Distinct Impact of Drought on Radial Growth at Different Heights and Parts of Populus euphratica in the Oasis at the Hinterland of the Taklimakan Desert
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Sample Collection
| Sample Trees | Longitude and Latitude | Height (m) | Diameter at Breast Height (cm) | Canopy (m) | Age (a) | |
|---|---|---|---|---|---|---|
| First tree | 38°21′23.19″ N 81°51′36.32″ E | 11.78 | 39.3 | 6.47 × 6.52 | 50 | |
| Second tree | 11.63 | 41.4 | 7.09 × 5.87 | 60 | ||
| Third tree | 11.51 | 48.6 | 7.43 × 6.02 | 56 | ||
| Different parts | Stem | Primary lateral branch | Secondary lateral branch | |||
| Sample trees | Sample size | Diameter range (cm) | Sample size | Diameter range (cm) | Sample size | Diameter range (cm) |
| First tree | 16 ① | 10.2~39.3 | 16 ② | 5~14 | 14 | 4~10 |
| Second tree | 16 | 15.5~41.4 | 16 | 6~19 | 12 | 4~7 |
| Third tree | 16 | 9.4~48.6 | 18 | 5~22 | 22 | 4~13 |
| Category | Full Title | Abbreviation |
| Characteristics of ring chronology | Tree-ring width | TRW |
| Ring width index | RWI | |
| Basal area increment | BAI | |
| Standardized chronology | STD | |
| Standard deviation | SD | |
| Different parts of Populus euphratica | Bottom of stem | STB |
| Primary lateral branches at the bottom of the stem | PLB | |
| Secondary lateral branches of primary lateral branches at the bottom of the stem | SLB | |
| Middle of stem | STM | |
| Primary lateral branches at the middle of the stem | PLM | |
| Secondary lateral branches of primary lateral branches at the middle of the stem | SLM | |
| Top of stem | STT | |
| Primary lateral branches at the top of the stem | PLT | |
| Soil | Soil water content | SWC |
| Drought index | Palmer drought severity index | PDSI |
| Indicators of drought response | Resistance | Rt |
| Recovery | Rc | |
| Resilience | Rs | |
| Relative resilience | RRs |
2.3. Gravimetric Soil Water Content, Groundwater Depth, and Meteorological Data
2.4. Tree-Ring Width, Chronology, and Basal Area Increment
2.5. Vulnerability to Drought
2.6. Data Analysis
3. Results
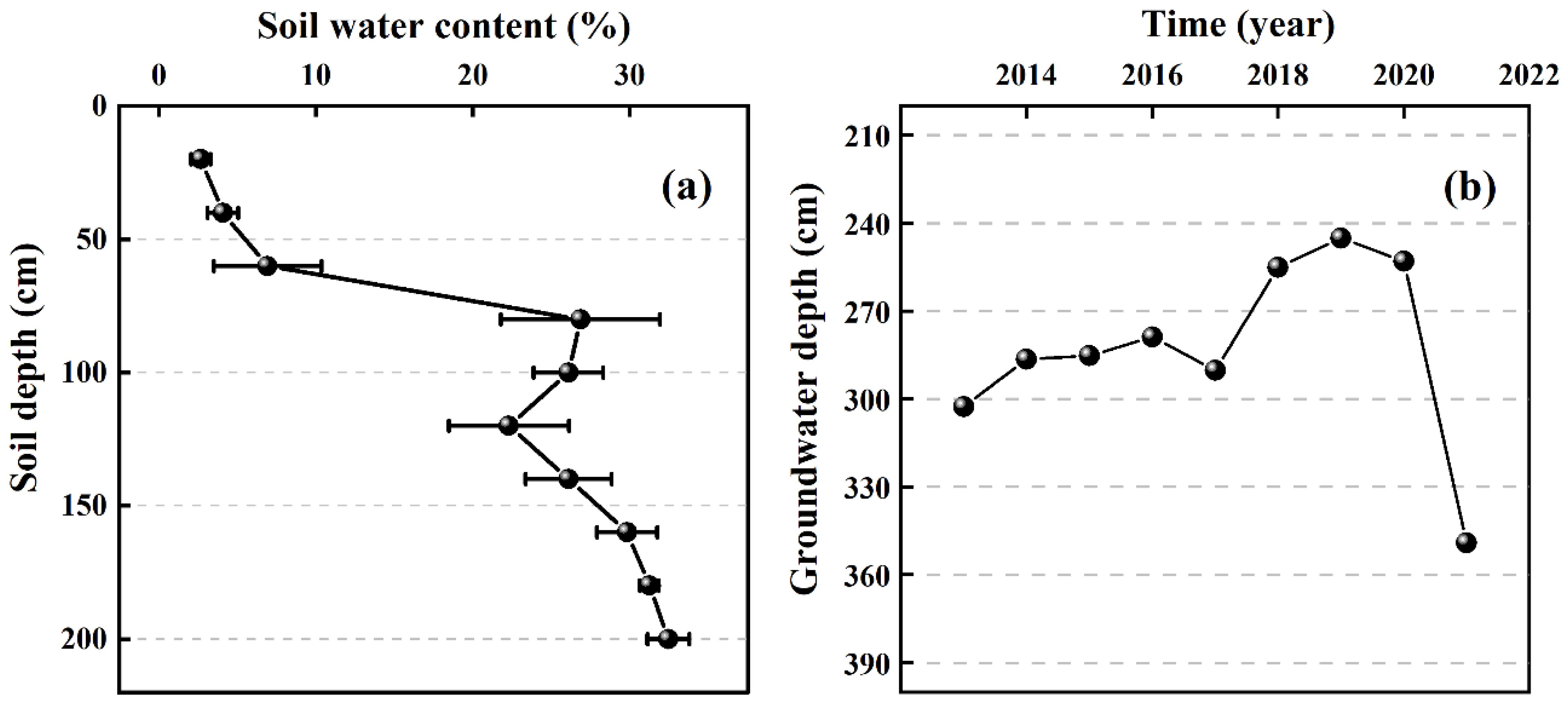
3.1. Soil Water Content and Groundwater Depth at Sampling Points
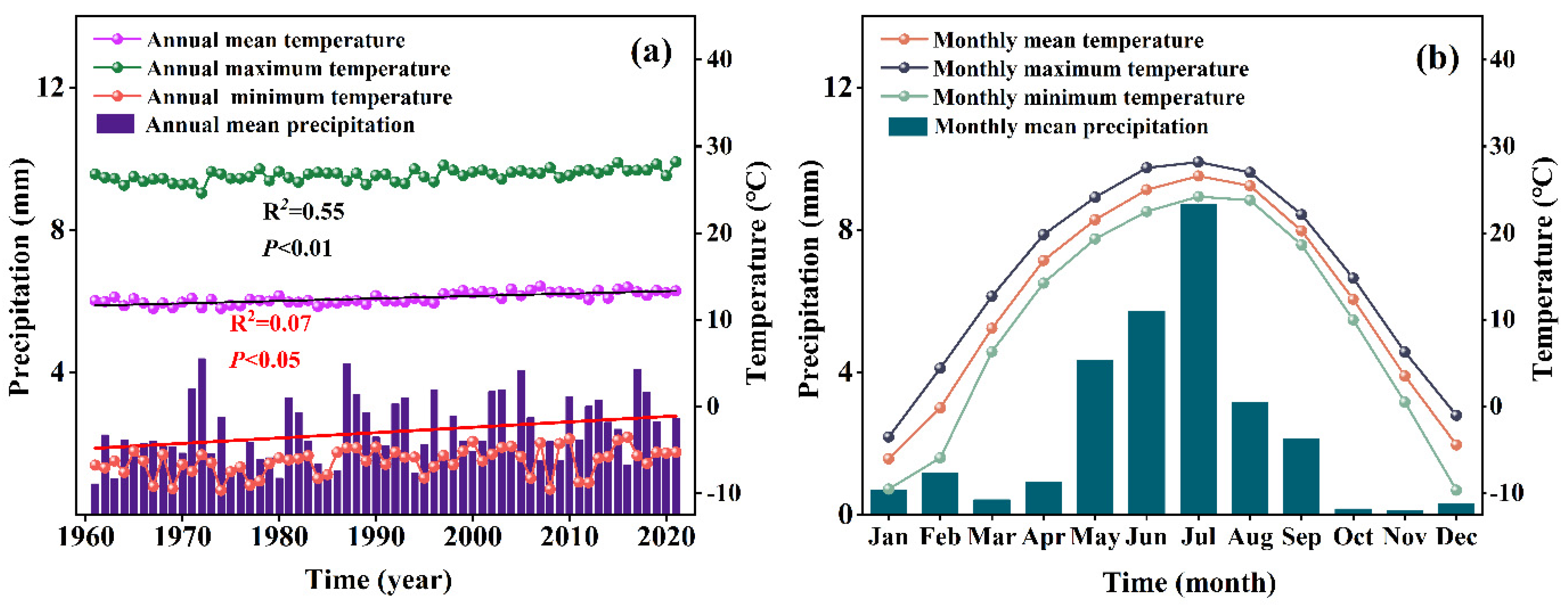
3.2. Changes in Meteorological Factors
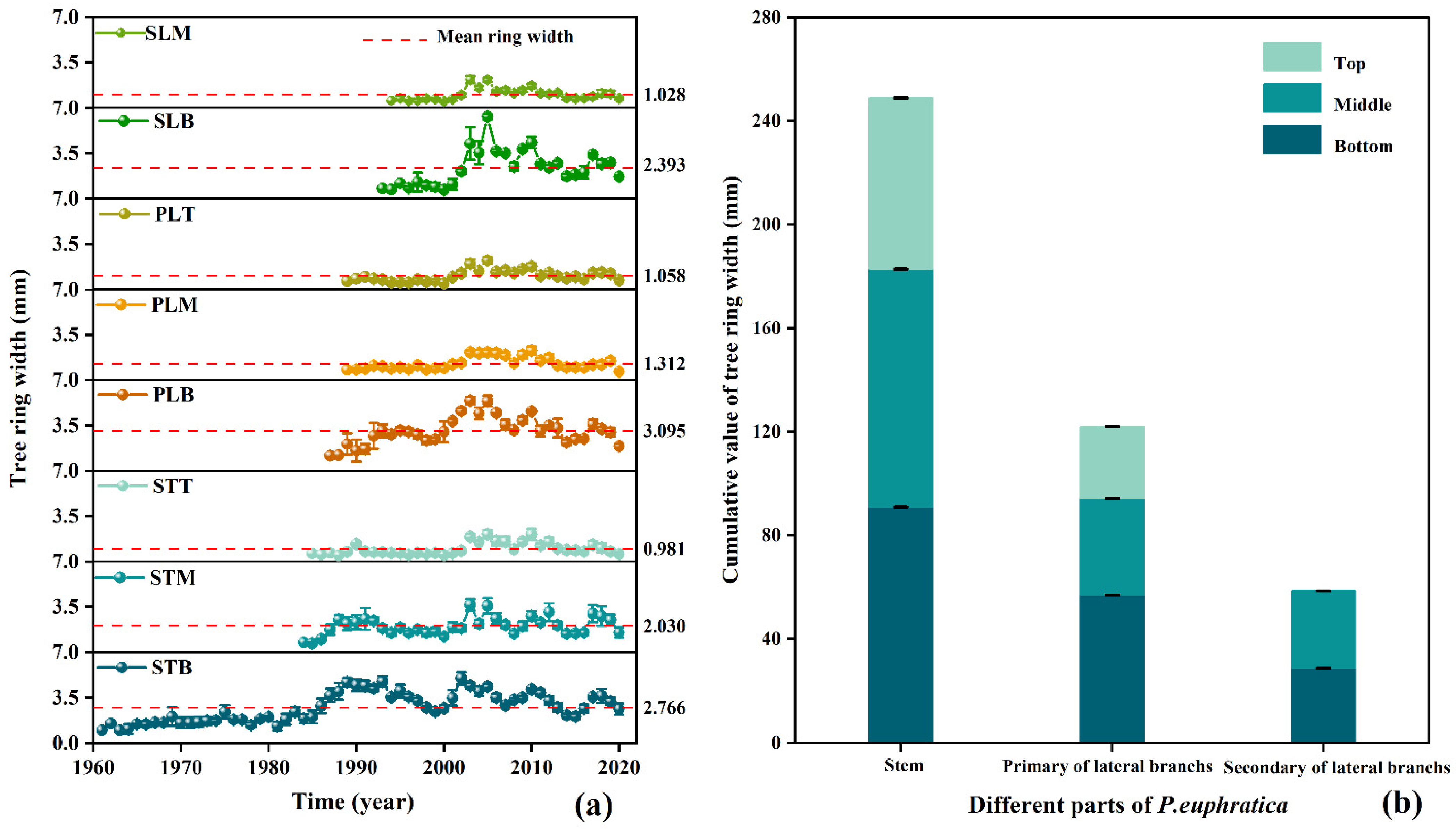
