Performance of Iron(II)-Sulphate-Treated Norway Spruce and Siberian Larch in Laboratory and Outdoor Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Treatment
2.2. Laboratory Experiments
2.2.1. Durability Test against Wood-Destroying Basidiomycetes
2.2.2. Water Performance Studies
2.2.3. Short-Term Water Uptake
2.2.4. Long-Term Water Uptake Test
2.2.5. Water Vapour Uptake in a Water-Saturated Atmosphere
2.2.6. Contact Angle of Water
2.3. Factor Approach to Quantifying the Resistance Dose
2.4. Outdoor Exposure
2.5. Statistical Evaluation
3. Results and Discussion
3.1. Durability Test against Wood-Destroying Basidiomycetes
3.2. Water Performance Studies
3.2.1. Contact Angle of Water
3.2.2. Short- and Long-Term Water Uptake
3.2.3. Water Vapour Uptake
3.3. Predicting Aboveground Performance
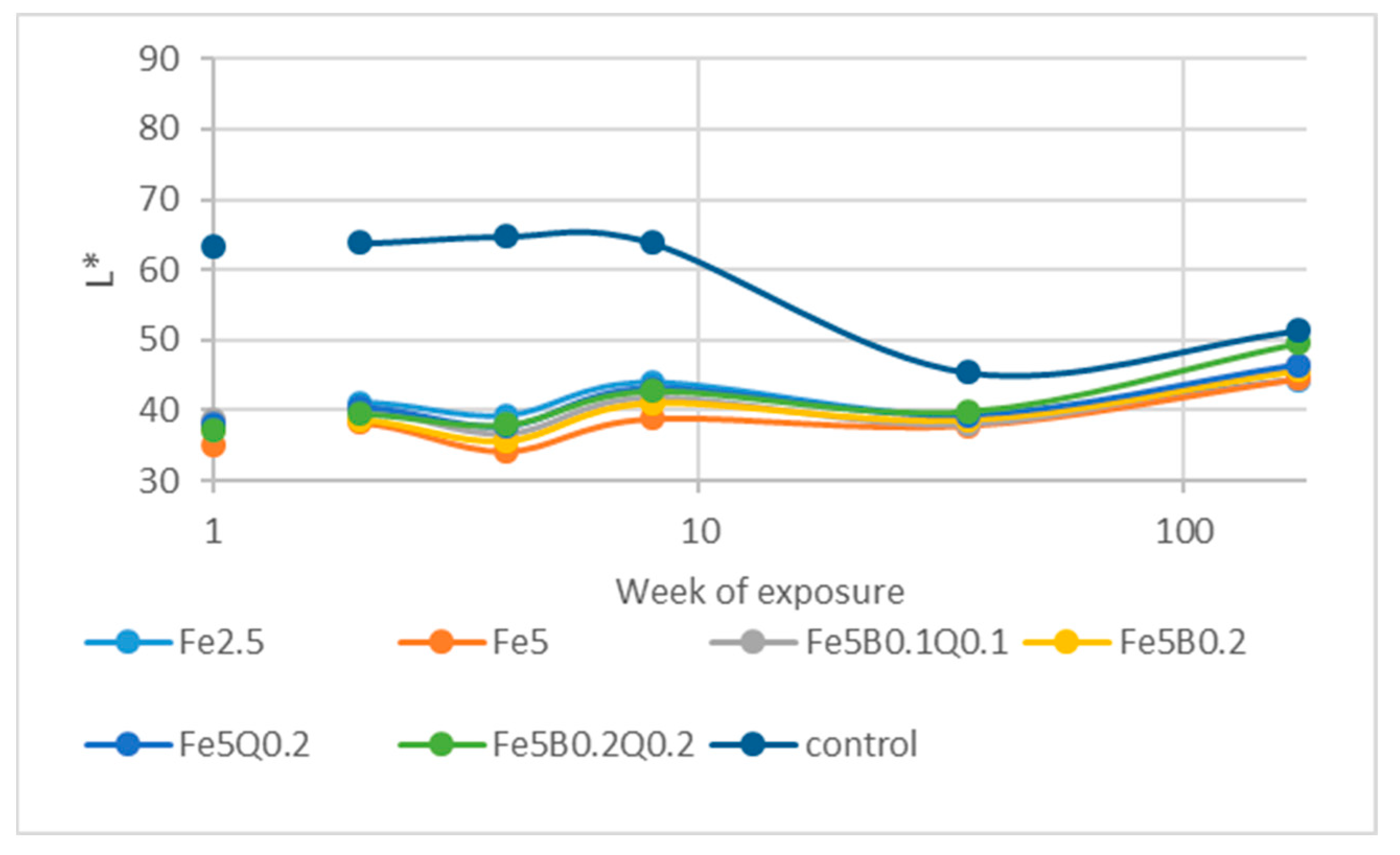
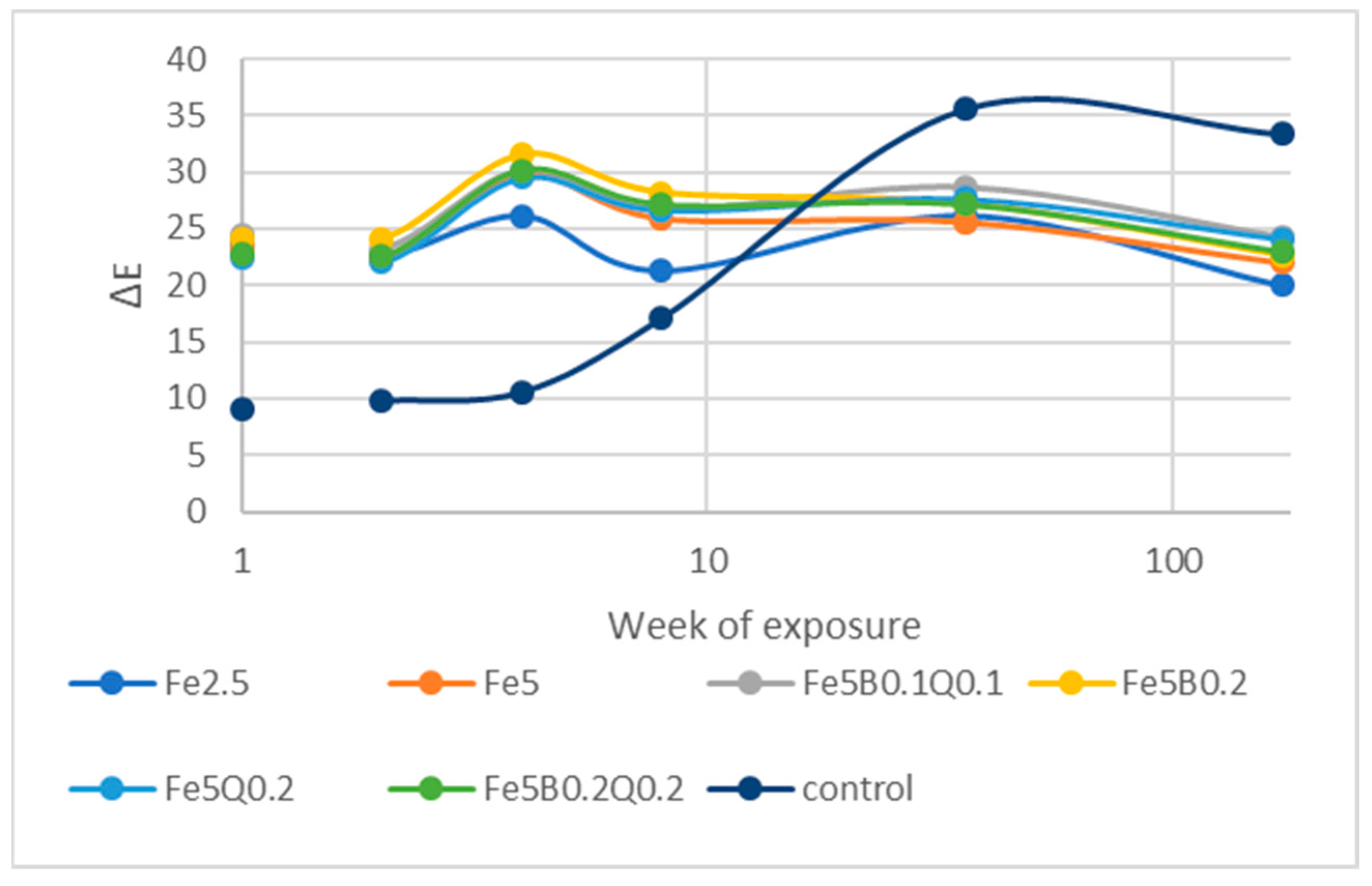
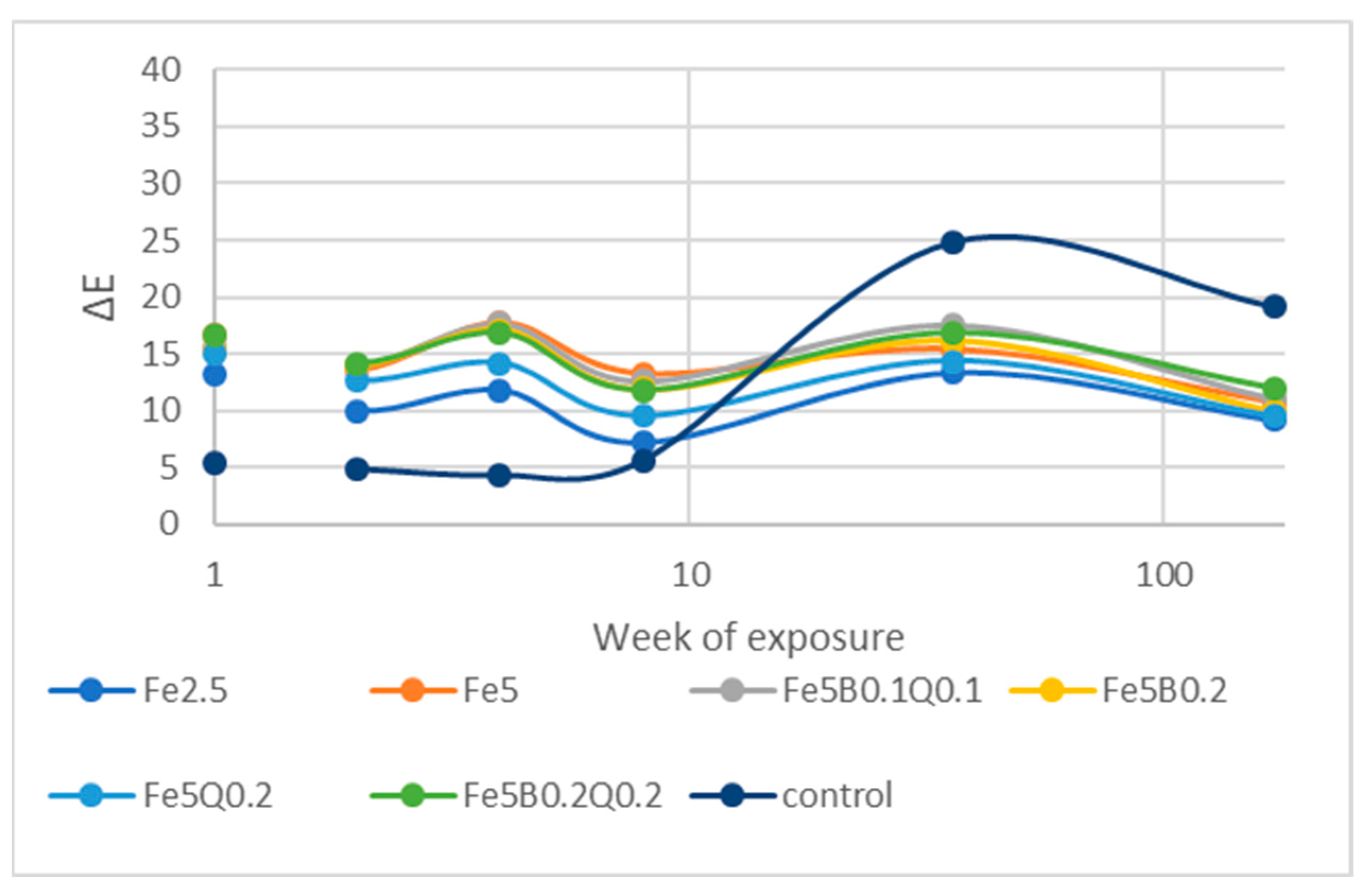
3.4. Outdoor Exposure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zabel, R.A.; Morrell, J.J. Changes in the strength and physical properties of wood caused by decay fungi. In Wood Microbiology; Academic Press: San Diego, CA, USA, 2020; pp. 271–291. [Google Scholar]
- Williams, R.S. Weathering of Wood. In Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; CRC PR ESS Boca: London, UK, 2005; pp. 155–202. ISBN 0849315883. [Google Scholar]
- Feist, W.C. Outdoor Wood Weathering and Protection. In Archaeological Wood: Properties, Chemistry, and Preservation. Proceedings of 196th Meeting of the American Chemical Society; Barbour, R.J., Rowell, R.M., Eds.; Advances in Chemistry Series 225; American Chemical Society: Los Angeles, CA, USA, 1988. [Google Scholar]
