The Influence of Physical Treatments on Seed Germination and Seedling Development of Spruce (Picea abies [L.] Karst.)
Abstract
1. Introduction
2. Materials and Methods
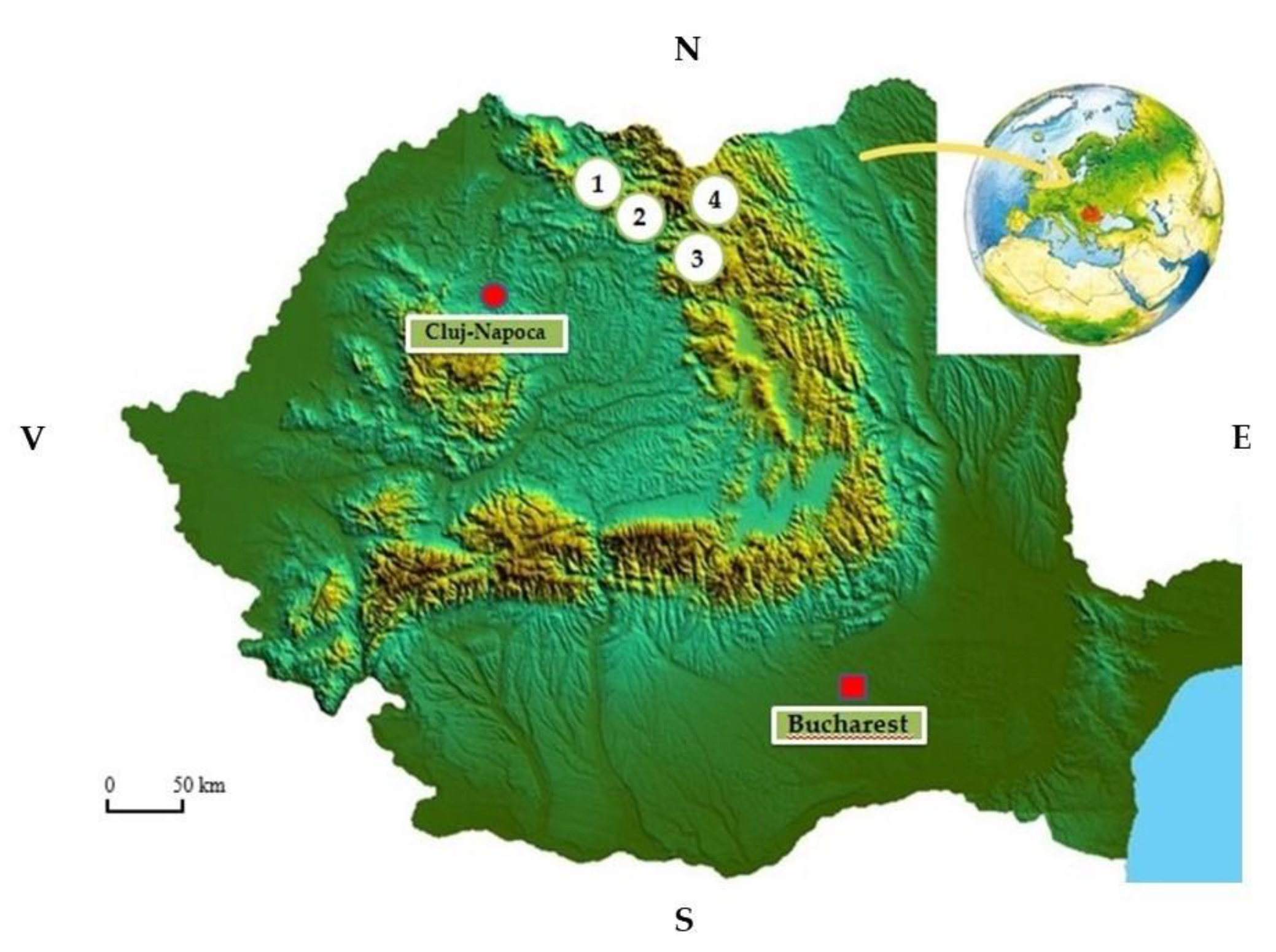
2.1. Biological Material
2.2. Exposure of the Seeds to Electric field (EF) Treatments
2.3. Exposure of Seeds to Gamma Irradiation (G) Treatments
2.4. Seeds and Seedlings Characteristics Measurements
2.5. Experimental Design and Data Analysis
3. Results
3.1. Morphological Traits of Spruce Seeds
3.2. Seed Germination after EF Treatments
3.3. Seedlings Development after EF Treatments Applied to the Seeds
3.4. Seed Germination after Gamma (G) Irradiation
3.5. Seedlings Development after G Treatments Applied to the Seeds
4. Discussions
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Le, K.C.; Weerasekara, A.B.; Ranade, S.S.; Egertsdotter, E.U. Evaluation of parameters to characterise germination-competent mature somatic embryos of Norway spruce (Picea abies). Biosyst. Eng. 2021, 203, 55–59. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Y.; Finzi, A.C. Carbon and nitrogen dynamics during forest stand development: A global synthesis. New Phytol. 2011, 190, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the world’s forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Mugloo, J.A.; Mir, N.A.; Khan, P.A.; Perray, G.N.; Kaiser, K.N. Determination of Effect of Cold Stratification Temperature and Duration on Germination of Spruce (Picea smithiana Wall. Boiss) under Laboratory Conditions. J. Exp. Agric. Int. 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Oszlányi, J.; Grodzińska, K.; Badea, O.; Shparyk, Y. Nature conservation in Central and Eastern Europe with a special emphasis on the Carpathian Mountains. Environ. Pollut. 2004, 130, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Kuemmerle, T. Where are Europe’s last primary forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Čermák, P.; Rybníček, M.; Žid, T.; Andreassen, K.; Børja, I.; Kolář, T. Impact of climate change on growth dynamics of Norway spruce in south-eastern Norway. Silva Fenn. 2017, 51, 1–16. [Google Scholar] [CrossRef]
- Ciocîrlan, E.; Șofletea, N.; Mihai, G.; Teodosiu, M.; Curtu, A.L. Comparative analysis of genetic diversity in Norway spruce (Picea abies) clonal seed orchards and seed stands. Not. Bot. HortiAgrobot. 2021, 49, 12575. [Google Scholar] [CrossRef]
