Dendroclimatological Analysis of Chinese Fir Using a Long-Term Provenance Trial in Southern China
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Materials
2.2. Tree-Ring Chronology Development and Calculation of Statistical Parameters
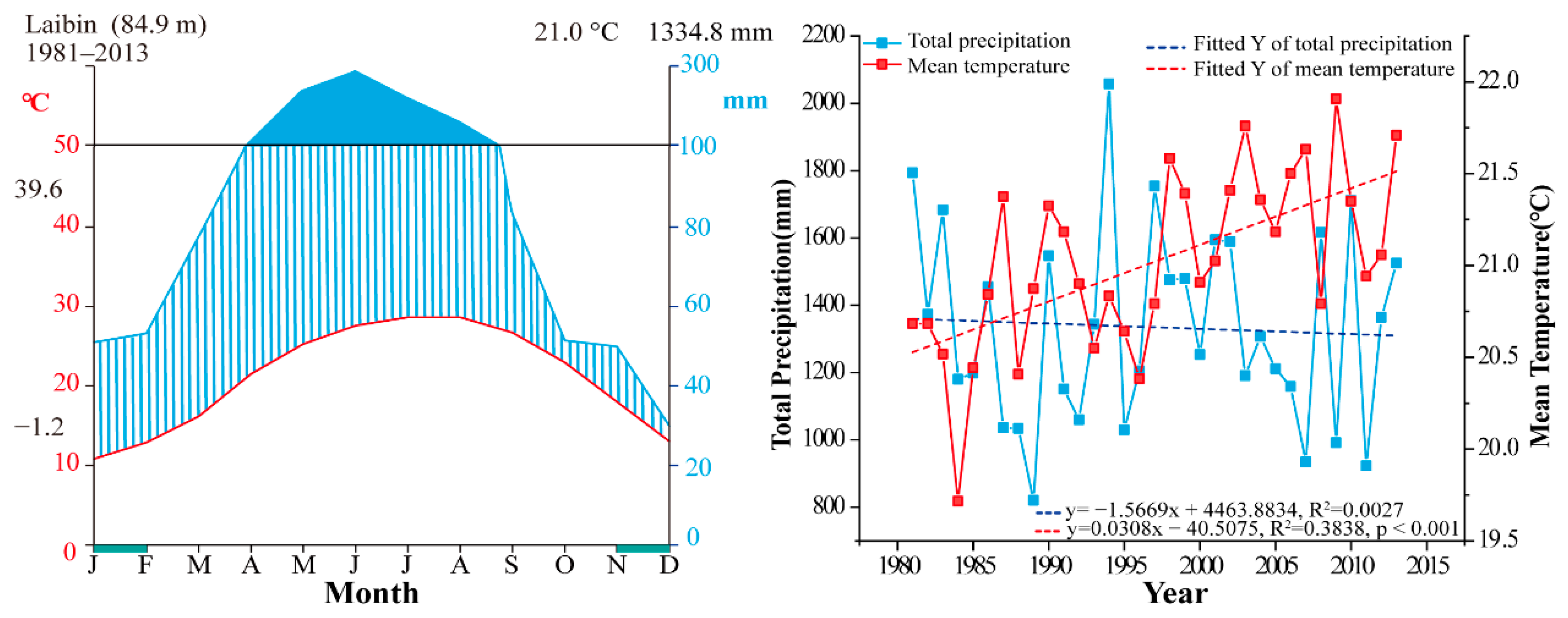
2.3. Meteorological Data
2.4. Statistical Analysis
3. Results
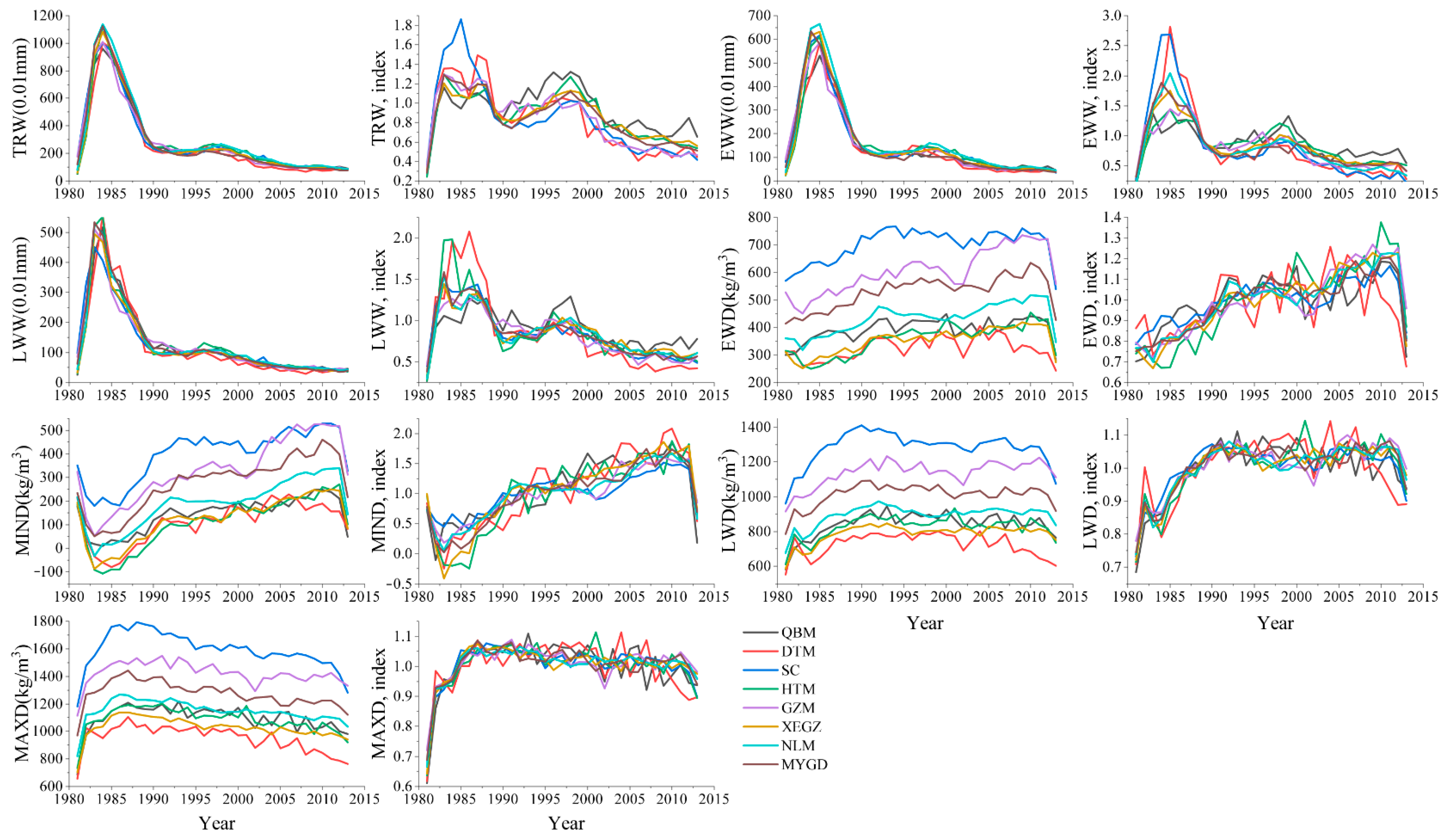
3.1. Width and Density Chronologies and General Features of Chronologies
3.2. Growth–Climate Relationships
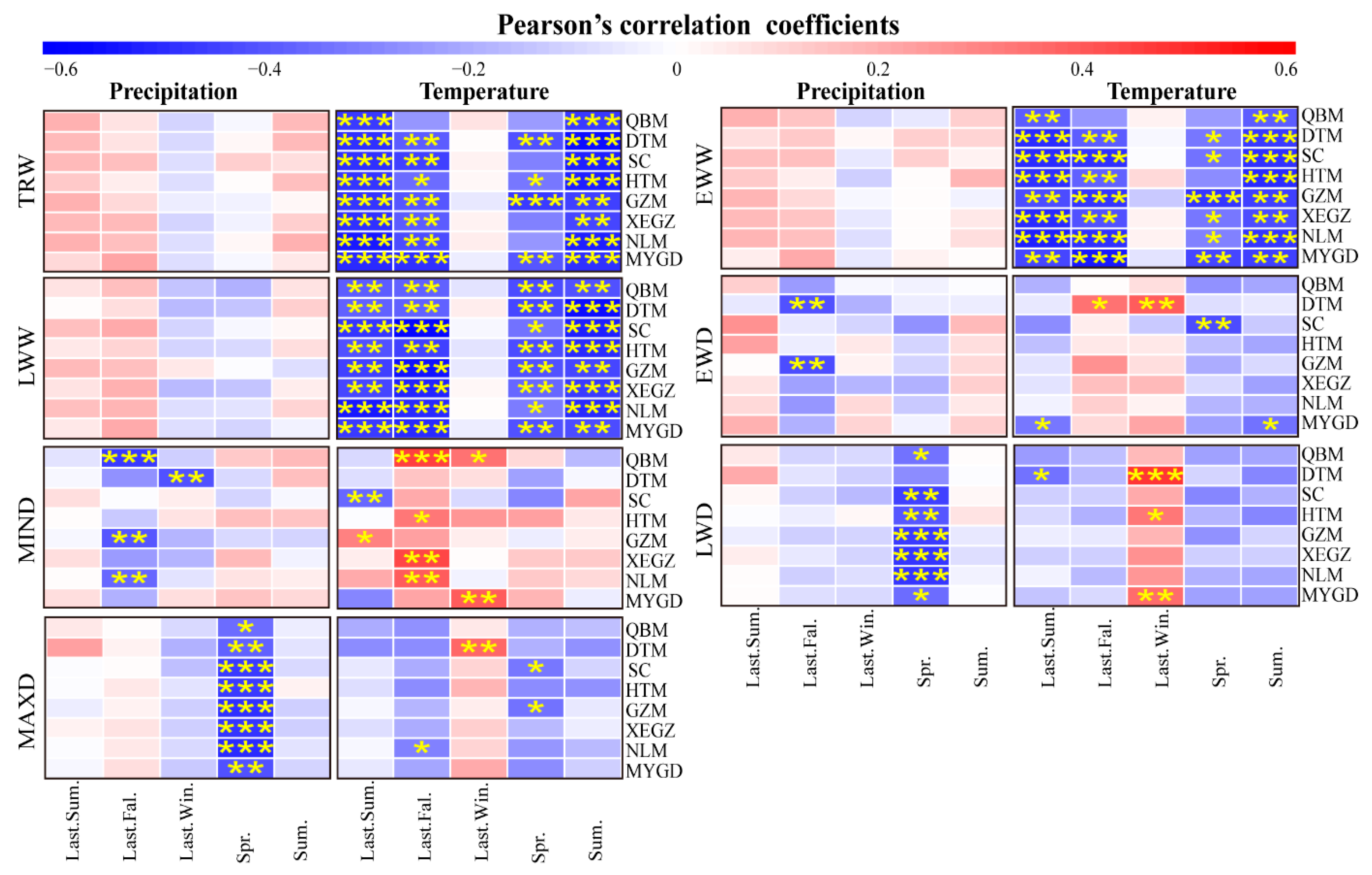
3.2.1. Relationships between Tree-Ring Chronologies and Seasonal Climate Factors
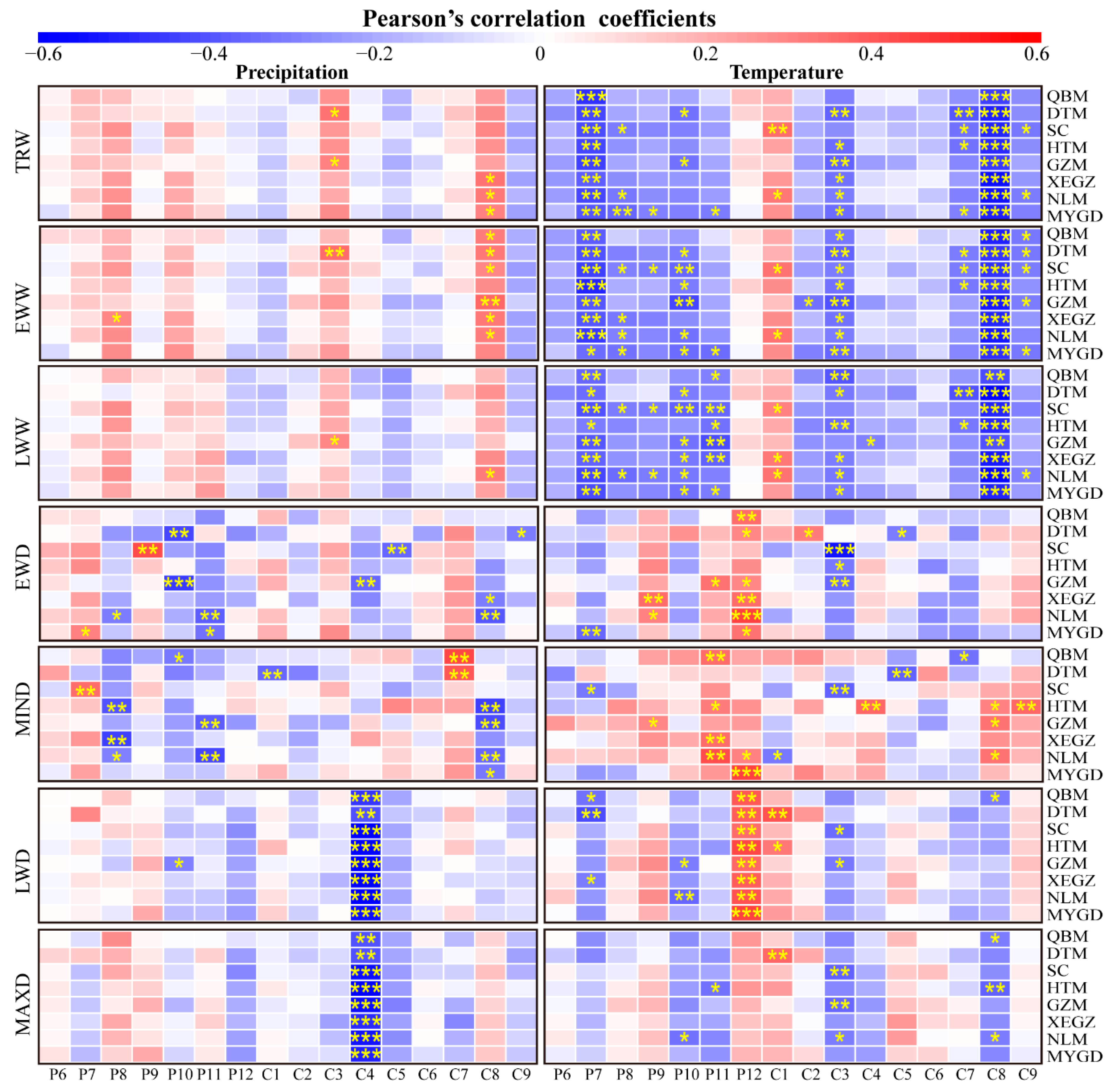
3.2.2. Relationships between Tree-Ring Chronologies and Monthly Climate Factors
4. Discussion
4.1. Tree-Ring Chronology Characteristics
4.2. Response of Chinese Fir Growth to Climate Variables
4.3. Variation in Tree-Ring Growth Responses to Climate Change for Different Provenance Regions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buras, A.; Sass-Klaassen, U.; Verbeek, I.; Copini, P. Provenance selection and site conditions determine growth performance of pedunculate oak. Dendrochronologia 2020, 61, 125705. [Google Scholar] [CrossRef]
- Klisz, M.; Ukalski, K.; Ukalska, J.; Jastrzębowski, S.; Puchałka, R.; Przybylski, P.; Mionskowski, M.; Matras, J. What can we learn from an early test on the adaptation of silver fir populations to marginal environments? Forests 2018, 9, 441. [Google Scholar] [CrossRef]
- Wu, H.; Lei, J.; Li, X.; Wang, H.; Duan, A.; Zhang, J. Aggregation distributions across stand age in provenances of Cunninghamia lanceolata (lamb.) hook. For. Ecol. Manag. 2021, 494, 119317. [Google Scholar] [CrossRef]
- Taeger, S.; Zang, C.; Liesebach, M.; Schneck, V.; Menzel, A. Impact of climate and drought events on the growth of Scots pine (Pinus sylvestris L.) provenances. For. Ecol. Manag. 2013, 307, 30–42. [Google Scholar] [CrossRef]
- Klisz, M.; Buras, A.; Sass-Klaassen, U.; Puchałka, R.; Koprowski, M.; Ukalska, J. Limitations at the limit? Diminishing of genetic effects in Norway spruce provenance trials. Front. Plant Sci. 2019, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Schmidtling, R. Use of provenance tests to predict response to climate change: Loblolly pine and Norway spruce. Tree Physiol. 1994, 14, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Burley, J.; Wood, J.; Adlard, P.; Andrew, I.; Greaves, A.; Hughes, J.; Kemp, R.; Lines, R.; Plumptre, R.; Styles, B. A Manual on Species and Provenance Research with Particular Reference to the Tropics; University of Oxford: Oxford, UK, 1976. [Google Scholar]