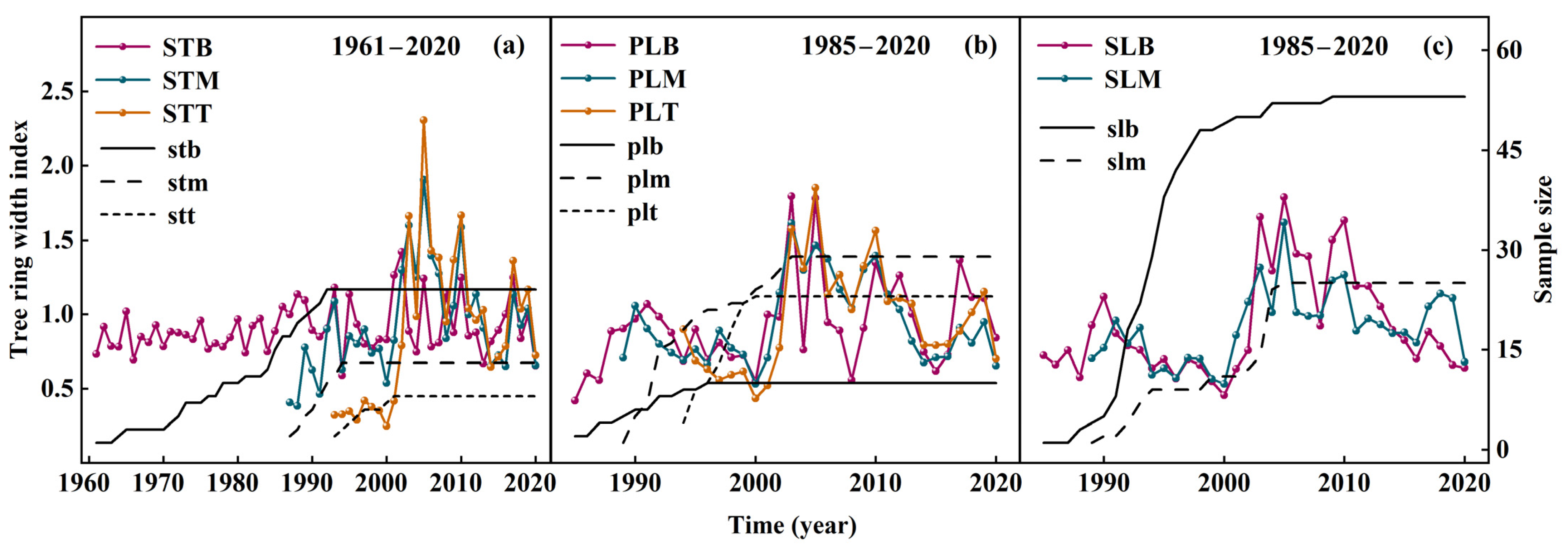
3.3. Tree-Ring Width and Chronology
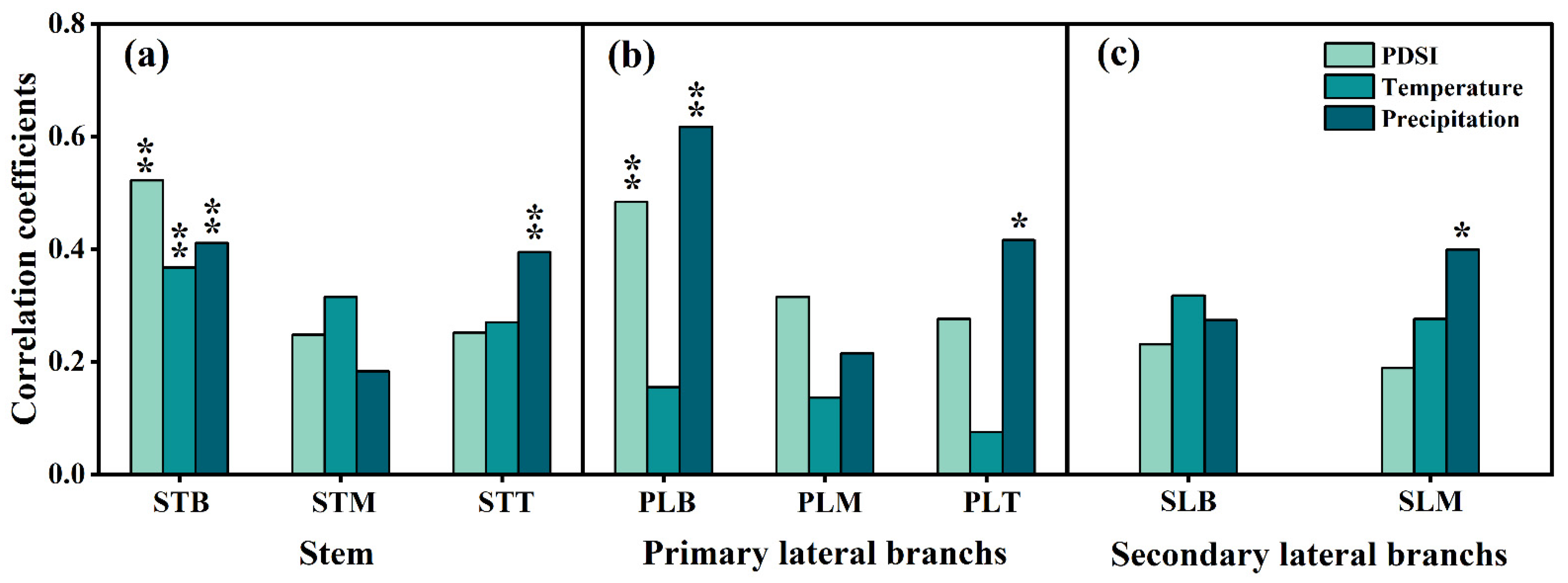
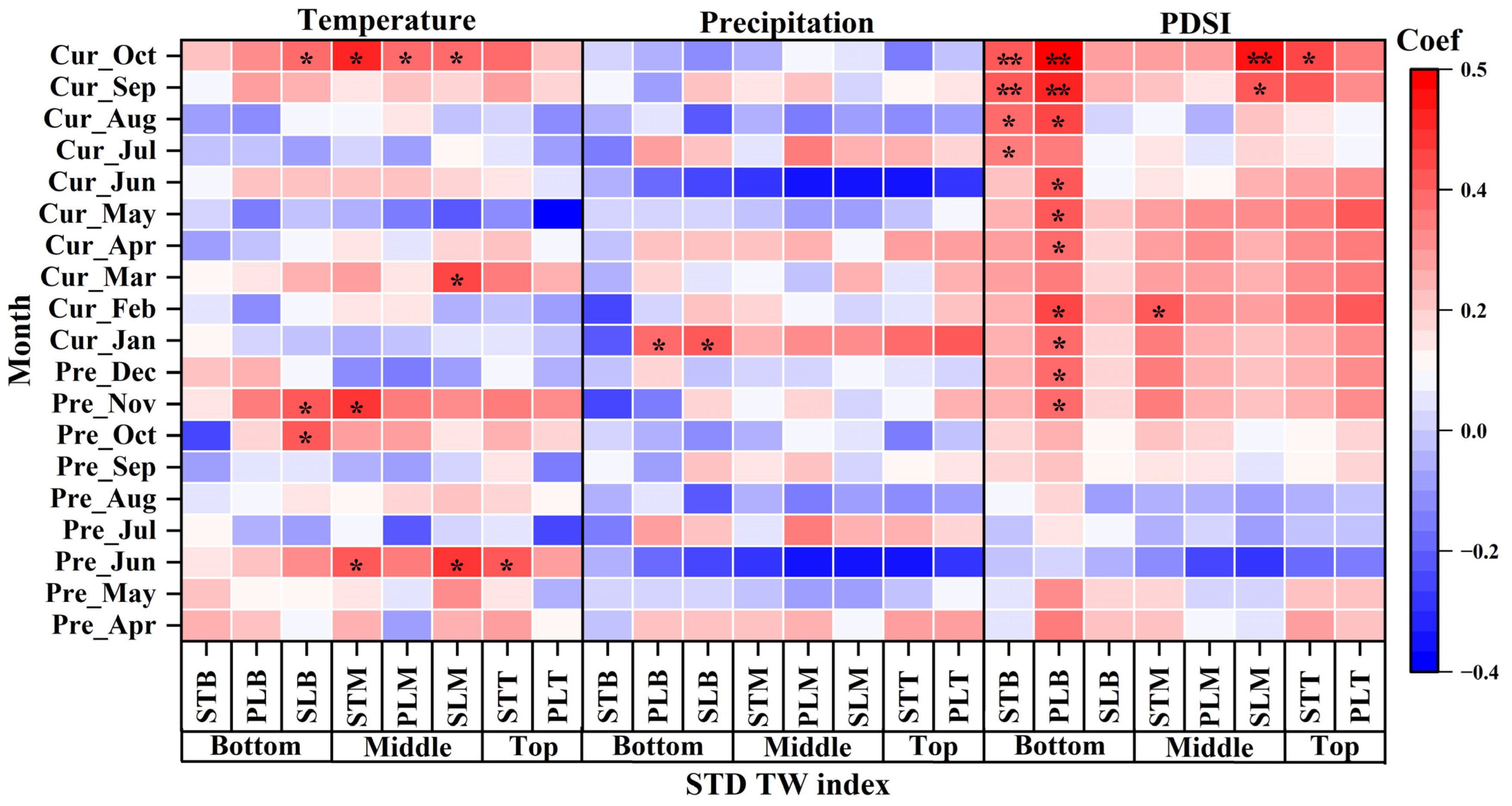
3.4. Radial Growth of P. euphratica in Relation to Climatic Variables
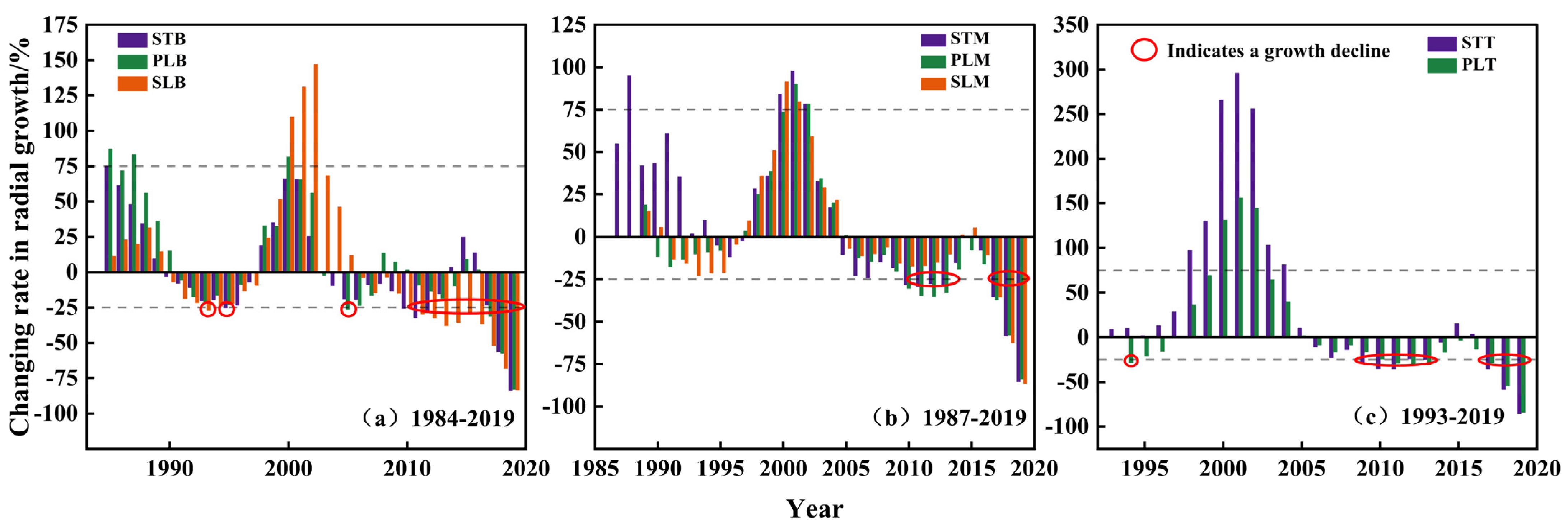
3.5. Growth Decline of P. euphratica at Different Heights and Parts
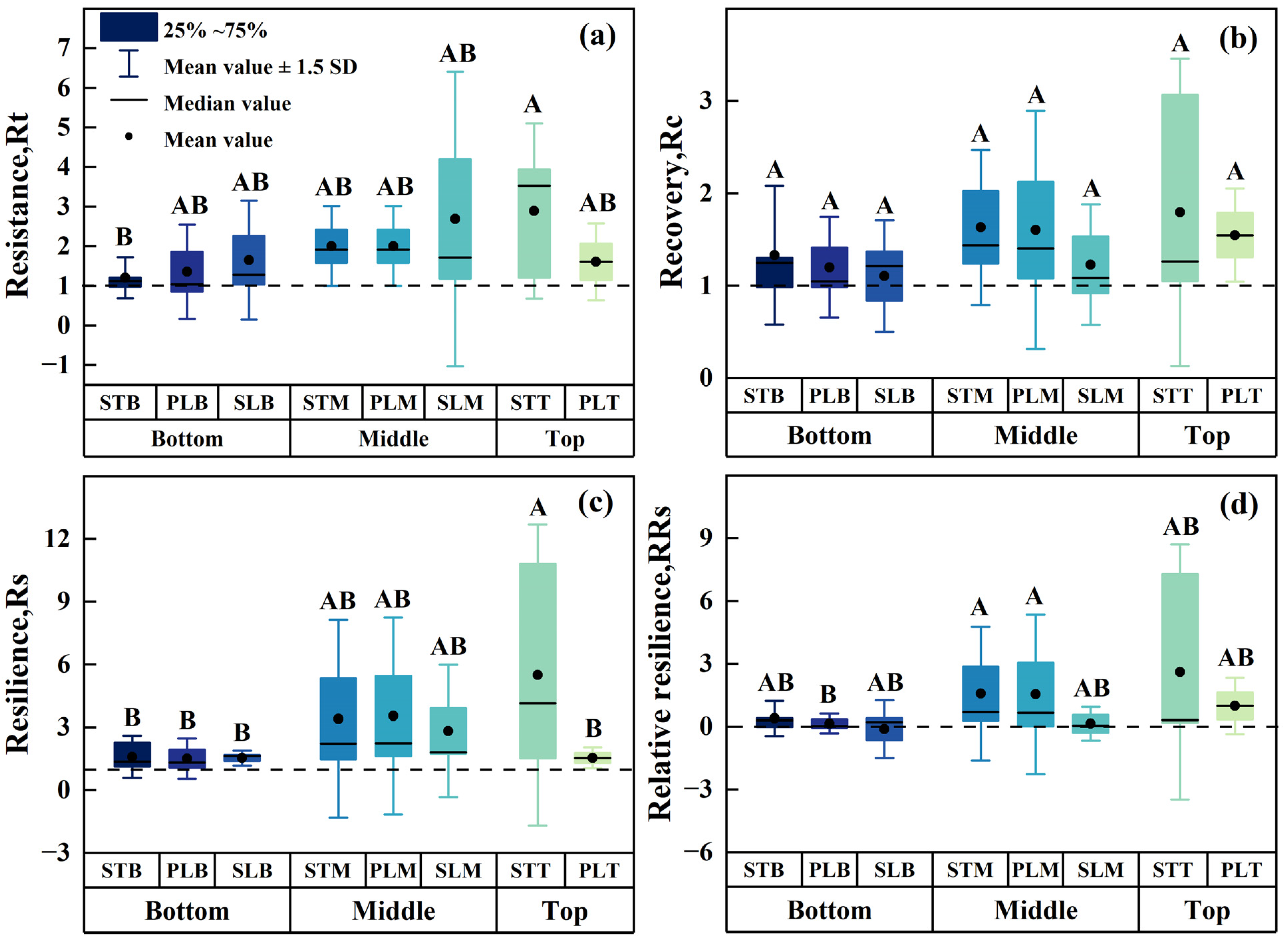
3.6. Adaptation of Different Parts of P. euphratica to Drought
4. Discussion
4.1. Meteorological Factors and Radial Growth
4.2. Relationship between Radial Growth and Meteorological Factors
4.3. Growth Decline and Response to Drought
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, C.; Macalady, A.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.; Hogg, E.; et al. A global overview of drought and heat–induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 4, 660–684. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Hu, M.; Li, Q.; Yan, Z.; Jones, P. Global land surface air temperature dynamics since 1880. Int. J. Climatol. 2018, 38, e466–e474. [Google Scholar] [CrossRef]
- IPCC. Climate change: Synthesis report. In Contribution of Working Groups, I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team; Pachauri, R., Meyer, L., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Liu, H.; Williams, A.; Allen, C.; Guo, D.; Wu, X.; Anenkhonov, O.; Badmaeva, N. Rapid warming accelerates tree growth decline in semi–arid forests of Inner Asia. Glob. Chang. Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, S.; Henrys, P.; Hemming, D.; Bell, J.; Botham, M.; Burthe, S.; Helaouet, P.; Johns, D.; Jones, I.; Leech, D.; et al. Phenological sensitivity to climate across taxa and trophic levels. Nature 2016, 535, 241–245. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, J.; Wang, W.; Ma, P.; Lu, G.; Liu, X.; Fang, F. Climatic Warming and Humidification in the Arid Region of Northwest China: Multi–Scale Characteristics and Impacts on Ecological Vegetation. J. Meteorol. Res. 2021, 35, 113–127. [Google Scholar] [CrossRef]
- Sun, S.; Lei, S.; Jia, H.; Li, C.; Zhang, J.; Meng, P. Tree–Ring Analysis Reveals Density–Dependent Vulnerability to Drought in Planted Mongolian Pines. Forests 2020, 11, 98. [Google Scholar] [CrossRef]
