Effects of Excess Nitrogen (N) on Fine Root Growth in Tropical Forests of Contrasting N Status
Abstract
:1. Introduction
2. Methods
2.1. Site Description
2.2. Experimental Design
2.3. Sampling
2.4. Chemical Analysis
2.5. Data Analysis
3. Results
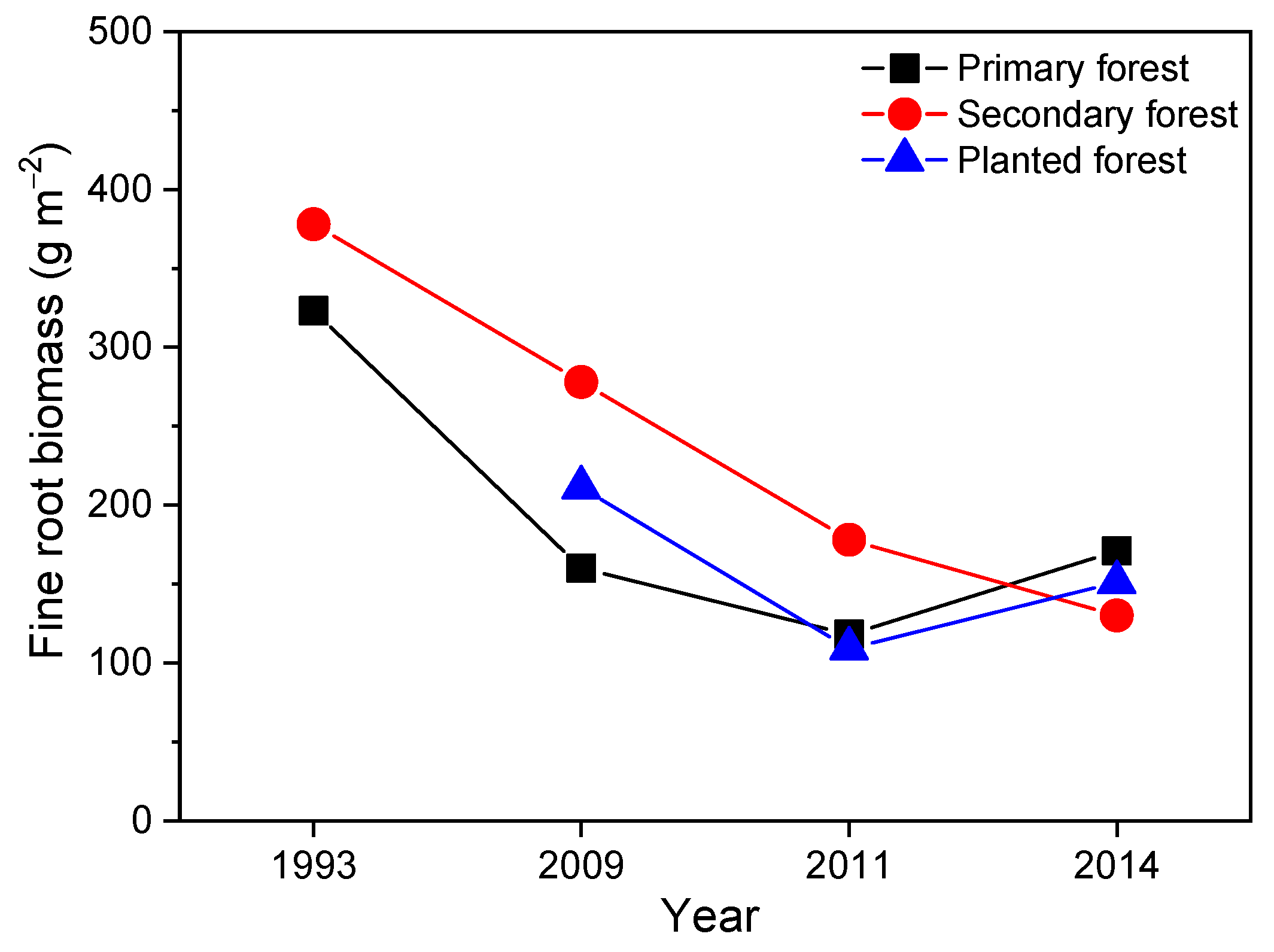
3.1. Historical Changes in Soil pH and Fine Root Biomass in the Three Forests and Role of Ambient N Deposition
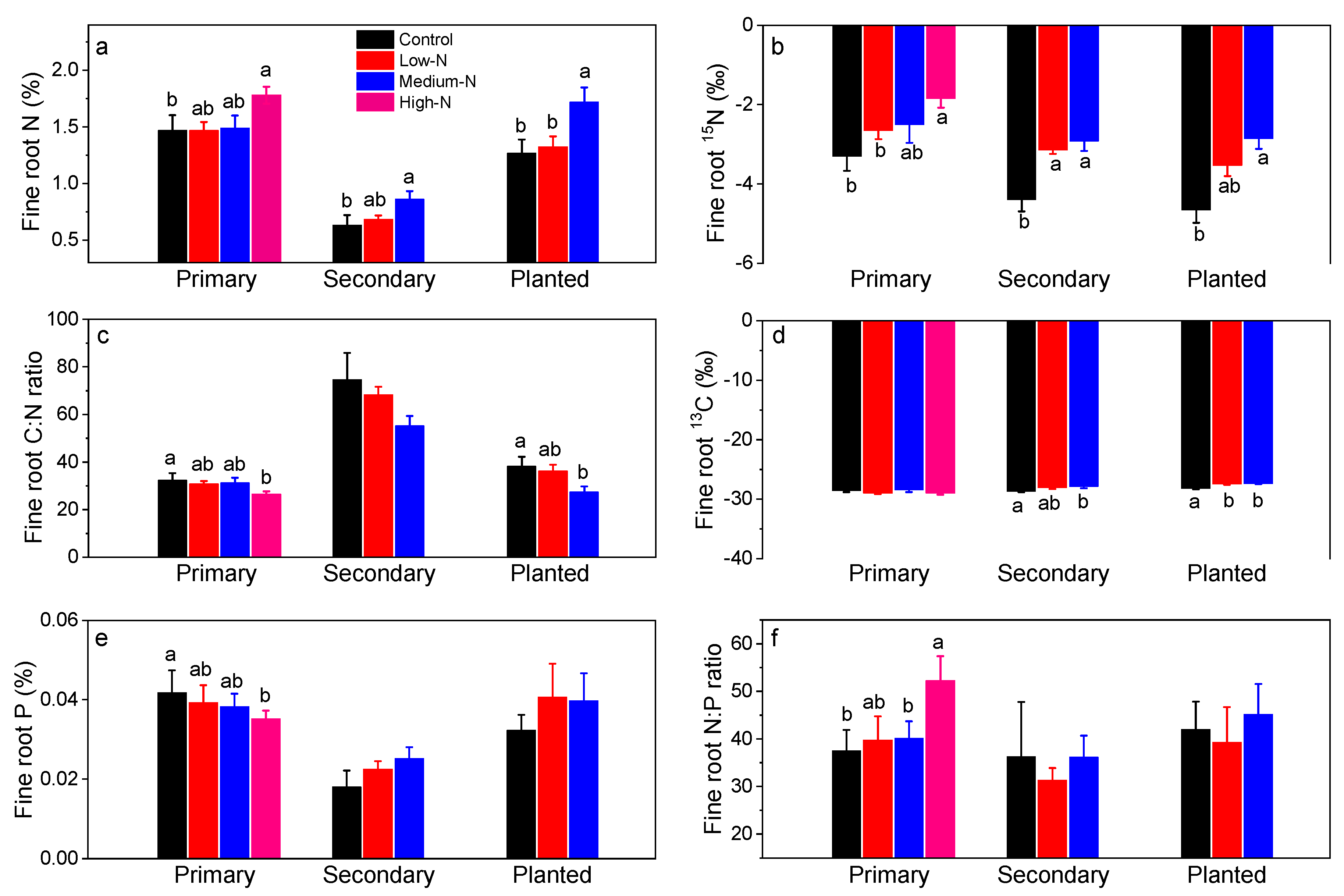
3.2. Fine Root Responses
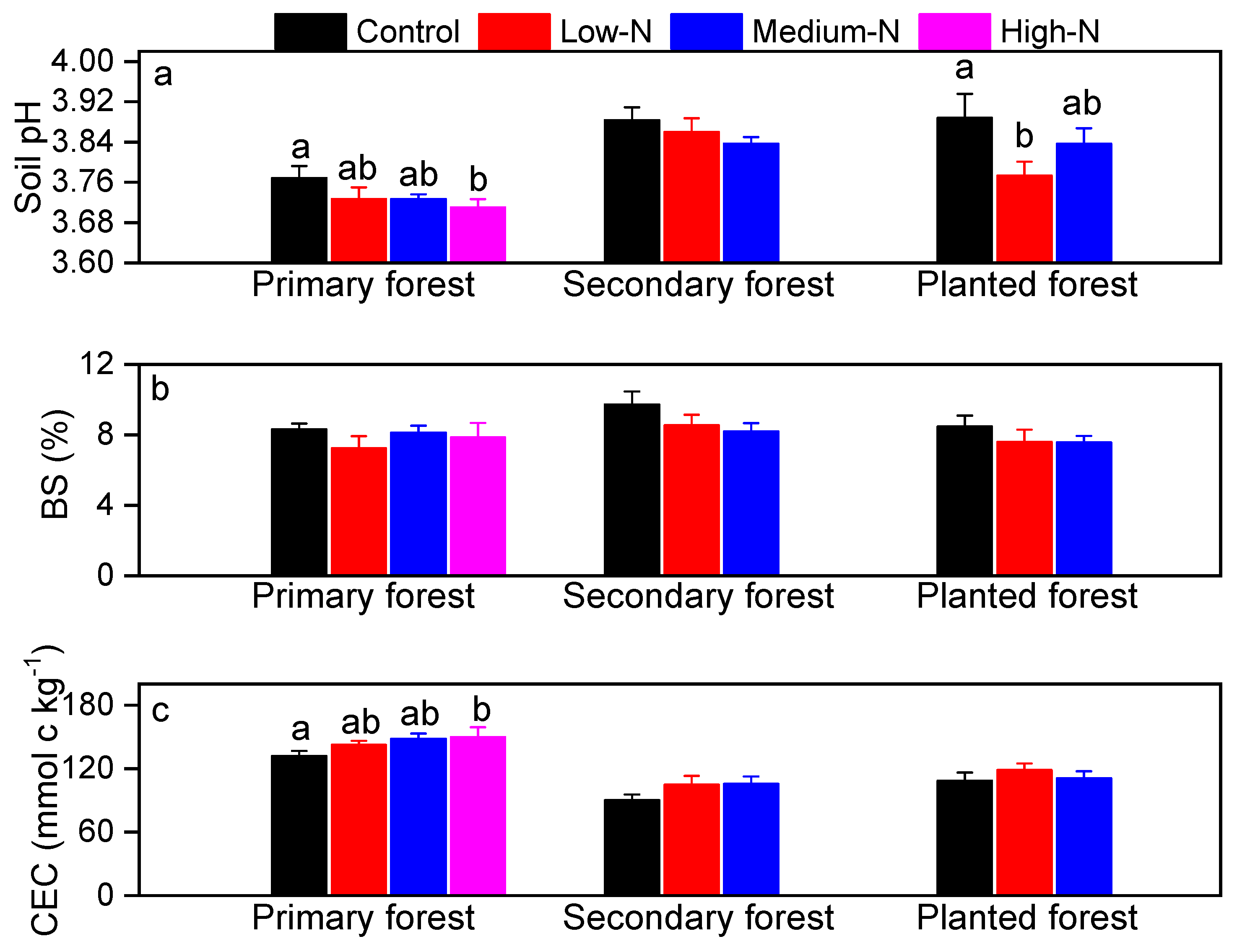
3.3. Soil Responses
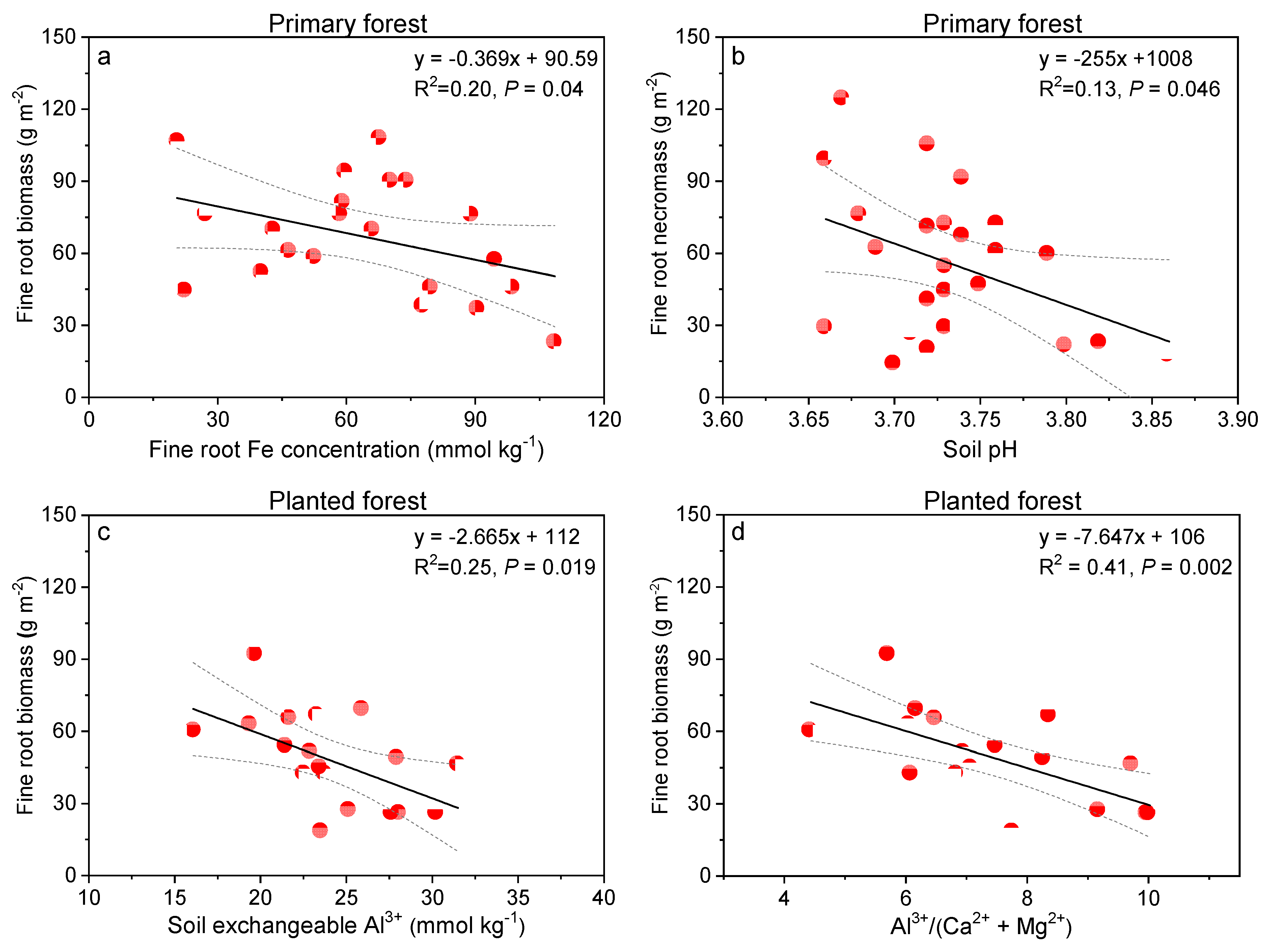
3.4. Relationships between Fine Root and Soil Responses
4. Discussion
4.1. Historical Trend in Soil pH Changes and Fine Root Biomass in the Three Tropical Forests
4.2. Effects of Long-Term N Additions on Fine Root Biomass and Vitality in the Primary Forest
4.3. Effects of Long-Term N Additions on Fine Root Biomass and Soil Cation Conditions in the Two Younger Forests
5. Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Forest | Treatment | Root N | Root P | Root K | Root Ca | Root Mg |
|---|---|---|---|---|---|---|
| Primary forest | Control | 100 | 3.0 (0.5) | 21.5 (2.6) | 16.1 (1.6) | 6.4 (0.9) |
| Low-N | 100 | 2.7 (0.4) | 28.8 (5.5) | 26.0 (3.6) | 6.0 (0.6) | |
| Medium-N | 100 | 2.6 (0.2) | 19.9 (3.1) | 17.2 (3.3) | 6.3 (0.7) | |
| High-N | 100 | 2.0 (0.2) | 12.7 (1.5) | 13.7 (4.1) | 4.4 (0.6) | |
| Secondary forest | Control | 100 | 3.9 (0.9) | 16.2 (5.2) | 37.5 (12.8) | 12.1 (4.0) |
| Low-N | 100 | 3.3 (0.3) | 26.8 (2.8) | 32.9 (6.2) | 11.3 (2.3) | |
| Medium-N | 100 | 3.0 (0.4) | 21.9 (4.8) | 28.0 (4.0) | 9.5 (1.1) | |
| Plantation forest | Control | 100 | 2.7 (0.5) | 20.2 (7.3) | 27.4 (6.6) | 9.5 (3.0) |
| Low-N | 100 | 3.1 (0.6) | 18.2 (3.6) | 30.0 (6.5) | 7.6 (1.6) | |
| Medium-N | 100 | 2.0 (0.5) | 18.8 (5.9) | 22.2 (5.7) | 7.1 (2.4) |
References
- Hendrick, R.L.; Pregitzer, K.S. Patterns of fine root mortality in two sugar maple forests. Nature 1993, 361, 59–61. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J. The potential effects of nitrogen deposition on fine-root production in forest ecosystems. New Phytol. 2000, 147, 131–139. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S., III; Mooney, H.A. Resource Limitation in Plants-An Economic Analogy. Annu. Rev. Ecol. Evol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Gower, S.T.; Vitousek, P.M. Effects of nutrient amendments on fine root biomass in a primary successional forest in Hawai’i. Oecologia 1989, 81, 566–568. [Google Scholar] [CrossRef]