- Walentowski, H.; Falk, W.; Mette, T.; Kunz, J.; Bräuning, A.; Meinardus, C.; Zang, C.; Sutcliffe, L.M.; Leuschner, C. Assessing future suitability of tree species under climate change by multiple methods: A case study in Southern Germany. Ann. For. Res. 2017, 60, 101–126. [Google Scholar] [CrossRef]
- Martín-Benito, D.; Cherubini, P.; Del Río, M.; Cañellas, I. Growth response to climate and drought in Pinus nigra Arn. trees of different crown classes. Trees 2008, 22, 363–373. [Google Scholar] [CrossRef]
- Griesbauer, H.P.; Klassen, H.; Saunders, S.C.; Spittlehouse, D.L. Variation in climate-growth relationships for Douglas-fir growth across spatial and temporal scales on Southern Vancouver Island, British Columbia. For. Ecol. Manag. 2019, 444, 30–41. [Google Scholar] [CrossRef]
- Matisons, R.; Adamovičs, A.; Jansone, D.; Bigača, Z.; Jansons, Ā. Climatic sensitivity of the top-performing provenances of Scots pine in Latvia. Balt. For. 2018, 24, 228–233. [Google Scholar]
- Fritts, H. Tree Rings and Climate; Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Zuidema, P.A.; Baker, P.J.; Groenendijk, P.; Schippers, P.; van der Sleen, P.; Vlam, M.; Sterck, F. Tropical forests and global change: Filling knowledge gaps. Trends Plant Sci. 2013, 18, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Growth Dynamics of Conifer Tree Rings: Images of Past and Future Environments; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; Volume 183. [Google Scholar]
- Camarero, J.J.; Fernández-Pérez, L.; Kirdyanov, A.V.; Shestakova, T.A.; Knorre, A.A.; Kukarskih, V.V.; Voltas, J. Minimum wood density of conifers portrays changes in early season precipitation at dry and cold Eurasian regions. Trees 2017, 31, 1423–1437. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, R.; Yin, J.; Tian, K.; Xiao, D.; Zhang, W.; Yin, D. Radial growth response of major conifers to climate change on Haba Snow Mountain, Southwestern China. Dendrochronologia 2020, 60, 125682. [Google Scholar] [CrossRef]
- Montwe, D.; Isaac-Renton, M.; Hamann, A.; Spiecker, H. Cold adaptation recorded in tree rings highlights risks associated with climate change and assisted migration. Nat. Commun. 2018, 9, 1574. [Google Scholar] [CrossRef] [PubMed]