- George, B.; Suttie, E.; Merlin, A.; Deglise, X. Photodegradation and photostabilisation of wood—The state of the art. Polym. Degrad. Stab. 2005, 88, 268–274. [Google Scholar] [CrossRef]
- Kržišnik, D.; Lesar, B.; Thaler, N.; Humar, M. Micro and material climate monitoring in wooden buildings in sub-Alpine environments. Constr. Build. Mater. 2018, 166, 188–195. [Google Scholar] [CrossRef]
- Evans, P.D.; Thay, P.D.; Schmalzl, K.J. Degradation of wood surfaces during natural weathering. Effects on lignin and cellulose and on the adhesion of acrylic latex primers. Wood Sci. Technol. 1996, 30, 411–422. [Google Scholar] [CrossRef]
- Gobakken, L.R.; Høibø, O.A. Aesthetic service life of coated and uncoated wooden cladding—Influencing factors and modelling. In Proceedings of the 42nd Annual Meeting of the International Research Group on Wood Protection, Queenstown, New Zealand, 8–12 May 2011. [Google Scholar]
- Zimmer, K.; Gobakken, L.R.; Flindall, O.; Nygaard, M. Colour changes in unpainted wooden façades—Fifty Shades of Grey Colour changes in unpainted wooden façades—Fifty Shades of Grey. In Proceedings of the IRG/WP 49th IRG Annual Meeting, Johannesburg, South Africa, 29 April–3 May 2018. [Google Scholar]
- Aloui, F.; Ahajji, A.; Irmouli, Y.; George, B.; Charrier, B.; Merlin, A. Inorganic UV absorbers for the photostabilisation of wood-clearcoating systems: Comparison with organic UV absorbers. Appl. Surf. Sci. 2007, 253, 3737–3745. [Google Scholar] [CrossRef]