- Albough, T.; Allen, H.; Dougherty, P.; Kress, L.; King, J. Leaf area and above- and belowground growth responses of loblolly pine to nutrient and water additions. For. Sci. 1998, 44, 317–328. [Google Scholar]
- Tateno, R.; Hishi, T.; Tkeda, H. Above-and below-ground biomass and net primary production in a cool-temperate deciduous forest in relation to topographical changes in soil nitrogen. For. Ecol. Manag. 2004, 193, 297–306. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.; Bergeron, Y. Influence of Environmental Variability on Root Dynamics in Northern Forests. Crit. Rev. Plant Sci. 2009, 28, 179–197. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.; Zhu, W.; Gundersen, P.; Fang, Y.; Li, D.; Wang, H. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Change Biol. 2007, 14, 403–412. [Google Scholar] [CrossRef]
- Van Breeman, N.; Mulder, J.; Driscoll, C. Acidification and alalizaiton of soils. Plant Soil 1983, 75, 283–308. [Google Scholar] [CrossRef]
- Godbold, D.L.; Fritz, E.; Hüttermann, A. Aluminum toxicity and forest decline. Proc. Natl. Acad. Sci. USA 1988, 85, 3888–3892. [Google Scholar] [CrossRef]
- Godbold, D.L.; Fritz, H.-W.; Jentschke, G.; Meesenburg, H.; Rademacher, P. Root turnover and root necromass accumulation of Norway spruce (Picea abies) are affected by soil acidity. Tree Physiol. 2003, 23, 915–921. [Google Scholar] [CrossRef]
- Persson, H.; Majdi, H.; Clemensson-Lindell, A. Effects of acid deposition on tree roots. Ecol. Bull. 1995, 44, 158–167. [Google Scholar]
- Persson, H.; Ahlström, K. Fine-root response to nitrogen supply in nitrogen manipulated Norway spruce catchment areas. For. Ecol. Manag. 2002, 168, 29–41. [Google Scholar] [CrossRef]
- Clemensson-Lindell, A.; Persson, H. The effects of nitrogen addition and removal on Norway spruce fine-root vitality and distribution in three catchment areas at Gårdsjön. For. Ecol. Manag. 1995, 71, 123–131. [Google Scholar] [CrossRef]
- Persson, H.; Stadenberg, I. Spatial distribution of fine-roots in boreal forests in eastern Sweden. Plant Soil 2008, 318, 1–14. [Google Scholar] [CrossRef]
- Widell, S.; Asp, H.; Jensen, P. Activities of plasma member-bound enzymes isolated from roots of spruce (Picea abies) grown in the presence of aluminum. Physiol. Plant. 1994, 92, 459–466. [Google Scholar] [CrossRef]
- Puhe, J. Growth and development of the root system of Norway spruce (Picea abies) in forest stands—A review. For. Ecol. Manag. 2003, 175, 253–273. [Google Scholar] [CrossRef]