- Eilmann, B.; Sterck, F.; Wegner, L.; de Vries, S.M.; von Arx, G.; Mohren, G.M.; den Ouden, J.; Sass-Klaassen, U. Wood structural differences between northern and southern beech provenances growing at a moderate site. Tree Physiol. 2014, 34, 882–893. [Google Scholar] [CrossRef]
- Evans, M.E.; Gugger, P.F.; Lynch, A.M.; Guiterman, C.H.; Fowler, J.C.; Klesse, S.; Riordan, E.C. Dendroecology meets genomics in the common garden: New insights into climate adaptation. New Phytol. 2018, 218, 401–403. [Google Scholar] [CrossRef]
- Fkiri, S.; Guibal, F.; Fady, B.; El Khorchani, A.; Khaldi, A.; Khouja, M.L.; Nasr, Z. Tree-rings to climate relationships in nineteen provenances of four black pines sub-species (Pinus nigra Arn.) growing in a common garden from Northwest Tunisia. Dendrochronologia 2018, 50, 44–51. [Google Scholar] [CrossRef]
- Isaac-Renton, M.G.; Roberts, D.R.; Hamann, A.; Spiecker, H. Douglas-fir plantations in Europe: A retrospective test of assisted migration to address climate change. Glob. Chang. Biol. 2014, 20, 2607–2617. [Google Scholar] [CrossRef]
- López, L.; Villalba, R. Climate influences on the radial growth of Centrolobium microchaete, a valuable timber species from the tropical dry forests in Bolivia. Biotropica 2011, 43, 41–49. [Google Scholar] [CrossRef]
- Arend, M.; Kuster, T.; Günthardt-Goerg, M.S.; Dobbertin, M. Provenance-specific growth responses to drought and air warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol. 2011, 31, 287–297. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Lamy, J.B.; Ducousso, A.; Musch, B.; Ehrenmann, F.; Delzon, S.; Cavers, S.; Chałupka, W.; Dağdaş, S.; Hansen, J.K. Adaptive and plastic responses of Quercus petraea populations to climate across Europe. Glob. Chang. Biol. 2017, 23, 2831–2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, C.; Puchi, P.; Profumo, L.; Ferreira, A.; Muñoz, A. Effect of climate on tree growth in the Pampa biome of Southeastern South America: First tree-ring chronologies from Uruguay. Dendrochronologia 2018, 52, 113–122. [Google Scholar] [CrossRef]
- Sheng, W. Chinese Plantation and Its Forest Cultivation System; Forestry Publishing House: Beijing, China, 2014. (In Chinese) [Google Scholar]
- Yu, X. Study on Chinese fir in China. J. Fujian Coll. For. 1988, 8, 203–220. [Google Scholar]
