Abstract
Wild medicinal mushrooms are known to contain significant amounts of essential biochemical compounds with potential health benefits. Therefore, this study aimed to investigate the metal elements and biochemical constituents of wild turkey tail (Trametes versicolor) mushrooms collected from the Shivalik foothills of the Himalayas, India. Mushroom samples were purposefully collected from eleven (11) sampling sites located in three (3) districts of North Indian states (Uttar Pradesh and Uttarakhand). The results of this study indicated that wild T. versicolor showed the presence of eight metal elements (Cd: 0.011–0.139, Cr: 0.225–0.680, Cu: 1.073–3.108, Fe: 4.273–8.467, Mn: 2.157–3.892, Zn: 3.069–4.478, Ni: 0.065–0.186, and Co: 0.035–0.120 mg/kg). The samples also showed a significant presence of total phenolics (51.81–70.13 mg GAE/g), flavonoids (9.02–14.01 mg QE/mg), lycopene (0.02–0.08 mg/g), and β-carotene (0.31–0.72 mg/g). The proximate analysis also showed that T. versicolor is a good source of carbohydrate (38.33%–41.94%), protein (8.12%–11.06%), fat (0.93%–1.26%), moisture (63.80%–70.64%), dietary fiber (9.59%–14.30%), and total ash (2.42%–3.48%). In addition, gas chromatography (GC-FID) analysis revealed the presence of the five most dominant fatty acids, including linoleic acid (18:2n6c), palmitic acid (C16:0), oleic acid (18:1n9c), linolenic acid (18:3n3), and stearic acid (C18:0). Principal component analysis (PCA) and hierarchical cluster analysis (HCA) were helpful in identifying variations and similarities among different constituents of T. versicolor at selected sampling sites. Due to its low metal element content and rich biochemical profile, T. versicolor was highlighted in this research for its significant potential as a functional food or nutraceutical ingredient. This work promotes its sustainable use in the healthcare and food industries and lays the groundwork for further research into its therapeutic applications.
1. Introduction
Wild mushrooms typically grow in shaded and edge-wooded areas. These mushrooms play a vital role in the sustainability of ecosystems by helping plants share and communicate life-sustaining nutrients through chemical signaling while recycling both organic and inorganic by-products [1]. Extreme weather fluctuations, i.e., severe droughts or unusually heavy rainfall, and the spread of pests are the main factors that inhibit/reduce the growth, proliferation, and mycelial activity of wild mushrooms [2,3]. The high availability of nutrients from the soil contributes to rapid mushroom growth [4]. However, the impact of climate change on the overall production and distribution of wild mushrooms is not uniform. Foraging for wild mushrooms (Agaricus spp., Lentinula spp., Pleurotus spp., Auriscalpium spp., Hericium spp., Hydnum spp., etc.) is a tradition in many communities, despite the expansion of indoor mushroom cultivation. Moreover, mushroom foraging is an important daily source of food in rural areas, filling the gap that the cultivation of numerous species of mushrooms is unable to fill [5].
Turkey tail (Trametes versicolor) is one of the most common mixed-wood forest fungi, growing abundantly in clusters on decaying trees and logs. It is a saprophytic white-rot fungus (Polyporaceae, Basidiomycota) that degrades wood lignin through its developed mycelium. Wild T. versicolor is native to European, Southeast Asian, and North American forests. It grows best at temperatures of 15–24 °C and 75%–90% humidity, and grows on over 70 different species of hardwoods and conifers [6]. This mushroom has tiny pores instead of gills. “Versicolor” refers to the multicolored feature of this mushroom, which is very attractive to look at. Its taste is considered slightly bitter, earthy, and rather mild compared to shiitake. Although its taste is not bad, most mushroom consumers find it somewhat woody (tough texture) with a chewy consistency. This makes it unpopular among edible mushrooms worldwide.
Wild T. versicolor is known as a medicinal mushroom with a good protein content (about 11.1 g/100 g dry weight). Mushroom protein can help alleviate malnutrition and could be a promising substitute for meat protein [7]. T. versicolor contains significant amounts of phenolic acids, i.e., homogentisic acid, p-hydroxybenzoic acid, protocatechuic acid, and vanillic acid (1.24–113.2 μg/g dry weight) [8]. Phenolic acids from medicinal mushrooms have several medicinal bioactivities, including anti-hyperglycemic, anti-inflammatory, antimicrobial, antioxidant, anti-osteoporotic, antitumor, and anti-tyrosinase actions [9]. In addition, this species has been reported to have anti-diabetic properties and to enhance immune and innate immune responses [10,11]. Essential and non-essential amino acids can be found in T. versicolor, including asparagine, glutamine, isoleucine, leucine, and tyrosine (15.5–72.4 mg/100 g dry weight) [8]. Non-essential amino acids have been shown to be important aspects of tumor metabolism, i.e., epigenetic regulatory mechanisms, maintenance of redox homeostasis, and biosynthesis of lipids and nucleotides [12], while essential amino acids are needed for protein synthesis. Nine such essential amino acids, which cannot be synthesized by the human body, are found in mushrooms. T. versicolor is also a good source of vitamins, mainly nicotinic acid (vitamin B3), nicotinamide (vitamin B3), and vitamin D (12.8–26.5 mg/100 g dry weight) [8]. Vitamins help the human body strengthen bones, regulate hormones, fight infections, and heal wounds [13]. The medical field has benefited from the high beta-glucans content of wild T. versicolor in clinical breast cancer trials, suggesting a further promising research area [14]. Several fatty acids have been found in T. versicolor, including oleic, linoleic, linolenic, palmitic, and stearic acids [8]. As a good source of unsaturated fatty acids, this species helps reduce blood cholesterol [15].