- Jump, A.; Ruiz-Benito, P.; Greenwood, S.; Allen, C.; Kitzberger, T.; Fensham, R.; Martínez–Vilalta, J.; Lloret, F. Structural overshoot of tree growth with climate variability and the global spectrum of drought–induced forest dieback. Glob. Chang. Biol. 2017, 23, 3742–3757. [Google Scholar] [CrossRef]
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: A review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Castagneri, D.; Battipaglia, G.; von Arx, G.; Pacheco, A.; Carrer, M. Tree–ring anatomy and carbon isotope ratio show both direct and legacy effects of climate on bimodal xylem formation in Pinus pinea. Tree Physiol. 2018, 38, 1098–1109. [Google Scholar] [CrossRef]
- Huang, M.; Wang, X.; Keenan, T.; Piao, S. Drought timing influences the legacy of tree growth recovery. Glob. Chang. Biol. 2018, 24, 3546–3559. [Google Scholar] [CrossRef]
- Gao, S.; Liu, R.; Zhou, T.; Fang, W.; Yi, C.; Lu, R.; Zhao, X.; Luo, H. Dynamic responses of tree–ring growth to multiple dimensions of drought. Glob. Chang. Biol. 2018, 24, 5380–5390. [Google Scholar] [CrossRef] [PubMed]
- Bottero, A.; D’Amato, A.; Palik, B.; Bradford, J.; Fraver, S.; Battaglia, M.; Asherin, L. Density–dependent vulnerability of forest ecosystems to drought. J. Appl. Ecol. 2017, 4, 1605–1614. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2012, 3, 30. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, A.; An, Y.; Lian, P.; Wu, D.; Zhu, J.; Meinzer, F.; Hao, G. Hydraulics play an important role in causing low growth rate and dieback of aging Pinus sylvestris var. Mongolia trees in plantations of Northeast China. Plant Cell Environ. 2018, 41, 1500–1511. [Google Scholar] [CrossRef]
- Salmon, Y.; Torres-Ruiz, J.; Poyatos, R.; Martinez-Vilalta, J.; Meir, P.; Cochard, H.; Mencuccini, M. Balancing the risks of hydraulic failure and carbon starvation: A twig scale analysis in declining Scots pine. Plant. Cell. Environ. 2015, 38, 2575–2588. [Google Scholar] [CrossRef]
- Guada, G.; Camarero, J.; Sánchez, S.; Cerrillo, R. Limited growth recovery after drought-induced forest dieback in very defoliated trees of two pine species. Front. Plant Sci. 2016, 7, 418. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.; Camarero, J.; Borghetti, M.; Gentilesca, T.; Oliva, J.; Redondo, M.; Ripullone, F. Drought and phytophthora are associated with the decline of Oak Species in Southern Italy. Front. Plant Sci. 2018, 9, 1595. [Google Scholar] [CrossRef]