- Horst, W. The role of the apoplast in aluminum toxicity and resistance of higher plants: A review. Z. Pflanz. Bodenk 1995, 158, 419–428. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Likens, G.E.; Driscoll, C.T.; Buso, D.C. Long-Term Effects of Acid Rain: Response and Recovery of a Forest Ecosystem. Science 1996, 272, 244–246. [Google Scholar] [CrossRef]
- Galloway, J.; Townsend, A.; Erisman, J.; Bekunda, M.; Cai, Z.; Freney, J.; Martinelli, L.; Seitzinger, S.; Sutton, M. Transfor-mation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Hietz, P.; Turner, B.L.; Wanek, W.; Richter, A.; Nock, C.A.; Wright, S.J. Long-Term Change in the Nitrogen Cycle of Tropical Forests. Science 2011, 334, 664–666. [Google Scholar] [CrossRef]
- Ulrich, B. Natural and anthropogenic components of soil acidification. Z. Pflanz. Bodenkd. 1986, 149, 702–717. [Google Scholar] [CrossRef]
- Fang, Y.T.; Gundersen, P.; Mo, J.M.; Zhu, W.X. Input and output of dissolved organic and inorganic nitrogen in subtropical forests of South China under high air pollution. Biogeosciences 2008, 5, 339–352. [Google Scholar] [CrossRef]
- Lu, X.; Mo, J.; Gundersen, P.; Zhu, W.; Zhou, G.; Li, D.; Zhang, X. Effects of simulated N deposition on soil exchangeable cat-ions in three forest types of subtropical China. Pedosphere 2009, 19, 189–198. [Google Scholar] [CrossRef]
- Lu, X.K.; Mo, J.M.; Gilliam, F.S.; Zhou, G.Y.; Fang, Y.T. Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Glob. Change Biol. 2010, 16, 2688–2700. [Google Scholar] [CrossRef]
- Lu, X.; Gilliam, F.; Yu, G.; Li, L.; Mao, Q.; Chen, H.; Mo, J. Long-term nitrogen addition decreases carbon leaching in nitro-gen-rich forest ecosystems. Biogeosciences 2013, 10, 3931–3941. [Google Scholar] [CrossRef]
- Brown, S.; Lenart, M.; Mo, J.; Kong, G. Structure and Organic Matter Dynamics of a Human-Impacted Pine Forest in a MAB Reserve of Subtropical China. Biotropica 1995, 27, 276. [Google Scholar] [CrossRef]
- Mo, J.; Brown, S.; Lenart, M.; Kong, G. Nutrient Dynamics of a Human-Impacted Pine Forest in a MAB Reserve of Subtropical China. Biotropica 1995, 27, 290. [Google Scholar] [CrossRef]
- Mo, J.; Brown, S.; Xue, J.; Fang, Y.; Li, Z. Response of litter decomposition to simulated N deposition in a disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 2006, 282, 135–151. [Google Scholar] [CrossRef]
- Fang, Y.; Gundersen, P.; Mo, J.; Zhu, W. Nitrogen leaching in response to increased nitrogen inputs in subtropical monsoon forests in southern China. For. Ecol. Manag. 2009, 257, 332–342. [Google Scholar] [CrossRef]
- Huang, Z.; Fan, Z. The climate of Ding Hu Shan. Trop. Subtrop. For. Ecosys. 1982, 1, 11–23. [Google Scholar]
- He, J.; Chen, Z.; Liang, Y. The soil of Ding Hu Shan. Trop. Subtrop. For. Ecosys. 1982, 1, 25–38. [Google Scholar]
