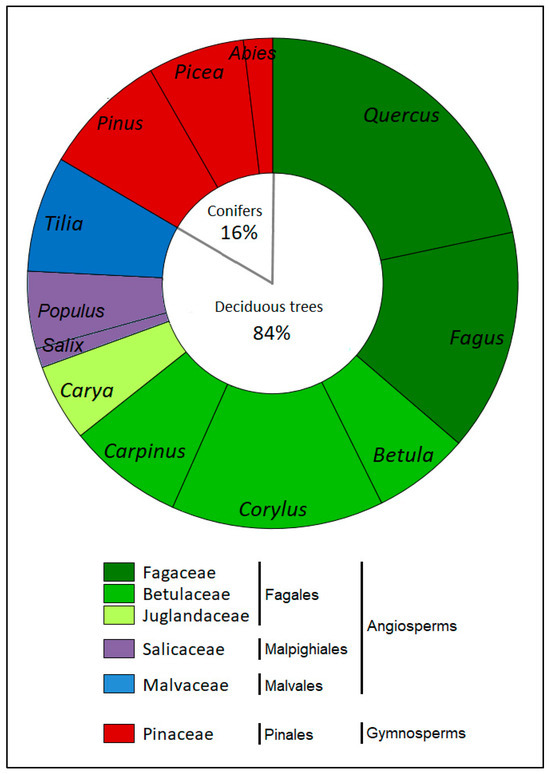
Figure 1.
Percentage share of symbiotic associations formed by identified native truffle species on the roots of different tree partners, according to the phylogenetic positions of tree taxa.
Identified truffle species belong to the seven phylogenetic lineages (hereafter clades) of truffles (Table 1). The highest numbers of ECM associations were noted for the clades /puberulum (52 associations); /maculatum and /rufum (29 and 34 associations); and /excavatum and /aestivum (17 and 18 associations, respectively) (Figure 2). All identified clades formed associations with Quercus (Fagaceae), six clades (all except /macrosporum) formed symbiosis with Corylus (Betulaceae), and five clades (all except /macrosporum and /melanosporum) formed symbiotic ECM associations with Fagus (Fagaceae) (Figure 2).

Table 1.
Identified truffle species with their conservation value and current status in Poland.
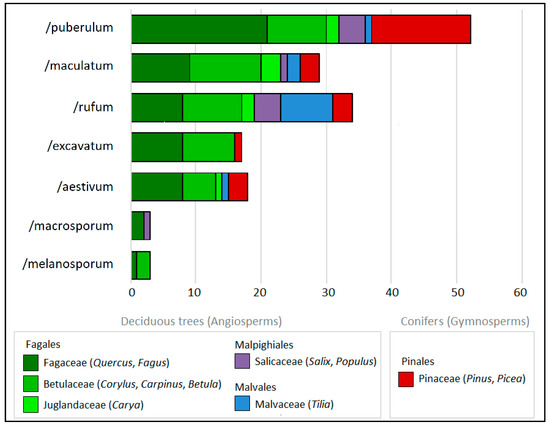
Figure 2.
The number of ectomycorrhizal associations formed by seven phylogenetic lineages (clades) of truffles according to the phylogenetic position of their tree partners at the family level.
The highest number of records and the widest range of tree partners were noted for T. rufum (27 records, associations with all tree genera listed in this study, except Larix). High numbers of records were noted for T. anniae (22 records), which had associations with similar shares of deciduous (55%) and coniferous trees (45% of associations), T. puberulum (18 records, 72% of associations with deciduous trees), and T. maculatum (18 records, 94% of associations with deciduous trees). Coniferous trees (Pinales) were identified as symbiotic partners of 10 out of 16 identified truffle species (Figure S2). The share of coniferous tree species in relation to all tree partners was around 30% for the /puberulum clade, and around 15% (+/−5%) for /maculatum, /rufum, /excavatum, and /aestivum (Figure 2). No truffle species associated exclusively with Pinales trees were identified (Figure S2).
Continental-scale distribution of 16 identified truffle species differed between the southern and northern regions of the continent. Considerably higher frequencies of T. anniae, T. rufum, T. maculatum, and T. rapaeodorum were noted in Central and Northern Europe, but higher frequencies of T. excavatum and two edible species, T. brumale and T. borchii, were identified in Southern Europe (Figure 3). Edible truffles had more records in Southern Europe, except for T. aestivum, which is cultivated in Central Europe [8,9,10] and was represented by a similar number of records in Southern and Northern Europe.
