Abstract
Emissions of dinitrogen (N2) and nitrous oxide (N2O) from soil are important components of the global nitrogen cycle. Soil N2O emissions from terrestrial ecosystems have been well studied. However, patterns and mechanisms of N2 emissions remain unclear due to the technical difficulty in measuring N2 production. In this study, an in situ 15N labeling method was employed to determine soil N2 and N2O emission rates from the lower, middle, and upper slopes, which correspond to different moisture conditions, in a temperate forest in Northeast China. We found that N2 emissions varied from 85 to 3442 μg N m−2 h−1 across the slopes and were dominated by denitrification. The emissions of bulk N2O (22 to 258 μg N m−2 h−1) and denitrification-derived N2O (14 to 246 μg N m−2 h−1) were significantly lower than N2 emissions from their corresponding slope positions. Both N2 and N2O emissions significantly increased when soils become wetter. The ratios of N2O/(N2O + N2) were significantly higher at the upper and middle slopes (0.22 and 0.20, respectively) compared with those at the lower slope (0.08 ± 0.01). At the catchment scale, N2 accounted for 85% of the total gaseous N losses (N2O + N2). Our study shows that soil moisture drives the patterns of N2 and N2O emissions and field quantification of N2O/(N2O + N2) ratio should further consider the effect of slope position of forest ecosystems to estimate total soil gaseous N losses.
1. Introduction
Gaseous nitrogen (N) emissions (e.g., NO, N2O, N2) from soils play a crucial role in the global N cycle and climate change [1] and have been proposed as an important mechanism of terrestrial ecosystem N limitation [2]. Nitrous oxide (N2O) and NO, as by-products of denitrification and nitrification, strongly impact global warming and atmospheric chemistry, respectively [3]. Dinitrogen (N2), the end product of denitrification, is relatively inert in the atmosphere [4]. Compared to N2O and NO, soil N2 emissions have not been well-quantified due to the high background atmospheric N2 concentration [5]. Presently, the acetylene (C2H2) inhibition method [6], 15N isotope trace technique [7,8,9,10], and gas–flow soil core method [11,12,13], have all been used to determine N2 emissions from the soil, and these methods contribute to our understanding of soil N2 dynamics. However, at a large ecosystem scale, the field quantification of N2 fluxes remains a huge challenge due to high spatial and temporal variations in soil environments [5,14].
Due to the above-mentioned difficulty in detecting soil N2, the N2O/(N2O + N2) ratios commonly obtained from the laboratory are applied to estimate soil N2 flux combined with field N2O flux [15,16,17]. Recently, for instance, soil N2 flux from a maize field was calculated based on laboratory-quantified N2O/(N2O + N2) ratios, in situ measured N2O emission rate, and soil factors [13]. However, some studies indicated that the physical transport of N2O and N2 from soil to the atmosphere in the laboratory incubation system did not realistically reflect in situ conditions [18,19,20]. This is because field soil environment is highly variable, where oxygen (O2) concentration, available substrates, and other soil properties change both spatially and temporally. Additionally, the changes in soil properties have different effects on the emissions of N2 and N2O [21,22,23], consequently affecting N2O/(N2O + N2) ratios. Two previous studies suggested a wide range of N2O/(N2O + N2) ratios in the terrestrial ecosystem through a synthesis of the relevant literature [16,24]. To date, few field studies from temperate forests investigated soil N2O/(N2O + N2) ratios using either the acetylene inhibition method [25,26] or the 15N isotope trace technique [27,28]. However, it is still unclear to researchers whether the pattern of in situ N2O/(N2O + N2) ratios is consistent with those in laboratory assays (i.e., Ref 9, 10 and 13). The lack of data obtained at the field scale creates great uncertainties on the N2 fluxes and global N cycle estimated by the existing models. Therefore, it is crucial to clarify the pattern of N2O/(N2O + N2) ratios in the field, which can be used as a promising tool to accurately estimate N2 fluxes and denitrification at the ecosystem scale.
Forests cover 31.7% of global land and play a vital role in regulating the N cycle and global climates [29]. Soil N2 and N2O emissions are mainly mediated by microorganisms. Environmental factors, such as N and carbon (C) availability and soil moisture, influence the populations and activities of nitrifier and denitrifier, consequently causing the changes in N2 and N2O emissions and N2O/(N2O + N2) ratios [21]. Studies on factors controlling N2O emissions from forest soils have attracted a great attention due to their global warming effect. In addition, previous studies indicated that N2O and N2 could be simultaneously emitted from the same soil aggregate because of the development of aerobic and anaerobic environments [13]. Thus, factors that control N2O emissions also regulate N2 emissions. For example, exogenous N addition in a tropical forest reduced soil N2O emissions but had an enhancing effect on N2 emissions under anaerobic conditions [30]; the snowmelt process promoted soil N2 and N2O emissions in a northern hardwood forest [9]. These studies suggest that the created anaerobic microsites in soil can enhance the proportion of denitrification to nitrification affecting the pattern of N2 and N2O emissions. Soil moisture may be a key driver in the development of the anaerobic conditions and the relative proportion of N2 and N2O emissions in the field since water controls prerequisite conditions (i.e., NO3− and O2 availability) for the occurrence of nitrification and denitrification [7,31]. Moreover, topography can affect denitrification rates by changing the distribution of the substrate (NO3− or C) in soil water [32]. However, the effects of soil water on N2 emissions and N2O/(N2O + N2) ratios remain unclear in the field under complex topography.
The purpose of the work reported was to evaluate the influence of slope position and associated soil moisture conditions on rates and ratios of N2O and N2 emissions in a temperate forest in Northeast China by employing an in situ 15N labeling technique. The specific objectives of the study were to: (1) Use an in situ 15N labeling method to determine soil N2 and N2O emission rates across three slopes (analogous to three moisture conditions) from a temperate forest in Northeast China; (2) explore the controlling factors of N2O/(N2O + N2) ratios; and (3) estimate the N2 flux at the current ecosystem scale by measuring N2O/(N2O + N2) ratios and N2O fluxes. We hypothesized that lower slopes would have greater conversion rates of N2O to N2 and lower N2O/(N2O + N2) ratios due to higher soil moisture condition.
2. Materials and Methods
2.1. Site Description
This study was conducted at the Qingyuan Forest Station (124°54′32.6″ E, 41°51′6.1″ N, 500–1100 m elevation) of Chinese Ecosystem Research Network (Qingyuan Forest CERN), located in the Liaoning Province, Northeast China (Figure 1). The climate of this region is a continental monsoon type. In this station, the annual average precipitation from 2014 to 2020 is 666 mm with approximately 80% falling during the growing season from April to October, and the annual average temperature is 4.7 °C [33,34]. Total inorganic N deposition (TIN) in precipitation at the study site is 15 to 21 kg ha−1 yr−1 (during 2014 to 2016) with NH4+ contributing 65% of the N input [34,35].