- Fang, Y.; Zhu, W.; Mo, J.; Zhou, G.; Gundersen, P. Dynamics of soil inorganic nitrogen and their responses to nitrogen additions in three subtropical forests, south China. J. Environ. Sci. 2006, 18, 752–759. [Google Scholar]
- Shen, C.; Liu, D.; Peng, S.; Sun, Y.; Jiang, M.; Yi, W.; Xing, C.; Gao, Q.; Li, Z.; Zhou, G. 14C measurement of forest soils in Ding hu shan Biosphere Reserve. Chin. Sci. Bull. 1999, 44, 251–256. [Google Scholar] [CrossRef]
- Gilliam, F.S. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J. Ecol. 2006, 94, 1176–1191. [Google Scholar] [CrossRef]
- Janssens, I.; Sampson, D.; Curiel-Yuste, J.; Carrara, A.; Ceulemans, R. The carbon cost of fine root turnover in a Scots pine forest. For. Ecol. Manag. 2002, 168, 231–240. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Liu, K.; Fang, Y.; Yu, F.; Liu, Q.; Li, F.; Peng, S. Soil acidification in response to acid deposition in three subtropical forests of subtropical China. Pedosphere 2010, 20, 399–408. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Wei, P.; Kong, G.; Ye, W. Production and turnover rate of fine roots in two lower subtropical forest sites at Ding hu shan. Acta Phytoecol. Sin. 1999, 23, 361–369. [Google Scholar]
- Lu, X.; Mao, Q.; Gilliam, F.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Change Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; De Vries, W.; Liu, X.; Zeng, M.; Hao, T.; Du, E.; Zhang, F.; Shen, J. The contribution of atmospheric deposition and forest harvesting to forest soil acidification in China since 1980. Atmos. Environ. 2016, 146, 215–222. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; He, H.; Zhao, X.; Datta, A.; Ma, W.; Zhang, Y.; Liu, X.; Han, W.; Wilson, M.C.; et al. Long-term changes in soil pH across major forest ecosystems in China. Geophys. Res. Lett. 2015, 42, 933–940. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H. Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J. Geophys. Res. Earth Surf. 2007, 112, D22S05. [Google Scholar] [CrossRef]
- Larssen, T.; Seip, H.; Semb, A.; Mulder, J.; Muniz, I.; Vogt, R.; Lydersen, E.; Angell, V.; Dagang, T.; Eilertsen, O. Acid rain in China. Environ. Sci. Technol. 2006, 40, 418–425. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Zhang, Y.; Shen, J.; Han, W.; Zhang, W.; Christie, P.; Goulding, K.; Zhang, F. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, J.; Ji, C.; Ma, W.; Mohammat, A.; Wang, S.; Wang, S.; Datta, A.; Robinson, D.; Smith, P. Widespread decreases in topsoil inorganic carbon stocks across China’s grasslands during 1980s–2000s. Glob. Change Biol. 2012, 18, 3672–3680. [Google Scholar] [CrossRef]
