Effects of Sewage Sludge Application on Plant Growth and Soil Characteristics at a Pinus sylvestris var. mongolica Plantation in Horqin Sandy Land
Abstract
1. Introduction
2. Materials and Methods
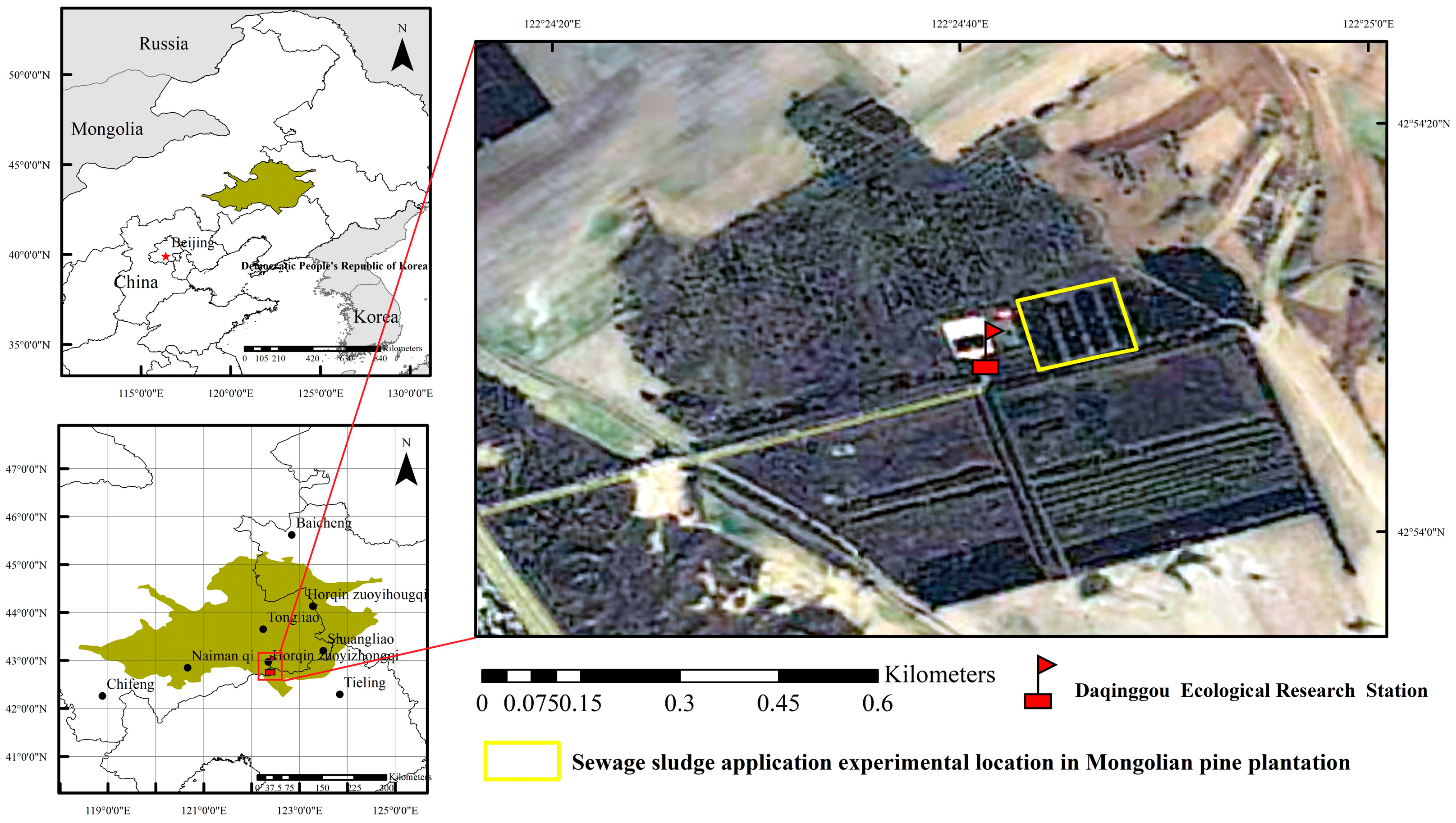
2.1. Site Description
2.2. Sewage Sludge
2.3. Experimental Design
2.4. Vegetation Survey
2.5. Soil Collection and Laboratory Analysis
2.6. Statistic Analysis
3. Results
3.1. Effects of SS Application on Tree Growth
3.2. Effects of SS Application on Species Diversity, Coverage, and Aboveground Biomass of the Understory
3.3. Effects of SS Application on Soil Physical and Chemical Properties
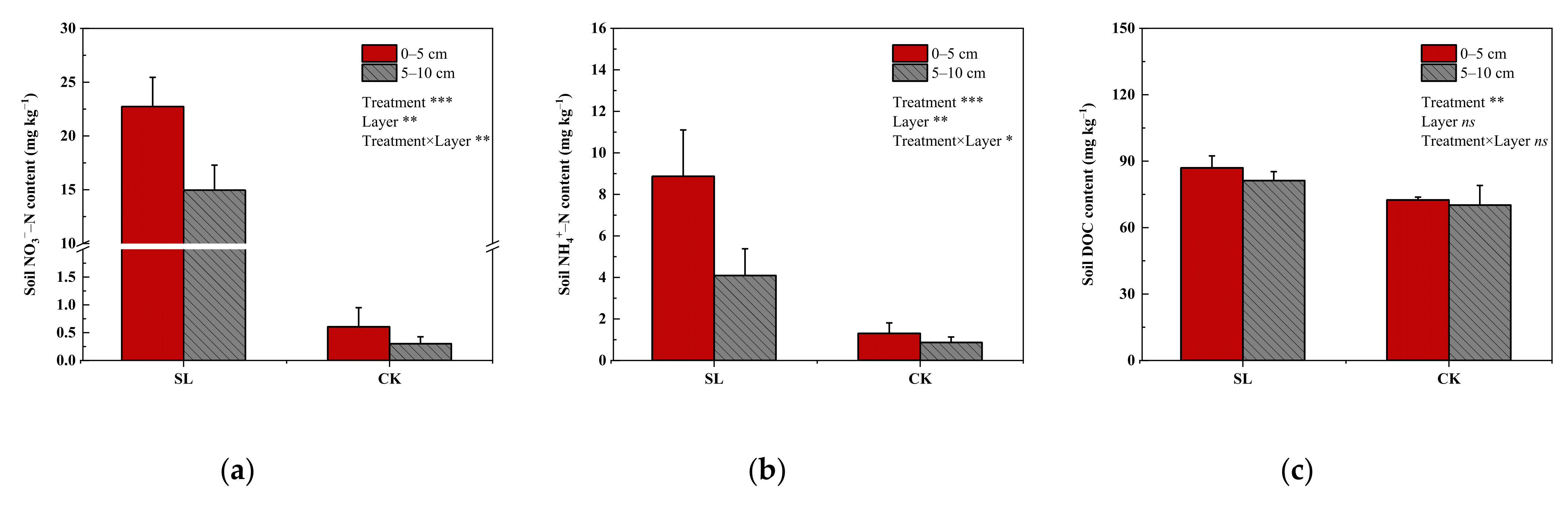
3.4. Effects of SS Application on Soil NO3−–N, NH4+–N, and DOC
3.5. Effects of SS Application on SOC and TN Stocks
4. Discussion
4.1. Responses of Plant Growth Performances to SS Application
4.2. Responses of Soil Properties to SS Application
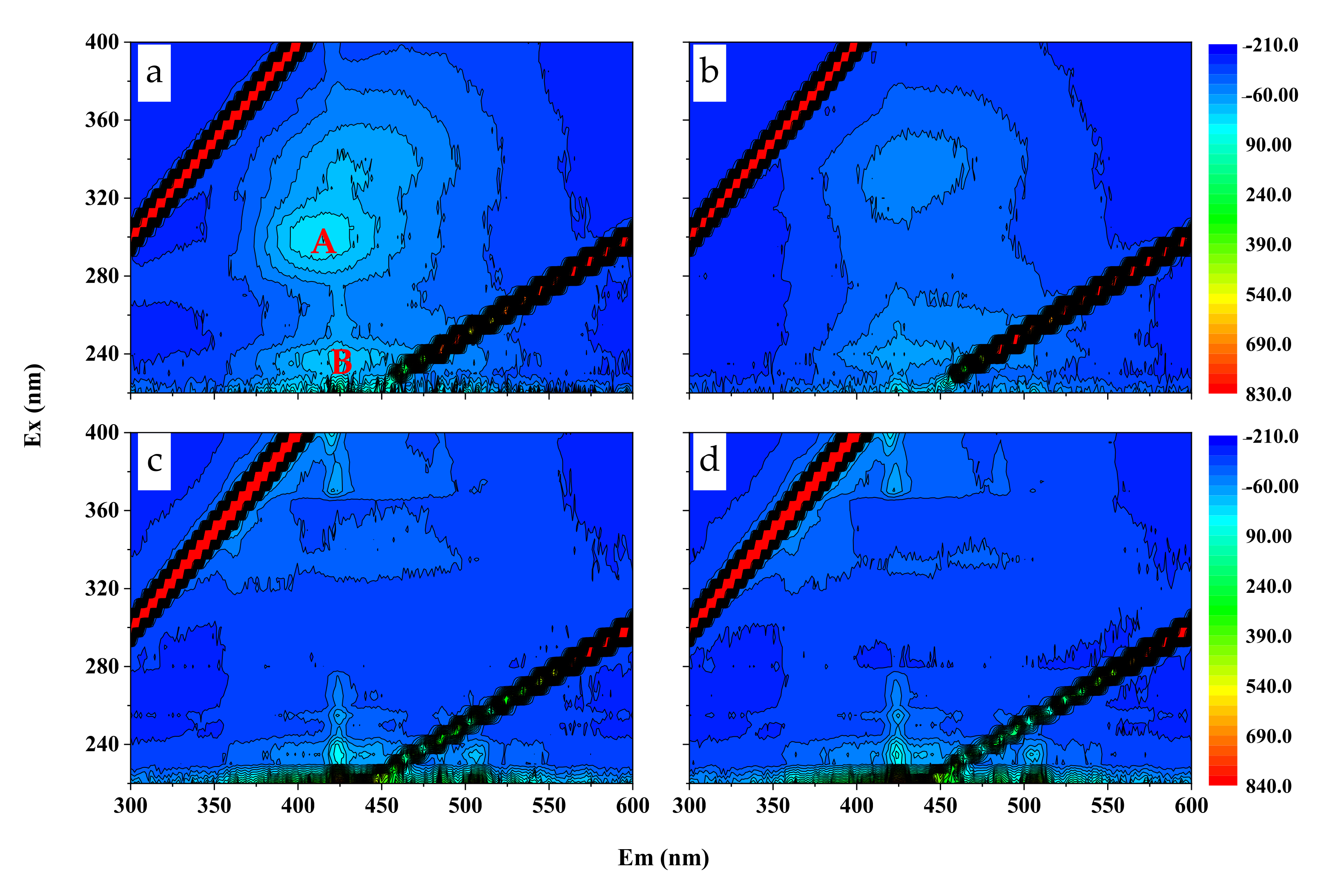
4.3. Responses of SOC, TN Stocks, and DOC Content to SS Application
4.4. Implications for Relationships between Soil Properties and Plant Performance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steele, J.C.; Meng, X.Z.; Venkatesan, A.K.; Halden, R.U. Comparative meta-analysis of organic contaminants in sewage sludge from the United States and China. Sci. Total Environ. 2022, 821, 153423. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Junior, C.H.; Brossi, M.J.D.; Monteiro, R.T.; Cardoso, P.H.S.; Mandu, T.D.; Nogueira, T.A.R.; Ganga, A.; Filzmoser, P.; de Oliveira, F.C.; Firme, L.P.; et al. Effects of sewage sludge application on unfertile tropical soils evaluated by multiple approaches: A field experiment in a commercial Eucalyptus plantation. Sci. Total Environ. 2019, 655, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.L.; Zhu, F.Y.; Li, Q.Y.; Xue, C.H.; Xia, X.H.; Yu, H.; Zhao, Q.L.; Jiang, J.Q.; Bai, S.W. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis. Environ. Int. 2020, 144, 106093. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Wang, X.M.; Liu, H.Q.; Yu, H.Q.; Li, W.W. Optimizing operation of municipal wastewater treatment plants in China: The remaining barriers and future implications. Environ. Int. 2019, 129, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Seiple, T.E.; Coleman, A.M.; Skaggs, R.L. Municipal wastewater sludge as a sustainable bioresource in the United States. J. Environ. Manag. 2017, 197, 673–680. [Google Scholar] [CrossRef]
- Patureau, D.; Mailler, R.; Delgenes, N.; Danel, A.; Vulliet, E.; Deshayes, S.; Moilleron, R.; Rocher, V.; Gasperi, J. Fate of emerging and priority micropollutants during the sewage sludge treatment—Part 2: Mass balances of organic contaminants on sludge treatments are challenging. Waste Manag. 2021, 125, 122–131. [Google Scholar] [CrossRef]
- Elmi, A.; Al-khaldy, A.; AlOlayan, M. Sewage sludge land application: Balancing act between agronomic benefits and environmental concerns. J. Clean. Prod. 2020, 250, 119512. [Google Scholar] [CrossRef]
- Hao, X.D.; Chen, Q.; van Loosdrecht, M.C.M.; Li, J.; Jiang, H. Sustainable disposal of excess sludge: Incineration without anaerobic digestion. Water Res. 2020, 170, 115298. [Google Scholar] [CrossRef]
- Sun, X.K.; Bai, J.; Dong, D.B.; Fan, Z.P. UV assisted photocatalytic remediation of polycyclic aromatic hydrocarbons (PAHs) in sewage sludge addition soils using synthesized nanometer mixed-crystal TiO2: Experiment and simulation. Soil Sediment Contam. 2022, 31, 572–585. [Google Scholar] [CrossRef]
- Rutgersson, C.; Ebmeyer, S.; Lassen, S.B.; Karkman, A.; Fickf, J.; Kristiansson, E.; Brandt, K.K.; Flach, C.F.; Larsson, D.G.J. Long-term application of Swedish sewage sludge on farmland does not cause clear changes in the soil bacterial resistome. Environ. Int. 2020, 137, 105339. [Google Scholar] [CrossRef]
- Cieślik, B.M.; Namieśnik, J.; Konieczka, P. Review of sewage sludge management: Standards, regulations and analytical methods. J. Clean. Prod. 2015, 90, 1–15. [Google Scholar] [CrossRef]