- Nejad, M.; Cooper, P. Exterior Wood Coatings. In Wood in Civil Engineering; IntechOpen: Vienna, Austria, 2017; pp. 111–129. [Google Scholar] [CrossRef]
- Markström, E.; Kitek Kuzman, M.; Bystedt, A.; Sandberg, D. Use of wood products in multi-storey residential buildings: Views of Swedish actors and suggested measures for an increased use. Wood Mater. Sci. Eng. 2019, 14, 404–419. [Google Scholar] [CrossRef]
- Zimmer, K.; Flindall, O.; Ross Gobakken, L.; Nygaard, M. Weathering of un Painted Wooden Façades—Experience and Examples; NIBIO Report; Norwegian Institute of Bioeconomy Research: Oslo, Norway, 2020. [Google Scholar]
- Høibø, O.; Nyrud, A.Q. Consumer perception of wood surfaces: The relationship between stated preferences and visual homogeneity. J. Wood Sci. 2010, 56, 276–283. [Google Scholar] [CrossRef]
- Podgorski, L.; Georges, V.; Garmendia, I.; Sarachu, B.S. A fast and economic method to produce grey wooden surfaces for decking and cladding: Preliminary results. In Proceedings of the RG/WP 40th IRG Anual Meeting, Beijing, China, 24–28 May 2009. [Google Scholar]
- Hundhausen, U.; Mai, C.; Slabohm, M.; Gschweidl, F.; Schwarzenbrunner, R. The staining effect of iron(II) Sulfate on nine different wooden substrates. Forests 2020, 11, 658. [Google Scholar] [CrossRef]
- CEN/TS 15083-1; Durability of wood and wood-based products—Determination of the natural durability of solid wood against wood-destroying fungi, test methods—Part 1: Basidiomycetes. European Committee for Standardization: Brussels, Belgium, 2005.