- Zhai, P.; Zhang, X.; Wan, H.; Pan, X. Trends in Total Precipitation and Frequency of Daily Precipitation Extremes over China. J. Clim. 2005, 18, 1096–1108. [Google Scholar] [CrossRef]
- Espeleta, J.F.; Clark, D.A. Multi-scale variation in fine-root biomass in a tropical rain forest: A seven-year study. Ecol. Monogr. 2007, 77, 377–404. [Google Scholar] [CrossRef]
- Cronan, C.S.; Grigal, D.F. Use of Calcium/Aluminum Ratios as Indicators of Stress in Forest Ecosystems. J. Environ. Qual. 1995, 24, 209–226. [Google Scholar] [CrossRef]
- Rost-Siebert, K. Untersuchungen zur H+—und Al-Ionentoxizit/it an Keimpflanzen von Fichte (Picea abies, Kartst.) und Buche (Fagus sylvatica, L.) in L/Ssingskultur. Ber. Forsch. Waldokosyst./Waldsterben Forsch. Wald. Göttingen 1985, 12, 1–219. [Google Scholar]
- Jorns, A.; Hecht-Buchholz, C. AlIg. Forstz 1985, 46, 1249–1252. [Google Scholar]
- Matson, P.; McDowell, W.; Townsend, A.; Vitousek, P. The globalization of nitrogen deposition: Ecosystem consequences in tropical environments. Biogeochemistry 1999, 46, 67–83. [Google Scholar] [CrossRef]
- Clemensson-Lindell, A.; Persson, H. Fine-root vitality in a Norway spruce stand subjected to various nutrient supplies. Plant Soil 1995, 168, 167–172. [Google Scholar] [CrossRef]
- Majdi, H.; Kangas, P. Demography of fine roots in response to nutrient applications in a Norway spruce stand in southwestern Sweden. Ecoscience 1997, 4, 199–205. [Google Scholar] [CrossRef]
- Fan, P.; Guo, D. Slow decomposition of lower order roots: A key mechanism of root carbon and nutrient retention in the soil. Oecologia 2010, 163, 509–515. [Google Scholar] [CrossRef]
- Guo, D.; Li, H.; Mitchell, R.; Han, W.; Hendricks, J.; Fahey, T.; Hendrick, R. Fine root heterogeneity by branch order: Exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytol. 2008, 177, 443–456. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter-Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin, Germany, 2003. [Google Scholar]
- Hobbie, S.E. Contrasting Effects of Substrate and Fertilizer Nitrogen on the Early Stages of Litter Decomposition. Ecosystems 2005, 8, 644–656. [Google Scholar] [CrossRef]
- Hobbie, S.E. Nitrogen effects on decomposition: A five-year experiment in eight temperate sites. Ecology 2008, 89, 2633–2644. [Google Scholar] [CrossRef]
- Guo, D.L.; Mitchell, R.J.; Hendricks, J.J. Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 2004, 140, 450–457. [Google Scholar] [CrossRef]