- Hong, J.; Li, X.; Chen, Y. Memoir of Chinese fir provenance test. For. Rec. 1994, 7. (In Chinese) [Google Scholar]
- Chen, P.; Welsh, C.; Hamann, A. Geographic variation in growth response of Douglas-fir to interannual climate variability and projected climate change. Glob. Chang. Biol. 2010, 16, 3374–3385. [Google Scholar] [CrossRef]
- Jensen, J.S. Provenance variation in phenotypic traits in Quercus robur and Quercus petraea in Danish provenance trials. Scand. J. For. Res. 2000, 15, 297–308. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, A.; Duan, A.; Wu, H.; Zhang, J. Responses to subtropical climate in radial growth and wood density of Chinese fir provenances, Southern China. For. Ecol. Manag. 2022, 521, 120428. [Google Scholar] [CrossRef]
- Stokes, M.A. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Schinker, M.G.; Hansen, N.; Spiecker, H. High-frequency densitometry—A new method for the rapid evaluation of wood density variations. IAWA J. 2003, 24, 231–239. [Google Scholar] [CrossRef]
- Koutsianitis, D.; Barboutis, I. Application of high frequency densitometry to determine wood density and ring width of beech trees. Pro Ligno 2017, 13, 78–83. [Google Scholar]
- Holmes, R.L. Computer assisted quality contro. Tree-Ring Bull 1983, 43, 69–78. [Google Scholar]
- Rinn, F. Lignovision™—Scanning of Wood and Tree-Ring Recognition; User Reference: Heidelberg, Germany, 2006. [Google Scholar]
- Fang, K.; Gou, X.; Peters, K.; Li, J.; Zhang, F. Removing Biological Trends from Tree-Ring Series: Testing Modified Hugershoff Curves. Tree-Ring Res. 2010, 66, 51–59. [Google Scholar] [CrossRef]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Thurm, E.A.; Uhl, E.; Pretzsch, H. Mixture reduces climate sensitivity of Douglas-fir stem growth. For. Ecol. Manag. 2016, 376, 205–220. [Google Scholar] [CrossRef]
- Wigley, T.M.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Biondi, F.; Qeadan, F. Inequality in paleorecords. Ecology 2008, 89, 1056–1067. [Google Scholar] [CrossRef] [Green Version]
- Klisz, M.; Puchałka, R.; Netsvetov, M.; Prokopuk, Y.; Vítková, M.; Sádlo, J.; Matisons, R.; Mionskowski, M.; Chakraborty, D.; Olszewski, P. Variability in climate-growth reaction of Robinia pseudoacacia in Eastern Europe indicates potential for acclimatisation to future climate. For. Ecol. Manag. 2021, 492, 119194. [Google Scholar] [CrossRef]