- Neimane, U.; Zadina, M.; Sisenis, L.; Dzerina, B.; Pobiarzens, A. Influence of lammas shoots on productivity of Norway spruce in Latvia. Agron. Res. 2015, 13, 354–360. [Google Scholar]
- Katrevičs, J.; Džeriņa, B.; Neimane, U.; Desaine, I.; Bigača, Z.; Jansons, Ā. Production and profitability of low density Norway spruce (Picea abies (L.) Karst.) plantation at 50 years of age: Case study from eastern Latvia. Agron. Res. 2018, 16, 113–121. [Google Scholar]
- Mania, P.; Fabisiak, E.; Skrodzka, E. Investigation of modal behaviour of resonance spruce wood samples (Picea abies L.). Arch. Acoust. 2017, 42, 23–28. [Google Scholar] [CrossRef][Green Version]
- Echard, J.P.; Lavédrine, B. Review on the characterisation of ancient stringed musical instruments varnishes and implementation of an analytical strategy. J. Cult. Herit. 2008, 9, 420–429. [Google Scholar] [CrossRef]
- Praciak, A.; Pasiecznik, N.; Sheil, D.; van Heist, M.; Sassen, M.; Correia, C.S.; Dixon, C.; Fyson, G.; Rushford, K.; Teeling, C. The CABI Encyclopedia of Forest Trees; CABI Oxfordshire: Wallingford, UK, 2013; ISBN 978-1-78064-236-9. [Google Scholar]
- Honkaniemi, J.; Rammer, W.; Seidl, R. Norway spruce at the trailing edge: The effect of landscape configuration and composition on climate resilience. Landsc. Ecol. 2020, 35, 591–606. [Google Scholar] [CrossRef]
- Koski, V.; Skrøppa, T.; Paule, L.; Wolf, H.; Turok, J. Technical Guidelines for Genetic Conservation of Norway Spruce Picea abies (L.) Karst.); Bioversity International: Rome, Italy, 1997. [Google Scholar]
- Feurdean, A.; Tanţău, I.; Fărcaş, S. Holocene variability in the range distribution and abundance of Pinus, Picea abies, and Quercus in Romania; implications for their current status. Quat. Sci. Rev. 2011, 30, 3060–3075. [Google Scholar] [CrossRef]
- Sofletea, N.; Curtu, A.L. Dendrologie; Editura Universitătii Transilvania: Brasov, Romania, 2007; ISBN 9789736358852. [Google Scholar]
- Jull, L.G.; Blazich, F.A. Seed germination of selected provenances of Atlantic white-cedar as influenced by stratification, temperature, and light. HortScience 2000, 35, 132–135. [Google Scholar] [CrossRef]
- Rîşca, I.M.; Ştiucă, P.; Leahu, A. Efectul unor tratamente cu radiaţii nucleare asupra germinaţiei seminţelor de molid (PiceaAbies (L.) Karsten). Analele Universității, Ștefan Cel Mare” Suceava Secțiunea Silvicultură Serie nouă–nr. 1/2006. 2006. Available online: http://www.silvic.usv.ro/anale/as_2006_1/as_rasca_2006_1.pdf (accessed on 1 February 2020).