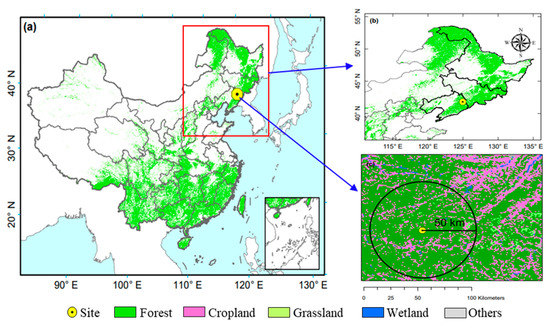
Figure 1.
Location of Qingyuan Forest station in Northeast China. (a,b) present the location of Qingyuan Forest station in the map of China; (c) presents the main land use types within a 50-km radius of Qingyuan Forest station.
The study site was originally occupied by a primary mixed broadleaved–Korean pine forest until the 1930s. Subsequently, the original forest was destroyed by a large fire in the early 1950s and replaced by a mixture of naturally regenerating broadleaved native tree species [36]. This natural secondary forest consists of Quercus mongolica, Juglans mandshurica, Fraxinus mandschurica, Phellodendron amurense, and Larix olgensis in the tree layer; Syringa amurensis, Acer tegmentosum, and Fraxinus rhynchophylla in the understory; and Equisetum hyemale, Arisaema amurense, and Polygonatum involucratum in the herbaceous layer [37]. In this forest, soil with the depth of 60 to 80 cm is developed from granite gneiss and categorized into Udalfs according to the definition of the second edition of U.S. Soil Taxonomy (1999) [36]. The soil texture in 0–10 cm mineral layer is clay loam (17.2% sand, 52.8% silt, and 30% clay) with a soil pH of 5.8, bulk density (BD) of 0.70 kg m−3, total organic carbon (TOC) of 2.1%, total nitrogen (TN) of 0.4%, and C/N ratio of 9.1 [34,38]. Due to the multiple effects of the topography and precipitation, the soil in lower places is usually in a water-saturated state compared with that in higher places, where soil properties can change with slope position in forests (Table 1).

Table 1.
Physical–chemical properties of the 0–20 cm mineral soil layer at different slope positions in the mixed forest of Qingyuan station in Northeast China.
2.2. Field 15N Labeling Experiment
To determine in situ soil N2 and N2O emissions, static chamber and 15N gas flux methods were adopted for rate measurements. In June 2015, one transect zone (site 1) along a 20 m (width) × 35 m (length) slope was chosen and divided into lower (20 m × 13 m), middle (20 m × 12 m), and upper (20 m × 10 m) slopes with three distinctive soil moisture conditions: high, intermediate, and low, respectively (Figure 2 and Figure S1). At each slope zone, four 2 m × 3 m plots were randomly established, and a collar of stainless steel (basal area 0.09 m2, 30 cm × 30 cm) was randomly anchored into the soil at 10 cm depth in each plot (Figure 2). All collars were finished one week before soil 15N labeling. The collar had a square groove of 3 cm × 3 cm depth for matching the stainless steel chambers, and the square groove could provide a gas-tight seal when filled with water. The chambers were equipped with a 3-way sampling port and a 3 mm diameter pressure equilibration tube (15 cm long) on the preinstalled frames. Solutions of labeled Na15NO3 (99.26 atom% 15N, Shanghai Research Institute of Chemical Industry, Shanghai, China) were evenly injected into the soil within the collars at a rate of 2.5 g 15N m−2 (diluted in 900 mL DI water, through 144 injections) by a syringe with a 10 cm long needle. The area inside the collar was then sprinkled with 300 mL DI water to wash the residual labeled solution on the litter into the soil. Subsequently, the collars were covered with the chambers, and a 50 mL gas sample was taken from the headspace with a gas-tight syringe at the time points of 4, 7, 24 and 30 h after the 15N tracer injection. The gas samples (50 mL) were transferred into the Tedlar gas bags (100 mL). At the end of incubation, five soil cores (0–20 cm) in each chamber were collected with a stainless steel sampler (2.5 cm diameter, 50 cm length) and composited to one sample. Field ambient air samples and composite soil samples (0–20 cm) were taken at the beginning of the incubations near each collar (within 15 cm distance).
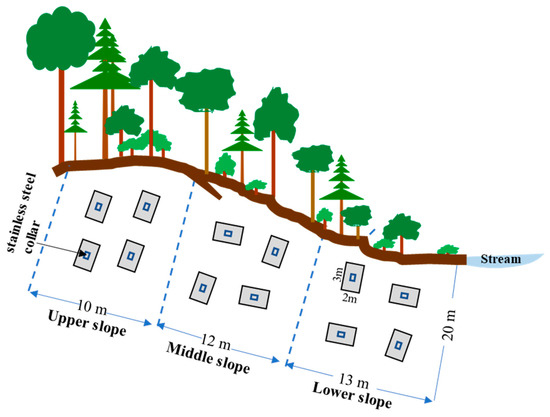
Figure 2.
Schematic diagram of experimental design along slopes within each site (3 sites) selected in the mixed forest of Qingyuan station in Northeast China. In total, three slopes in each site were selected and used for gas emission measurements.
In June 2017, an additional two transects (site 2 and site 3, respectively), where the aspect, vegetation composition and soil texture were similar to those in site 1, were established in the same forest and divided into lower, middle, and upper slopes as three different soil moisture zones. Using the same procedures as mentioned above, four stainless steel collars on each slope of site 1, site 2, and site 3 were randomly inserted into the soil one week before soil 15N labeling, and the same dose of 15N tracer solution was injected into the soil. The gas and soil samples were collected in the same way as described above.
2.3. Soil N2O, N2 Analysis and Flux Calculation
A 5 mL gas sample was injected into the gas chromatography for analyzing N2O concentrations (C). The gas chromatography was fitted with a Porapak Q column (30 m length, 0.53 mm id) and equipped with an electron capture detector (ECD) (GC-2014, Shimadzu, Kyoto, Japan). Three standard N2O samples with concentrations of 0.35, 5, 20 ppm (National Center for Standard Matters, Beijing, China) were used to calibrate the sample N2O concentration. The bulk N2O flux (, µg∙m−2∙h−1) was calculated from the linear change in N2O concentration over time and the following Equation (1):
where bulk N2O flux includes the fluxes from 15N-labeled and non-labeled sources; is the N2O concentration in the mixed gases at the incubation time T (ppm); ρN2O is the N2O density at an air temperature of 20 °C in the field; V and A are the volume (m3) and basal area (m2) of the chamber.
The enrichment of 15N in N2O was measured by an IsoPrime trace gas analyzer (TG) coupled with an auto-sampler of 112 plots (Gilson GX-271, Dunstable, UK) and a continuous-flow isotope ratio mass spectrometer (IRMS, IsoPrime 100, Cheadle, UK). The peak areas for major (44N2O), minor 1 (45N2O), and minor 2 (46N2O) from IRMS, as well as the ratios 45R (45N2O/44N2O) and 46R (46N2O/44N2O), were reported in all the gas samples from enriched ( = 4, 7, 24, 30 h) and ambient air samples (). Previous studies showed that the non-random 15N distribution in N2O was observed due to the high 15N enrichment in the source pool [8]. Hence, the 15N enrichment of N2O () at each time point was calculated according to the ratios of 45R and 46R with the following Equation (2), assuming 17R (17O/16O) = 3.8861 × 10−4 and 18R (18O/16O) = 2.0947 × 10−3 [8,39]. The 15N2O flux () was estimated through bulk N2O flux and the 15N difference of N2O enrichment between enriched and air samples. Furthermore, the N2O flux produced from denitrification () was calculated by dividing the 15N2O flux by 15N enrichment of the soil-labeled NO3− pool () [8,40].