- Heuret, P.; Meredieu, C.; Coudurier, T.; Courdier, F.; Barthélémy, D. Ontogenetic trends in the morphological features of main stem annual shoots of Pinus pinater Ait (Pinaceae). Am. J. Bot. 2006, 93, 1577–1587. [Google Scholar] [CrossRef]
- Yu, M.; Cheng, X.; He, Z.; Wu, T.; Yin, Z. Longitudinal Variation of Ring Width, Wood Density and Basal Area Increment in 26–Year–Old Loblolly Pine (Pinus taeda) Trees. Tree-Ring Res. 2014, 70, 137–144. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, L.; Zhang, R.; Gao, Y.; Hu, D.; Yu, S.; Jiang, S. The impacts of climatic factors on radial growth patterns at different stem heights in Schrenk spruce (Picea schrenkiana). Trees 2020, 34, 163–175. [Google Scholar] [CrossRef]
- Maaten, M.; Bouriaud, O. Climate–growth relationships at different stem heights in silver fir and Norway spruce. Can. J. For. Res. 2012, 42, 958–969. [Google Scholar]
- Meng, S.; Lieffers, V.; Reid, D. Reducing stem bending increases the height growth of tall pines. J. Exp. Bot. 2006, 57, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Chhin, S.; Hogg, E.H.; Lieffers, V.J.; Huang, S. Growth–climate relationships vary with height along the stem in lodgepole pine. Tree Physiol. 2010, 30, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Pedà, G.; Rocca, N. Trade–offs between leaf hydraulic capacity and drought vulnerability: Morpho–anatomical bases, carbon costs and ecological consequences. New Phytolog. 2012, 196, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Simcha, L.; Roni, A. Vascular differentiation in branch junctions of circular patterns and functional significance. Trees 1990, 4, 49–54. [Google Scholar]
- Zhou, H.; Chen, Y.; Li, W.; Ayup, M. Xylem hydraulic conductivity and embolism in riparian plants and their responses to drought stress in desert of Northwest China. Ecohydrology 2013, 6, 984–993. [Google Scholar] [CrossRef]
- Rice, K.; Matzner, S.; Byer, W.; Brown, J. Patterns of tree dieback in Queensland, Australia: The importance of drought stress and the role of resistance to cavitation. Oecologia 2004, 139, 190–198. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Q.; Zhai, L. Study of the height growth dynamic based on tree-ring data in Populus euphratica from the lower reach of the Heihe River, China. Dendrochronologia 2010, 28, 49–64. [Google Scholar]