- Team, R.C. The R Foundation for Statistical Computing Platform. R Version 2020, 3. [Google Scholar]
- Revelle, W.R. psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2017. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 21 June 2022).
- Matyas, C. Climatic adaptation of trees: Rediscovering provenance tests. Euphytica 1996, 92, 45–54. [Google Scholar] [CrossRef]
- Liu, S.; Gao, P.; Liu, P.; Niu, X.; Wang, B. The Response of Chinese Fir Forest Tree Ring Growth to Climate Change in China’s Dagangshan Region. Pol. J. Environ. Stud. 2019, 28, 2371–2379. [Google Scholar]
- Hevia, A.; Campelo, F.; Chambel, R.; Vieira, J.; Alía, R.; Majada, J.; Sánchez-Salguero, R. Which matters more for wood traits in Pinus halepensis Mill., provenance or climate? Ann. For. Sci. 2020, 77, 55. [Google Scholar] [CrossRef]
- Andreu, L.; Planells, O.; Gutiérrez, E.; Helle, G.; Schleser, G.H. Climatic significance of tree-ring width and δ13C in a Spanish pine forest network. Tellus B Chem. Phys. Meteorol. 2008, 60, 771–781. [Google Scholar] [CrossRef]
- Savva, Y.V.; Schweingruber, F.; Vaganov, E.; Milyutin, L. Influence of climate changes on tree-ring characteristics of Scots pine provenances in Southern Siberia (forest-steppe). IAWA J. 2003, 24, 371–383. [Google Scholar] [CrossRef]
- Suvanto, S.; Nöjd, P.; Henttonen, H.M.; Beuker, E.; Mäkinen, H. Geographical patterns in the radial growth response of Norway spruce provenances to climatic variation. Agric. For. Meteorol. 2016, 222, 10–20. [Google Scholar] [CrossRef]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar]
- Shi, J.; Li, J.; Cook, E.R.; Zhang, X.; Lu, H. Growth response of Pinus tabulaeformis to climate along an elevation gradient in the eastern Qinling Mountains, central China. Clim. Res. 2012, 53, 157–167. [Google Scholar] [CrossRef]
- Peng, J.; Li, J.; Wang, T.; Huo, J.; Yang, L. Effect of altitude on climate–growth relationships of Chinese white pine (Pinus armandii) in the northern Funiu Mountain, central China. Clim. Chang. 2019, 154, 273–288. [Google Scholar] [CrossRef]
- Shimamoto, C.Y.; Botosso, P.C.; Amano, E.; Marques, M.C. Stem growth rhythms in trees of a tropical rainforest in Southern Brazil. Trees 2016, 30, 99–111. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Griçar, J.; Seo, J.W.; Rathgeber, C.B.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Maherali, H.; Pockman, W.T.; Jackson, R.B. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 2004, 85, 2184–2199. [Google Scholar] [CrossRef]