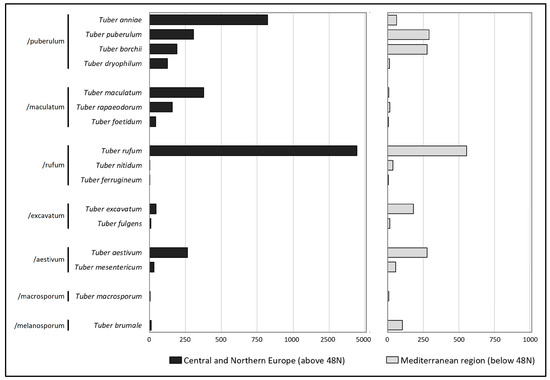
Figure 3.
Records of Tuber in Europe (the Global Biodiversity Information Facility database; GBIF.org [4]) in the northern and southern regions of Europe, divided by the 48th parallel north.
4. Discussion
4.1. Distribution of ECM Fungi as Ectomycorrhizas
Ectomycorrhizal fungi are heterotrophic organisms that, throughout mutually beneficial ectomycorrhizal symbiosis, receive the photosynthetically fixed carbon directly from the tree species [53]. Therefore, ectomycorrhizal tips on the roots of trees are constantly renewed, regardless of the actual weather conditions. On the contrary, fruit bodies (ascomata for Ascomycota and basidiomata for Basidiomycota) are reproductive structures of the sexual phase of fungal life cycles. Ascomata and basidiomata production is a facultative and ephemeral phase and depends on environmental factors such as soil moisture and the amplitude of the daily temperature [54]. Consequently, a lack of truffle ascomata does not imply the absence of truffle species in a forest habitat [30,31,36,55,56,57,58]. For example, ectomycorrhizal root tips of T. melanosporum survived about 500 km further north [6] than its fruit bodies have ever been found [7]. This reflects the scale of differences in minimum ecological requirements for fruit body metabolism, contrary to the requirements for the development of mycelium and ectomycorrhizas [59]. Long-lasting studies on deciduous and coniferous trees in forest nurseries [32,60,61] have shown that species of the genus Tuber are among the most widespread and abundant ECM symbionts on the roots of young native trees in forest nurseries, where truffle ascomata were never found (Professor Maria Rudawska, personal communication, 2022). Truffle species belong to Ascomycota, in which it is more difficult to isolate DNA from ECM roots than in Basidiomycota [41]. Therefore, it is likely that ectomycorrhizas formed by truffle species are more common in native forest ecosystems in Poland than has been reported.
4.2. Specificity between Truffle Species and Tree Partners for Symbiosis
Ectomycorrhizas formed by truffle species are often reported from the roots of deciduous trees [30,35,36] but are rarely found on the roots of coniferous tree species [30,33,40]. However, this study showed that coniferous trees indeed constitute one-sixth of all ECM associations between truffles and tree species. Concurrently, 10 out of 16 identified truffle species formed ECM associations with the roots of both coniferous and deciduous tree species. However, the habitat requirements of truffle species seem to overlap with the ecological niches of deciduous trees more than with the niches of coniferous tree species. Truffles generally prefer neutral and alkaline soils (pH 6–8) and are infrequent or absent in soils of pH < 5 [62,63]. In Poland, coniferous forests are grown mostly in acidic soils (pH 3–5), while neutral and alkaline soils are inhabited by deciduous trees. It can be concluded that different habitat requirements, not an inability to enter symbiosis, explain the low number of identified ECM associations between truffles and coniferous trees.
No truffle species associated specifically with coniferous tree species were found. On the contrary, a few truffle species, such as T. macrosporum (/macrosporum) and T. brumale (/melanosporum), were associated only with deciduous tree species. Nevertheless, these truffles were represented by a low number of records, which can result in a lack of detected coniferous tree partners. Molecular studies based on ITS rDNA, a barcode region for fungi, have revealed that coniferous trees represent a high share of symbiotic ECM associations formed by /puberulum, the most frequent clade among all identified clades of truffles. Coniferous trees formed up to 35% of ECM associations of T. anniae and about 30% of ECM associations of T. puberulum and T. borchii (Figure S2). It is likely that further records on the ECM associations of T. macrosporum and T. brumale will provide data on their coniferous tree partners. For instance, T. melanosporum, a highly valuable truffle species closely related to T. brumale (clade /melanosporum), was found to be a symbiotic partner of Pinus nigra [15].