Wild mushroom species can bioaccumulate significant amounts of potentially toxic elements (metal elements) from the environment in which they grow [16]. Recent studies have shown that wild mushroom caps have a greater ability than stalks to bioaccumulate metal elements [17]. These elements, when exceeding the standard safe limits set by local and international standards, can pose serious health risks, such as kidney, liver, skeletal, and cardiovascular issues. Therefore, bioaccumulation capacities and health risk assessments should be evaluated for safe human consumption. Pacheco et al. [18] reported the capacities of T. versicolor in the biosorption and bioaccumulation of 0.25–2.0 mg/L of lead ion (Pb II). Additionally, this species was able to accumulate 62.8 mg/g, 5 mg/g, 40–80 mg/L, and 30–400 mg/L of copper (Cu II), cadmium (Cd II), arsenic (As III), and chromium (Cr VI) ions, respectively, from different sources of pollution [19,20,21]. These reports revealed that T. versicolor is a potential metal element bioaccumulator.
The Shivalik Hills are an outer Himalayan mountain range occupying a total area of 2400 km, which includes uncoordinated rivers originating from the main Himalayan mountains. The Shivalik range is considered the main source of sediments for the Terai Plain and is home to diverse flora and fauna species. This region is solely dependent on agriculture; however, with the increased use of chemical fertilizers and pesticides, these products are mixed with drained water, causing deterioration of soil quality [22]. While the state of cultivated crops in this area has been denoted, the state of the wild vegetation, which constitutes the majority of flora present, remains scantly investigated. More particularly, wild mushroom populations in Shivalik Hills have been poorly explored. Therefore, the current study aims to investigate the biochemical and metal element contents of wild T. versicolor.
2. Materials and Methods
2.1. Description of the Study Area and Sample Collection
The present study was conducted in a total of three districts, namely Haridwar (H), Dehradun (D), and Saharanpur (S), located in the Shivalik foothills of the Indian Himalayan region (IHR). Of these, the Haridwar and Dehradun districts are located in Uttarakhand, while Saharanpur is located in Uttar Pradesh in northern India (Figure 1). A total of 11 sampling sites were purposively selected based on mushroom availability and the accessibility of the area, as listed in Table 1. The selected region is a mixture of different types of ecological landscapes, including mountains, dense forests, and the Ganga–Yamuna plains. In particular, the Shivalik foothills in this region are home to diverse wildlife, including several endangered species. Playing a vital role in maintaining the ecological balance of the region, the Shivalik foothills serve as an important corridor for wildlife movement. The region is also home to several species of wild mushrooms due to its favorable environmental conditions, such as rainfall, humidity, and temperature, especially during the monsoon season. Out of these, T. versicolor is a saprophytic mushroom that grows abundantly on dead wood logs and tree stumps (mostly the stumps of Casearia graveolens, Terminalia arjuna, Dalbergia sissoo, Mallotus philippensis, etc.) in this area.
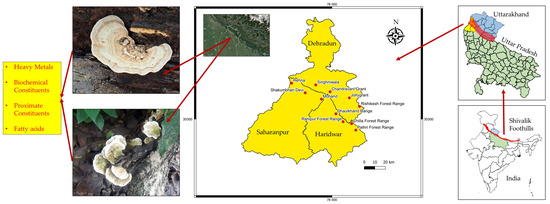
Figure 1.
Map of the study area showing selected locations for sampling of turkey tail (T. versicolor) mushroom.

Table 1.
List of sampling locations in selected states and districts used for collection of turkey tail (T. versicolor) mushroom samples collection.
For this research, T. versicolor samples were collected during the post-monsoon season in the study region, i.e., September to October 2022. A total of three (n = 3) healthy and fresh fruiting body samples of T. versicolor were collected from each sampling site. Thus, a total of 33 mushroom samples were collected (3 from each site). Mushroom fruiting bodies were carefully cut from the substrate material using a sharp knife, packed in transparent polyethylene zipper bags (100 g capacity), and placed in a polyurethane foam insulated ice cooler box (11 L, PinnacleThermo, Ahmedabad, Gujrat, India) for immediate transportation to the laboratory for further analysis.
2.2. Metal Element Analysis Using ICP-OES
T. versicolor samples collected from the selected sites were analyzed for eight metal elements using inductively coupled plasma optical emission spectroscopy (ICP-OES, 7300 DV, Perkin Elmer, Boston, MA, USA). For this purpose, fungal fruiting bodies were oven-dried at 60 °C until constant biomass was reached and then ground to a fine powder using a mechanical grinder. A total of 2 g of dried and powdered sample was then mixed with 20 mL of a diacid mixture containing a 3:1 ratio of HNO3 and HClO4, respectively. The mixture was allowed to self-digest overnight (12 h) in a 250 mL conical flask. The contents were heat digested using a hot plate at 150 °C for 1 h until a clear solution was obtained. The contents were further cooled, and the final volume was adjusted to 50 mL by the addition of 3% HNO3 solution. This content was filtered through Whatman no. 41 filter paper and finally used for metal element analysis by ICP-OES, as previously described by Kumar et al. [23].
2.3. Methods for Biochemical Analyses
The T. versicolor samples collected in this study were analyzed for selected biochemical (total phenolics: mg GAE/g, flavonoids: mg QE/mg, lycopene: mg/g, and β-carotene: mg/g) and proximate (carbohydrates, proteins, fat, moisture content, fiber, and total ash: %) parameters. For biochemical determinations, a total of 5 g of sun-dried mushroom sample was used to prepare a methanolic extract by continuous extraction in a Soxhlet apparatus. Total phenolic compounds were determined from the methanolic extract using Folin and Ciocalteu’s phenol reagent, followed by spectrophotometric determination at 725 nm (Cary 60, UV-Vis, Agilent, Santa Clara, CA, USA). The flavonoid content in the mushroom samples was determined using the aluminum nitrate colorimetric method, as previously described by Pękal et al. [24]. Similarly, lycopene and β-carotene contents were determined using 100 mg of methanolic extract continuously mixed with 10 mL of acetone–hexane solution (4:6) followed by filtration through Whatman filter paper number 4. The filtrate was used for spectroscopic determination of lycopene and β-carotene at 453, 505, 645, and 663 nm, as previously outlined by Barros et al. [25]. The fatty acid profiles of the mushroom samples were determined by extraction of the lipids using an n-hexane-based Soxhlet apparatus, followed by characterization by gas chromatography (GC) equipped with a flame ionizing detector (FID), as previously adopted by Kumari et al. [26]. All instrument-based analytical measurements were validated using certified reagent materials (CRM; BCR679, Merck, Mumbai, India) and standard reference materials (SRM; 1000 mg/L stock, Merck, India), as per the manufacturer’s instruction.