- Raspor, P.; Smole-Možina, S.; Podjavoršek, J.; Pohleven, F.; Gogala, N.; Nekrep, F.V.H. ZIM: Collection of Industrial Microorganisms. Catalogue of Cultures; University of Ljubljana, Biotechnical Faculty: Ljubljana, Slovenia, 1995. [Google Scholar]
- ENV 1250-2:1994; Wood Preservatives—Methods of Measuring Losses of Active Ingredients and Other Preservative Ingredients from Treated Timber Part 2: Laboratory Method for Obtaining Samples for Analysis to Measure Losses by Leaching into Water or Synthetic Sea Water. European Committee for Standardization: Brussels, Belgium, 1994.
- Meyer-Veltrup, L.; Brischke, C.; Alfredsen, G.; Humar, M.; Flæte, P.O.; Isaksson, T.; Brelid, P.L.; Westin, M.; Jermer, J. The combined effect of wetting ability and durability on outdoor performance of wood: Development and verification of a new prediction approach. Wood Sci. Technol. 2017, 51, 615–637. [Google Scholar] [CrossRef]
- Isaksson, T.; Brischke, C.; Thelandersson, S. Development of decay performance models for outdoor timber structures. Mater. Struct. Constr. 2013, 46, 1209–1225. [Google Scholar] [CrossRef]
- CEN EN 335; Durability of wood and wood-based products—Use classes: Definitions, application to solid wood and wood-based products. European Committee for Standardization: Brussels, Belgium, 2013.
- Archer, K.; Lebow, S. Wood preservation. Prim. Wood Process. Princ. Pract. 2006, 9781402043, 297–338. [Google Scholar] [CrossRef]
- EN 350:2016; Durability of wood and wood-based products—Testing and classification of the resistance to biological agents, the permeability to water and the performance of wood and wood- based materials. European Committee for Standardization: Brussels, Belgium, 2016.
- Jung, Y.H.; Kim, H.K.; Park, H.M.; Park, Y.C.; Park, K.; Seo, J.H.; Kim, K.H. Mimicking the Fenton reaction-induced wood decay by fungi for pretreatment of lignocellulose. Bioresour. Technol. 2015, 179, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Seesuriyachan, P.; Kuntiya, A.; Kawee-ai, A.; Techapun, C.; Chaiyaso, T.; Leksawasdi, N. Improvement in efficiency of lignin degradation by Fenton reaction using synergistic catalytic action. Ecol. Eng. 2015, 85, 283–287. [Google Scholar] [CrossRef]