- Li, W.; Jin, C.; Guan, D.; Wang, Q.; Wang, A.; Yuan, F.; Wu, J. The effects of simulated nitrogen deposition on plant root traits: A meta-analysis. Soil Biol. Biochem. 2015, 82, 112–118. [Google Scholar] [CrossRef]
- Bowman, W.; Cleveland, C.; Halada, L.; Hreško, J.; Baron, J. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci. 2008, 1, 767–770. [Google Scholar] [CrossRef]
- Foy, C.D.; Chaney, R.L.; White, M.C. The Physiology of Metal Toxicity in Plants. Annu. Rev. Plant Physiol. 1978, 29, 511–566. [Google Scholar] [CrossRef]
- Adamek, M.; Corre, M.D.; Hölscher, D. Responses of fine roots to experimental nitrogen addition in a tropical lower montane rain forest, Panama. J. Trop. Ecol. 2010, 27, 73–81. [Google Scholar] [CrossRef]
- Braun, S.; Cantaluppi, L.; Fluckiger, W. Fine roots in stands of Fagus sylvatica and Picea abies along a gradient of soil acidifica-tion. Environ. Pollut. 2005, 137, 574–579. [Google Scholar] [CrossRef]
- Majdi, H.; Persson, H. A study on fine-root dynamics in response to nutrient applications in a Norway spruce stand using the minirhizotron technique. J. Plant Nutr. Soil Sci. 1995, 158, 429–433. [Google Scholar] [CrossRef]
- Lu, X.; Mo, J.; Gilliam, F.; Fang, H.; Zhu, F.; Fang, Y.; Zhang, W.; Huang, J. Nitrogen addition shapes soil phosphorus availability in two reforested tropical forests in southern China. Biotropica 2012, 44, 302–311. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D.; Currie, W.S.; Nadelhoffer, K.J.; Martin, M.E.; McDowell, W.H.; Melillo, J.M.; Steudler, P. Ecosystem response to 15 years of chronic nitrogen additions at the Harvard Forest LTER, Massachusetts, USA. For. Ecol. Manag. 2004, 196, 7–28. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, S.; Li, Z.; Zhang, D.; Tang, X.; Zhou, C.; Yan, J.; Mo, J. Old-Growth Forests Can Accumulate Carbon in Soils. Science 2006, 314, 1417. [Google Scholar] [CrossRef] [PubMed]
- Yavitt, J.; Harms, K.; Garcia, M.; Mirabello, A.; Wright, S. Soil fertility and fine root dynamics in response to 4 years of nutrient (N, P, K) fertilization in a lowland tropical moist forest, Panama. Austral Ecol. 2011, 36, 433–445. [Google Scholar] [CrossRef]



| Primary Forest | Secondary Forest | Planted Forest | |
|---|---|---|---|
| Aboveground | |||
| Stem density (DBH > 2 cm, stems ha−1) | 1013 | 1933 | 767 |
| Basal area (m2 ha−1) | 26 | 13.8 | 14.0 |
| Litter production (t ha−1 yr−1) in 2005 | 6.6 | 5.0 | 4.3 |
| Mineral soil (0–10 cm) | |||
| Bulk density (g cm−3) | 1.0 | 1.2 | 1.2 |
| pH (H2O) | 3.8 | 3.9 | 3.9 |
| Total C concentration (%) | 4.5 | 2.6 | 2.8 |
| Total N concentration (%) | 0.2 | 0.1 | 0.1 |