2.4. Methods for Proximate Analyses
For proximate determination, total carbohydrate fat and fiber contents were determined by following the standard methods adopted by Uju and Obiakor [27]. Protein contents were estimated by following Kjeldahl’s digestion method, as outlined by James [28]. Finally, total ash was determined using a muffle furnace.
2.5. Data Analysis and Software
The data obtained in this study were analyzed using Microsoft Excel (Version 2019, Microsoft Corp., Redmond, WA, USA) and OriginPro (Version 2023, OriginLab, Northampton, MA, USA) software packages. The data was processed using Pearson correction, principal component analysis (PCA), and hierarchically clustered heatmap tools. The level of statistical significance was p < 0.05.
3. Results and Discussion
3.1. Results of Metal Element Content Analysis in Turkey Tail (T. versicolor)
The results of the metal element content analysis in T. versicolor collected from different sampling locations of the Shivalik foothills of the Himalayas are shown in Table 2. Very low coefficients of variance (CV) were observed (0.12%–1.04%) among different analyzed metal elements, enabling a precise estimate of the metal elements. Cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), nickel (Ni), and cobalt (Co) contents in mushrooms collected from different locations were in the ranges of 0.011–0.139, 0.225–0.680, 1.073–3.108, 4.273–8.467, 0.065–0.186, and 0.035–0.120 mg/kg, respectively. Therefore, the metal element bioaccumulation order in collected mushrooms was as follows: Fe > Zn > Mn > Cu > Cr > Ni > Co > Cd. By contrast, Bulam et al. [29] mentioned a metal element accumulation in wild T. versicolor in the following order: Ni > Cu > Cr > Cd > Co. This difference could be attributed to the difference in soil composition among the sampling sites. However, there is also the possibility of metal element incorporation into the soil through air pollution. Moreover, variations may be due to the fact that different tree species accumulate different levels of metal elements, ultimately affecting the bioavailable concentration of mushroom species growing on them. It was also observed that different sampling sites significantly (p < 0.05) influenced the content of metal elements in T. versicolor.

Table 2.
The concentration of metal elements (mg/kg dwt.) in turkey tail (T. versicolor) mushroom collected from selected sampling locations in the Shivalik foothills of Himalaya, India.
Most sampling sites showed Cd content (0.011–0.035 mg/kg) below the safe limit set by Indian standards (0.10 mg/kg) [30]. Only H2 (Ranipur Forest Range) and D3 (Chandravani Grant) mushrooms showed slightly higher Cd values (0.139 and 0.0114 mg/kg, respectively). H2 has faced large-scale human activities in the last few years, i.e., mining, unsustainable construction, and infrastructure development, which has naturally resulted in toxic element dispersal. By contrast, the sources of pollutants in a small village such as D3 are mainly related to agricultural activities (i.e., pesticides, fertilizers, waste burning). High Cd exposure can lead to cardiovascular diseases, chronic kidney disease, and diabetes [31]. Nevertheless, Cd content in all collected T. versicolor was in accordance with the value denoted by Bulam et al. [29] (0.00258 mg/kg), and lower than that reported by Keskin et al. [32] (0.19 mg/kg). T. versicolor was considered an effective bioaccumulator for Cd, as it succeeded in absorbing 100–350 mg/kg of Cd from polluted soils within 2–7 days [21]. The H2 site showed the highest bioaccumulation of metal elements (Cd, Cu, Fe, Mn, Zn, and Ni), whereas D3 mushrooms had the highest bioaccumulation of Cr and Co. Encouragingly, Cr, Cu, Fe, Mn, Zn, Ni, and Co contents in all collected mushrooms were well below the safe limits set by Indian standards [30], USEPA [33], and FAO/WHO [34,35]. All these elements, when found in certain concentrations, are essential for the human body’s growth and development except for Cd. Cd is considered a toxic metal and could have detrimental impacts on the consumer’s health, making its biomonitoring essential before the mushroom can be considered for edible purposes. Fe, Zn, and Cu contents in all collected mushrooms from the Shivalik foothills of Himalaya were lower than observed by Akgul et al. [36] and Keskin et al. [32] (73.9–154.34, 11.4–15.68, and 6.80–8.94 mg/kg, respectively) in T. versicolor collected from the Kastamonu and Muğla provinces, Turkey.
The analysis of the Kurtosis and Skewness test results revealed both positive and negative values (−1.46 to 2.29 and −0.41 to 1.88, respectively). The positive values had random symmetric distribution throughout the sampling locations, and the negative values were represented by a few outliers. The vector length of the PCA plot in Figure 2a is the relationship between metal elements and sampling locations. The vector length showed the highest Fe, Mn, Cu, Zn, and Cr contents in H2, D3, and S3, followed by H1 and S1. Figure 2b outlines the hierarchal cluster dendrogram with a heatmap for metal elements contents in T. versicolor collected from different sampling locations of the Shivalik foothills of Himalaya, India. Analysis of this dendrogram indicates that Cd–Cu and Ni–Co contents had the highest similarities within collected mushrooms. Moreover, similarities between sampling locations were identified, with D3 and H2 showing the highest similarity.
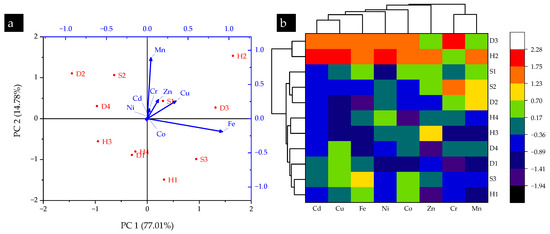
Figure 2.
(a) PCA and (b) HCA results of metal elements in turkey tail (T. versicolor) mushroom collected from selected sampling locations in the Shivalik foothills of Himalaya, India.