- Jain, M.S.; Paul, S.; Kalamdhad, A.S. Utilization of Biochar as an amendment during lignocellulose waste composting: Impact on composting physics and Realization (probability) amongst physical properties. Process Saf. Environ. 2019, 121, 229–238. [Google Scholar] [CrossRef]
- Liu, J.Y.; Fu, J.W.; Ning, X.A.; Sun, S.Y.; Wang, Y.J.; Xie, W.M.; Huang, S.S.; Zhong, S. An experimental and thermodynamic equilibrium investigation of the Pb, Zn, Cr, Cu, Mn and Ni partitioning during sewage sludge incineration. J. Environ. Sci. 2015, 35, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.Y.; Long, G.C.; Zhou, J.L.; Ma, C. Valorization of sewage sludge in the fabrication of construction and building materials: A review. Resour. Conserv. Recycl. 2020, 154, 104606. [Google Scholar] [CrossRef]
- Alvarenga, P.; Mourinha, C.; Farto, M.; Santos, T.; Palma, P.; Sengo, J.; Morais, M.C.; Cunha-Queda, C. Sewage sludge, compost and other representative organic wastes as agricultural soil amendments: Benefits versus limiting factors. Waste Manag. 2015, 40, 44–52. [Google Scholar] [CrossRef]
- Mañas, P.; Castro, E.; de las Heras, J. Application of treated wastewater and digested sewage sludge to obtain biomass from Cynara cardunculus L. J. Clean. Prod. 2014, 67, 72–78. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Epa Unable to Assess the Impact of Hundreds of Unregulated Pollutants in Land-Applied Biosolids on Human Health and the Environment Report No. 19–p–0002; National Service Center for Environmental Publications: Washington, DC, USA, 2018; pp. 6–7. [Google Scholar]
- Santos, A.F.; Vaz, T.E.; Lopes, D.V.; Cardoso, O.; Quina, M.J. Beneficial use of lime mud from kraft pulp industry for drying and microbiological decontamination of sewage sludge. J. Environ. Manag. 2021, 296, 113255. [Google Scholar] [CrossRef]
- Alaoui-Sossé, B.; Bourioug, M.; Benbrahim, M.; Carnus, J.M.; Clert, S.; Bourgeade, P.; Aleya, L. Influence of different sludge compositions on understorey vegetation in an amended Pinus pinatser forest plantation. Sci. Total Environ. 2018, 640–641, 1082–1087. [Google Scholar] [CrossRef]
- Gutiérrez-Ginés, M.J.; Robinson, B.H.; Esperschuetz, J.; Madejón, E.; Horswell, J.; McLenaghen, R. Potential use of biosolids to reforest degraded areas with New Zealand native vegetation. J. Environ. Qual. 2017, 46, 906–914. [Google Scholar] [CrossRef]
- Zhu, T.S.; Shao, T.Y.; Liu, J.Y.; Li, N.; Long, X.H.; Gao, X.M.; Rengel, Z. Improvement of physico-chemical properties and microbiome in different salinity soils by incorporating Jerusalem artichoke residues. Appl. Soil Ecol. 2021, 158, 103791. [Google Scholar] [CrossRef]
- Mosquera-Losada, M.R.; Rigueiro-Rodríguez, A.; Fernández-Núñez, E. Deciduous plantations established on former agricultural land in northwest of Spain as silvopastoralism: Tree growth; pasture production and vascular plant biodiversity. Catena 2018, 169, 1–10. [Google Scholar] [CrossRef]
- Denaix, L.; Anne-Laure Thomas-Chéry, A.L.; Balet, J.; Benbrahim, M.; Carnus, J.M. Effects of municipal sewage sludge application on soil and purple moor-grass (Molinia caerulea) contamination by metals in a maritime pine forest. Water Air Soil Pollut. 2011, 219, 239–249. [Google Scholar] [CrossRef]
- Rodriguez, D.R.O.; Andrade, G.D.; Bellote, A.F.J.; Tomazello, M. Effect of pulp and paper mill sludge on the development of 17-year-old loblolly pine (Pinus taeda L.) trees in Southern Brazil. Forest Ecol. Manag. 2018, 422, 179–189. [Google Scholar] [CrossRef]
- Wang, M.H.; Xue, J.M.; Horswell, J.Q.; Kimberley, M.O.; Huang, Z.Q. Long-term biosolids application alters the composition of soil microbial groups and nutrient status in a pine plantation. Biol. Fertil. Soils 2017, 53, 799–809. [Google Scholar] [CrossRef]