| C/N | 22 | 26 | 28 |
| Base saturation (%) | 4.5 | 3.2 | 5.5 |
| Net N mineralization (mg N kg−1 mon−1) | 6.7 | 3.9 | 8.2 |
| Net nitrification (mg N kg−1 mon−1) | 6.1 | 1.1 | 7.8 |
| Inorganic N leaching in 2005 (kg N ha−1 yr−1, 20 cm Below the organic layer) | 41.7 | 9.5 | 15.5 |
| Forest | Treatment | K | Na | Ca | Mg | Al | Fe | Ca:Al Molar Ratio |
|---|---|---|---|---|---|---|---|---|
| Primary forest | Control | 3.0 (0.2) | 0.2 (0.1) | 2.3 (0.1) | 0.9 (0.1) | 3.6 (0.5) | 2.6 (0.4) a | 0.5 (0.1) |
| Low-N | 4.1 (0.7) | 0.2 (0.1) | 2.7 (0.4) | 0.9 (0.1) | 5.8 (1.5) | 3.3 (0.6) ab | 0.7 (0.2) | |
| Medium-N | 3.0 (0.6) | 0.3 (0.1) | 2.5 (0.4) | 0.9 (0.1) | 4.6 (0.8) | 3.6 (0.4) ab | 0.5 (0.2) | |
| High-N | 2.3 (0.3) | 0.4 (0.1) | 2.4 (0.6) | 0.8 (0.1) | 3.2 (0.6) | 4.9 (0.3) b | 0.6 (0.2) | |
| Secondary forest | Control | 0.9 (0.2) a | 0.2 (0.1) | 1.8 (0.5) | 0.6 (0.1) | 1.9 (0.5) a | 0.6 (0.2) a | 1.0 (0.4) |
| Low-N | 1.8 (0.2) b | 0.4 (0.1) | 2.2 (0.4) | 0.7 (0.1) | 4.0 (0.3) b | 1.7 (0.2) b | 0.4 (0.1) | |
| Medium-N | 1.8 (0.3) b | 0.3 (0.1) | 2.3 (0.2) | 0.8 (0.1) | 2.6 (0.4) ab | 1.2 (0.3) ab | 0.7 (0.1) | |
| Planted forest | Control | 2.3 (0.6) | 0.4 (0.1) | 3.3 (0.6) | 1.1 (0.2) | 3.8 (1.0) | 1.5 (0.1) | 0.7 (0.1) |
| Low-N | 2.5 (0.6) | 0.6 (0.1) | 3.9 (0.8) | 1.0 (0.2) | 4.8 (1.2) | 2.6 (0.5) | 0.6 (0.1) | |
| Medium-N | 3.6 (0.8) | 0.8 (0.3) | 4.3 (0.7) | 1.3 (0.2) | 4.4 (0.8) | 2.1 (0.6) | 0.7 (0.1) |
| Forests | Treatment | H+ | K+ | Na+ | Ca2+ | Mg2+ | Al3+ | Fe3+ | Ca:Al Molar Ratio | Al/ (Ca + Mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Primary forest | Control | 28.3 (3.9) a | 2.6 (0.6) | 1.0 (0.1) | 2.8 (0.3) a | 0.8 (0.08) a | 29.1 (2.1) | 1.8 (0.1) a | 0.10 (0.01) | 8.2 (0.6) |
| Low-N | 35.0 (4.1) ab | 3.4 (0.4) | 0.8 (0.1) | 2.4 (0.3) ab | 0.7 (0.08) ab | 30.2(1.8) | 2.1 (0.1) ab | 0.08 (0.01) | 10.3 (1.0) | |
| Medium-N | 42.6 (4.3) b | 3.4 (0.3) | 0.9 (0.1) | 1.3 (0.1) b | 0.4 (0.03) b | 28.9 (1.6) | 2.3 (0.2) ab | 0.10 (0.01) | 7.6 (0.3) | |
| High-N | 44.0 (5.1) b | 3.2 (0.4) | 0.9 (0.1) | 1.0 (0.2) b | 0.3 (0.06) b | 31.5 (2.2) | 2.5 (0.2) b | 0.09 (0.01) | 8.8 (0.9) | |
| Secondary forest | Control | 23.7 (4.6) | 2.0 (0.1) | 0.7 (0.1) | 2.4 (0.2) | 0.6 (0.06) | 20.8 (1.5) | 1.1 (0.1) | 0.12 (0.01) | 6.9 (0.4) |
| Low-N | 30.4 (5.1) | 2.0 (0.1) | 0.8 (0.1) | 2.3 (0.1) | 0.6 (0.05) | 20.7 (1.0) | 1.2 (0.1) | 0.11 (0.01) | 7.1 (0.4) | |
| Medium-N | 35.5 (2.9) | 2.1 (0.1) | 0.8 (0.1) | 2.2 (0.2) | 0.6 (0.05) | 21.3 (1.6) | 1.2 (0.1) | 0.11 (0.01) | 7.8 (1.1) | |