- Houšková, K.; Klepárník, J.; Mauer, O. How to accelerate the germination of Scots pine and Norway spruce seeds? J. For. Sci. 2021, 67, 134–142. [Google Scholar] [CrossRef]
- Bergsten, U. Temperature tolerance of invigorated seeds of Pinus sylvestris L., and Picea abies (L.) Karst. using TTGP-test. For. Suppl. 1989, 62, 107–115. [Google Scholar]
- Leinonen, K.; Nygren, M.; Rita, H. Temperature control of germination in the seeds of Picea abies. Scandinavian. J. For. Res. 1992, 8, 107–117. [Google Scholar]
- Truta, A.M.; Viman, O.; Dohotar, V.D.; Sîngeorzan, S.; Truța, P.; Holonec, L. The Influence of Certain Types of Substrate and Biochemical Substances in Seed Germination and Plant Development of Spruce (Picea abies). Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Hortic 2022, 77, 128–135. [Google Scholar]
- Rikala, R.; Jozefek, H.J. Effect of dolomite lime and wood ash on peat substrate and development of tree seedlings. Silva Fenn. 1990, 24, 323–334. [Google Scholar] [CrossRef][Green Version]
- Tomášková, I.; Vítámvás, J.; Korecký, J. Testing of germination of spruce, pine and larch seed after 10 years from collection. J. For. Sci. 2014, 60, 540–543. [Google Scholar] [CrossRef]
- Bezděčková, L.; Matějka, K. Influence of weather conditions on the quality of Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) Karst.) seeds. Zprávy Lesn. Výzkumu 2018, 63, 1–9. [Google Scholar]
- Das, R.; Bhattacharya, R. Impact of electromagnetic field on seed germination. In Proceedings of the international Conference on Modern Electrostatics, Beijing, China, 25 May 2006; Volume 141, p. 145. [Google Scholar]
- Aladjadjiyan, A. Influence of stationary magnetic field on lentil seeds. Int. Agrophys 2010, 24, 321–324. [Google Scholar]
- Molamofrad, F.; Lotfi, M.; Khazaei, J.; Tavakkol-Afshari Shaiegani-Akmald, A.A. The effect of electric field on seed germination and growth parameters of onion seeds (Alium cepa). Adv. Crop Sci. 2013, 3, 291–298. [Google Scholar]
- Mamlic, Z.; Maksimovic, I.; Canak, P.; Mamlic, G.; Djukic, V.; Vasiljevic, S.; Dozet, G. The use of electrostatic field to improve soybean seed germination in organic production. Agronomy 2021, 11, 1473. [Google Scholar] [CrossRef]
- Mahajan, T.S.; Pandey, O.P. Magnetic-time model at off-season germination. Int. Agrophys 2014, 28, 57–62. [Google Scholar] [CrossRef]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Liu, S. Magnetic field (MF) applications in plants: An overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Golijan, J.; Dimitrijevi’c, B. Global organic food market. Acta Agric. Serb. 2018, 23, 125–140. [Google Scholar] [CrossRef]
- Aladjadjiyan, A. The use of physical methods for plant growing stimulation in Bulgaria. J. Cent. Eur. Agric. 2007, 8, 369–380. Available online: https://hrcak.srce.hr/19607 (accessed on 1 February 2020).