- Zhou, H.; Chen, Y.; Zhu, C.; Li, Z.; Fu, A. Climate change may accelerate the decline of desert riparian forest in the lower Tarim River, Northwestern China: Evidence from tree–rings of Populus euphratica. Ecol. Indicat. 2020, 111, 105997. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, F.; Shi, Q.; Li, M.; Dai, Y.; Zhang, Z.; Zhu, C. Responses of arid plant species diversity and composition to environmental factors. J. For. Res. 2023, 34, 1723–1734. [Google Scholar] [CrossRef]
- Imin, B.; Dai, Y.; Shi, Q.; Guo, Y.; Li, H.; Nijat, M. Responses of two dominant desert plant species to the changes in groundwater depth in hinterland natural oasis, Tarim Basin. Ecol. Evol. 2021, 11, 9460–9471. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shi, Q.; Zhou, X.; Bilal, E.; Li, H.; Zhang, W.; Yasin, K. Effect of the competition mechanism of between co–dominant species on the ecological characteristics of Populus euphratica under a water gradient in a desert oasis. Glob. Ecol. Conserv. 2021, 27, e01611. [Google Scholar] [CrossRef]
- Havens, A.V.; Snow, W.B.; Horowitz, J.L.; Liu, C.S. Drought Frequency, Intensity, and Duration: Its Correlation to Streamflow and Its Impact Upon Synthetic Hydrology; New Jersey Water Resources Research Institute: New Brunswick, NJ, USA, 1968; p. 49. [Google Scholar]
- Draper, S.H.; Palmer, R.N.; Lettenmaier, D.P.; Burges, S.J. Water Resource System Reliability under Drought Conditions: The Seattle Water Supply System as a Case Study; Charles W. Harris Hydraulics Laboratory Technical Report No. 72; University of Washington: Seattle, DC, USA, 1981; p. 72. [Google Scholar]
- Alley, W.M. The Palmer Drought Severity Index: Limitations and Assumptions. J. Clim. Appl. Meteor. 1984, 23, 1100–1109. [Google Scholar] [CrossRef]
- Alley, W.M. The palmer drought severity index as a measure of hydrologic drought. J. Am. Water Resour. Assoc. 1985, 21, 105–114. [Google Scholar] [CrossRef]
- GB/T 20481–2017; Classification of Meteorological Drought. China Meteorological Administration: Beijing, China; Standards Press of China: Beijing, China, 2017.