Recent studies have adopted multivariate statistical analyses to analyze the metal element profile of collected mushrooms in different world regions. For instance, PCA and HCA were effective in depicting the variability of metal element contents in Russula and parasol mushrooms collected from Turkey, Morocco, and Spain, respectively [37,38]. Moreover, PCA and HCA adequately identified the heavy metal contamination levels of Agaricus bisporus mushrooms collected from different sites in Uttarakhand state, India [39] and Pleurotus spp. collected from different sites in Rajaji National Park, Haridwar, India [16]. Corroborating the evidence of all these studies, PCA and HCA successfully achieved the localization of metal element contamination levels in the Shivalik foothills of the Himalayas, India.
3.2. Results of Analysis of Biochemical Constituents in Turkey Tail (T. versicolor)
The results of the biochemical analysis of T. versicolor fruiting bodies collected from different locations of the Shivalik foothills of Himalaya are reported in Table 3. CV values were all very low (0.11%–0.37%), enabling a precise estimate of the elemental contents. H2 mushrooms showed the highest total phenolic and β-carotene contents (70.13 ± 5.01 mg GAE/g and 0.72 ± 0.05 mg/g, respectively), whereas D3 mushrooms had the highest flavonoid and lycopene contents (14.01 ± 1.00 mg QE/mg and 0.08 ± 0.03 mg/g, respectively). It is worth noting that although H2 and D3 mushrooms showed the highest metal element bioaccumulation, they also had the best medicinal properties. T. versicolor collected in the present investigation had lower total phenolic content than those analyzed by Bulam et al. [29] in Turkey (51.81 ± 3.70–70.13 ± 5.01 and 77.41 ± 1.10 mg GAE/g, respectively), and higher than those reported by Pop et al. (2018) in Romania (8.18–46.22 mg GAE/g) and Bains and Chawla [40] in Himachal Pradesh, India (48.71 mg GAE/g).

Table 3.
Contents of selected biochemical constituents in turkey tail (T. versicolor) mushroom collected from selected sampling locations of Shivalik foothills of Himalaya, India.
Phenolic compounds are well known for their free radical inhibition, metal inactivation, prevention of negative reactivity of undesired reactive oxygen, peroxide decomposition, and oxidative disease burden [41,42]. The flavonoid content found in T. versicolor collected from Turkey (13.82 ± 0.2 mg QE/mg) [29] and Himachal Pradesh, India (13.13 mg QE/mg) [40] fell within the range observed in the current study (9.02 ± 0.64–14.01 ± 1.00 mg QE/mg), whereas Szwajkowska-Michałek et al. [43] found poor flavonoid content in T. versicolor collected from Poland, and Pop et al. [44] failed to identify any flavonoid content. Flavonoids can help reduce blood platelet aggregation, lipoprotein oxidation, and cardiovascular mortality, and can regulate vascular reactivity [45]. The lycopene contents found in the collected T. versicolor of the present study (0.02 ± 0.01–0.08 ± 0.03 mg/g) corroborate the finding of Bains and Chawla [40]. The inhibition of cancer cell growth, modulation of immune function, and prevention of DNA, lipid, and protein oxidative damage could all be achieved with lycopene-rich products [46,47,48]. The β-carotene content in T. versicolor collected from Poland in a previous study was reported to be 1.01 mg/g [43], which is higher than the range observed in the present study (0.31–0.72 mg/g). It has been reported that both lycopene and β-carotene can stabilize or inhibit oxidation reactions [49]. Moreover, Grune et al. [50] mentioned that β-carotene is a good source of vitamin A, which is needed for human body growth and development, reproduction, and immune-system boosting. The results of the Kurtosis test showed negative values (−1.62 to −0.15), which indicated low extreme outliers, whereas those of the Skewness test denoted both positive and negative values (−0.19 to 0.62), which indicated few outliers. Skewness values were all between −2 and +2, which, according to George [51], indicates normal univariate distribution and acceptable asymmetry.
The PCA plot in Figure 3a outlined a strong relation between total phenolics and flavonoids at the H1, H2, H4, D1, and D3 sampling locations. The variance of identified components was noted as PC1: 95.11% and PC2: 4.87%. The vector length showed the highest total phenolic and flavonoid contents in H2 and D3, which confirms the results in Table 3. Figure 3b depicts a hierarchal cluster dendrogram with a heatmap for biochemical elemental contents in T. versicolor collected from different sampling locations of the Shivalik foothills of Himalaya, India. Analysis of this dendrogram indicates that TP–F and BC–L contents had potential similarities among the collected mushrooms. Furthermore, D3 and H2 sampling locations showed very high similarities in terms of the biochemical contents of the collected mushrooms, which confirms the findings of Table 3 and Figure 3a. Tokul-Olmez et al. [52] successfully used both PCA and HCA for the classification of four Ganoderma spp. based on the analysis of fifteen fatty acids. Earlier, Heinke et al. [53] adopted the same methodology for the identification and classification of four Suillus spp. based on their metabolite profiles. They not only reported the suitability of the PCA–HCA combination for the aforementioned task, but also proved it as a powerful tool for the chemotaxonomy of fungi. However, this is the first study to successfully combine PCA and HCA tools for the identification and quantification of total phenolic, flavonoid, lycopene, and β-carotene contents in mushrooms.
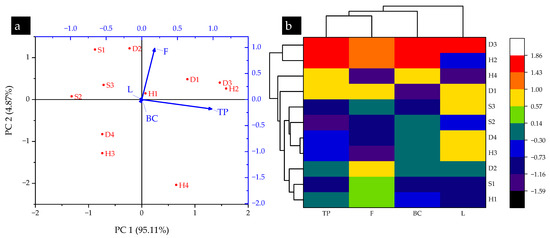
Figure 3.
(a) PCA and (b) HCA results of biochemical constituents in turkey tail (T. versicolor) mushroom collected from selected sampling locations in the Shivalik foothills of Himalaya, India.