4.3. Protection Requirements of Truffle Species in Poland
Only four truffle species (T. aestivum, T. brumale, T. mesentericum, and T. borchii) out of all 16 truffle species are classified as tasty and can be commercially used. However, all except T. aestivum are known from a limited number of localities in Poland, and with the increasing pressure of truffle hunting [10,64], may become endangered in the wild.
The principles of truffle harvest from wild stands (i.e., truffle hunting) have not yet been determined by legal regulations in Poland. Only one truffle species T. mesentericum is a protected fungal species in Poland and five other truffle species were placed on the Red List of Macrofungi in Poland (T. rapaeodorum, T. dryophilum, T. maculatum, T. nitidum, and T. aestivum [52]). Another six truffle species are known from a few records in Poland (T. borchii, T. foetidum, T. ferrugineum, T. nitidum, T. macrosporum, and T. brumale), but are not indexed on the mentioned Red List of Macrofungi in Poland [52]. In total, 12 of 16 native truffle species belong to conservation-relevant fungal species in Poland, and five of them are also classified as rare fungal species in Europe (Table 1). The available data indicate that the legal protection of truffles in Poland requires urgent action to prevent potential threats to their occurrence, the data on truffle distribution need to be upgraded.
Tuber anniae, T. puberulum, T. rufum, and T. maculatum are among the most widespread native truffle species and are frequently observed as ectomycorrhiza tips on the roots of native tree species in various ecosystems across the country, including managed forests and forest reserves, national parks, forest nurseries, botanical gardens, and plantations of alien tree species in forest conditions [30,32,35,36]. Mentioned four truffles were detected in numerous studies on ECM fungal communities, among others, in old-growth oak–hornbeam, beech, and oak–pine forests in Central and Northern Poland. These truffles are widespread enough, to be used in truffle cultivation with no threat to their distribution in the wild, but unfortunately, they are classified as inedible due to their pungent or bitter taste.
5. Conclusions
Native truffle species in Poland exhibit low specificity to their ectomycorrhizal tree partners, and will probably be less affected by the predicted changes in the tree cover in Central Europe [20,21] than by changes in climate conditions, soil properties, and habitat disturbances caused by human activity.
All but a few native truffle species in Poland belong to conservation-relevant species. Truffles are classified as rare species in Poland (T. foetidum, T. nitidum, T. ferrugineum, T. macrosporum, and the edible truffles T. borchii and T. brumale), and rare species in Europe (fewer than 50 records; T. ferrugineum, T. foetidum, T. fulgens, T. nitidum, and T. macrosporum). In addition, edible truffles are threatened by truffle hunting (T. borchi, T. brumale, T. aestivum, and T. mesentericum). Admittedly, Tuber mesentericum is a protected species in Poland but is often confused with T. aestivum and harvested from wild stands, despite that the harvest of T. mesentericum is prohibited by law and can incur fines up to USD 1250.
In view of the above-listed conservation-relevant species, further studies on the anthropogenic and natural factors that influence the distribution of truffle species in forest ecosystems in Poland are required for managing these rare fungi. The results of further studies will lead to the identification of the determining factors necessary for the maintenance of truffles in Poland, and in particular, for the management of truffle-producing forests and orchards, that can support truffle species with high economic value.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/1999-4907/14/12/2407/s1, Figure S1: The study stands gathered in 12 regions distributed across the country; Figure S2: Percentage share of ectomycorrhizal associations formed by truffle species of the seven phylogenetic lineages (clades) according to the identity of their tree partners.
Funding
This study was supported by the Institute of Dendrology of the Polish Academy of Sciences and the National Science Center of Poland, grant number 2020/37/N/NZ8/01403.
Data Availability Statement
This study was conducted using data on species distribution from the Global Biodiversity Information Facility (GBIF) database, which provides open access to the biodiversity data (available in https://www.gbif.org/species/8282501; accessed on 1 December 2023).