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Sciences; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1990; p. 394. [Google Scholar]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree–Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1968. [Google Scholar]
- Silva, L.C.; Anand, M.; Leithead, M.D. Recent widespread tree growth decline despite increasing atmospheric CO2. PLoS ONE 2010, 5, e11543. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Nowacki, G.; Abrams, M. Radial–growth averaging criteria for reconstructing disturbance histories from presettlement–origin oaks. Ecol. Monogr. 1997, 67, 225–249. [Google Scholar] [CrossRef]
- Shen, J.; Li, S.; Huang, X.; Wang, S.; Su, J. Ecological resilience and growth degradation of Pinus Yunnanensis at different altitudes in Jinsha River Basin. Sci. Silvae Sin. 2019, 56, 1–11. [Google Scholar]
- Gartner, B.; North, E.; Johonson, G.; Singleton, R. Effects of live crown on vertical patterns of wood density and growth in Douglas-fir. Can. J. For. Res. 2002, 32, 439–447. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, L.; Qiu, Y.; Guo, J. Characteristics of Korean pine (Pinus koraiensis) radial growth at different heights and its response to climate change on Changbai Mountain. Acta Ecol. Sinic. 2015, 5, 2978–2984. [Google Scholar]
- Peterson, D.; Arbaugh, M.; Robinson, L. Effects of ozone and climate on ponderosa pine (Pinus ponderosa) growth in the Colorado Rocky Mountains. Can. J. For. Res. 1993, 23, 1750–1759. [Google Scholar] [CrossRef]
- Farrar, J. Longitudinal variation in the thickness of the annual ring. For. Chron. 1961, 37, 323–349. [Google Scholar] [CrossRef][Green Version]
- Larson, P. Stem Form Development of Forest Trees. For. Sci. 1963, 9 (Suppl. S2), a0001-42. [Google Scholar] [CrossRef]
- Ford, E.; Avery, A.; Ford, R. Simulation of branch growth in the Pinaceae: Interactions of morphology, phenology, foliage productivity, and the requirement for structural support, on the export of carbon. J. Theor. Biol. 1990, 146, 15–36. [Google Scholar] [CrossRef]
- Lehnebach, R.; Beyer, R.; Letort, V.; Heuret, P. The pipe model theory half a century on: A review. Ann. Bot. 2018, 121, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xiao, S.; Cheng, G.; Xiao, H.; Tian, Q.; Zhang, Q. Human activity impacts on the stem radial growth of Populus euphratica riparian forests in China’s Ejina Oasis, using tree-ring analysis. Trees 2017, 31, 379–392. [Google Scholar] [CrossRef]