- Pederson, N.; Cook, E.R.; Jacoby, G.C.; Peteet, D.M.; Griffin, K.L. The influence of winter temperatures on the annual radial growth of six northern range margin tree species. Dendrochronologia 2004, 22, 7–29. [Google Scholar] [CrossRef]
- King, G.M.; Gugerli, F.; Fonti, P.; Frank, D.C. Tree growth response along an elevational gradient: Climate or genetics? Oecologia 2013, 173, 1587–1600. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Desplanque, C.; Rolland, C.; Michalet, R. Dendroécologie comparée du sapin blanc (Abies alba) et de l’épicéa commun (Picea abies) dans une vallée alpine de France. Can. J. For. Res. 1998, 28, 737–748. [Google Scholar] [CrossRef]
- Camarero, J.J.; Rozas, V.; Olano, J.M.; Fernández-Palacios, J.M. Minimum wood density of Juniperus thurifera is a robust proxy of spring water availability in a continental Mediterranean climate. J. Biogeogr. 2014, 41, 1105–1114. [Google Scholar] [CrossRef]
- Klisz, M.; Ukalska, J.; Koprowski, M.; Tereba, A.; Puchałka, R.; Przybylski, P.; Jastrzębowski, S.; Nabais, C. Effect of provenance and climate on intra-annual density fluctuations of Norway spruce Picea abies (L.) Karst. in Poland. Agric. For. Meteorol. 2019, 269, 145–156. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Rathgeber, C.B.; Ulrich, E. Sensitivity of French temperate coniferous forests to climate variability and extreme events (Abies alba, Picea abies and Pinus sylvestris). J. Veg. Sci. 2010, 21, 364–376. [Google Scholar] [CrossRef]
- Camarero, J.J.; Hevia, A. Links between climate, drought and minimum wood density in conifers. IAWA J. 2020, 41, 236–255. [Google Scholar] [CrossRef]
- Balzano, A.; Battipaglia, G.; De Micco, V. Wood-trait analysis to understand climatic factors triggering intra-annual density-fluctuations in co-occurring Mediterranean trees. IAWA J. 2019, 40, 241–258. [Google Scholar] [CrossRef] [Green Version]
- Latreille, A.; Davi, H.; Huard, F.; Pichot, C. Variability of the climate-radial growth relationship among Abies alba trees and populations along altitudinal gradients. For. Ecol. Manag. 2017, 396, 150–159. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Yanchuk, A.; O’neill, G.; Aitken, S. Use of response functions in selecting lodgepole pine populations for future climates. Glob. Chang. Biol. 2006, 12, 2404–2416. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Camarero, J.J.; Olano, J.M.; Parras, A. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytol. 2010, 185, 471–480. [Google Scholar] [CrossRef] [PubMed]