Acknowledgments
I would like to thank Maria Rudawska and Tomasz Leski for the discussions on the details of the previous studies conducted in the last two decades in the Department of Symbiotic Associations, and for providing access to the archival data on the distribution of ECM fungi stored in the Department of Symbiotic Associations of the Institute of Dendrology, Polish Academy of Sciences. I am also grateful to the employees of the Regional Directorates of the State Forests in Poland for their constant willingness to help and assistance during the fieldwork.
Conflicts of Interest
The author declare that he has no competing or financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Correction Statement
This article has been republished with a minor correction to the reference 4. This change does not affect the scientific content of the article.
References
- Hall, I.; Brown, G.; Zambonelli, A. Taming the Truffle: The History, Lore, and Science of the Ultimate Mushroom; Timber Press: Portland, OR, USA, 2008; ISBN 978-0-88162-860-0. [Google Scholar]
- Lefevre, C. Native and Cultivated Truffles of North America. In Edible Ectomycorrhizal Mushrooms; Zambonelli, A., Bonito, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 209–226. [Google Scholar]
- Berch, S.M.; Bonito, G. Cultivation of Mediterranean Species of Tuber (Tuberaceae) in British Columbia, Canada. Mycorrhiza 2014, 24, 473–479. [Google Scholar] [CrossRef] [PubMed]
- GBIF.org (2023) GBIF Occurrence of Tuber P.Micheli ex F.H.Wigg. Available online: https://www.gbif.org/occurrence/download/0016714-230828120925497 (accessed on 1 December 2023). [CrossRef]
- Wedén, C.; Chevalier, G.; Danell, E. Tuber Aestivum (Syn. T. Uncinatum) Biotopes and Their History on Gotland, Sweden. Mycol. Res. 2004, 108, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Otsing, E.; Tedersoo, L. Temporal Dynamics of Ectomycorrhizal Fungi and Persistence of Tuber melanosporum in Inoculated Quercus Robur Seedlings in North Europe. Mycorrhiza 2015, 25, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Büntgen, U. First Harvest of Périgord Black Truffle in the UK as a Result of Climate Change. Clim. Res. 2017, 74, 67–70. [Google Scholar] [CrossRef]
- Hilszczańska, D.; Sierota, Z.; Palenzona, M. New Tuber Species Found in Poland. Mycorrhiza 2008, 18, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Stobbe, U.; Büntgen, U.; Sproll, L.; Tegel, W.; Egli, S.; Fink, S. Spatial Distribution and Ecological Variation of Re-Discovered German Truffle Habitats. Fungal Ecol. 2012, 5, 591–599. [Google Scholar] [CrossRef]
- Rosa-Gruszecka, A.; Hilszczańska, D.; Gil, W.; Kosel, B. Truffle Renaissance in Poland—History, Present and Prospects. J. Ethnobiol. Ethnomed. 2017, 13, 36. [Google Scholar] [CrossRef]
- Stobbe, U.; Egli, S.; Tegel, W.; Peter, M.; Sproll, L.; Büntgen, U. Potential and Limitations of Burgundy Truffle Cultivation. Appl. Microbiol. Biotechnol. 2013, 97, 5215–5224. [Google Scholar] [CrossRef]
- Moser, B.; Büntgen, U.; Molinier, V.; Peter, M.; Sproll, L.; Stobbe, U.; Tegel, W.; Egli, S. Ecological Indicators of Tuber Aestivum Habitats in Temperate European Beech Forests. Fungal Ecol. 2017, 29, 59–66. [Google Scholar] [CrossRef]
- Lefevre, C.K.; Hall, I.R. The Status of Truffle Cultivation: A Global Perspective. Acta Hortic. 2001, 556, 513–520. [Google Scholar] [CrossRef]
- Stobbe, U.; Stobbe, A.; Sproll, L.; Tegel, W.; Peter, M.; Büntgen, U.; Egli, S. New Evidence for the Symbiosis between Tuber Aestivum and Picea Abies. Mycorrhiza 2013, 23, 669–673. [Google Scholar] [CrossRef]