The content and rate of N2 in samples were also determined using the TG-IRMS system. Gas sample (0.5 mL) was manually injected into the sample loop (50 μL) using a gas-tight syringe and the peak areas for major (28N2), minor 1 (29N2), and minor 2 (30N2) from IRMS as well as the ratios (29N2/28N2) and (30N2/28N2) were measured in both enriched ( = 4, 7, 24, 30 h) and ambient air samples (). According to the difference in the ratios and , respectively, between the sample and normal air, the 15N mole fraction () and 15N flux () of N2 at each time point were calculated using the following Equations (5) and (6). Then, the N2 flux produced from denitrification () at each time point was calculated using Equation (7) by dividing the 15N2 flux by 15N enrichment of soil NO3− pool. The total N2 flux () was estimated by dividing 15N flux by the 15N enrichment of N2O pool (), assuming that N2 production is from both denitrification-derived N2O and NH4+ oxidation-derived N2O [8].
where pN2 is the density of N2 at air temperature of 20 °C in the field; V and A are the volume (m3) and basal area (m2) of the chamber.
We did not directly measure the 15N abundance of soil NO3− pool in this study. However, it can be calculated based on the ratios of and in N2O, assuming that there is the same uniformly 15N-labeled pool of NO3− for 15N2O and 15N2 [41]. The detailed calculations can be found in Buchen et al. [40] and Spott et al. [42]. We further calculated N2O/(N2O + N2) ratios from denitrification (RN2O) according to N2Odenitrification and N2denitrification fluxes. The denitrification rates were present by the sum of N2Odenitrification plus N2denitrification fluxes. The total N2O/(N2O + N2) ratios (RN2O-total) were also calculated by total N2 and bulk N2O fluxes.
In this study, we defined three times the standard deviation as the minimum detectable change for N2 and N2O measurements [8]. Ultrahigh-purity N2 gas (−1.63‰ δ15N) was manually injected (n = 60), and N2O standard gas (0.37 ppm, n = 30) was automatically injected, in every batch as quality controls. The detection limit was 4.6 × 10−7 for , 3.9 × 10−7 for , 0.11‰ for δ15N or 4 × 10−5 atom% 15N in N2, and 7.8 × 10−6 for , 3.8 × 10−5 for in N2O. These values were applied to determine if the sample at each time point was significantly different from reference samples (T = 0 h), and if not, they were defined as having no 15N-N2 or N2O production at this time point.
2.4. Soil Parameter Analysis
The soil samples were analyzed for bulk density (BD), soil moisture, pH, total nitrogen (TN), total organic carbon (TOC), and inorganic nitrogen (NH4+, NO3−) contents. Soil NH4+ and NO3− were extracted with 2 M KCl and measured by an auto discrete analyzer (Smartchem 200, Rome, Italy). The soil pH was determined by a pH meter (PHS-3E, INESA Scientific Instrument Co., Ltd., Shanghai, China) in a 1:2.5 soil water suspension. The TN and TOC contents were determined using an elemental analyzer (Micro Isotope Cube, Langenselbold, Germany). Soil BD was measured using a known volume metal container. Soil gravity water content (SGWC) was quantified by drying in oven at 105 °C for 24 h to a constant weight. The corresponding soil water-filled pore space (WFPS) was calculated based on BD and SGWC (). The main soil characteristics were shown in Table 1.
2.5. Statistical Analyses
Prior to statistical analysis, data were checked for normality and homogeneity of variance with the Kolmogorov–Smirnov test and Levene’s test, respectively. All statistical analyses were performed with SPSS software (Version 16.0; SPSS Inc., Chicago, IL, USA). One-way ANOVA was used to check the differences in rates of N2 and N2O emissions, N2O/(N2O + N2) ratios, and soil properties among slopes followed by multiple comparisons using the LSD method. Paired-samples t-test was applied to determine the differences in soil moisture, NH4+, and NO3− contents between before and after 15N labeled incubation. The relationships between rates and ratios of gaseous N emissions and soil properties were examined using Pearson correlation analysis and regression analysis. To identify the dominant factors regulating soil N2 and N2O emissions as well as N2O/(N2O + N2) ratios, a stepwise regression analysis was conducted. The statistically significant differences were set at a 0.05 level unless otherwise stated.
3. Results
3.1. Soil Physical-Chemical Parameters
The soil water-filled pore space (WFPS) varied from 28% to 128% and was distinctly different among slopes, with higher values at the lower slope (Table 1). In contrast, soil pH had no significant variation among slopes, except site 1 in 2015. The total average soil BD increased from the lower (0.47 ± 0.03 g cm−3) to the upper slope (0.59 ± 0.02 g cm−3). Higher average contents of TOC, TN, NH4+ and ratios of C/N, NH4+/NO3− were observed at the lower slope. The total average NO3− contents were significantly higher at the upper and middle slopes than at the lower slope, although there were no significant differences among slopes within sites (Table 1). After soil 15N was labeled for 30 h, no significant differences were found in soil moisture and NH4+ content, except NO3− content (Figure S2).
3.2. Soil N2O and N2 Emissions
Soil bulk N2O emissions (except site 1 in 2015) peaked at 7 h and then decreased with incubation time (Figure 3A). The average bulk N2O emission rates from 7 to 30 h decreased more at the upper slope (62%) than the lower and middle slope (8% and 15%, respectively). However, the 15N2O emissions at the upper slope did not follow the same pattern as bulk N2O emissions (Figure 3B). Over the 30 h incubation, the mean bulk N2O fluxes varied significantly across slopes, with higher values at the lower slope (Figure 4A, Table 2). A similar pattern was also observed in the denitrification-derived N2O fluxes (Figure 4B).
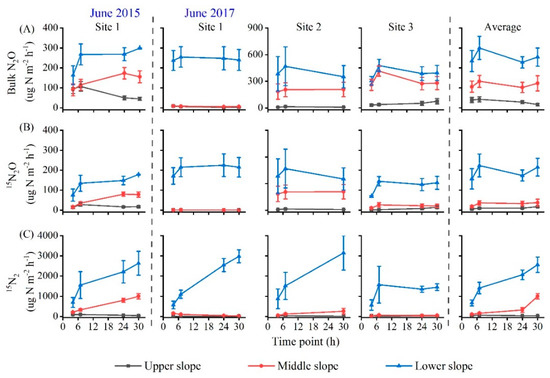
Figure 3.