- Amoroso, M.; Daniels, L.; Larson, B. Temporal patterns of radial growth in declining Austrocedrus chilensis forests in Northern Patagonia: The use of tree-rings as an indicator of forest decline. For. Ecol. Manag. 2012, 265, 62–70. [Google Scholar] [CrossRef]
- Liang, E.; Leuschner, C.; Dulamsuren, C.; Wagner, B.; Hauck, M. Global warming-related tree growth decline and mortality on the north-eastern Tibetan plateau. Climat. Chang. 2015, 134, 163–176. [Google Scholar] [CrossRef]
- Ren, P.; Rossi, S.; Gricar, J.; Liang, E.; Cufar, K. Is precipitation a trigger for the onset of Xylo genesis in Juniperus przewalskii on the north-eastern Tibetan Plateau? Ann. Bot. 2015, 115, 629–639. [Google Scholar] [CrossRef]
- Fang, O.; Alfaro, R.; Zhang, Q. Tree rings reveal a major episode of forest mortality in the late 18th century on the Tibetan Plateau. Glob. Planet Chang. 2018, 163, 44–50. [Google Scholar] [CrossRef]
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.; Schepaschenko, D. Boreal Forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Arend, M.; Sever, K.; Pflug, E.; Gessler, A.; Schaub, M. Seasonal photosynthetic response of European beech to severe summer drought: Limitation, recovery and post–drought stimulation. Agric. For. Meteorol. 2016, 220, 83–89. [Google Scholar] [CrossRef]
- Backhaus, S.; Kreyling, J.; Grant, K.; Beierkuhnlein, C.; Walter, J.; Jentsch, A. Recurrent mild drought events increase resistance toward extreme drought stress. Ecosystems 2014, 17, 1068–1081. [Google Scholar] [CrossRef]
- Rubio–Cuadrado, Á.; Camarero, J.; Aspizua, R.; Sánchez–González, M.; Gil, L.; Montes, F. Abiotic factors modulate post-drought growth resilience of Scots pine plantations and rear-edge Scots pine and oak forests. Dendrochronologia 2018, 51, 54–65. [Google Scholar] [CrossRef]
- Sánchez, S.R.; Camarero, J.J.; Rozas, V.; Génova, M.O.; José, M.; Arzac, A. Resist, recover or both? growth plasticity in response to drought is geographically structured and linked to intraspecific variability in pinus pinaster. J. Biogeogr. 2018, 45, 1126–1139. [Google Scholar] [CrossRef]