- García-Montero, L.G.; Manjón, J.L.; Martín-Fernández, S.; Di Massimo, G. Problems of Using Pines in Tuber melanosporum Culture: Soils and Truffle Harvest Associated with Pinus Nigra and P. Sylvestris. Agrofor. Syst. 2007, 70, 243–249. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the Impacts of Climate Change on the Distribution of Species: Are Bioclimate Envelope Models Useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Taccoen, A.; Piedallu, C.; Seynave, I.; Perez, V.; Gégout-Petit, A.; Nageleisen, L.M.; Bontemps, J.D.; Gégout, J.C. Background Mortality Drivers of European Tree Species: Climate Change Matters. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190386. [Google Scholar] [CrossRef] [PubMed]
- Gryndler, M.; Šmilauer, P.; Šťovíček, V.; Nováková, K.; Hršelová, H.; Jansa, J. Truffle biogeography—A Case Study Revealing Ecological Niche Separation of Different Tuber Species. Ecol. Evol. 2017, 7, 4275–4288. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Büntgen, U. A Risk Assessment of Europe’s Black Truffle Sector under Predicted Climate Change. Sci. Total Environ. 2019, 655, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How Much Does Climate Change Threaten European Forest Tree Species Distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Thurm, E.A.; Hernandez, L.; Baltensweiler, A.; Ayan, S.; Rasztovits, E.; Bielak, K.; Zlatanov, T.M.; Hladnik, D.; Balic, B.; Freudenschuss, A.; et al. Alternative Tree Species under Climate Warming in Managed European Forests. For. Ecol. Manag. 2018, 430, 485–497. [Google Scholar] [CrossRef]
- Pietras, M. First Record of North American Fungus Rhizopogon Pseudoroseolus in Australia and Prediction of Its Occurrence Based on Climatic Niche and Symbiotic Partner Preferences. Mycorrhiza 2019, 29, 397–401. [Google Scholar] [CrossRef]
- Pietras, M.; Kolanowska, M. Predicted Potential Occurrence of the North American False Truffle Rhizopogon Salebrosus in Europe. Fungal Ecol. 2019, 39, 225–230. [Google Scholar] [CrossRef]
- Andrew, C.; Heegaard, E.; Halvorsen, R.; Martinez-Peña, F.; Egli, S.; Kirk, P.M.; Bässler, C.; Büntgen, U.; Aldea, J.; Høiland, K.; et al. Climate Impacts on Fungal Community and Trait Dynamics. Fungal Ecol. 2016, 22, 17–25. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate Change and European Forests: What Do We Know, What Are the Uncertainties, and What Are the Implications for Forest Management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Spathelf, P.; Van Der Maaten, E.; Van Der Maaten-Theunissen, M.; Campioli, M.; Dobrowolska, D. Climate Change Impacts in European Forests: The Expert Views of Local Observers. Ann. For. Sci. 2014, 71, 131–137. [Google Scholar] [CrossRef]
- Guiot, J.; Cramer, W. Climate Change: The 2015 Paris Agreement Thresholds and Mediterranean Basin Ecosystems. Science 2016, 354, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, C.R.; Glendon, S.; Duffy, P.B. RCP8.5 Tracks Cumulative CO2 Emissions. Proc. Natl. Acad. Sci. USA 2020, 117, 19656–19657. [Google Scholar] [CrossRef]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The Projected Timing of Abrupt Ecological Disruption from Climate Change. Nature 2020, 580, 496–501. [Google Scholar] [CrossRef]
- Leski, T.; Rudawska, M.; Kujawska, M.; Stasińska, M.; Janowski, D.; Karliński, L.; Wilgan, R. Both Forest Reserves and Managed Forests Help Maintain Ectomycorrhizal Fungal Diversity. Biol. Conserv. 2019, 238, 108206. [Google Scholar] [CrossRef]
- Rudawska, M.; Leski, T.; Stasińska, M.; Karliński, L.; Wilgan, R.; Kujawska, M. The Contribution of Forest Reserves and Managed Forests to the Diversity of Macrofungi of Different Trophic Groups in European Mixed Coniferous Forest Ecosystem. For. Ecol. Manag. 2022, 518, 120274. [Google Scholar] [CrossRef]