- Li, Y.S.; Sun, B.; Deng, T.Y.; Lian, P.; Chen, J.H.; Peng, X.W. Safety and efficiency of sewage sludge and garden waste compost as a soil amendment based on the field application in woodland. Ecotox. Environ. Safe 2021, 222, 112497. [Google Scholar] [CrossRef]
- Zhao, Q.; Chu, S.S.; He, D.; Wu, D.M.; Mo, Q.F.; Zeng, S.C. Sewage sludge application alters the composition and co-occurrence pattern of the soil bacterial community in southern China forestlands. Appl. Soil Ecol. 2021, 157, 103744. [Google Scholar] [CrossRef]
- Abreu, C.H.; Firme, L.P.; Maldonado, C.A.B.; Neto, S.P.D.; Alves, M.C.; Muraoka, T.; Boaretto, A.E.; Gava, J.L.; He, Z.L.; Nogueira, T.A.R.; et al. Fertilization using sewage sludge in unfertile tropical soils increased wood production in Eucalyptus plantations. J. Environ. Manag. 2017, 203, 51–58. [Google Scholar] [CrossRef]
- Sass, A.L.; Bassaco, M.V.M.; Motta, A.C.V.; Maeda, S.; Barbosa, J.Z.; Bognola, I.A.; Bosco, J.V.G.; Goularte, G.D.; Prior, S.A. Cellulosic industrial waste to enhance Pinus taeda nutrition and growth: A study in subtropical Brazil. Sci. For. 2020, 48, e3165. [Google Scholar] [CrossRef]
- Song, L.N.; Zhu, J.J.; Zheng, X.; Wang, K.; Lü, L.Y.; Zhang, X.L.; Hao, G.Y. Transpiration and canopy conductance dynamics of Pinus sylvestris var. mongolica in its natural range and in an introduced region in the sandy plains of Northern China. Agric. Forest Meteorol. 2020, 281, 107830. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, J.J.; Yan, Q.L.; Song, L.N. Effects of land use changes on the groundwater table and the decline of Pinus sylvestris var. mongolica plantations in southern Horqin Sandy Land, Northeast China. Agric. Water Manag. 2012, 109, 94–106. [Google Scholar] [CrossRef]
- Song, L.N.; Zhu, J.J.; Li, M.C.; Zhang, J.X.; Lv, L.Y. Sources of water used by Pinus sylvestris var. mongolica trees based on stable isotope measurements in a semiarid sandy region of Northeast China. Agric. Water Manag. 2016, 164, 281–290. [Google Scholar] [CrossRef]
- Song, L.N.; Zhu, J.J.; Li, M.C.; Yu, Z.Y. Water utilization of Pinus sylvestris var. mongolica in a sparse wood grassland in the semiarid sandy region of Northeast China. Trees-Struct. Funct. 2014, 28, 971–982. [Google Scholar] [CrossRef]
- Sun, S.J.; Lei, S.; Jia, H.S.; Li, C.Y.; Zhang, J.S.; Meng, P. Tree-ring analysis reveals density-dependent vulnerability to drought in planted Mongolian pines. Forests 2020, 11, 98. [Google Scholar] [CrossRef]
- Shi, S.N.; Yu, Z.Y.; Zhao, Q. Responses of plant diversity and species composition to the cessation of fertilization in a sandy grassland. J. Forestry Res. 2014, 25, 337–342. [Google Scholar] [CrossRef]
- Song, L.N.; Zhu, J.J.; Li, M.C.; Yan, Q.L. Intrinsic water use efficiency in wet and dry years at young and old plantations of Pinus sylvestris var. mongolica in semiarid China. J. Forest Res. 2015, 20, 263–271. [Google Scholar] [CrossRef]
- Huang, L.R.; Li, X.; Tang, F.D.; Yan, C.F.; Chen, Z.L.; You, G.C.; Li, L.D.; Ai, J. Effect of municipal sewage sludge on the biomass and heavy metal accumulation of Mongolian pine (Pinus sylvestris var. mongolica) and heavy metal availability in the soil. Acta Sci. Circumstantiae 2010, 30, 2450–2456. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, L.L.; Zhao, Q.; Sun, Q.Y.; Liu, L.; Zeng, D.H. Nitrogen addition elevated autumn phosphorus retranslocation of living needles but not resorption in a nutrient-poor Pinus sylvestris var. Mongolica plantation. Forest Ecol. Manag. 2020, 468, 118174. [Google Scholar] [CrossRef]
- Zhao, Q.; Zeng, D.H. Nitrogen addition effects on tree growth and soil properties mediated by soil phosphorus availability and tree species identity. For. Ecol. Manag. 2019, 449, 117478. [Google Scholar] [CrossRef]
- Fan, Z.P.; Tu, Z.H.; Li, F.Y.; Qin, Y.B.; Deng, D.Z.; Zeng, D.H.; Sun, X.K.; Zhao, Q.; Hu, Y.L. Experimental manipulation of precipitation affects soil nitrogen availability in semiarid Mongolian pine (Pinus sylvestris var. mongolica) Plantation. Water 2017, 9, 208. [Google Scholar] [CrossRef]