3.3. Results of Analysis of Proximate Constituents in Turkey Tail (T. versicolor)
Table 4 shows the contents of proximate parameters of T. versicolor samples collected from 11 locations in the Shivalik foothills of the Himalayas, India. With these results, it was observed that the carbohydrate contents of T. versicolor ranged between 38.33 and 41.94%, with the maximum value observed at the D4 site. Carbohydrate acts as a major constituent of the mushroom body, serving functions such as the formation of cell wall structures and storage reserves, which are necessary for mushrooms to thrive in nature [54]. The protein contents of the samples ranged between 8.12 and 11.06%, with maximum levels recorded at the H4 site. The fat contents were maximal at the H2 site (1.26 ± 0.09%), with an overall range of 0.93%–1.26%, respectively. Although most wild edible mushrooms have a higher moisture content (>80%), the turkey tail showed lower contents ranging from 63.80 to 70.64%. This might be because turkey tails have adapted to fluctuating environmental conditions by minimizing moisture content, which allows better shelf-life and prevents excessive water loss during dry periods [55]. Due to this feature, turkey tail has been found to survive at greater rates both in domestic and wild habitats. The total ash contents of the T. versicolor were observed within a range of 2.42%–3.48%, indicating optimum levels of inorganic material. The variation in the ash content might have been affected by the availability of different metal elements present in the substrate (usually wood logs) which were absorbed by the fungal mycelia and then transported to the upper body parts of the T. versicolor. The results of the Kurtosis test showed negative values (−1.44 to −0.82) for most parameters, indicating low extreme outliers, except for protein (1.12). However, the Skewness test denoted negative values (−0.48 to −0.04) for most parameters except for total ash, which had a positive value (0.25).

Table 4.
Contents of selected proximate constituents in turkey tail (T. versicolor) mushroom collected from selected sampling locations in the Shivalik foothills of Himalaya, India.
Moreover, the results of the PCA in Figure 4a show the data on two axes, PC1 and PC2, having a variance of 57.67% and 30.10%, with eigenvalues of 6.10 and 3.18, respectively. The vector length shows that moisture content was maximally correlated with the H2 site, while fiber content was correlated with the H1 site. These results are in line with those reported in Table 4. Similarly, the clustered heatmap dendrogram shown in Figure 4b indicates that S2 and H3 sites showed the highest similarities in terms of proximate properties of T. versicolor. Likewise, S3 and H2 formed the second most similar pair. T. versicolor has been widely analyzed for proximate constituents by researchers around the world. However, its characterization in the wild, particularly in the Indian Himalayan region, has been limited. A previous report by Upadhyaya et al. [56] found that T. versicolor collected from Nepal had significant contents of carbohydrates (54%), moisture (12%), ash (18%), fiber (20%), fat (2%), and protein (11%) contents, in line with those reported in the current investigation. Similarly, Kıvrak et al. [8] also found that T. versicolor had significant contents of moisture (87.21 g/100 g fw), protein (11.07 g/100 g dw), and fat (1.35 g/100 g dw). It was likewise observed that wild T. versicolor had significant contents of selected proximate constituents in the studied region.
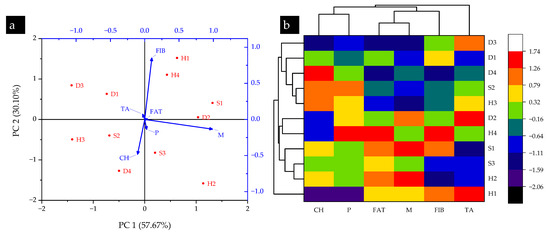
Figure 4.
(a) PCA and (b) HCA results of proximate constituents in turkey tail (T. versicolor) mushroom collected from selected sampling locations in the Shivalik foothills of Himalaya, India (CH: carbohydrate; P: protein; FAT: fat; M: moisture; FIB: fiber; TA: total ash).
3.4. Fatty Acids in Turkey Tail (T. versicolor)
In the identification and qualification results using GC-FID, the T. versicolor extract showed the presence of various fatty acids, as listed in Table 5. The contents of five fatty acids (i.e., linoleic acid: 18:2n6c; palmitic acid: C16:0; oleic acid: 18:1n9c; linolenic acid: 18:3n3; and stearic acid: C18:0) were observed in varying ranges across the selected sampling locations. However, linoleic and palmitic acids were observed in the highest levels, at 13.94%–23.38% and 14.08%–18.29%, respectively. Linoleic acid is an omega-6 unsaturated fatty acid helpful in reducing cholesterol, while palmitic acid is a saturated fatty acid that is widely used as an emollient [57]. Oleic acid (4.92%–8.13%) and stearic acid (1.74%–2.72%) were found at moderate levels, while linolenic acid (0.90%–2.02%) was found at minimum levels. However, the fatty acid composition of turkey tail might be influenced by several internal and external factors, such as the type of substrate on which they are grown and the climatic conditions supporting the synthesis of several biochemical constituents. Diets with selected saturated and unsaturated fatty acids are known to improve health benefits [58].

Table 5.
Contents of selected fatty acids (%) in turkey tail (T. versicolor) mushroom collected from selected sampling locations in the Shivalik foothills of Himalaya, India.
The Kurtosis test showed mostly negative values (−1.50 to −0.31) for all fatty acids except for stearic acid (0.14), while the Skewness test showed mostly positive values (0.10 to 0.21) except for oleic and linolenic acids (−0.35 and −0.26). Moreover, the PCA results given in Figure 5a show that the vector lengths of linoleic acid were mostly correlated, with D2, H3, and S3 the highest concentrations. For HCA, the S3 and D4 sampling sites showed the highest similarity in the occurrence of fatty acids in T. versicolor, followed by S1-H4 and D1-H1, respectively. This indicates that most of the sites had similar agroclimatic conditions, resulting in high similarities for fatty acid contents in wild T. versicolor.
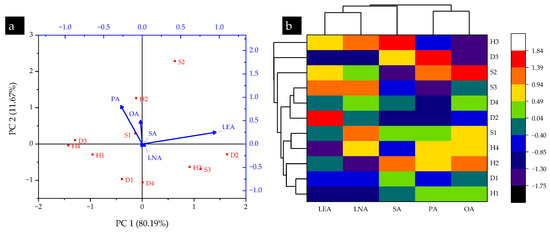
Figure 5.
(a) PCA and (b) HCA results of fatty acids in turkey tail (T. versicolor) mushroom collected from selected sampling locations in the Shivalik foothills of Himalaya, India (LEA: linoleic acid; LNA: linolenic acid; SA: stearic acid; PA: palmitic acid; OA: oleic acid).