Emission rates of soil bulk N2O (A), 15N2O (B) and 15N2 (C) with incubation time after in situ 15NO3− addition (2.5 g 15N m−2) at different slope positions in the mixed forest of Qingyuan station in Northeast China. Values from different sites (1, 2, 3) and the overall average are shown.
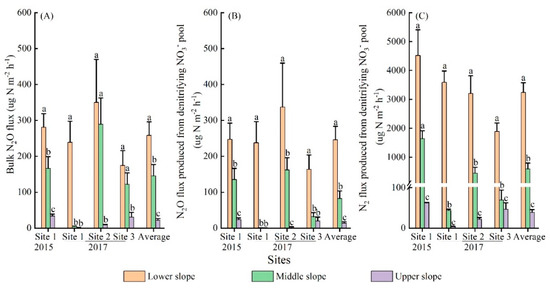
Figure 4.
Mean fluxes of bulk N2O (A), N2O (B) and N2 (C) produced from denitrifying NO3− pool over a 30 h incubation after in situ 15NO3− addition (2.5 g 15N m−2) at different slope positions in the mixed forest of Qingyuan station in Northeast China. Values from different sites (1, 2, 3) and overall average are shown. Different lowercase letters indicate significant differences among slopes (p < 0.05).

Table 2.
The area-weighed N2 and N2O fluxes and ratios in the mixed forest of Qingyuan station in Northeast China.
Similar to 15N2O, the 15N2 emissions with incubation time at each slope position also had different patterns among sites (Figure 3C). The average 15N2 emissions at the lower and middle slopes significantly increased with incubation time, while a slight downward trend was observed at the upper slope (Figure 3C). Furthermore, the N2 flux produced from denitrifying NO3− pool (N2denitrification) varied from 4 to 4517 μg N m−2 h−1 among sites, being much higher than N2O emissions (Figure 4). A large variation in denitrification rate (N2 + N2O) was also observed among sites (Figure S3A). Overall, the mean fluxes of N2denitrification and total N2, as well as denitrification rates, significantly decreased from the lower to the upper slope (Table 2, Figure 4C and Figure S3A).
3.3. Soil N2O/(N2O + N2) Ratio
Significant variations in RN2O (ratio of N2Odenitrification to N2Odenitrification plus N2denitrification) among slopes were observed in all sites (Figure S3B). This ratio at the lower slope was significantly lower than those at the middle and upper slopes (Table 2). Based on the areas of different soil moisture zones occupied in the study forest, the weighted average RN2O was 0.16 ± 0.02, being similar to RN2O-total (ratio of bulk N2O to bulk N2O plus total N2, 0.15 ± 0.02) (Table 2). Further, the area-weighted RN2O and RN2O-total were much lower than the value of soil N2O/(N2O + N2) ratio (0.30) from natural forests recompiled by those previously reported literature (Table S1).
3.4. Relationships between Gaseous N Rates, Ratios, and Soil Properties
Soil N2, N2Odenitrification, bulk N2O and denitrification rates were all positively correlated with soil WFPS, TOC, C/N and NH4+/NO3− ratio, while they had a negative correlation with BD (Table 3). The RN2O was negatively correlated with soil WFPS and C/N ratio (Table 3). A stepwise regression analysis showed that soil WFPS was the key factor regulating N2denitrification, denitrification rate and RN2O, accounting for 63%, 63% and 13% of variation, respectively (Table 4). In contrast, the C/N and NH4+/NO3− ratios mainly affected the emissions of bulk N2O and N2Odenitrification (Table 4).

Table 3.
Pearson correlations between soil properties and fluxes, ratios of N2 and N2O emissions in the mixed forest of Qingyuan station in Northeast China.

Table 4.
Multiple linear regression models between fluxes, ratios of N2 and N2O from denitrification, bulk N2O flux and selected soil properties in the mixed forest of Qingyuan station in Northeast China.
4. Discussion
4.1. Variations of Soil N2 and N2O Emissions
The 15N isotope tracer technique allows us to determine in situ N2 as well as N2O emissions due to denitrification. In our study, soil N2 emissions were mainly produced by denitrification (area-weighted: 282 μg N m−2 h−1) and denitrification accounted for average 75% of total N2 emissions (area-weighted: 375 μg N m−2 h−1, Table 2), which was consistent with our previous laboratory findings at the same study site [37]. The mean N2 fluxes compared well with the rates reported from a woodland forest in the UK [28], a forested wetland and a northern hardwood forest in the US [7,27], using the similar 15N tracer approach. Our result was also comparable to the rates reported in other temperate forests and maize soils using the gas–flow soil core method [11,13]. Furthermore, the fluxes of both bulk N2O and N2Odenitrification were significantly lower in comparison with the N2 fluxes (Table 2, Figure 3 and Figure 4), which was consistent with the results reported from the 15N tracer studies in temperate forests [27], tropical forests [8,30], and other upland soils [43]. Therefore, our results, together with those of previous studies, suggest that N2 emissions are likely the main gaseous N loss from terrestrial ecosystems. Moreover, the total denitrification rate (N2 + N2O) (54 to 3488 μg N m−2 h−1, Table 2) was in the range reported in the laboratory 15N tracer assay [7]. In contrast, this rate was significantly higher than those in other temperate forests from in situ 15N tracer studies [27,28]. This may be partly explained by the differences in NO3− and water input. In previous studies, the added NO3− and water only adjusted within 10% and 5% of the ambient soil NO3− pool and volumetric water, respectively. In our study, however, soil–extracted NO3− significantly increased by more than 5 times (Figure S2C). Moreover, about 11 mm precipitation was deposited into the soil, potentially leading to a 10% increase in soil volumetric, although no significant change was observed in soil gravity water content (Figure S2A). One laboratory 15N study from a tropical rainforest showed an increase in N2O emission with increasing soil NO3− [8]. Additionally, high water input to soil may promote the formation of an anaerobic environment, potentially promoting the further reduction of the produced N2O to N2 during denitrification [30]. The significantly higher N2 rather than N2O flux from denitrification, was confirmed in our study (Table 2, Figure 3 and Figure 4).
In this study, we found the highest N2 and N2O emissions or denitrification rates occurring at the lower slope (Table 2, Figure 4 and Figure S3A). This was partly in line with our expectations. Topography plays an important role in the redistribution of water and substrates (available N or C) which can influence gaseous N emissions [17]. Substrates are prone to accumulate in the bottom position with the transportation of water [31,44]. In the present study, the lower slope had higher soil WFPS, TOC, TN, NH4+ contents and lower soil BD compared to the upper slope, and the variation in soil WFPS across slopes was significantly larger than other soil parameters (Table 1). Previous studies indicated that soil water could affect the production of N gas from nitrification and denitrification by regulating O2 and/or substrate availability [31,45]. In this study, we found that soil WFPS was significantly positively correlated with TOC and C/N ratio (Table S2). Furthermore, good positive relationships between soil WFPS and N2denitrification flux, denitrification rate were observed, although soil BD had a negative contribution to denitrification rate and N2denitrification flux (Table 4). It suggests that the change in soil water associated with bulk density is important factor driving the difference in denitrification across slopes. Additionally, it was noted that the underestimated N2O and N2 fluxes may occur in the current study due to the gas diffusion from soil surface to subsoil. Previous study demonstrated a large underestimation of denitrification rate from the soil surface using static chamber method and the underestimated extent could decrease with the increase in soil moisture [46]. In the present study, we speculated that the N2 and N2O fluxes measured at the lower slope were likely to reflect real values since soil water was oversaturated in this location (110% WFPS). However, these fluxes will be significantly underestimated at the middle and upper slopes, where soil WFPS were 40% and 60%, respectively, slightly lowering the values reported by Well et al. [46]. Nevertheless, our field results are consistent with previous laboratory findings, where high soil moisture corresponded to high N2 and N2O emissions [7,9]. These results confirm that the change in soil moisture could moderate the variations of N2 and N2O emissions.