- Reinprecht, L.; Panek, M. Fungicide efficacy of boron in the wood preservative containing ammonium salts. Acta Fac. Xylologiae TU Zvolen 2007, 49, 53–62. [Google Scholar]
- Lesar, B.; Kralj, P.; Humar, M. Montan wax improves performance of boron-based wood preservatives. Int. Biodeterior. Biodegrad. 2009, 63, 306–310. [Google Scholar] [CrossRef]
- Mckeen, L. Quaternary Ammonium Compound Introduction to Food Irradiation and Medical Sterilization. In The Effect of Sterilization on Plastics and Elastomers; Studies in Natural Products Chemistry; Elsevier Science: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Ramos, A.M.; Jorge, F.C.; Botelho, C. Boron fixation in wood: Studies of fixation mechanisms using model compounds and maritime pine. Holz Roh-und Werkst. 2006, 64, 445–450. [Google Scholar] [CrossRef]
- Lesar, B.; Gorišek, Ž.; Humar, M. Sorption properties of wood impregnated with boron compounds, sodium chloride and glucose. Dry. Technol. 2009, 27, 94–102. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Glass, S.V. Water vapor sorption isotherms for southern pine treated with several waterborne preservatives. J. Test. Eval. 2010, 38, 5. [Google Scholar] [CrossRef]
- Humar, M.; Kržišnik, D.; Lesar, B.; Brischke, C. The performance of wood decking after five years of exposure: Verification of the combined effect of wetting ability and durability. Forests 2019, 10, 903. [Google Scholar] [CrossRef]
- Uzunovic, A.; Byrne, T.; Gignac, M.; Yang, D.Q. Wood Discolourations and Their Prevention with an Emphasis on Bluestain. Special Publication SP-50; FPInnovations: Quebec City, QU, Canada, 2008; pp. 5–15. [Google Scholar]
- Krpat, M.; Hubbe, M.A.; Laleicke, F. Natural, Accelerated, and Simulated Weathering of Wood: A Review. Bioresurces 2020, 15, 9998–10062. [Google Scholar] [CrossRef]