- Chen, H.H.; Chang, H.C.; Chen, Y.K.; Hung, C.L.; Lin, S.Y.; Chen, Y.S. An improved process for high nutrition of germinated brown rice production: Low-pressure plasma. Food Chem. 2016, 191, 120–127. [Google Scholar] [CrossRef]
- Holonec, R.; Viman, O.; Morar, I.M.; Sîngeorzan, S.; Scheau, C.; Vlasin, H.D.; Holonec, L.; Truta, A.M. Non-chemical treatments to improve the seeds germination and plantlets growth of sessile oak. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12401. [Google Scholar] [CrossRef]
- Iglesias-Andreu, L.G.; Octavio-Aguilar, P.; Bello-Bello, J. Current importance and potential use of low doses of gamma radiation in forest species. Gamma Radiat. 2012, 265–280. [Google Scholar] [CrossRef]
- Jan, S.; Parween, T.; Hameed, R.; Siddiqi, T.O. Effects of presowing gamma irradiation on the photosynthetic pigments, sugar content and carbon gain of Cullen corylifolium (L.) Medik. Chil. J. Agric. Res. 2013, 73, 345–350. [Google Scholar] [CrossRef]
- Macovei, A.; Garg, B.; Raikwar, S.; Balestrazzi, A.; Carbonera, D.; Buttafava, A.; Bremont, J.F.; Gill, S.S.; Tuteja, N. Synergistic exposure of rice seeds to different doses of γ-ray and salinity stress resulted in increased antioxidant enzyme activities and gene-specific modulation of TC-NER pathway. Biomed Res. Int. 2014, 2014, 676934. [Google Scholar] [CrossRef]
- Rajendra, P.; Sujatha, D.H.; Devendranath, B.; Gunasekaran, R.; Sashidhar, C. Subramanyam, Channakeshava. Biological effects of power frequency magnetic fi elds: Neurochemical and toxicological changes in developing chick embryos. Biomagn. Res. Technol. 2004, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stefa, L.; Pozeliene, A. Effect of Electrical Field on Barley Seed Germination Stimulation. Agric. Eng. Int. 2003, 2, 3–7. [Google Scholar]
- Lynikiene, S.; Pozeliene, A. Influence of corona discharge field on seed viability and dynamics of germination. Int. Agrophysics 2006, 20, 195–200. [Google Scholar]
- Kerdonfag, P.; Klinsa-ard, C.; Khan-ngern, W.; Ketjaew, S. Effect of electric field from the electric field Rice grain separation unit on growth stages of the rice plant. Fac. Eng. EMC Lab. 2002, 5, 250–253. [Google Scholar]