| Provenance Zone | Number of Provenances | Number of Discs | DBH (cm) | MAP (mm) | MAT (°C) | MINT (°C) | MAXT (°C) | RH (%) |
|---|---|---|---|---|---|---|---|---|
| QBM | 2 | 22 | 18.55 | 1067.0 | 16.3 | −2.7 | 37.2 | 78.8 |
| DTM | 2 | 22 | 17.25 | 1123.6 | 16.3 | −6.1 | 37.8 | 74.6 |
| SC | 5 | 57 | 19.30 | 1078.6 | 15.2 | −3.0 | 34.3 | 78.6 |
| HTM | 4 | 45 | 20.28 | 1336.8 | 16.6 | −6.2 | 37.9 | 74.9 |
| GZM | 3 | 38 | 21.10 | 1096.7 | 16.8 | −1.3 | 37.2 | 76.7 |
| XEGZ | 11 | 133 | 19.65 | 1530.8 | 17.7 | −3.4 | 38.5 | 77.7 |
| NLM | 16 | 191 | 22.97 | 1771.4 | 19.9 | −0.8 | 37.3 | 77.0 |
| MYGD | 9 | 106 | 19.62 | 1090.7 | 16.8 | −1.7 | 33.7 | 76.9 |
| Provenance Zone | TRW | EWW | LWW | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rbar | EPS | SNR | Ginicoef | AC1 | Rbar | EPS | SNR | Ginicoef | AC1 | Rbar | EPS | SNR | Ginicoef | AC1 | Sampdepth | |
| QBM | 0.167 | 0.861 | 6.215 | 0.320 | 0.417 | 0.141 | 0.836 | 5.082 | 0.399 | 0.308 | 0.147 | 0.843 | 5.354 | 0.366 | 0.295 | 31 |
| DTM | 0.295 | 0.936 | 14.644 | 0.445 | 0.515 | 0.255 | 0.923 | 11.977 | 0.525 | 0.473 | 0.289 | 0.934 | 14.231 | 0.480 | 0.397 | 35 |
| SC | 0.238 | 0.970 | 32.160 | 0.462 | 0.506 | 0.235 | 0.969 | 31.285 | 0.546 | 0.458 | 0.161 | 0.951 | 19.566 | 0.454 | 0.334 | 103 |
| HTM | 0.215 | 0.940 | 15.606 | 0.385 | 0.451 | 0.214 | 0.939 | 15.479 | 0.438 | 0.363 | 0.209 | 0.938 | 15.069 | 0.443 | 0.341 | 57 |
| GZM | 0.248 | 0.942 | 16.154 | 0.437 | 0.448 | 0.166 | 0.907 | 9.787 | 0.483 | 0.349 | 0.189 | 0.919 | 11.409 | 0.438 | 0.307 | 49 |
| XEGZ | 0.202 | 0.980 | 47.999 | 0.393 | 0.436 | 0.200 | 0.979 | 47.375 | 0.485 | 0.385 | 0.166 | 0.974 | 37.690 | 0.447 | 0.301 | 190 |
| NLM | 0.216 | 0.988 | 82.285 | 0.430 | 0.474 | 0.236 | 0.989 | 91.896 | 0.508 | 0.441 | 0.168 | 0.984 | 59.968 | 0.439 | 0.331 | 298 |
| MYGD | 0.180 | 0.972 | 34.901 | 0.432 | 0.470 | 0.166 | 0.969 | 31.548 | 0.505 | 0.419 | 0.167 | 0.971 | 33.088 | 0.455 | 0.332 | 159 |
| Provenance Zone | EWD | MIND | LWD | MAXD | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rbar | EPS | SNR | Ginicoef | AC1 | Rbar | EPS | SNR | Ginicoef | AC1 | Rbar | EPS | SNR | Ginicoef | AC1 | Rbar | EPS | SNR | Ginicoef | AC1 | Sampdepth | |