Previously, researchers have analyzed T. versicolor for the presence of saturated and unsaturated fatty acids. Among them, Kıvrak et al. [8] found that wild T. versicolor had significant contents of linoleic, oleic, palmitic, stearic, and linolenic acids. Similarly, Günç et al. [59] investigated the fatty acid profiles of six wild edible mushroom species, including Boletus reticulatus, Flammulina velutipes, Lactarius salmonicolor, Pleurotus ostreatus, Polyporus squamosus, and Russula anthracina. They found that the most dominating fatty acids were cis-linoleic acid, cis-oleic acid, palmitic acid, and stearic acid, in line with the findings of the current study. The presence of a diverse range of fatty acids in wild T. versicolor found in the Shivalik foothills signifies their nutritional importance and health benefits. Particularly promising is the presence of significant amounts of unsaturated fatty acids, among which the omega-3 and omega-6 fatty acids are well-known for their beneficial effects on cardiovascular health, immune function, and the regulation of inflammation [15].
4. Conclusions
The results of this study confirm that wild T. versicolor samples collected from the Shivalik foothills of the Himalayas, India, showed the occurrence of eight metal elements (Cd, Cr, Cu, Fe, Mn, Zn, Ni, and Co). However, the levels of metal elements were relatively low, providing valuable insights into the safety and potential health benefits of this unique fungal species. Furthermore, the investigation of the biochemical, proximate, and fatty acid constituents of T. versicolor indicated that it can be used as a potential functional food or nutraceutical. However, it is essential to monitor the levels of metal elements to ensure consumer safety. This study underscores the potential of wild T. versicolor for various applications, including in the healthcare and the food industries. However, it is essential to emphasize that further validation is required to establish the safety of this species for the food industry. Further studies on the identification and quantification of other biochemical constituents, as well as on the domestication and commercial exploitation of wild strains of T. versicolor, are highly recommended.
Author Contributions
Conceptualization, Y.S.M., P.K., S.A.F., R.S. and E.M.E.; data curation, I.Š., S.A.M.A., S.A.A., S.A.F. and S.Z.; formal analysis, P.K. and R.S.; funding acquisition, Y.S.M. and E.M.E.; investigation, P.K. and R.S.; methodology, Y.S.M., I.Š., P.K., R.S. and E.M.E.; project administration, Y.S.M. and E.M.E.; resources, P.K.; Software, S.A.M.A., S.A.A. and S.Z.; supervision, E.M.E.; validation, I.Š., S.A.M.A., S.A.A., S.A.F. and S.Z.; visualization, I.Š., S.A.M.A., S.A.A., S.A.F. and S.Z.; writing—original draft, P.K. and S.A.F.; writing—review and editing, Y.S.M., I.Š., S.A.M.A., S.A.A., S.Z., R.S. and E.M.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at King Khalid University, Abha, Saudi Arabia (grant number RGP.2/223/44).
Data Availability Statement
All data generated in this study is already provided within the article.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Group Research Project under grant number R.G.P. 2/223/44. All individuals included in this section have consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Devkota, S.; Fang, W.; Arunachalam, K.; Phyo, K.M.M.; Shakya, B. Systematic Review of Fungi, Their Diversity and Role in Ecosystem Services from the Far Eastern Himalayan Landscape (FHL). Heliyon 2023, 9, e12756. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, R.; Kaiser, A.; Sardella, A.; De Nuntiis, P.; Drdácký, M.; Hanus, C.; Bonazza, A. Climate Change-Induced Disasters and Cultural Heritage: Optimizing Management Strategies in Central Europe. Clim. Risk Manag. 2021, 32, 100301. [Google Scholar] [CrossRef]
- Procházka, P.; Soukupová, J.; Tomšík, K.; Mullen, K.J.; Čábelková, I. Climatic Factors Affecting Wild Mushroom Foraging in Central Europe. Forests 2023, 14, 382. [Google Scholar] [CrossRef]
- Stojek, K.; Gillerot, L.; Jaroszewicz, B. Predictors of Mushroom Production in the European Temperate Mixed Deciduous Forest. For. Ecol. Manag. 2022, 522, 120451. [Google Scholar] [CrossRef]
- Savoie, J.-M.; Largeteau, M.L. Production of Edible Mushrooms in Forests: Trends in Development of a Mycosilviculture. Appl. Microbiol. Biotechnol. 2011, 89, 971–979. [Google Scholar] [CrossRef]
- Hardin, A. Biotic Inventory: Documenting Diversity at the Katharine Ordway Natural History Study Area. Available online: https://www.macalester.edu/ordway/biodiversity/inventory/turkeytailfungus/#:~:text=TheTurkeyTailfungusis,conifersintheUnitedStates (accessed on 7 July 2023).