- Chen, L.F.; He, Z.B.; Zhu, X.; Du, J.; Yang, J.J.; Li, J. Impacts of afforestation on plant diversity, soil properties, and soil organic carbon storage in a semi-arid grassland of northwestern China. Catena 2016, 147, 300–307. [Google Scholar] [CrossRef]
- Hu, Y.L.; Zeng, D.H.; Liu, Y.X.; Zhang, Y.L.; Chen, Z.H.; Wang, Z.Q. Responses of soil chemical and biological properties to nitrogen addition in a Dahurian larch plantation in Northeast China. Plant Soil 2010, 333, 81–92. [Google Scholar] [CrossRef]
- Ohno, T.; Fernandez, I.J.; Hiradate, S.; Sherman, J.F. Effects of soil acidification and forest type on water soluble soil organic matter properties. Geoderma 2007, 140, 176–187. [Google Scholar] [CrossRef]
- Corrêa1, R.S.; White, R.E.; Weatherley, A.J. Effects of sewage sludge stabilization on organic-N mineralization in two soils. Soil Use Manag. 2012, 28, 12–18. [Google Scholar] [CrossRef]
- Shukla, O.P.; Juwarkar, A.A.; Singh, S.K.; Khan, S.; Rai, U.N. Growth responses and metal accumulation capabilities of woody plants during the phytoremediation of tannery sludge. Waste Manag. 2011, 31, 115–123. [Google Scholar] [CrossRef]
- Rigueiro-Rodríguez, A.; Mosquera-Losada, M.R.; Ferreiro-Domínguez, N. Use of sewage sludge in silvopastoral systems under Pinus radiata D. Don: Soil, tree growth, and pasture production. Agroforest Syst. 2021, 95, 867–880. [Google Scholar] [CrossRef]
- Xu, G.Q.; Cao, X.Q.; Bai, L.P.; Qi, H.T.; Lu, H.B. Absorption, accumulation and distribution of metals and nutrient elements in poplars planted in land amended with composted sewage sludge: A field trial. Ecotox. Environ. Safe 2019, 182, 109360. [Google Scholar] [CrossRef]
- Hu, Y.L.; Niu, Z.X.; Zeng, D.H.; Wang, C.Y. Soil amendment improves tree growth and soil carbon and nitrogen pools in Mongolian pine plantations on post-mining land in Northeast China. Land Degrad. Develop. 2015, 26, 807–812. [Google Scholar] [CrossRef]
- Meyer, V.F.; Redente, E.F.; Barbarick, K.A.; Brobst, R.B.; Paschke, M.W.; Miller, A.L. Plant and soil responses to biosolids application following forest fire. J. Environ. Qual. 2004, 33, 873–881. [Google Scholar] [CrossRef]
- Mosquera-Losada, M.R.; Cuiñ-Cotarelo, R.; Rigueiro-Rodríguez, A. Effect of understory vegetation management through liming and sewage sludge fertilisation on soil fertility and Pinus radiate D. Don growth after reforestation. Eur. J. Forest Res. 2011, 130, 997–1008. [Google Scholar] [CrossRef][Green Version]
- Ferreiro-Domínguez, N.; Rigueiro-Rodríguez, A.; Bianchetto, E.; Mosquera-Losada, M.R. Effect of lime and sewage sludge fertilisation on tree and understory interaction in a silvopastoral system. Agric. Ecosyst. Environ. 2014, 188, 72–79. [Google Scholar] [CrossRef]
- Velayoudon, P.; Pagand, P.; Winterton, P.; Guiresse, M. Sewage sludge application for spontaneous plant restoration of a New Caledonian Ferralsol. Soil Res. 2014, 52, 76–86. [Google Scholar] [CrossRef]
- Tandy, S.; Wallace, H.L.; Jones, D.L.; Nason, M.A.; Williamson, J.C.; Healey, J.R. Can a mesotrophic grassland community be restored on a post-industrial sandy site with compost made from waste materials? Biol. Conserv. 2011, 144, 500–510. [Google Scholar] [CrossRef]
- Basta, N.T.; Busalacchi, D.M.; Hundal, L.S.; Kumar, K.; Dick, R.P.; Lanno, R.P.; Carlson, J.; Cox, A.E.; Granato, T.C. Restoring ecosystem function in degraded urban soil using biosolids, biosolids blend, and compost. J. Environ. Qual. 2016, 45, 74–83. [Google Scholar] [CrossRef]
- Gagnon, A.; Fenton, N.J.; Sirois, P.; Boucher, J.F. Plant community diversity at two reclaimed mine tailing storage facilities in Québec, Canada. Land 2021, 10, 1191. [Google Scholar] [CrossRef]