In addition to soil moisture, the relative proportion of N2 and N2O was affected by N substrates [7,8]. The N availability, such as NO3− and NH4+, is the primary requirement for denitrification and nitrification [17]. The high nitrification rates and NO3− contents were reported at lower slope sites in a temperate coniferous forest [44]. Previous assays indicated the simultaneous increase in N2 and N2O production with the increase in NO3− concentration [8,12]. However, our study showed a high NO3− content at the middle and upper slopes, which did not have the highest gaseous N emissions (Table 1, Figure 4). Alternatively, we found that soil N2O emissions were affected by the NH4+/NO3− ratio (Table 3 and Table 4), which was inconsistent with previous findings. It was likely that the relative proportion of substrate N species in comparison to their contents may be more important to N2O rather than N2 emissions. Moreover, the C/N ratio significantly affected soil bulk N2O and N2Odenitrification fluxes (Table 4), although soil TOC, C/N, or bulk density was individually observed to correlate with these fluxes (Table 3). These relationships indicate that the changes in soil parameters may have the different effects on N2 and N2O emissions. In addition, the temporal variation in N2O and N2 emissions was larger at the upper and middle slopes within the site (Figure 4). This suggests that the in situ denitrification rates may change with the seasons or years [28]. Therefore, the dynamics of in situ soil N2 and N2O fluxes should be addressed in the future.
4.2. Comparison of Soil N2O/(N2O + N2) Ratios from Denitrification
Spatial variations of N2 and N2O emissions in this study were expected. Commonly measured N2O fluxes are widely available, yet to accurately estimate denitrification rates requires a better understanding of the controls on N2O/(N2O + N2) ratios from denitrification (RN2O). In the current study, lower RN2O were associated with high soil moisture (Table 2, Figure S3B). Similar findings were also observed from other temperate forests in laboratory assays using 15N labeling [7] or the direct gas flux method [11]. Increasing soil moisture will decrease O2 diffusion, most likely forming anaerobic conditions for denitrification, and it is expected that the RN2O will decrease [47]. Besides, NO3− availability, as the electron acceptor of denitrification, also affected its product ratio [13]. However, our results demonstrated that this ratio was mainly regulated by soil moisture (Table 4), further supporting our previous hypothesis. Moreover, the area-weighted average RN2O from the entire forest (0.16, Table 2) was similar to the value from the 15N gas flux method reviewed by Scheer et al. [24]. In contrast, our result was significantly lower than previously reviewed global ratios in upland soils based on approaches of 15N trace and acetylene inhibition [16], while it was higher compared to previous field 15N trace studies on temperate forests (e.g., 0.008 and 0.01 in Refs. [27,28], respectively). We further calculated the RN2O from forest soils, with an average value of 0.30 (Table S1), by compiling these data from previous research [16,24] and our study. This reflected the great spatial variability of RN2O, and its dynamic change urgently requires to be clarified. Nevertheless, these results suggest that N2 fluxes from forest ecosystems are likely to be underestimated by around one-third when using the N2O/(N2O + N2) ratio in nature soils reported by Schlesinger [16].
Previous studies indicated the variations in the N2O/(N2O + N2) ratio due to the difference in N2 measurement among methods [41]. Based on the literature compiled with the current study, we observed a significant difference in N2O/(N2O + N2) ratios among methods, with the highest values observed by the acetylene inhibition method and the lowest values by the 15N tracer method (Figure S4). As reported in previous studies, each method has a special application range and its advantage or disadvantage [11,48]. For instance, the 15N-gas flux method requires reasonable amounts of labeled NO3− introduced to soil systems, which may disturb the soil micro−environment and stimulate microbial N turnover [11], affecting soil N2 emission. Therefore, the approach used in this study also had some bias for N2 measurement. These bias may be linked to the amount of added NO3− and water, labeled soil depth, enclosure time and volume of the chamber, and even the precision of the instrument [8,41,46,48]. In our study, soil NO3− and moisture were higher than the common conditions (Figure S2A,C), although a high NO3− content (i.e., >20 mg N kg−1) was often observed in some natural temperate forests in the same region as our study [49]. As discussed above, the potential interaction of NO3− and water regulated the proportion of N2 and N2O emissions, leading to a lower N2O/(N2O + N2) ratio than the real ratios. As a result, it was likely to overestimate N2 emission and underestimate RN2O. In the preliminary experiment, we did not find a significant change in the δ15N-N2 value between the labeled sample (24 h) and ambient air (0 h) after the soil was labeled with 15NO3− solution at a rate of 0.25 g 15N m−2 (data not shown). Furthermore, an unknown proportion of downward diffusion of 15N−labeled gases to the non−labeled subsoil might cause the bias in N2 and N2O fluxes [41,46]. The extend closure time of chamber would further add the variations. Although the fluxes of 15N2 and 15N2O at all the slopes were found to increase during 30 h incubation (Figure 3B,C), the 15N gas diffusion to the subsoil (below 10 cm) could occur, especially on the middle and upper slopes with larger air-filled porosity (Table 1). Moreover, the large volume of the chamber (18 L) likely diluted the produced 15N2 with air 14N. Consequently, it could lead to an underestimation of N2 fluxes. These were thus responsible for the observation of low or undetectable N2 flux explained in part due to these effects (Figure S5). In addition, quantifying the limit of detection for the 15N gas flux method is still a challenge in low N2 emissions in nature forest ecosystems, although the detection sensitivity of our IRMS (0.79 μg 15N m−2 h−1) is better compared to previous studies [8,27,28]. As addressed above, undoubtedly, there are still some other bias regarding the accuracy of N2 loss due to the interactions of multiple and unpredictable factors. Therefore, some improvements could be explored to reduce these bias for the in situ 15N tracer method. For example, one way to improve detection is to reduce the N2 background concentration in the field by flushing chambers with a N2–free air [20]. Additionally, based on this, shorting chamber height and sampling time can further improve the N2 analytical accuracy. Overall, more field studies on N2 emissions and the N2O/(N2O + N2) ratios from forest ecosystems with 15N tracer method are required in the future.