| Planted forest | Control | 33.3 (6.1) | 1.7 (0.1) a | 0.7 (0.1) a | 2.7 (0.1) | 0.6 (0.02) a | 24.9 (2.4) | 0.9 (0.1) | 0.12 (0.02) | 7.8 (0.9) |
| Low-N | 37.4 (6.3) | 1.6 (0.1) b | 0.6 (0.1) ab | 2.8 (0.2) | 0.5 (0.02) ab | 25.3 (0.9) | 1.0 (0.1) | 0.11 (0.01) | 7.8 (0.6) | |
| Medium-N | 40.7 (6.4) | 1.6 (0.1) b | 0.5 (0.1) b | 2.6 (0.1) | 0.5 (0.01) b | 22.1 (0.9) | 0.8 (0.1) | 0.12 (0.01) | 7.2 (0.5) |
| pH | Soil Ca2+ | Soil Mg2+ | Soil Al3+ | Soil Fe3+ | Ca:Al Ratio | Al/ (Ca + Mg) | Root Ca | Root Mg | Root Al | Root Fe | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary forest | |||||||||||
| Biomass | 0.031 | 0.061 | 0.007 | 0.067 | −0.159 | −0.036 | 0.122 | 0.052 | 0.386 | 0.140 | −0.463 * |
| Necromass | −0.410 * | 0.467 * | 0.512 * | 0.302 | 0.111 | 0.231 | −0.294 | 0.216 | 0.033 | 0.004 | −0.038 |
| Live proportion | 0.301 | −0.411 | −0.462 * | −0.229 | −0.117 | −0.267 | 0.318 | −0.217 | 0.247 | 0.114 | −0.248 |
| Secondary forest | |||||||||||
| Biomass | −0.088 | −0.367 | -0.314 | 0.08 | 0.23 | −0.442 | 0.449 | −0.366 | 0.145 | 0.346 | 0.65 ** |
| Necromass | −0.456 | −0.05 | 0.015 | 0.176 | −0.165 | −0.271 | 0.124 | −0.191 | −0.539 * | −0.113 | −0.23 |
| Live proportion | 0.421 | −0.214 | −0.252 | −0.083 | 0.151 | −0.112 | 0.17 | −0.172 | 0.428 | 0.194 | 0.436 |
| Planted forest | |||||||||||
| Biomass | 0.300 | 0.525 ** | 0.015 | −0.546 * | 0.372 | 0.625 ** | −0.669 ** | −0.034 | −0.423 | −0.269 | −0.321 |
| Necromass | −0.32 | −0.183 | −0.04 | −0.113 | 0.181 | −0.054 | −0.009 | −0.423 | 0.145 | −0.035 | −0.08 |
| Live proportion | 0.296 | 0.515 * | 0.097 | −0.219 | 0.253 | 0.419 | −0.401 | 0.326 | −0.357 | −0.168 | −0.104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, F.; Gilliam, F.S.; Mulder, J.; Yoh, M.; Mo, J.; Lu, X. Effects of Excess Nitrogen (N) on Fine Root Growth in Tropical Forests of Contrasting N Status. Forests 2022, 13, 1328. https://doi.org/10.3390/f13081328
Zhu F, Gilliam FS, Mulder J, Yoh M, Mo J, Lu X. Effects of Excess Nitrogen (N) on Fine Root Growth in Tropical Forests of Contrasting N Status. Forests. 2022; 13(8):1328. https://doi.org/10.3390/f13081328
Chicago/Turabian StyleZhu, Feifei, Frank S. Gilliam, Jan Mulder, Muneoki Yoh, Jiangming Mo, and Xiankai Lu. 2022. "Effects of Excess Nitrogen (N) on Fine Root Growth in Tropical Forests of Contrasting N Status" Forests 13, no. 8: 1328. https://doi.org/10.3390/f13081328
APA StyleZhu, F., Gilliam, F. S., Mulder, J., Yoh, M., Mo, J., & Lu, X. (2022). Effects of Excess Nitrogen (N) on Fine Root Growth in Tropical Forests of Contrasting N Status. Forests, 13(8), 1328. https://doi.org/10.3390/f13081328