- Mohamed, B.; Olivier, G.; Francois, G.; Laurence, A.S.; Bourgeade, P.; Badr, A.S.; Lotfi, A. Sewage sludge as a soil amendment in a Larix decidua plantation: Effects on tree growth and floristic diversity. Sci. Total Environ. 2018, 621, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Y.; Zeng, D.H.; Jiang, F.Q.; Zhao, Q. Responses of biomass to the addition of water, nitrogen and phosphorus in Keerqin sandy grassland, Inner Mongolia, China. J. Forestry Res. 2009, 20, 23–26. [Google Scholar] [CrossRef]
- Chen, Y.; Högberg, P. Gross nitrogen mineralization rates still high 14 years after suspension of N input to a N-saturated forest. Soil Biol. Biochem. 2006, 38, 2001–2003. [Google Scholar] [CrossRef]
- Huberty, L.E.; Gross, K.L.; Miller, C.J. Effects of nitrogen addition on successional dynamics and species diversity in Michigan old-fields. J. Ecol. 1998, 86, 794–803. [Google Scholar] [CrossRef]
- Stevens, M.H.H.; Carson, W.P. Plant density determines species richness along an experimental fertility gradient. Ecology 1999, 80, 455–465. [Google Scholar] [CrossRef]
- Ritter, E. Carbon, nitrogen and phosphorus in volcanic soils following afforestation with native birch (Betula pubescens) and introduced larch (Larix sibirica) in Iceland. Plant Soil 2007, 295, 239–251. [Google Scholar] [CrossRef]
- Asensio, V.; Vega, F.A.; Andrade, M.L.; Covelo, E.F. Tree vegetation and waste amendments to improve the physical condition of copper mine soils. Chemosphere 2013, 90, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Beaudet, L.; Galopin, G.; Grosbellet, C. Effect of organic amendment for the construction of favourable urban soils for tree growth. Eur. J. Hortic. Sci. 2018, 83, 173–186. [Google Scholar] [CrossRef]
- Page-Dumroese, D.S.; Ott, M.R.; Strawn, D.G.; Tirocke, J.M. Using organic amendments to restore soil physical and chemical properties of a mine site in Northeastern Oregon, USA. Appl. Eng. Agric. 2018, 34, 43–55. [Google Scholar] [CrossRef]
- Song, U.; Lee, E.J. Environmental and economical assessment of sewage sludge compost application on soil and plants in a landfill. Resour. Conserv. Recy. 2010, 54, 1109–1116. [Google Scholar] [CrossRef]
- Eden, M.; Gerke, H.H.; Houot, S. Organic waste recycling in agriculture and related effects on soil water retention and plant available water: A review. Agron. Sustain. Dev. 2017, 37, 11. [Google Scholar] [CrossRef]
- Dhanker, R.; Chaudhary, S.; Goyal, S.; Garg, V.K. Influence of urban sewage sludge amendment on agricultural soil parameters. Environ. Technol. Innov. 2021, 23, 101642. [Google Scholar] [CrossRef]
- Ding, Z.L.; Kheir, A.M.S.; Ali, M.G.M.; Ali, O.A.M.; Abdelaal, A.I.N.; Lin, X.E.; Zhou, Z.X.; Wang, B.Z.; Liu, B.B.; He, Z.L. The integrated effect of salinity, organic amendments, phosphorus fertilizers, and deficit irrigation on soil properties, phosphorus fractionation and wheat productivity. Sci. Rep. 2020, 10, 2736. [Google Scholar] [CrossRef]
- Chen, H.M.; Ma, J.Y.; Wang, X.J.; Xu, P.P.; Zheng, S.; Zhao, Y.W. Effects of biochar and sludge on carbon storage of urban green roofs. Forests 2018, 9, 413. [Google Scholar] [CrossRef]
- Carabassa, V.; Domene, X.; Díaz, E.; Alcañiz, J.M. Mid-term effects on ecosystem services of quarry restoration with Technosols under Mediterranean conditions: 10-year impacts on soil organic carbon and vegetation development. J. Restor. Ecol. 2020, 28, 960–970. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Hechmi, S.; Hamdi, H.; Mokni-Tlili, S.; Zoghlami, I.R.; Khelil, M.N.; Benzarti, S.; Hassen, A.; Jedidi, N. Carbon mineralization, biological indicators, and phytotoxicity to assess the impact of urban sewage sludge on two light-textured soils in a microcosm. J. Environ. Qual. 2020, 49, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Higashikawa, F.S.; Silva, C.A.; Nunes, C.A.; Bettiol, W.; Guerreiro, M.C. Physico-chemical evaluation of organic wastes compost-based substrates for Eucalyptus seedlings growth. Commun. Soil Sci. Plan. 2016, 47, 581–592. [Google Scholar] [CrossRef]