4.3. Implications for Ecosystem N Loss
In this study, about 5.1 kg N ha−1 yr−1 of total N2 flux was estimated based on the area–weighted total N2O/(N2O + N2) ratio (0.15, Table 2) and the field average N2O flux with 0.90 kg N ha−1 yr−1 monitored in 2019 to 2021 (Huang K. et al., unpublished). The actual N2 fluxes were probably higher than this estimate because the chambers for monitoring field N2O flux were mostly set at the upper and middle slope positions, where N2O emissions were relatively lower than those at the lower slope position (Table 2). The total gaseous N emissions (N2 + N2O) were calculated at about 6.0 kg N ha−1 yr−1, accounting for 30% of the inorganic N (NH4+ plus NO3−, 20 kg N ha−1 yr−1) deposition in precipitation, as reported in this study forest by Huang et al. [35]. This result was comparable to those from Hawaiian rainforests [50,51] and temperate forests [52] estimated by the natural nitrogen and/or oxygen stable isotope approach. In all, these findings suggest that the gaseous N losses from the forest ecosystems may be larger than commonly thought, and the N cycle is likely to be more open.
The present study still have some uncertainties in estimating soil N2 flux with measured N2O/(N2O + N2) ratio, including the bias from the 15N–gas flux method as mentioned above. For instance, previous studies indicated the variations in N2O/(N2O + N2) ratios during different growing periods [26,28]. In the current study, we carried out the 15N trace experiment in the middle period of the growing season (April to October) and monitored the N gas emission once. In this period, soil temperature was high which stimulated microbial activity to consume considerable substrates for nitrification and denitrification. These processes in turn promoted gaseous N losses from soil. In the early or later phase, however, plant growth and microbial activities may decrease due to temperature limitations. Low available substrates could affect microbial enzyme activity and community composition, causing an increase or decrease in N2O/(N2O + N2) ratios [17]. As a result, the calculated N2 loss was under– or over–estimated. Moreover, the change in soil moisture could affect the diffusion of N2 and N2O produced in soil pore space [41], leading to the differences in the N2O/(N2O + N2) ratios among different periods. It was found that field soil WFPS changed from 24% to 73% throughout the growing season in our study site [34]. Previous studies showed a low N2O/(N2O + N2) ratio with high soil moisture [7,15], being similar to our result. The effect extent of soil moisture on N2O/(N2O + N2) ratio remains unclear and should be further clarified in the following studies. Additionally, the unquantified other microbial processes from N2 and N2O emissions are also contributed to the uncertainty in N2O/(N2O + N2) ratios. More field research on differentiating production pathways of N2 and N2O emissions from forest ecosystems are urgently required for more robust estimates of gaseous N losses [53].
5. Conclusions
The results of this study showed that in situ soil N2 and N2O emissions significantly varied across slopes and were strongly affected by soil moisture. Soil N2 emissions were significantly higher compared to N2O emissions, accounting for 85% of gaseous N losses (N2 + N2O). The combined field N2O flux and N2O/(N2O + N2) ratio is likely to be a promising tool to quantify soil N2 flux and even denitrification rate for a given forest ecosystem. In addition, we recognize that there are some bias for determining in situ soil N2 emission and therefore the calculated N2O/(N2O + N2) ratio in the current 15N trace study, such as the added 15NO3− amount, enclosure time of the chamber, and gas diffusion to the subsoil. Overall, to further elucidate the dynamic of soil N2 and N2O emissions associated with N2O/(N2O + N2) ratios, in situ 15N–labeled experiments in different seasons from different forest ecosystems should be performed. Meanwhile, we also need to refine and combine established methodologies using models for better predicting ecosystem denitrification rate and gaseous N losses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13091347/s1, Figure S1: The sampling photos at the three slope positions in site 1 in the mixed forest of Qingyuan station in Northeast China; Figure S2: Changes of 0–20 cm in soil moisture (A, express as gravity water content), ammonium (NH4+, B), and nitrate (NO3−, C) contents before and after 15N labeling at different slope positions in the mixed forest of Qingyuan station in Northeast China. Differences were analyzed by paired-samples t-test (p < 0.05); Figure S3: The rates (A) and N2O(N2O + N2) ratios (B) from denitrification over a 30 h incubation after in situ 15NO3− addition (2.5 g 15N m−2) at different slope positions in the mixed forest of Qingyuan station in Northeast China. Values from different sites (1, 2, 3) and overall average were shown. Different lowercase letters indicate significant differences among slopes (p < 0.05); Figure S4. Comparison of N2O/(N2O + N2) ratios obtained by different methods for forest soils. Figure S5: Changes of δ15N-N2 (‰) value with incubation time at the lower (A), middle (B), and upper (C) slope in site 1, respectively, after soil was labeled with NO3− addition (2.5 g 15N m−2) in the mixed forest of Qingyuan station in Northeast China. The red dotted line indicates the minimum detectable change for δ15N (0.11‰) and the error bars represent standard errors; Table S1: Recompilation of the values of N2O/(N2 + N2O) ratio in natural forest soils; Table S2: Pearson correlation between soil properties in the mixed forest of Qingyuan station in Northeast China.
Author Contributions
Conceptualization, Y.F. and W.Z.; Investigation, D.X.; Writing—original draft, D.X.; Writing—review and editing, Y.F. and W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Key Research and Development Program of China (Grant Nos. 2016YFA0600802, 2017YFC0212704-01), the National Natural Science Foundation of China (Grant Nos. 41703068, 41773094), the Key Research Program of Frontier Sciences of Chinese Academy of Sciences (Grant No. QYZDB-SSW-DQC002), Research and Development Project of Scientific Instruments and Equipment of Chinese academy of sciences (Grant No. YJKYYQ20190054), and the Project of Forestry Peak Discipline at Fujian Agriculture and Forestry University, China (Grant No. 118—71201800724).