- Rudawska, M.; Kujawska, M.; Leski, T.; Janowski, D.; Karliński, L.; Wilgan, R. Ectomycorrhizal Community Structure of the Admixture Tree Species Betula pendula, Carpinus betulus, and Tilia cordata Grown in Bare-Root Forest Nurseries. For. Ecol. Manag. 2019, 473, 113–125. [Google Scholar] [CrossRef]
- Rudawska, M.; Wilgan, R.; Janowski, D.; Iwański, M.; Leski, T. Shifts in Taxonomical and Functional Structure of Ectomycorrhizal Fungal Community of Scots Pine (Pinus sylvestris L.) Underpinned by Partner Tree Ageing. Pedobiologia 2018, 71, 20–30. [Google Scholar] [CrossRef]
- Kujawska, M.; Rudawska, M.; Wilgan, R.; Banach, J.; Leski, T. Comparable Ectomycorrhizal Fungal Species Richness but Low Species Similarity among Native Abies alba and Alien abies Grandis from Provenance Trials in Poland. For. Ecol. Manag. 2023, 546, 121355. [Google Scholar] [CrossRef]
- Wilgan, R.; Leski, T. Ectomycorrhizal Assemblages of Invasive Quercus rubra L. and Non-Invasive Carya Nutt. Trees under Common Garden Conditions in Europe. Forests 2022, 13, 676. [Google Scholar] [CrossRef]
- Wilgan, R.; Leski, T.; Kujawska, M.; Karliński, L.; Janowski, D.; Rudawska, M. Ectomycorrhizal Fungi of Exotic Carya ovata in the Context of Surrounding Native Forests on Central European Sites. Fungal Ecol. 2020, 44, 100908. [Google Scholar] [CrossRef]
- Rudawska, M.; Leski, T.; Wilgan, R.; Karliński, L.; Kujawska, M.; Janowski, D. Mycorrhizal Associations of the Exotic Hickory Trees, Carya laciniosa and Carya cordiformis, Grown in Kórnik Arboretum in Poland. Mycorrhiza 2018, 28, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Leski, T. Grzyby. In Wyniki Inwentaryzacji Elementów Przyrodniczych i Kulturowych Puszczy Białowieskiej; Matuszkiewicz, J.M., Tabor, J., Eds.; Instytut Badawczy Leśnictwa: Sękocin Stary, Poland, 2023. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List; Bolchacova, E.; et al. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Region as a Universal DNA Barcode Marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Aučina, A.; Rudawska, M.; Wilgan, R.; Janowski, D.; Skridaila, A.; Dapkūnienė, S.; Leski, T. Functional Diversity of Ectomycorrhizal Fungal Communities along a Peatland–forest Gradient. Pedobiologia 2019, 74, 15–23. [Google Scholar] [CrossRef]
- Janowski, D.; Wilgan, R.; Leski, T.; Karlinski, L.; Rudawska, M. Effective Molecular Identification of Ectomycorrhizal Fungi: Revisiting DNA Isolation Methods. Forests 2019, 10, 218. [Google Scholar] [CrossRef]
- Tedersoo, L.; Smith, M.E. Lineages of Ectomycorrhizal Fungi Revisited: Foraging Strategies and Novel Lineages Revealed by Sequences from Belowground. Fungal Biol. Rev. 2013, 27, 83–99. [Google Scholar] [CrossRef]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal Lifestyle in Fungi: Global Diversity, Distribution, and Evolution of Phylogenetic Lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Wurzburger, N.; Brookshire, E.N.J.; McCormack, M.L.; Lankau, R.A. Mycorrhizal Fungi as Drivers and Modulators of Terrestrial Ecosystem Processes. New Phytol. 2017, 213, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C. Coevolution of Roots and Mycorrhiza of Land Plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Agerer, R. Exploration Types of Ectomycorrhizae: A Proposal to Classify Ectomycorrhizal Mycelial Systems according to Their Patterns of Differentiation and Putative Ecological Importance. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Mrak, T.; Gričar, J. Atlas of Woody Plant Roots: Morphology and Anatomy with Special Emphasis on Fine Roots; The Silva Slovenica Publishing Centre: Ljubljana, Slovenia, 2016. [Google Scholar]