- Asik, B.B.; Aydinalp, C.; Katkat, A.V.; Sagban, F.O.T. Effect of the application of various wastewater sludges on the properties of sandy soil. Environ. Monit. Assess. 2015, 187, 30. [Google Scholar] [CrossRef] [PubMed]
- Moreno, T.; Graziella, M.; Elena, B.; Maurizio, Q.; Giovambattista, S.; Enrico, M.; Angelo, I.; Lamberto, D. Agro-industry sludge as a potential organic fertilizer for a prompt nitrogen release. Commun. Soil. Sci. Plant 2017, 48, 999–1007. [Google Scholar] [CrossRef]
- Mitchell, D.S.; Edwards, A.C.; Ferrier, R.C. Changes in fluxes of N and P in water draining a stand of Scots pine treated with sewage sludge. Forest Ecol. Manag. 2000, 139, 203–213. [Google Scholar] [CrossRef]
- Cavalcante, J.S.; Favaretto, N.; Dieckow, J.; Cherobim, V.F.; Barth, G. Long-term surface application of dairy liquid manure to soil under no-till improves carbon and nitrogen stocks. Eur. J. Soil Sci. 2020, 71, 1132–1143. [Google Scholar] [CrossRef]
- Yang, X.M.; Reynolds, W.D.; Drury, C.F.; Fleming, R.; Tan, C.S.; Denholm, K.; Yang, J.Y. Organic carbon and nitrogen stocks in a clay loam soil 10 years after a single compost application. Can. J. Soil Sci. 2014, 94, 357–363. [Google Scholar] [CrossRef]
- Kätterera, T.; Börjesson, G.; Kirchmann, H. Changes in organic carbon in topsoil and subsoil and microbial community composition caused by repeated additions of organic amendments and N fertilisation in a long-term field experiment in Sweden. Agric. Ecosyst. Environ. 2014, 189, 110–118. [Google Scholar] [CrossRef]
- Koutroubas, S.D.; Antoniadis, V.; Fotiadis, S.; Damalas, C.A. Growth, grain yield and nitrogen use efficiency of Mediterranean wheat in soils amended with municipal sewage sludge. Nutr. Cycl. Agroecosys. 2014, 100, 227–243. [Google Scholar] [CrossRef]
- Silva, J.R.; Silva, D.J.; Gava, C.A.T.; de Oliveira, T.C.T.; de Freitas, M.D.C. Carbon in humic fractions of organic matter in soil treated with organic composts under mango cultivation. Rev. Bras. Cienc. Solo 2016, 40, e0150095. [Google Scholar] [CrossRef]
- Placek-Lapaj, A.; Grobelak, A.; Fijalkowski, K.; Singh, B.R.; Almas, A.R.; Kacprzak, M. Post—Mining soil as carbon storehouse under polish conditions. J. Environ. Manag. 2019, 238, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Paetsch, L.; Mueller, C.W.; Rumpel, C.; Houot, S.; Kögel-Knabner, I. Urban waste composts enhance OC and N stocks after long-term amendment but do not alter organic matter composition. Agric. Ecosyst. Environ. 2016, 223, 211–222. [Google Scholar] [CrossRef]
- Pegoraro, R.F.; de Souza, A.R.; Sampaio, R.A.; Frazão, L.A.; Rodrigues, M.N.; Maia, V.M. Carbon and nitrogen balance in an Oxisol influenced by sewage sludge and mineral fertilization. Commun. Soil Sci. Plant Anal. 2020, 51, 2441–2451. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Gang, Q.; Chang, S.X.; Lin, G.G.; Zhao, Q.; Mao, B.; Zeng, D.H. Exogenous and endogenous nitrogen differentially affect the decomposition of fine roots of different diameter classes of Mongolian pine in semi-arid northeast China. Plant Soil 2019, 436, 109–122. [Google Scholar] [CrossRef]
- Schaeffer, S.M.; Homyak, P.M.; Boot, C.M.; Roux-Michollet, D.; Schimel, J.P. Soil carbon and nitrogen dynamics throughout the summer drought in a California annual grassland. Soil Biol. Biochem. 2017, 115, 54–62. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Shangguan, Z.P.; Deng, L. Soil aggregate stability and aggregate-associated carbon and nitrogen in natural restoration grassland and Chinese red pine plantation on the Loess Plateau. Catena 2017, 149, 253–260. [Google Scholar] [CrossRef]
- Kalisz, B.; Lachacz, A.; Glazewski, R.; Klasa, A. Effect of municipal sewage sludge under Salix plantations on dissolved soil organic carbon pools. Arch. Environ. Prot. 2012, 38, 87–97. [Google Scholar] [CrossRef]
- Toosi, E.R.; Doane, T.A.; Horwath, W.R. Abiotic solubilization of soil organic matter, a less-seen aspect of dissolved organic matter production. Soil Biol. Biochem. 2012, 50, 12–21. [Google Scholar] [CrossRef]