- Brodribb, T.; Powers, J.; Cochard, H.; Choat, B. Hanging by a thread? Forests and drought. Science 2020, 368, 261–266. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C. Regime Shifts, Resilience, and Biodiversity in Ecosystem Management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- De Grandpré, L.; Kneeshaw, D.; Perigon, S.; Boucher, D.; Marchand, M.; Pureswaran, D.; Girardin, M. Adverse climatic periods precede and amplify defoliator-induced tree mortality in eastern boreal North America. J. Ecol. 2019, 107, 452–467. [Google Scholar] [CrossRef]
- Engelbrecht, B.M.; Comita, L.S.; Condit, R.; Kursar, T.A.; Tyree, M.T.; Turner, B.L.; Hubbell, S.P. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 2007, 447, 80. [Google Scholar] [CrossRef]
- Li, D.; Si, J.; Zhang, X. Comparison of Branch Water Relations in Two Riparian Species: Populus euphratica and Tamarix ramosissima. Sustainability 2019, 11, 5461. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901. [Google Scholar] [CrossRef] [PubMed]
- Cochard, H.; Herbette, S.; Hernández, E.; Hölttä, T.; Mencuccini, M. The effects of sap ionic composition on xylem vulnerability to cavitation. J. Exp. Bot. 2009, 61, 275–285. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdureyim, A.; Dai, Y.; Shi, Q.; Zhang, F.; Wan, Y.; Shi, H.; Peng, L. Distinct Impact of Drought on Radial Growth at Different Heights and Parts of Populus euphratica in the Oasis at the Hinterland of the Taklimakan Desert. Forests 2023, 14, 2338. https://doi.org/10.3390/f14122338
Abdureyim A, Dai Y, Shi Q, Zhang F, Wan Y, Shi H, Peng L. Distinct Impact of Drought on Radial Growth at Different Heights and Parts of Populus euphratica in the Oasis at the Hinterland of the Taklimakan Desert. Forests. 2023; 14(12):2338. https://doi.org/10.3390/f14122338
Chicago/Turabian StyleAbdureyim, Anwar, Yue Dai, Qingdong Shi, Feng Zhang, Yanbo Wan, Haobo Shi, and Lei Peng. 2023. "Distinct Impact of Drought on Radial Growth at Different Heights and Parts of Populus euphratica in the Oasis at the Hinterland of the Taklimakan Desert" Forests 14, no. 12: 2338. https://doi.org/10.3390/f14122338
APA StyleAbdureyim, A., Dai, Y., Shi, Q., Zhang, F., Wan, Y., Shi, H., & Peng, L. (2023). Distinct Impact of Drought on Radial Growth at Different Heights and Parts of Populus euphratica in the Oasis at the Hinterland of the Taklimakan Desert. Forests, 14(12), 2338. https://doi.org/10.3390/f14122338