- Kanyago, G.A.; Kuria, K.P. Effect of Electric Field In The Soil On The Germination And Growth Rate Of Rosecoco Beans Plant. J. Agric. Res. 2020, 7, 1–10. [Google Scholar]
- Carbonell, M.V.; Martinez, E.; Amaya, J.M. Stimulation of germination in rice (Oryza sative L.) by a static magnetic field. Electro-Magnetobiol. 2000, 19, 121–128. [Google Scholar] [CrossRef]
- Yin, J.; Finno, R.J.; Feldkamp, J.R.; Chung, K. Coefficient of permeability from ac electroosmosis experiments. I: Theory. J. Geotech. Eng. 1996, 122, 346–354. [Google Scholar] [CrossRef]
- Olszanowski, A.; Piechowiak, K. The Use of an Electric Field to Enhance Bacterial Movement and Hydrocarbon Biodegradation in Soils. Pol. J. Environ. Stud. 2006, 15, 2. [Google Scholar]
- Pârnuță, G.; Budeanu, M.; Stuparu, E.; Scărlătescu, V.; Cheșnoiu, E.-N.; Tudoroiu, M.; Filat, M.; Nica, M.-S.; Teodosiu, M.; Lorenț, A.; et al. Catalogul Național al Materialelor de Bază Pentru Producerea Materialelor Forestiere de Reproducere (National Catalogue of Basic Materials for Production of Forest Reproductive Materials); Silvică Publishing House: Bucharest, Romania, 2012; p. 304. (In Romanian) [Google Scholar]
- Sestras, A.F. Biostatistica si Tehnica Experimentala Forestiera: Manual Didactic; Editura Academic Press: Cluj-Napoca, Romania, 2018. [Google Scholar]
- Rajora, O.P.; Mosseler, A. Challenges and opportunities for conservation of forest genetic resources. Euphytica 2001, 118, 197–212. [Google Scholar] [CrossRef]
- Roman, A.M.; Truta, A.M.; Viman, O.; Morar, I.M.; Spalevic, V.; Dan, C.; Sestras, R.; Holonec, L.; Sestras, A.F. Seed Germination and Seedling Growth of Robinia pseudoacacia Depending on the Origin of Different Geographic Provenances. Diversity 2022, 14, 34. [Google Scholar] [CrossRef]
- Cordazzo, C.V. Effect of seed mass on germination and growth in three dominant species in southern Brazilian coastal dunes. Braz. J. Biol. 2002, 62, 427–435. [Google Scholar]
- Murali, K.S. Patterns of Seed Size, Germination and Seed Viability of tropical Tree Species in Southern India. Biotropica 1997, 29, 271–279. [Google Scholar] [CrossRef]