- Andreasson, F.; Bergkvist, B.; Bååth, E. Bioavailability of DOC in leachates, soil matrix solution and soil water extracts from beech forest floors. Soil Biol. Biochem. 2009, 41, 1652–1658. [Google Scholar] [CrossRef]
- Roig, N.; Sierra, J.; Martí, E.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Long-term amendment of Spanish soils with sewage sludge: Effects on soil functioning. Agric. Ecosyst. Environ. 2012, 158, 41–48. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Tian, G. Chemical structure of humic acids in biosolids–amended soils as revealed by NMR spectroscopy. Appl. Soil Ecol. 2011, 49, 76–80. [Google Scholar] [CrossRef]
- Song, N.H.; Chen, L.; Yang, H. Effect of dissolved organic matter on mobility and activation of chlorotoluron in soil and wheat. Geoderma 2008, 146, 344–352. [Google Scholar] [CrossRef]
- Katsuyama, M.; Ohte, N. Determining the sources of stormflow from the fluorescence properties of dissolved organic carbon in a forested headwater catchment. J. Hydrol. 2002, 268, 192–202. [Google Scholar] [CrossRef]
- Xiao, K.K.; Horn, H.; Abbt-Braun, G. “Humic substances” measurement in sludge dissolved organic matter: A critical assessment of current methods. Chemosphere 2022, 293, 133608. [Google Scholar] [CrossRef]
- Li, W.T.; Chen, S.Y.; Xu, Z.X.; Li, Y.; Shuang, C.D.; Li, A.M. Characterization of dissolved organic matter in municipal wastewater using fluorescence PARAFAC analysis and chromatography multi-excitation/emission scan: A comparative study. Environ. Sci. Technol. 2014, 48, 2603–2609. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.F.; Li, D.M.; Xu, C.X.; Wu, M.; Liu, M.; Li, P.F.; Li, G.L.; Zhang, T.L.; Li, Z.P. Variation of soil dissolved organic carbon under long-term different fertilizations and its correlation with maize yields. J. Soil Sediment. 2020, 20, 2761–2770. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Shima, K. Composting of sewage sludge with a simple aeration method and its utilization as a soil fertilizer. Environ. Manag. 2019, 63, 455–465. [Google Scholar] [CrossRef]
- Duan, M.; House, J.; Chang, S.X. Understory plant communities vary with tree productivity in two reclaimed boreal upland forest types in Canada. Forest Ecol. Manag. 2019, 453, 117577. [Google Scholar] [CrossRef]
- Song, L.N.; Zhu, J.J.; Li, M.C.; Zhang, J.X.; Zheng, X.; Wang, K. Canopy transpiration of Pinus sylvestris var. mongolica in a sparse wood grassland in the semiarid sandy region of Northeast China. Agric. Forest Meteorol. 2018, 250–251, 192–201. [Google Scholar] [CrossRef]
- Song, L.N.; Zhu, J.J.; Zhang, J.X.; Zhang, T.; Wang, K.; Wang, G.C.; Liu, J.H. Effect of drought and topographic position on depth of soil water extraction of Pinus sylvestris L. var. Mongolica Litv. trees in a semiarid sandy region, Northeast China. Forests 2019, 10, 370. [Google Scholar] [CrossRef]
- Zhu, J.J.; Li, F.Q.; Xu, M.L.; Kang, H.Z.; Wu, X.Y. The role of ectomycorrhizal fungi in alleviating pine decline in semiarid sandy soil of northern China: An experimental approach. Ann. For. Sci. 2008, 65, 304. [Google Scholar] [CrossRef]
- Reich, P.B.; Frelich, L.E.; Voldseth, R.A.; Bakken, P.; Adair, E.C. Understorey diversity in southern boreal forests is regulated by productivity and its indirect impacts on resource availability and heterogeneity. J. Ecol. 2012, 100, 539–545. [Google Scholar] [CrossRef]



| Parameter | Unit | Mean Concentration in the SS | Limits |
|---|---|---|---|
| pH | 7.4 | ||
| Dry matter | % | 13.3 | |
| Organic matter | % | 77.7 | |
| Total nitrogen | % | 2.6 | |
| Total phosphorus | % | 1.0 | |
| Total potassium | % | 0.6 | |
| NH4+–N | mg kg−1 | 1198.6 | |
| NH3−–N | mg kg−1 | 12.9 | |
| Zn | mg kg−1 | 341 | <3000 |
| Cu | mg kg−1 | 65.7 | <1500 |
| Cr | mg kg−1 | 69.9 | <1000 |
| Cd | mg kg−1 | 0.5 | <20 |
| Ni | mg kg−1 | 16.6 | <200 |
| Pb | mg kg−1 | 11.9 | <1000 |
| As | mg kg−1 | 63.4 | <75 |