| QBM | 0.060 | 0.666 | 1.994 | 0.250 | 0.228 | 0.100 | 0.776 | 3.461 | 1.045 | 0.382 | 0.107 | 0.788 | 3.711 | 0.126 | 0.127 | 0.112 | 0.796 | 3.907 | 0.114 | 0.109 | 31 |
| DTM | 0.050 | 0.646 | 1.823 | 0.352 | 0.338 | 0.076 | 0.741 | 2.864 | 2.195 | 0.482 | 0.088 | 0.772 | 3.388 | 0.160 | 0.179 | 0.064 | 0.706 | 2.399 | 0.146 | 0.137 | 35 |
| SC | 0.059 | 0.865 | 6.389 | 0.303 | 0.262 | 0.114 | 0.929 | 13.178 | 4.716 | 0.436 | 0.091 | 0.911 | 10.243 | 0.119 | 0.184 | 0.082 | 0.901 | 9.100 | 0.111 | 0.156 | 103 |
| HTM | 0.119 | 0.885 | 7.720 | 0.391 | 0.365 | 0.114 | 0.880 | 7.337 | 3.732 | 0.512 | 0.109 | 0.875 | 6.970 | 0.129 | 0.254 | 0.088 | 0.846 | 5.510 | 0.114 | 0.186 | 57 |
| GZM | 0.117 | 0.866 | 6.478 | 0.254 | 0.316 | 0.186 | 0.918 | 11.193 | 1.180 | 0.490 | 0.100 | 0.844 | 5.423 | 0.118 | 0.226 | 0.067 | 0.778 | 3.502 | 0.109 | 0.148 | 49 |
| XEGZ | 0.092 | 0.951 | 19.233 | 0.408 | 0.320 | 0.103 | 0.956 | 21.740 | 2.135 | 0.381 | 0.096 | 0.953 | 20.224 | 0.135 | 0.181 | 0.074 | 0.938 | 15.172 | 0.121 | 0.119 | 190 |
| NLM | 0.097 | 0.970 | 32.177 | 1.971 | 0.336 | 0.106 | 0.972 | 35.336 | 5.120 | 0.478 | 0.102 | 0.971 | 33.854 | 0.131 | 0.235 | 0.073 | 0.959 | 23.296 | 0.119 | 0.170 | 298 |
| MYGD | 0.093 | 0.942 | 16.388 | 0.277 | 0.310 | 0.165 | 0.969 | 31.446 | 3.900 | 0.474 | 0.101 | 0.947 | 17.942 | 0.136 | 0.219 | 0.070 | 0.923 | 12.058 | 0.123 | 0.142 | 159 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Sun, J.; Duan, A.; Zhu, A.; Wu, H.; Zhang, J. Dendroclimatological Analysis of Chinese Fir Using a Long-Term Provenance Trial in Southern China. Forests 2022, 13, 1348. https://doi.org/10.3390/f13091348
Wang H, Sun J, Duan A, Zhu A, Wu H, Zhang J. Dendroclimatological Analysis of Chinese Fir Using a Long-Term Provenance Trial in Southern China. Forests. 2022; 13(9):1348. https://doi.org/10.3390/f13091348
Chicago/Turabian StyleWang, Hong, Jianjun Sun, Aiguo Duan, Anming Zhu, Hanbin Wu, and Jianguo Zhang. 2022. "Dendroclimatological Analysis of Chinese Fir Using a Long-Term Provenance Trial in Southern China" Forests 13, no. 9: 1348. https://doi.org/10.3390/f13091348
APA StyleWang, H., Sun, J., Duan, A., Zhu, A., Wu, H., & Zhang, J. (2022). Dendroclimatological Analysis of Chinese Fir Using a Long-Term Provenance Trial in Southern China. Forests, 13(9), 1348. https://doi.org/10.3390/f13091348