- Kržišnik, D.; Lesar, B.; Thaler, N.; Humar, M. Influence of natural and artificial weathering on the colour change of different wood and wood-based materials. Forests 2018, 9, 488. [Google Scholar] [CrossRef]
- Richardson, H.W. Handbook of Copper Compounds and Applications; Marcel Dekker: New York, NY, USA, 1997; ISBN 0-8247-8998-9. [Google Scholar]
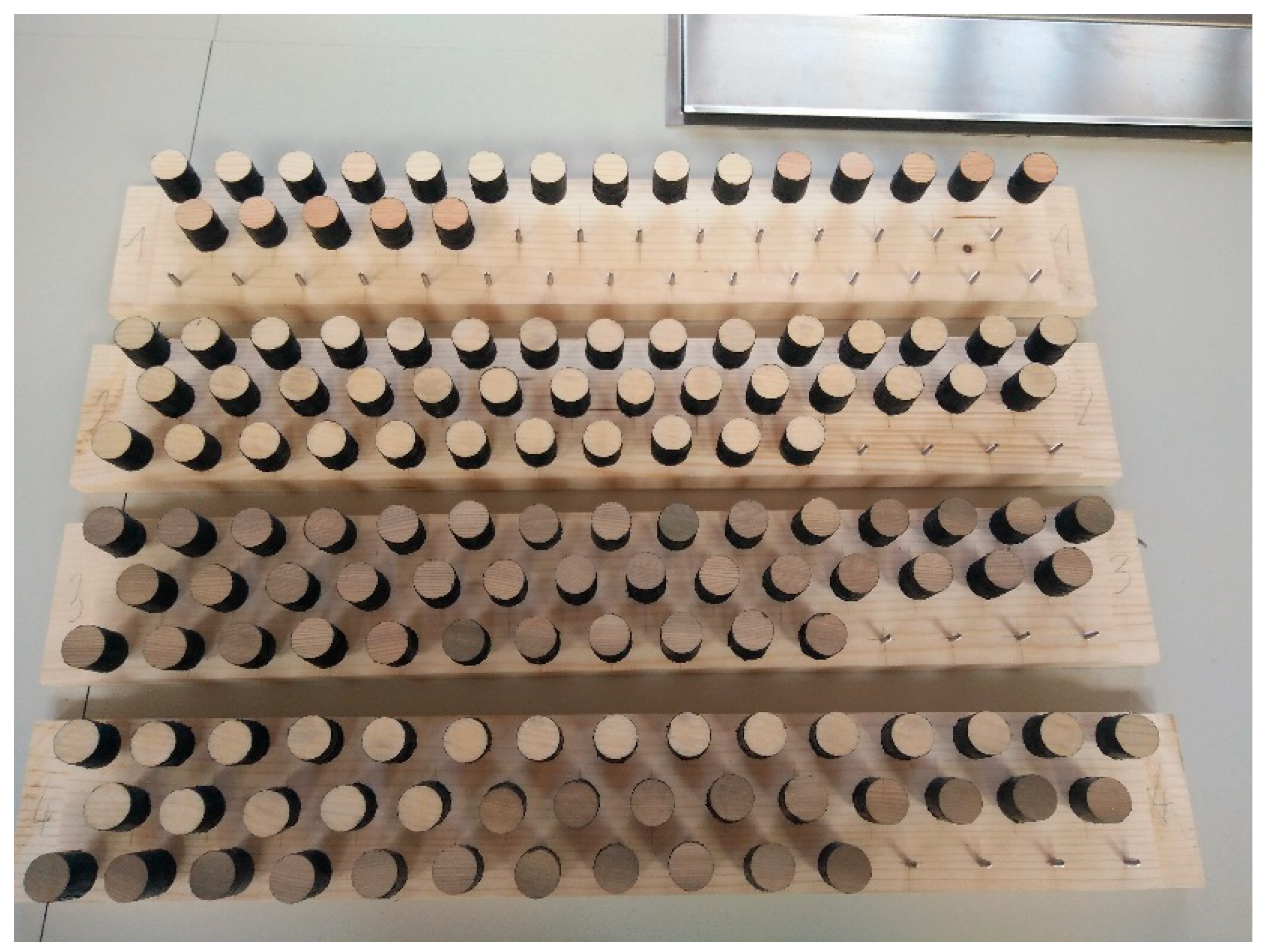

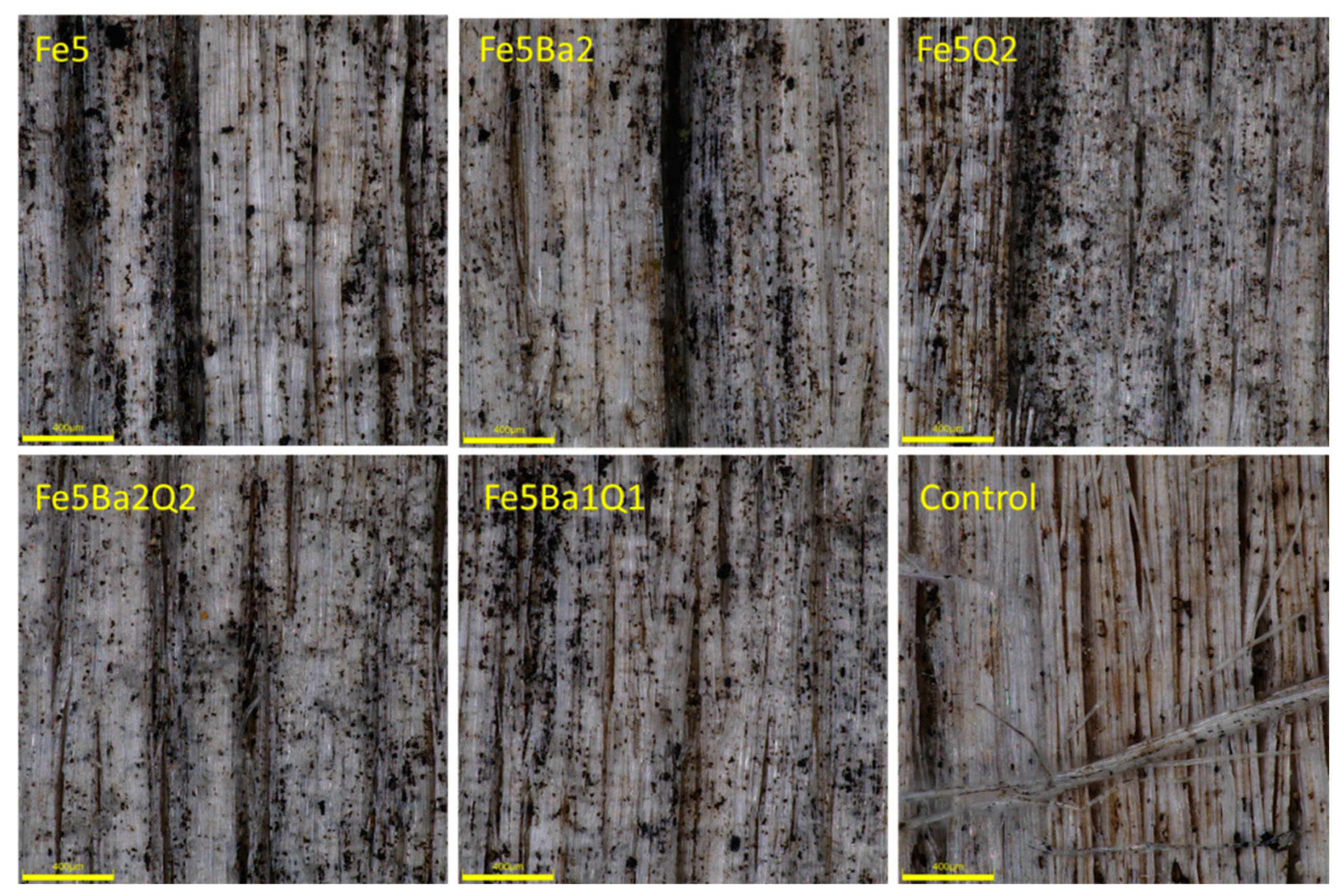
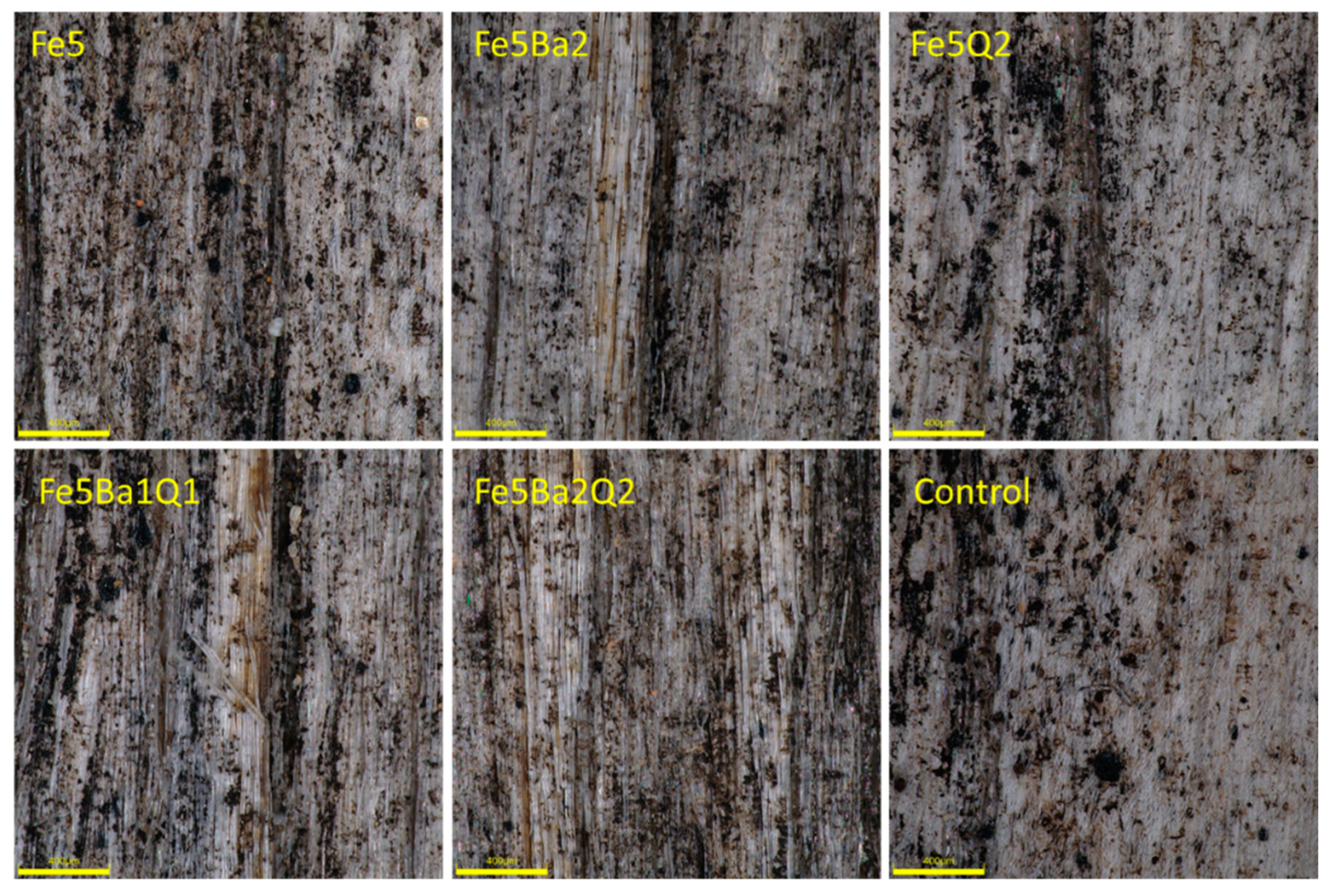

| Treatment | Type of Test | ||||||
|---|---|---|---|---|---|---|---|
| Mark | FeSO4 Conc.% (wt/wt) | Boric Acid Conc.% (wt/wt) | QUAT * Conc.% (wt/wt) | Durability | Water Performance | Leaching | Field Test |
| Fe2.5 | 2.5 | x | x | x | x | ||
| Fe5 | 5 | x | x | x | x | ||
| Fe5B0.1 | 5 | 0.1 | x | x | |||
| Fe5Q0.1 | 5 | 0.1 | x | x | |||
| Fe5B0.1Q0.1 | 5 | 0.1 | 0.1 | x | x | x | |
| Fe5Ba0.2 | 5 | 0.2 | x | x | x | ||
| Fe5Q0.2 | 5 | 0.2 | x | x | x | ||
| Fe5B0.2Q0.2 | 5 | 0.2 | 0.2 | x | x | x | |
| Fe10 | 10 | x | x | x | |||
| Control | x | x | x | ||||
| Mark | Control | Fe2.5 | Fe5 | Fe5B0.1 | Fe5Q0.1 | Fe5B0.1Q0.1 | Fe5B0.2 | Fe5Q0.2 | Fe5B0.2Q0.2 | Fe10 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solution uptake | (kg/m3) | Avg. | 0 c | 32 a | 35 a | 32 a | 36 a | 32 a | 40 b | 37 b | 37 b | 34 a | |
| Std. dev. | 0.0 | 6.1 | 10.6 | 6.1 | 6.6 | 4.8 | 8.5 | 10.0 | 7.8 | 6.2 | |||
| Wood Decay Fungi | |||||||||||||
| Mass loss (%) | G. trabeum | Avg. | 39.8 a | 31.3 b | 32.3 b | 31.0 b | 32.4 b | 21.5 c | 29.3 b | 19.5 d | 21.0 d | 29.4 b | |
| Std. dev. | 8.0 | 10.0 | 4.2 | 4.0 | 6.6 | 9.0 | 3.5 | 5.2 | 3.5 | 7.3 | |||
| F. vaillanti | Avg. | 12.3 a | 7.5 b | 4.7 b | 5.4 b | 6.0 b | 6.8 b | 4.8 b | 5.5 b | 6.1 b | 4.9 b | ||
| Std. dev. | 3.7 | 2.8 | 1.7 | 1.4 | 1.7 | 2.6 | 2.0 | 2.1 | 2.2 | 2.3 | |||
| Durability class | 5 | 4 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 | |||
| Time of Contact | |||||||||||||
| Contact angle (°) | 1 s | Avg. | 57 a | 91 c | 78 b | 63 a | 40 d | 39 d | 90 c | 24 e | 22 e | 52 a | |
| Std. dev. | 10 | 11 | 15 | 15 | 19 | 7 | 22 | 10 | 6 | 15 | |||