| Benzo (a) pyrene | mg kg−1 | 0.08 | <3 |
| PAHs | mg kg−1 | 1.2 | <6 |
| Treatment | Simpson’s Diversity Index | Species Richness | Coverage (%) | Aboveground Biomass (g m−2) |
|---|---|---|---|---|
| SL | 0.29 ± 0.21 | 3.00 ± 0.87 | 48.33 ± 22.68 | 176.27 ± 31.11 |
| CK | 0.51 ± 0.15 | 4.33 ± 1.89 | 36.33 ± 10.97 | 98.59 ± 32.51 |
| Student’s t-test | ns | ns | ns | * |
| Treatment | Soil Layer (cm) | SBD (g cm−3) | SWC (%) | pH | SOC (g kg−1) | TN (g kg−1) | TP (g kg−1) | C/N | N/P |
|---|---|---|---|---|---|---|---|---|---|
| SL | 0–5 | 1.43 ± 0.03 | 6.14 ± 0.99 | 6.87 ± 0.11 | 5.61 ± 1.35 | 0.51 ± 0.12 | 0.15 ± 0.03 | 10.9 ± 1.1 | 3.4 ± 0.3 |
| 5–10 | 1.49 ± 0.05 | 5.01 ± 0.58 | 6.99 ± 0.09 | 3.11 ± 0.51 | 0.36 ± 0.08 | 0.09 ± 0.01 | 8.8 ± 0.7 | 4.0 ± 0.4 | |
| CK | 0–5 | 1.52 ± 0.02 | 4.84 ± 0.72 | 6.55 ± 0.13 | 3.68 ± 0.15 | 0.31 ± 0.06 | 0.11 ± 0.02 | 12.2 ± 2.7 | 2.8 ± 0.4 |
| 5–10 | 1.53 ± 0.07 | 4.34 ± 0.15 | 6.71 ± 0.09 | 2.59 ± 0.02 | 0.29 ± 0.05 | 0.08 ± 0.02 | 9.2 ± 1.4 | 3.5 ± 0.2 | |
| Two-way ANOVA analysis | |||||||||
| Treatment (T) | * | * | ** | * | * | ns | ns | * | |
| Soil layer (L) | ns | ns | * | ** | ns | * | * | * | |
| T × L | ns | ns | ns | ns | ns | ns | ns | ns | |
| Hi | BDi | DBHi | D | R | C | AB | pH | SBD | SWC | SOC | TN | TP | NO3−–N | NH4+–N | DOC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hi | 1 | 0.500 * | 0.564 * | −0.675 ** | −0.539 * | 0.500 | 0.326 | 0.253 | −0.077 | 0.003 | 0.130 | −0.049 | 0.083 | 0.269 | 0.106 | 0.145 |
| BDi | 1 | 0.604 ** | −0.308 | −0.330 | −0.458 | −0.342 | 0.152 | −0.106 | −0.148 | −0.107 | −0.052 | −0.102 | 0.056 | −0.180 | 0.185 | |
| DBHi | 1 | −0.399 | −0.261 | 0.001 | −0.122 | 0.213 | −0.262 | 0.039 | 0.070 | 0.078 | 0.073 | 0.248 | 0.056 | 0.436 | ||
| D | 1 | 0.850 ** | −0.433 | −0.331 | −0.179 | 0.038 | 0.116 | 0.015 | 0.137 | 0.185 | −0.311 | −0.374 | −0.202 | |||
| R | 1 | −0.280 | −0.350 | −0.058 | −0.170 | 0.063 | −0.089 | 0.128 | 0.188 | −0.272 | −0.309 | −0.199 | ||||
| C | 1 | 0.449 | 0.207 | −0.017 | 0.149 | 0.219 | 0.170 | 0.268 | 0.274 | 0.294 | 0.089 | |||||
| AB | 1 | 0.649 * | −0.205 | 0.356 | 0.229 | 0.281 | 0.161 | 0.600 * | 0.427 | 0.442 | ||||||
| pH | 1 | −0.266 | 0.494 | 0.205 | 0.525 | 0.219 | 0.713 ** | 0.474 | 0.394 | |||||||
| SBD | 1 | −0.725 ** | −0.684 * | −0.795 ** | −0.737 ** | −0.709 ** | −0.656 * | −0.815 ** | ||||||||
| SWC | 1 | 0.881 ** | 0.950 ** | 0.897 ** | 0.738 ** | 0.640 * | 0.586 * | |||||||||
| SOC | 1 | 0.875 ** | 0.910 ** | 0.708 ** | 0.647 * | 0.645 * | ||||||||||
| TN | 1 | 0.881 ** | 0.789 ** | 0.673 * | 0.625 * | |||||||||||
| TP | 1 | 0.594 * | 0.533 | 0.549 | ||||||||||||
| NO3−–N | 1 | 0.881 ** | 0.836 ** | |||||||||||||
| NH4+–N | 1 | 0.723 ** | ||||||||||||||
| DOC | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Sun, X.; Xu, C.; Ma, X.; Huang, Y.; Fan, Z.; Cao, X. Effects of Sewage Sludge Application on Plant Growth and Soil Characteristics at a Pinus sylvestris var. mongolica Plantation in Horqin Sandy Land. Forests 2022, 13, 984. https://doi.org/10.3390/f13070984
Bai J, Sun X, Xu C, Ma X, Huang Y, Fan Z, Cao X. Effects of Sewage Sludge Application on Plant Growth and Soil Characteristics at a Pinus sylvestris var. mongolica Plantation in Horqin Sandy Land. Forests. 2022; 13(7):984. https://doi.org/10.3390/f13070984
Chicago/Turabian StyleBai, Jie, Xuekai Sun, Chengbin Xu, Xiping Ma, Yue Huang, Zhiping Fan, and Xiangyu Cao. 2022. "Effects of Sewage Sludge Application on Plant Growth and Soil Characteristics at a Pinus sylvestris var. mongolica Plantation in Horqin Sandy Land" Forests 13, no. 7: 984. https://doi.org/10.3390/f13070984
APA StyleBai, J., Sun, X., Xu, C., Ma, X., Huang, Y., Fan, Z., & Cao, X. (2022). Effects of Sewage Sludge Application on Plant Growth and Soil Characteristics at a Pinus sylvestris var. mongolica Plantation in Horqin Sandy Land. Forests, 13(7), 984. https://doi.org/10.3390/f13070984