- Ryberg, M.; Andreasen, M.; Björk, R.G. Weak Habitat Specificity in Ectomycorrhizal Communities Associated with Salix Herbacea and Salix Polaris in Alpine Tundra. Mycorrhiza 2011, 21, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.; Millot, S.; Gardes, M.; Selosse, M.A. Diversity and Specificity of Ectomycorrhizal Fungi Retrieved from an Old-Growth Mediterranean Forest Dominated by Quercus Ilex. New Phytol. 2005, 166, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, S.M.; Sakakibara, S.M.; Durall, D.M. The Potential for Woody Understory Plants to Provide Refuge for Ectomycorrhizal Inoculum at an Interior Douglas-Fir Forest after Clear-Cut Logging. Can. J. For. Res. 2001, 31, 711–721. [Google Scholar] [CrossRef]
- Wojewoda, W.; Ławrynowicz, M. Czerwona Lista Roślin I Grzybów Polski. In Czerwona Lista Roślin i Grzybów Polski; Zarzycki, K., Mirek, Z., Eds.; Instytut Botaniki im. W. Szafera PAN: Kraków, Poland, 2006. [Google Scholar]
- Nehls, U.; Göhringer, F.; Wittulsky, S.; Dietz, S. Fungal Carbohydrate Support in the Ectomycorrhizal Symbiosis: A Review. Plant Biol. 2010, 12, 292–301. [Google Scholar] [CrossRef]
- Boddy, L.; Büntgen, U.; Egli, S.; Gange, A.C.; Heegaard, E.; Kirk, P.M.; Mohammad, A.; Kauserud, H. Climate Variation Effects on Fungal Fruiting. Fungal Ecol. 2014, 10, 20–33. [Google Scholar] [CrossRef]
- De la Varga, H.; Águeda, B.; Martínez-Peña, F.; Parladé, J.; Pera, J. Quantification of Extraradical Soil Mycelium and Ectomycorrhizas of Boletus Edulis in a Scots Pine Forest with Variable Sporocarp Productivity. Mycorrhiza 2012, 22, 59–68. [Google Scholar] [CrossRef]
- Queralt, M.; Parladé, J.; Pera, J.; De Miguel, A.M. Seasonal Dynamics of Extraradical Mycelium and Mycorrhizas in a Black Truffle (Tuber melanosporum) Plantation. Mycorrhiza 2017, 27, 565–576. [Google Scholar] [CrossRef]
- Oliach, D.; Colinas, C.; Castaño, C.; Fischer, C.R.; Bolaño, F.; Bonet, J.A.; Oliva, J. The Influence of Forest Surroundings on the Soil Fungal Community of Black Truffle (Tuber melanosporum) Plantations. For. Ecol. Manage. 2020, 470–471, 118212. [Google Scholar] [CrossRef]
- Kujawska, M.B.; Rudawska, M.; Wilgan, R.; Leski, T. Similarities and Differences among Soil Fungal Assemblages in Managed Forests and Formerly Managed Forest Reserves. Forests 2021, 12, 353. [Google Scholar] [CrossRef]
- Pacioni, G.; Comandini, O. Tuber. In Ectomycorrhizal Fungi Key Genera in Profile; Springer Science & Business Media: Berlin/Heidelberg, Germay, 1999. [Google Scholar]
- Leski, T.; Pietras, M.; Rudawska, M. Ectomycorrhizal Fungal Communities of Pedunculate and Sessile Oak Seedlings from Bare-Root Forest Nurseries. Mycorrhiza 2010, 20, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Pietras, M.; Rudawska, M.; Leski, T.; Karliński, L. Diversity of Ectomycorrhizal Fungus Assemblages on Nursery Grown European Beech Seedlings. Ann. For. Sci. 2013, 70, 115–121. [Google Scholar] [CrossRef]
- Iotti, M.; Lancellotti, E.; Hall, I.; Zambonelli, A. The Ectomycorrhizal Community in Natural Tuber Borchii Grounds. FEMS Microbiol. Ecol. 2010, 72, 250–260. [Google Scholar] [CrossRef]
- Ge, Z.W.; Brenneman, T.; Bonito, G.; Smith, M.E. Soil pH and Mineral Nutrients Strongly Influence Truffles and Other Ectomycorrhizal Fungi Associated with Commercial Pecans (Carya illinoinensis). Plant Soil 2017, 418, 493–505. [Google Scholar] [CrossRef]
- Rosa-Gruszecka, A.; Hilszczańska, D.; Pacioni, G. Virtual Truffle Hunting—A New Method of Burgundy Truffle (Tuber aestivum Vittad.) Site Typing. Forests 2021, 12, 1239. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).