| 5 s | Avg. | 52 a | 86 c | 73 b | 58 a | 26 d | 27 d | 83 c | 12 e | 14 e | 47 a | ||
| Std. dev. | 12 | 10 | 15 | 15 | 17 | 7 | 24 | 7 | 5 | 15 | |||
| 30 s | Avg. | 47 a | 75 c | 62 b | 49 a | 15 d | 11 | 71 c | N.A. | N.A. | 44 a | ||
| Std. dev. | 12 | 10 | 13 | 16 | 8 | 10 | 26 | N.A. | N.A. | 14 | |||
| 60 s | Avg. | 40 a | 72 c | 53 b | 39 a | 12 d | N.A. | 47 b | N.A. | N.A. | 39 a | ||
| Std. dev. | 10 | 1 | 12 | 10 | 5 | N.A. | N.A. | N.A. | N.A. | 14 | |||
| Time of immersion | |||||||||||||
| Short-term water uptake (g/cm2) | 50 s | Avg. | 0.083 a | 0.122 c | 0.084 a | 0.117 c | 0.054 b | 0.063 b | 0.104 c | 0.052 b | 0.042 d | 0.079 a | |
| Std. dev. | 0.020 | 0.017 | 0.010 | 0.047 | 0.031 | 0.031 | 0.051 | 0.028 | 0.023 | 0.024 | |||
| 100 s | Avg. | 0.096 a | 0.139 c | 0.099 a | 0.135 c | 0.067 b | 0.075 b | 0.126 c | 0.069 b | 0.052 d | 0.099 a | ||
| Std. dev. | 0.023 | 0.018 | 0.013 | 0.052 | 0.034 | 0.036 | 0.059 | 0.034 | 0.029 | 0.031 | |||
| 200 s | Avg. | 0.109 a | 0.153 c | 0.113 a | 0.150 c | 0.084 b | 0.092 b | 0.152 c | 0.092 b | 0.067 d | 0.118 a | ||
| Std. dev. | 0.026 | 0.018 | 0.016 | 0.057 | 0.037 | 0.041 | 0.069 | 0.041 | 0.035 | 0.039 | |||
| Time of immersion | |||||||||||||
| Long-term water uptake (%) | 1 h | Avg. | 20.6 a | 14.0 a | 15.6 a | 21.2 a | 16.3 a | 16.0 a | 22.0 a | 20.3 a | 15.5 a | 26.5 b | |
| Std. dev. | 1.6 | 1.4 | 1.1 | 6.7 | 2.0 | 5.0 | 9.8 | 7.3 | 1.9 | 8.6 | |||
| 24 h | Avg. | 45.2 a | 42.0 a | 44.0a | 50.8 b | 44.9 a | 43.7 a | 50.5 b | 49.4 b | 40.9 a | 58.0 c | ||
| Std. dev. | 2.4 | 1.9 | 1.7 | 8.6 | 3.1 | 6.7 | 9.2 | 7.3 | 2.5 | 8.1 | |||
| 48 h | Avg. | 51.1 a | 48.7 a | 50.7 a | 56.1 b | 51.5 a | 50.7 a | 55.7 b | 55.9 b | 47.6 a | 62.8 c | ||
| Std. dev. | 1.9 | 1.6 | 1.5 | 7.7 | 2.5 | 5.0 | 7.4 | 6.6 | 2.0 | 6.5 | |||
| Time of condition | |||||||||||||
| Water vapour uptake (%) | 24 h | Avg. | 13.5 a | 14.4 b | 14.0 b | 14.1 b | 14.3 b | 14.3 b | 13.5 a | 14.0 b | 14.3 b | 13.7 b | 14.0 b |
| Std. dev. | 0.13 | 0.35 | 0.42 | 0.82 | 0.81 | 0.75 | 0.41 | 0.45 | 0.54 | 0.43 | 0.42 | ||