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
We greatly thank Shasha Zhang, Shanlong Li, Xiaoming Fang, Shaonan Huang, for their help on the field gas sampling, Feifei Zhu, Dongwei Liu, and Kai Huang for suggestions on data analysis and the presentation of results of the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Green, P.A.; Holland, E.A. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Stange, C.F.; Spott, O.; Arriaga, H.; Menendez, S.; Estavillo, J.M.; Merino, P. Use of the inverse abundance approach to identify the sources of NO and N2O release from Spanish forest soils under oxic and hypoxic conditions. Soil Biol. Biochem. 2013, 57, 451–458. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Myrold, D.D.; Firestone, M.; Voytek, M. Environmental controls on denitrifying communities and denitrification rates: Insights from molecular methods. Ecol. Appl. 2006, 16, 2143–2152. [Google Scholar] [CrossRef]
- Groffman, P.M.; Altabet, M.A.; Böhlke, J.K.; Butterbach-Bahl, K.; David, M.B.; Firestone, M.K.; Giblin, A.E.; Kana, T.M.; Nielsen, L.P.; Voytek, M.A. Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecol. Appl. 2006, 16, 2091–2122. [Google Scholar] [CrossRef]
- Yoshinari, T.; Hynes, R.; Knowles, R. Acetylene inhibition of nitrous oxide reduction and measurement of denitrification and nitrogen fixation in soil. Soil Biol. Biochem. 1977, 9, 177–183. [Google Scholar] [CrossRef]
- Morse, J.L.; Bernhardt, E.S. Using 15N tracers to estimate N2O and N2 emissions from nitrification and denitrification in coastal plain wetlands under contrasting land-uses. Soil Biol. Biochem. 2013, 57, 635–643. [Google Scholar] [CrossRef]
- Yang, W.H.; McDowell, A.C.; Brooks, P.D.; Silver, W.L. New high precision approach for measuring 15N-N2 gas fluxes from terrestrial ecosystems. Soil Biol. Biochem. 2014, 69, 234–241. [Google Scholar] [CrossRef]
- Morse, J.L.; Durán, J.; Groffman, P.M. Soil denitrification fluxes in a northern Hardwood forest: The importance of snowmelt and implications for ecosystem N budgets. Ecosystems 2015, 18, 520–532. [Google Scholar] [CrossRef]
- Morse, J.L.; Durán, J.; Beall, F.; Enanga, E.M.; Creed, I.F.; Fernandez, I.; Groffman, P.M. Soil denitrification fluxes from three northeastern North American forests across a range of nitrogen deposition. Oecologia 2015, 177, 17–27. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Willibald, G.; Papen, H. Soil core method for direct simultaneous determination of N2 and N2O emissions from forest soils. Plant Soil 2002, 240, 105–116. [Google Scholar] [CrossRef]
- Wang, R.; Feng, Q.; Liao, T.T.; Zheng, X.H.; Butterbach-Bahl, K.; Zhang, W.; Jin, C.Y. Effects of nitrate concentration on the denitrification potential of a calcic cambisol and its fractions of N2, N2O and NO. Plant Soil 2013, 363, 175–189. [Google Scholar] [CrossRef]
- Wang, R.; Pan, Z.L.; Zheng, X.H.; Ju, X.T.; Yao, Z.S.; Butterbach-Bahl, K.; Zhang, C.; Wei, H.H.; Huang, B.X. Using field-measured soil N2O fluxes and laboratory scale parameterization of N2O/(N2O + N2) ratios to quantify field-scale soil N2 emissions. Soil Biol. Biochem. 2020, 148, 107904. [Google Scholar] [CrossRef]
- Boyer, E.W.; Alexander, R.B.; Parton, W.J.; Li, C.S.; Butterbach-Bahl, K.; Donner, S.D.; Skaggs, R.W.; Del Gross, S.J. Modeling denitrification in terrestrial and aquatic ecosystems at regional scales. Ecol. Appl. 2006, 16, 2123–2142. [Google Scholar] [CrossRef]
- Weier, K.L.; Doran, J.W.; Power, J.F.; Walters, D.T. Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water; available carbon; and nitrate. Soil Sci. Soc. Am. J. 1993, 57, 66–72. [Google Scholar] [CrossRef]
- Schlesinger, W.H. On the fate of anthropogenic nitrogen. Proc. Natl. Acad. Sci. USA 2009, 106, 203–208. [Google Scholar] [CrossRef]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.G.; Zaman, M.; Tillman, R.W. Denitrification and N2O:N2 production in temperate grasslands: Processes; measurements; modelling and mitigating negative impacts. Sci. Total Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Groffman, P.M.; Yavitt, J.B.; Goodale, C.L. Complex controls of denitrification at ecosystem; landscape and regional scales in northern hardwood forests. Ecol. Model. 2015, 298, 39–52. [Google Scholar] [CrossRef]
- Liptzin, D.; Silver, W.L. Spatial patterns in oxygen and redox sensitive biogeochemistry in tropical forest soils. Ecosphere 2015, 6, 211. [Google Scholar] [CrossRef]
- Well, R.; Burkart, S.; Giesemann, A.; Grosz, B.; Koester, J.R.; Lewicka-Szczebak, D. Improvement of the 15N gas flux method for in situ measurement of soil denitrification and its product stoichiometry. Rapid Commun. Mass Spectrom. 2019, 33, 437–448. [Google Scholar] [CrossRef]
- Burgin, A.J.; Groffman, P.M. Soil O2 controls denitrification rates and N2O yield in a riparian wetland. J. Geophys. Res. 2012, 117, 1010. [Google Scholar] [CrossRef]
- Lai, T.V.; Denton, M.D. N2O and N2 emissions from denitrification respond differently to temperature and nitrogen supply. J. Soils Sediment 2018, 18, 1548–1557. [Google Scholar] [CrossRef]
- Senbayram, M.; Well, R.; Bol, R.; Chadwick, D.R.; Jones, D.L.; Wu, D. Interaction of straw amendment and soil NO3− content controls fungal denitrification and denitrification product stoichiometry in a sandy soil. Soil Biol. Biochem. 2018, 126, 204–212. [Google Scholar] [CrossRef]
- Scheer, C.; Fuchs, K.; Pelster, D.E.; Butterbach-Bahl, K. Estimating global terrestrial denitrification from measured N2O:(N2O + N2) product ratios. Curr. Opin. Env. Sust. 2020, 47, 72–80. [Google Scholar] [CrossRef]
- Mogge, B.; Kaiser, E.A.; Munch, J.C. Nitrous oxide emissions and denitrification N-losses from forest soils in the Bornhöved Lake region (Northern Germany). Soil Biol. Biochem. 1998, 30, 703–710. [Google Scholar] [CrossRef]
- Bai, E.; Li, W.; Li, S.L.; Sun, J.F.; Peng, B.; Dai, W.W.; Jiang, P.; Han, S.J. Pulse increase of soil N2O emission in response to N addition in a temperate forest on Mt Changbai; Northeast China. PLoS ONE 2014, 9, e102765. [Google Scholar]
- Kulkarni, M.V.; Burgin, A.J.; Groffman, P.M.; Yavitt, J.B. Direct flux and 15N tracer methods for measuring denitrification in forest soils. Biogeochemistry 2014, 117, 359–373. [Google Scholar] [CrossRef]