- Ayimbila, F.; Keawsompong, S. Nutritional Quality and Biological Application of Mushroom Protein as a Novel Protein Alternative. Curr. Nutr. Rep. 2023, 12, 290–307. [Google Scholar] [CrossRef]
- Kıvrak, I.; Kivrak, S.; Karababa, E. Assessment of Bioactive Compounds and Antioxidant Activity of Turkey Tail Medicinal Mushroom Trametes versicolor (Agaricomycetes). Int. J. Med. Mushrooms 2020, 22, 559–571. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A Comprehensive Review on Phenolic Compounds from Edible Mushrooms: Occurrence, Biological Activity, Application and Future Prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef]
- Im, K.; Nguyen, T.; Choi, J.; Lee, T. In Vitro Antioxidant, Anti-Diabetes, Anti-Dementia, and Inflammation Inhibitory Effect of Trametes pubescens Fruiting Body Extracts. Molecules 2016, 21, 639. [Google Scholar] [CrossRef]
- Benson, K.F.; Stamets, P.; Davis, R.; Nally, R.; Taylor, A.; Slater, S.; Jensen, G.S. The Mycelium of the Trametes versicolor (Turkey Tail) Mushroom and Its Fermented Substrate Each Show Potent and Complementary Immune Activating Properties in Vitro. BMC Complement. Altern. Med. 2019, 19, 342. [Google Scholar] [CrossRef]
- Choi, B.-H.; Coloff, J.L. The Diverse Functions of Non-Essential Amino Acids in Cancer. Cancers 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Ofoedu, C.E.; Iwouno, J.O.; Ofoedu, E.O.; Ogueke, C.C.; Igwe, V.S.; Agunwah, I.M.; Ofoedum, A.F.; Chacha, J.S.; Muobike, O.P.; Agunbiade, A.O.; et al. Revisiting Food-Sourced Vitamins for Consumer Diet and Health Needs: A Perspective Review, from Vitamin Classification, Metabolic Functions, Absorption, Utilization, to Balancing Nutritional Requirements. PeerJ 2021, 9, e11940. [Google Scholar] [CrossRef] [PubMed]
- Stamets, P. Trametes versicolor (Turkey Tail Mushrooms) and the Treatment of Breast Cancer. Glob. Adv. Health Med. 2012, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Sande, D.; de Oliveira, G.P.; Moura, M.A.F.E.; Martins, B.d.A.; Lima, M.T.N.S.; Takahashi, J.A. Edible Mushrooms as a Ubiquitous Source of Essential Fatty Acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef] [PubMed]
- Širić, I.; Kumar, P.; Adelodun, B.; Abou Fayssal, S.; Bachheti, R.K.; Bachheti, A.; Ajibade, F.O.; Kumar, V.; Taher, M.A.; Eid, E.M. Risk Assessment of Heavy Metals Occurrence in Two Wild Edible Oyster Mushrooms (Pleurotus spp.) Collected from Rajaji National Park. J. Fungi 2022, 8, 1007. [Google Scholar] [CrossRef]
- Širić, I.; Rukavina, K.; Mioč, B.; Držaić, V.; Kumar, P.; Taher, M.A.; Eid, E.M. Bioaccumulation and Health Risk Assessment of Nickel Uptake by Five Wild Edible Saprotrophic Mushroom Species Collected from Croatia. Forests 2023, 14, 879. [Google Scholar] [CrossRef]
- Pacheco, J.S.; Santana, M.; Guadalupe, M.; Uscanga, A.; Cavazos, A.; Niño, J.S.; Gómez, H.; Uscanga, B.A. Ability of Phanerochaete Chrysosporium and Trametes Versicolor to Remove Zn2+, Cr3+, Pb2+ Metal Ions. Terra Latinoam. 2015, 33, 189–198. [Google Scholar]
- Aksu, Z.; Kılıç, N.K.; Ertuğrul, S.; Dönmez, G. Inhibitory Effects of Chromium(VI) and Remazol Black B on Chromium(VI) and Dyestuff Removals by Trametes versicolor. Enzym. Microb. Technol. 2007, 40, 1167–1174. [Google Scholar] [CrossRef]
- Akar, S.T.; Akar, T.; Kaynak, Z.; Anilan, B.; Cabuk, A.; Tabak, Ö.; Demir, T.A.; Gedikbey, T. Removal of Copper(II) Ions from Synthetic Solution and Real Wastewater by the Combined Action of Dried Trametes versicolor Cells and Montmorillonite. Hydrometallurgy 2009, 97, 98–104. [Google Scholar] [CrossRef]
- Manna, A.; Sundaram, E.; Amutha, C.; Vasantha, V.S. Efficient Removal of Cadmium Using Edible Fungus and Its Quantitative Fluorimetric Estimation Using (Z)-2-(4 H -1,2,4-Triazol-4-Yl)Iminomethylphenol. ACS Omega 2018, 3, 6243–6250. [Google Scholar] [CrossRef]
- Kaur, T.; Sehgal, S.K.; Singh, S.; Sharma, S.; Dhaliwal, S.S.; Sharma, V. Assessment of Seasonal Variability in Soil Nutrients and Its Impact on Soil Quality under Different Land Use Systems of Lower Shiwalik Foothills of Himalaya, India. Sustainability 2021, 13, 1398. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Adelodun, B.; Bedeković, D.; Kos, I.; Širić, I.; Alamri, S.A.M.; Alrumman, S.A.; Eid, E.M.; Abou Fayssal, S.; et al. Sustainable Use of Sewage Sludge as a Casing Material for Button Mushroom (Agaricus bisporus) Cultivation: Experimental and Prediction Modeling Studies for Uptake of Metal Elements. J. Fungi 2022, 8, 112. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Barros, L.; Ferreira, M.-J.; Queirós, B.; Ferreira, I.C.F.R.; Baptista, P. Total Phenols, Ascorbic Acid, β-Carotene and Lycopene in Portuguese Wild Edible Mushrooms and Their Antioxidant Activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, V.; Kothari, R.; Kumar, P. Experimental and Optimization Studies on Phycoremediation of Dairy Wastewater and Biomass Production Efficiency of Chlorella vulgaris Isolated from Ganga River, Haridwar, India. Environ. Sci. Pollut. Res. 2022, 29, 74643–74654. [Google Scholar] [CrossRef] [PubMed]
- Uju, N.L.; Obiakor, O.P. Nutritional Profile of Three Different Mushroom Varieties Consumed in Amaifeke, Orlu Local Government Area, Imo State, Nigeria. Food Sci. Qual. Manag. 2014, 31, 70–78. [Google Scholar]
- James, C.S. Analytical Chemistry of Foods; Chapman and Hall: New York, NY, USA, 1995; Volume 309. [Google Scholar]
- Bulam, S.; Karadeniz, M.; Bakir, T.K.; Ünal, S. Assessment of Total Phenolic, Total Flavonoid, Metal Contents and Antioxidant Activities of Trametes versicolor and Laetiporus sulphureus. Acta Sci. Pol. Hortorum Cultus 2022, 21, 39–47. [Google Scholar] [CrossRef]
- Sinha, S.K.; Upadhyay, T.K.; Sharma, S.K. Heavy Metals Detection in White Button Mushroom (Agaricus bisporus) Cultivated in State of Maharashtra, India. Biochem. Cell Arch. 2019, 19, 3501–3506. [Google Scholar]
- Chunhabundit, R. Cadmium Exposure and Potential Health Risk from Foods in Contaminated Area, Thailand. Toxicol. Res. 2016, 32, 65–72. [Google Scholar] [CrossRef]
- Keskin, F.; Sarikurkcu, C.; Akata, I.; Tepe, B. Element Concentration, Daily Intake of Elements, and Health Risk Indices of Wild Mushrooms Collected from Belgrad Forest and Ilgaz Mountain National Park (Turkey). Environ. Sci. Pollut. Res. 2021, 28, 51544–51555. [Google Scholar] [CrossRef]
- USEPA Integrated Risk Information System US EPA. 2018.
- Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme CODEX Committee on Contaminants in Foods; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- FAO/WHO Codex Alimentarius Commission. Food Additives and Contaminants. Joint FAO/WHO Food Standards Programme; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Akgul, H.; Sevindik, M.; Coban, C.; Alli, H.; Selamoglu, Z. New Approaches in Traditional and Complementary Alternative Medicine Practices: Auricularia auricula and Trametes versicolor. J. Tradit. Med. Clin. Nat. 2017, 6, 239. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Akata, I.; Tepe, B. Metal Concentration and Health Risk Assessment of Eight Russula Mushrooms Collected from Kizilcahamam-Ankara, Turkey. Environ. Sci. Pollut. Res. 2021, 28, 15743–15754. [Google Scholar] [CrossRef] [PubMed]
- Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; Bouziane, H.; López-Castillo, J.G.; Palma, M.; Barbero, G.F. Toxic Elements and Trace Elements in Macrolepiota procera Mushrooms from Southern Spain and Northern Morocco. J. Food Compos. Anal. 2022, 108, 104419. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Eid, E.M.; AL-Huqail, A.A.; Adelodun, B.; Abou Fayssal, S.; Goala, M.; Arya, A.K.; Bachheti, A.; Andabaka, Ž.; et al. Spatial Assessment of Potentially Toxic Elements (PTE) Concentration in Agaricus bisporus Mushroom Collected from Local Vegetable Markets of Uttarakhand State, India. J. Fungi 2022, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Bains, A.; Chawla, P. In Vitro Bioactivity, Antimicrobial and Anti-Inflammatory Efficacy of Modified Solvent Evaporation Assisted Trametes versicolor Extract. 3 Biotech 2020, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K. Therapeutic and Nutraceutical Potential of Bioactive Compounds Extracted from Fruit Residues. Crit. Rev. Food Sci. Nutr. 2015, 55, 319–337. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Szwajkowska-Michałek, L.; Stuper-Szablewska, K.; Krzyżaniak, M.; Łakomy, P. A Bioactive Compounds Profile Present in the Selected Wood Rot. Forests 2022, 13, 1242. [Google Scholar] [CrossRef]
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Leopold, N.; Bocsan, I.C.; Buzoianu, A.D. Characterization of Trametes versicolor: Medicinal Mushroom with Important Health Benefits. Not. Bot. Horti. Agrobot. Cluj Napoca 2018, 46, 343–349. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef]
- Rao, A.V.; Ray, M.R.; Rao, L.G. Lycopene. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2006; Volume 51, pp. 99–164. ISBN 0120164515. [Google Scholar]
- Rao, L.G.; Mackinnon, E.S.; Josse, R.G.; Murray, T.M.; Strauss, A.; Rao, A.V. Lycopene Consumption Decreases Oxidative Stress and Bone Resorption Markers in Postmenopausal Women. Osteoporos. Int. 2007, 18, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Simone, R.; Catalano, A.; Boninsegna, A.; Böhm, V.; Fröhlich, K.; Mele, M.C.; Monego, G.; Ranelletti, F.O. Lycopene Prevents 7-Ketocholesterol-Induced Oxidative Stress, Cell Cycle Arrest and Apoptosis in Human Macrophages. J. Nutr. Biochem. 2010, 21, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Rao, L. Carotenoids and Human Health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.; Biesalski, H.K. β-Carotene Is an Important Vitamin A Source for Humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Mallery, P. SPSS for Windows Step by Step: A Simple Guide and Reference. 11.0 Update; Pearson Education: London, UK, 2003; Volume 10. [Google Scholar]
- Tokul-Olmez, O.; Kaplaner, E.; Ozturk, M.; Ullah, Z.; Duru, M.E. Fatty Acid Profile of Four Ganoderma Species Collected from Various Host Trees with Chemometric Approach. Biochem. Syst. Ecol. 2018, 78, 91–97. [Google Scholar] [CrossRef]
- Heinke, R.; Schöne, P.; Arnold, N.; Wessjohann, L.; Schmidt, J. Metabolite Profiling and Fingerprinting of Suillus Species (Basidiomycetes) by Electrospray Mass Spectrometry. Eur. J. Mass Spectrom. 2014, 20, 85–97. [Google Scholar] [CrossRef]
- Alzand, K.I.; Bofaris, M.S.M.; Ugis, A. Chemical Composition and Nutritional Value of Edible Wild Growing Mushrooms: A Review. World J. Pharm. Res. 2019, 8, 31–46. [Google Scholar]
- Hobbs, C. Medicinal Value of Turkey Tail Fungus Trametes versicolor (L.:Fr.) Pilat (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2005, 7, 346–347. [Google Scholar] [CrossRef]
- Upadhyaya, J. Analysis of Nutritional and Nutraceutical Properties of Wild-Grown Mushrooms of Nepal. Planta Medica Int. Open 2018, 5, FF09P. [Google Scholar]
- Bazinet, R.P.; Chu, M.W.A. Omega-6 Polyunsaturated Fatty Acids: Is a Broad Cholesterol-Lowering Health Claim Appropriate? Can. Med. Assoc. J. 2014, 186, 434–439. [Google Scholar] [CrossRef]
- Yehuda, S. Omega-6/Omega-3 Ratio and Brain-Related Functions. World Rev. Nutr. Diet 2003, 92, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Günç Ergönül, P.; Akata, I.; Kalyoncu, F.; Ergönül, B. Fatty Acid Compositions of Six Wild Edible Mushroom Species. Sci. World J. 2013, 2013, 163964. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).