- Mölken, T.; Jorritsma-Wienk, L.D.; Hoek, P.H.; Kroon, W.H. Only Seed Size Matters for Germination in Different Populations of the Dimorphic Tragopogonp ratensiss subsp. pratensis (Asteraceae). Am. J. Bot. 2005, 92, 432–437. [Google Scholar] [CrossRef]
- Yanlong, H.; Mantang, W.; Shujun, W.; Yanhui, Z.; Tao, M.; Guozhen, D. Seed Size Effect on Seedling Growth under Different Light Conditions in the Clonal Herb Ligularia virgaurea in Qinghai-Tibet Plateau. Acta Ecol. Sin. 2007, 27, 3091–3108. [Google Scholar]
- Souza, M.L.; Fagundes, M. Seed size as key factor in germination and seedling development of Copaifera langsdorffii (Fabaceae). Am. J. Plant Sci. 2014, 5, 2566–2573. [Google Scholar] [CrossRef]
- Kheloufi, A.; Mansouri, L.; Aziz, N.; Sahnoune, M.; Boukemiche, S.; Ababsa, B. Breaking seed coat dormancy of six tree species. Reforesta 2018, 5, 4–14. [Google Scholar] [CrossRef]
- Mtambalika, K.; Munthali, C.; Gondwe, D.; Missanjo, E. Effect of Seed Size of Afzelia Quanzensis on Germination and Seedling Growth. Int. J. For. Res. 2014, 2014, 384565. [Google Scholar] [CrossRef]
- Kaliniewicz, Z.; Zuk, Z.; Kusińska, E. Physical properties of seeds of eleven spruce species. Forests 2018, 9, 617. [Google Scholar] [CrossRef]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef]
- Gätjens-Boniche, O.; Díaz, C.; Hernández-Vásquez, L.; Chavarría-Rodríguez, P.; Martínez-Ávila, E. Effect of Electrical Current Applied in Soaking Conditions on Germination of Acacia and Maize Seeds. J. Agric. Vet. Sci. 2017, 10, 11–18. [Google Scholar]
- Rezaei-Zarchi, S.; Imani, S.; Mehrjerdi, A.H.; Mohebbifar, R.M. The effect of electric field on the germination and growth of Medicago sativa planet, as a native Iranian alfalfa seed. Acta Agric. Serbica 2012, 17, 105–115. [Google Scholar]
- Marcu, D.; Besenyei, E.; Cristea, V. Radiosensitivity of maize to gamma radiation based on physiological responses. Muzeul Olteniei Craiova. Oltenia. Studii sicomunicări. Stiinele Nat. 2014, 30, 1. [Google Scholar]