- Sgouridis, F.; Ullah, S. Relative magnitude and controls of in situ N2 and N2O fluxes due to denitrification in natural and seminatural terrestrial ecosystems using 15N tracers. Environ. Sci. Technol. 2015, 49, 14110–14119. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.A. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Tang, W.G.; Chen, D.X.; Phillips, O.L.; Liu, X.; Zhou, Z.; Li, Y.D.; Xi, D.; Zhu, F.F.; Fang, J.Y.; Zhang, L.M.; et al. Effects of long-term increased N deposition on tropical montane forest soil N2 and N2O emissions. Soil Biol. Biochem. 2018, 126, 194–203. [Google Scholar] [CrossRef]
- Friedl, J.; Scheer, C.; Rowlings, D.W.; McIntosh, H.V.; Strazzabosco, A.; Warner, D.I.; Grace, P.R. Denitrification losses from an intensively managed sub-tropical pasture–Impact of soil moisture on the partitioning of N2 and N2O emissions. Soil Biol. Biochem. 2016, 92, 58–66. [Google Scholar] [CrossRef]
- Luo, J.; Tillman, R.W.; Ball, P.R. Nitrogen loss through denitrification in a soil under pasture in New Zealand. Soil Biol. Biochem. 2000, 32, 497–509. [Google Scholar] [CrossRef]
- Zhu, J.J.; Mao, Z.H.; Hu, L.L.; Zhang, J.X. Plant diversity of secondary forests in response to anthropogenic disturbance levels in montane regions of northeastern China. J. Forest Res. 2007, 12, 403–416. [Google Scholar] [CrossRef]
- Huang, K.; Su, C.X.; Liu, D.W.; Duan, Y.H.; Kang, R.H.; Yu, H.M.; Liu, Y.Q.; Li, X.; Gurmesa, G.A.; Quan, Z.; et al. A strong temperature dependence of soil nitric oxide emission from a temperate forest in Northeast China. Agric. For. Meteorol. 2022, 323, 109035. [Google Scholar] [CrossRef]
- Huang, S.N.; Elliott, M.M.; Felix, J.D.; Pan, Y.P.; Liu, D.W.; Li, S.L.; Li, Z.J.; Zhu, F.F.; Zhang, N.; Fu, P.P.; et al. Seasonal pattern of ammonium 15N natural abundance in precipitation at a rural forested site and implications for NH3 source partitioning. Environ. Pollut. 2019, 247, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, J.J.; Yan, Q.L.; Sun, O.J. Changes in soil P chemistry as affected by conversion of natural secondary forests to larch plantations. Forest Ecol. Manag. 2010, 260, 422–428. [Google Scholar] [CrossRef]
- Xi, D.; Bai, R.; Zhang, L.M.; Fang, Y.T. Contribution of anammox to nitrogen removal in two temperate forest soils. Appl. Environ. Microbiol. 2016, 82, 4602–4612. [Google Scholar] [CrossRef]
- Li, S.L.; Gurmesa, G.A.; Zhu, W.X.; Gundersen, R.P.; Zhang, S.S.; Xi, D.; Huang, S.N.; Wang, A.; Zhu, F.F.; Jiang, Y.; et al. Fate of atmospherically deposited NH4+ and NO3− in two temperate forests in China: Temporal pattern and redistribution. Ecol. Appl. 2019, 29, e01920. [Google Scholar] [CrossRef]
- Kaiser, J.; Röckmann, T.; Brenninkmeijer, C.A.M. Complete and accurate mass spectrometric isotope analysis of tropospheric nitrous oxide. J. Geophys. Res. 2003, 108, 4476. [Google Scholar] [CrossRef]
- Buchen, C.; Lewicka-Szczebak, D.; Fuss, R.; Helfrich, M.; Flessa, H.; Well, R. Fluxes of N2 and N2O and contributing processes in summer after grassland renewal and grassland conversion to maize cropping on a Plaggic Anthrosol and a Histic Gleysol. Soil Biol. Biochem. 2016, 101, 6–19. [Google Scholar] [CrossRef]
- Sgouridis, F.; Stott, A.; Ullah, S. Application of the 15N gas-flux method for measuring in situ N2 and N2O fluxes due to denitrification in natural and semi-natural terrestrial ecosystems and comparison with the acetylene inhibition technique. Biogeosciences 2016, 13, 1821–1835. [Google Scholar] [CrossRef] [Green Version]
- Spott, O.; Russow, R.; Apelt, B.; Stange, C.F. A 15N-aided artificial atmosphere gas flow technique for online determination of soil N2 release using the zeolite Kostrolith SX6 (R). Rapid Commun. Mass Spectrom. 2006, 20, 3267–3274. [Google Scholar] [CrossRef] [PubMed]
- Baily, A.; Watson, C.J.; Laughlin, R.; Matthews, D.; McGeough, K.; Jordan, P. Use of the 15N gas flux method to measure the source and level of N2O and N2 emissions from grazed grassland. Nutr. Cycl. Agroecosyst. 2012, 94, 287–298. [Google Scholar] [CrossRef]
- Koba, K.; Hirobe, M.; Koyama, L.; Kohzu, A.; Tokuchi, N.; Nadelhoffer, K.J.; Wada, E.; Takeda, H. Natural 15N abundance of plants and soil N in a temperate coniferous forest. Ecosystems 2003, 6, 457–469. [Google Scholar] [CrossRef]
- Ullah, S.; Breitenbeck, G.A.; Faulkner, S.P. Denitrification and N2O emission from forested and cultivated alluvial clay soil. Biogeochemistry 2005, 73, 499–513. [Google Scholar] [CrossRef]
- Well, R.; Maier, M.; Lewicka-Szczebak, D.; Koster, J.R.; Ruoss, N. Underestimation of denitrification rates from field application of the 15N gas flux method and its correction by gas diffusion modelling. Biogeosciences 2019, 16, 2233–2246. [Google Scholar] [CrossRef]
- Scholefield, D.; Hawkins, J.M.B.; Jackson, S.M. Use of a flowing helium atmosphere incubation technique to measure the effects of denitrification controls applied to intact cores of a clay soil. Soil Biol. Biochem. 1997, 29, 1337–1344. [Google Scholar] [CrossRef]
- Zaman, M.; Kleineidam, K.; Bakken, L.; Berendt, J.; Bracken, C.; Butterbach-Bahl, K.; Cai, Z.; Chang, S.X.; Clough, T.; Dawar, K.; et al. Isotopic techniques to measure N2O, N2 and their sources. In Measuring Emission of Agricultural Greenhouse Gases and Developing Mitigation Options Using Nuclear and Related Techniques: Applications of Nuclear Techniques for GHGs; Zaman, M., Heng, L., Müller, C., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 213–301. [Google Scholar]
- Zhang, J.B.; Zhu, T.B.; Cai, Z.C.; Müller, C. Nitrogen cycling in forest soils across climate gradients in Eastern China. Plant Soil 2011, 342, 419–432. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Sigman, D.M.; Hedin, L.O. Isotopic evidence for large gaseous nitrogen losses from tropical rainforests. Proc. Natl. Acad. Sci. USA 2006, 103, 8745–8750. [Google Scholar] [CrossRef]
- Bai, E.; Houlton, B.Z. Coupled isotopic and process-based modeling of gaseous nitrogen losses from tropical rain forests. Global Biogeochem. Cycles 2009, 23, GB2011. [Google Scholar] [CrossRef]
- Fang, Y.T.; Koba, K.; Makabe, A.; Takahashi, C.; Zhu, W.X.; Hayashi, T.; Hokari, A.A.; Urakawa, R.; Bai, E.; Houlton, B.Z.; et al. Microbial denitrification dominates nitrate losses from forest ecosystems. Proc. Natl. Acad. Sci. USA 2015, 115, 1470–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.J.; Xi, D.; Fang, Y.T.; Wang, A.; Sha, L.Q.; Song, Q.H.; Liu, Y.T.; Zhou, L.G.; Zhou, R.W.; Lin, Y.X.; et al. Microbial processes responsible for soil N2O production in a tropical rainforest, illustrated using an in situ 15N labeling approach. CATENA 2021, 202, 105214. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).