| 4 w | Avg. | 25.5 a | 26.4 b | 26.5 b | 26.9 b | 26.7 b | 26.1 b | 25.7 b | 26.1 b | 26.2 b | 25.6 a | 26.5 b | |
| Std. dev. | 0.95 | 0.22 | 0.41 | 0.25 | 0.22 | 0.62 | 0.53 | 0.39 | 0.31 | 0.14 | 0.41 | ||
| Time of condition | |||||||||||||
| Drying above silica gel (%) | 24 h | Avg. | 17.0 a | 16.9 a | 17.3 a | 18.0 b | 16.8 a | 16.9 a | 17.5 a | 16.7 a | 17.4 a | 17.4 a | |
| Std. dev. | 0.66 | 0.41 | 0.41 | 0.67 | 0.22 | 0.50 | 0.40 | 0.56 | 0.60 | 0.45 | |||
| Factors that determine the service life of wood* | kinh | 1 | 1.5 | 1.9 | 1.8 | 1.6 | 1.8 | 2.0 | 2.1 | 2.0 | 1.9 | ||
| kwa | 1 | 1.1 | 1.1 | 0.9 | 1.2 | 1.1 | 0.9 | 1.1 | 1.2 | 0.9 | |||
| DRd (d) | 325 | 502 | 682 | 531 | 613 | 672 | 580 | 739 | 781 | 586 | |||
| Drd rel | 1 | 1.5 | 2.1 | 1.6 | 1.9 | 2.1 | 1.8 | 2.3 | 2.4 | 1.8 | |||
| Wood | Fe2.5 | Fe5 | Fe5B0.1Q1 | Fe5B0.2 | Fe5Q0.2 | Fe5B0.2Q0.2 | ||
|---|---|---|---|---|---|---|---|---|
| Solution uptake (kg/m3) | Norway spruce | Avg. | 58 a | 61 a | 59 a | 67 b | 63 a | 67 b |
| Std. Dev. | 3.7 | 3.2 | 6.1 | 5.2 | 2.6 | 5.0 | ||
| Siberian larch | Avg. | 18 a | 19 a | 16 b | 19 a | 18 a | 16 b | |
| Std. Dev. | 1.0 | 2.2 | 1.6 | 1.1 | 1.5 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesar, B.; Humar, M. Performance of Iron(II)-Sulphate-Treated Norway Spruce and Siberian Larch in Laboratory and Outdoor Tests. Forests 2022, 13, 1497. https://doi.org/10.3390/f13091497
Lesar B, Humar M. Performance of Iron(II)-Sulphate-Treated Norway Spruce and Siberian Larch in Laboratory and Outdoor Tests. Forests. 2022; 13(9):1497. https://doi.org/10.3390/f13091497
Chicago/Turabian StyleLesar, Boštjan, and Miha Humar. 2022. "Performance of Iron(II)-Sulphate-Treated Norway Spruce and Siberian Larch in Laboratory and Outdoor Tests" Forests 13, no. 9: 1497. https://doi.org/10.3390/f13091497
APA StyleLesar, B., & Humar, M. (2022). Performance of Iron(II)-Sulphate-Treated Norway Spruce and Siberian Larch in Laboratory and Outdoor Tests. Forests, 13(9), 1497. https://doi.org/10.3390/f13091497