- Singh, B.; Datta, P.S. Gamma irradiation to improve plant vigour, grain development, and yield attributes of wheat. Radiat. Phys. Chem. 2010, 79, 139–143. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, H.S.; Pai, H.S. Expression Patterns of Diverse Genes in Response to Gamma Irradiation in Nicotiana tabacum. J. Plant Biol. 2000, 43, 82–87. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, L.; Wang, L.; Xu, H.; Jin, Q.; Jiao, Z. Pretreatment with low-dose gamma irradiation enhances tolerance to the stress of cadmium and lead in Arabidopsis thaliana seedlings. Ecotoxicol. Environ. Saf. 2015, 115, 243–249. [Google Scholar] [CrossRef]
- Maity, J.; Mishra, D.; Chakraborty, A.; Saha, A.; Santra, S.; Chanda, S. Modulation of some quantitative and qualitative characteristics in rice (Oryza sativa L.) and mung (Phaseolus mungo L.) by ionizing radiation. Radiat. Phys. Chem. 2005, 74, 391–394. [Google Scholar] [CrossRef]
- Araújo Sde, S.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical methods for seed invigoration: Advents and challenges in seed technology. Front. Plant Sci. 2016, 7, 646. [Google Scholar] [CrossRef] [PubMed]




| Treatment | Provenance | |||
|---|---|---|---|---|
| Măria Mică (P1) | Făina (P2) | Aluniș (P3) | Putnișoara (P4) | |
| T1EF (Control) | 60.00 a–c | 63.33 a–d | 56.67 ab | 50.00 a |
| T2EF (10 V-15 min) | 80.00 bd | 76.67 a–d | 73.33 a–d | 63.33 a–d |
| T3EF (10 V-35 min) | 86.67 cd | 83.33 b–d | 63.33 a–d | 63.33 a–d |
| T4EF (30 V-15 min) | 86.67 cd | 90.00 d | 76.67 a–d | 70.00 a–d |
| T5EF (30 V-35 min) | 76.67 a–d | 76.67 a–d | 70.00 a–d | 60.00 a–c |
| T6EF (50 V-15 min) | 73.33 a–d | 60.00 a–c | 63.33 a–d | 50.00 a |
| T7EF (50 V-35 min) | 73.33 a–d | 70.00 a–d | 56.67 ab | 56.67 ab |
| Treatment | Provenance | |||
|---|---|---|---|---|
| Măria Mică (P1) | Făina (P2) | Aluniș (P3) | Putnișoara (P4) | |
| T1EF (Control) | 2.89 d–f | 3.08 e–g | 2.59 b–e | 2.79 b–e |
| T2EF (10 V-15 min) | 3.66 gh | 3.57 f–h | 3.11 e–g | 3.13 e–g |
| T3EF (10 V-35 min) | 2.70 b–e | 2.78 b–e | 3.00 e–g | 2.83 c–e |
| T4EF (30 V-15 min) | 4.18 h | 4.00 h | 3.54 f–h | 3.91 h |
| T5EF (30 V-35 min) | 2.99 d–g | 2.63 b–e | 3.09 e–g | 2.90 d–f |
| T6EF (50 V-15 min) | 1.93 a | 2.17a–c | 1.66 a | 1.82 a |
| T7EF (50 V-35 min) | 2.3 a–d | 2.66 b–e | 2.62 b–e | 2.14 ab |
| Treatment | Provenance | |||
|---|---|---|---|---|
| Măria Mică (P1) | Făina (P2) | Aluniș (P3) | Putnișoara (P4) | |
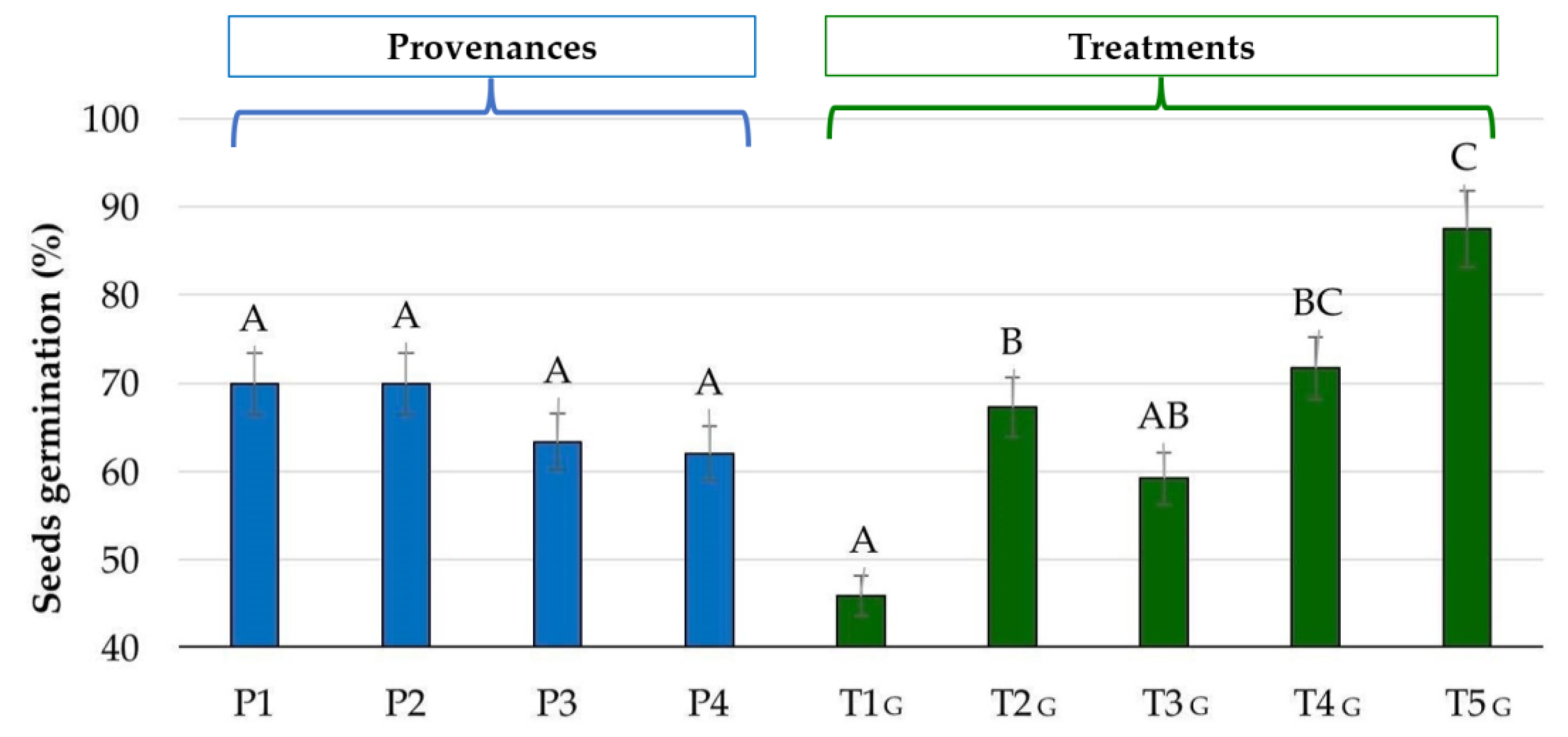
| T1G (Control) | 43.33 a | 50.00 a,b | 43.33 a | 46.67 ab |
| T2G (1 Gy-31 min) | 66.00 a–d | 66.33 a–d | 70.00 a–d | 66.67 a–d |
| T3G (1,5 Gy-46 min) | 63.33 a–d | 63.33 a–d | 53.33ab | 56.67 a–c |
| T4G (2 Gy-62 min) | 80.00 a–d | 76.67 a–d | 66.67a–d | 63.33 a–d |
| T5G (6 Gy-186 min) | 96.67 d | 93.33 c–d | 83.33 b–d | 76.67 a–d |
| Treatment | Provenance | |||
|---|---|---|---|---|
| Măria Mică (P1) | Făina (P2) | Aluniș (P3) | Putnișoara (P4) | |
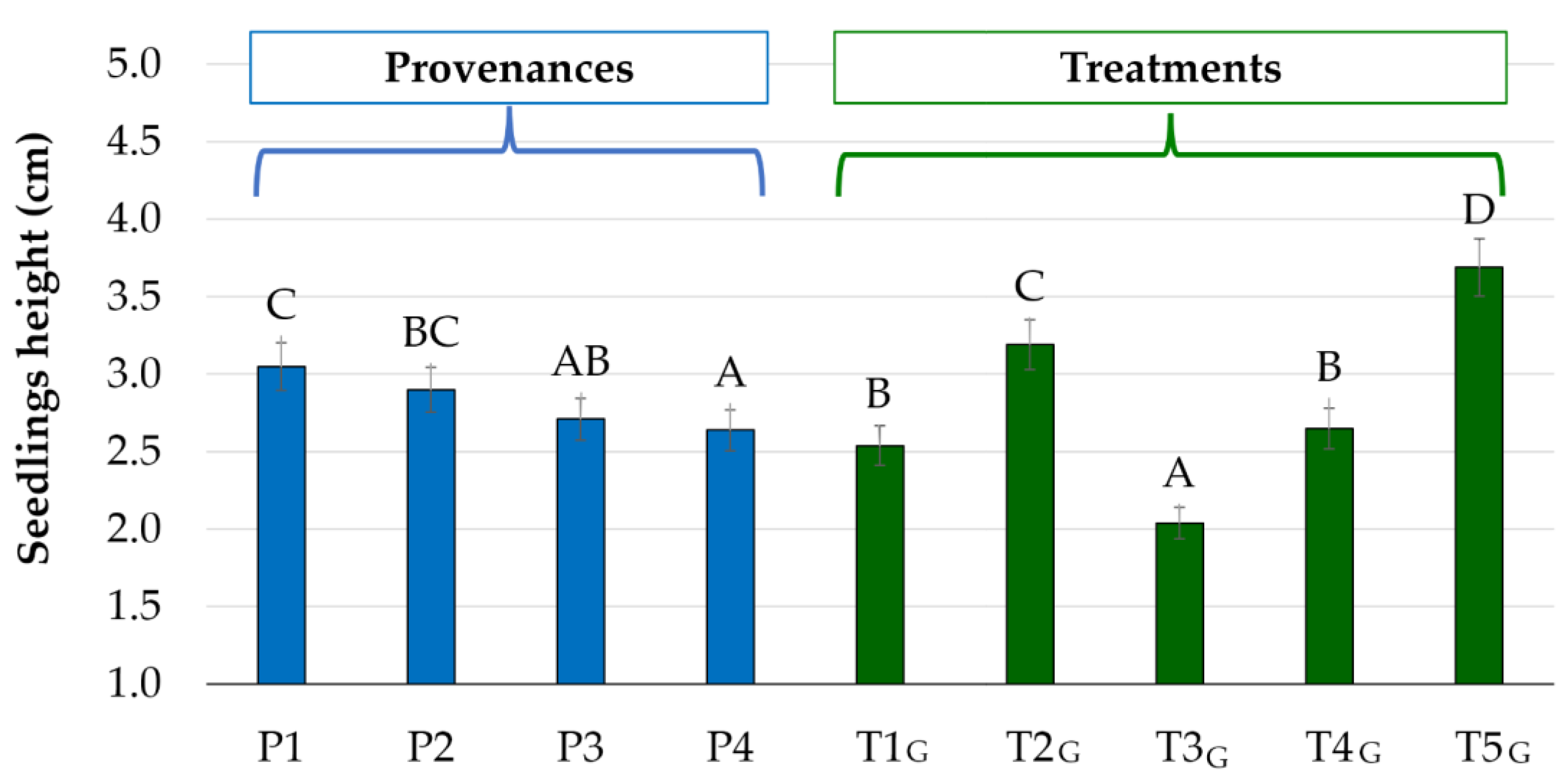
| T1G (Control) | 2.55 a–c | 2.47 a–c | 2.52 a–c | 2.61 b–d |
| T2G (1 Gy-31 min) | 3.63 f–h | 3.21d–f | 2.96 c–e | 2.97 c–e |
| T3G (1,5 Gy-46 min) | 2.10 ab | 2.03 ab | 2.12 ab | 1.92 a |
| T4G (2 Gy-62 min) | 2.83 c–e | 2.87 c–e | 2.49 a–c | 2.41 a–c |
| T5G (6 Gy-186 min) | 4.13 h | 3.91gh | 3.45 e–g | 3.28 ef |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sîngeorzan, S.-M.; Holonec, L.; Truta, A.M.; Morar, I.M.; Dan, C.; Colișar, A.; Viman, O.; Negrușier, C.; Borsai, O.; Criveanu, H.; et al. The Influence of Physical Treatments on Seed Germination and Seedling Development of Spruce (Picea abies [L.] Karst.). Forests 2022, 13, 1498. https://doi.org/10.3390/f13091498
Sîngeorzan S-M, Holonec L, Truta AM, Morar IM, Dan C, Colișar A, Viman O, Negrușier C, Borsai O, Criveanu H, et al. The Influence of Physical Treatments on Seed Germination and Seedling Development of Spruce (Picea abies [L.] Karst.). Forests. 2022; 13(9):1498. https://doi.org/10.3390/f13091498
Chicago/Turabian StyleSîngeorzan, Steluța-Maria, Liviu Holonec, Alina M. Truta, Irina M. Morar, Catalina Dan, Alexandru Colișar, Oana Viman, Cornel Negrușier, Orsolya Borsai, Horia Criveanu, and et al. 2022. "The Influence of Physical Treatments on Seed Germination and Seedling Development of Spruce (Picea abies [L.] Karst.)" Forests 13, no. 9: 1498. https://doi.org/10.3390/f13091498
APA StyleSîngeorzan, S.-M., Holonec, L., Truta, A. M., Morar, I. M., Dan, C., Colișar, A., Viman, O., Negrușier, C., Borsai, O., Criveanu, H., Vlasin, H. D., & Păcurar, I. (2022). The Influence of Physical Treatments on Seed Germination and Seedling Development of Spruce (Picea abies [L.] Karst.). Forests, 13(9), 1498. https://doi.org/10.3390/f13091498







