Effects of Habitat Filtering on Tree Growth and Mortality across Life Stages in an Old-Growth Temperate Forest
Abstract
:1. Introduction
- How does the relationship between the growth rate and the mortality rate of trees change at different life stages?
- Which environmental factors affect the growth and mortality rates of temperate tree species in northeast China?
- Is there evidence of habitat filtering from the sapling stage through the large tree stage? If there is, how do the trends of growth and mortality of the temperate trees change along environmental gradients?
2. Materials and methods
2.1. Study Site and Data Collection
2.2. Topographic Indices
2.3. Soil-Based Indices
2.4. Data Analysis
3. Results
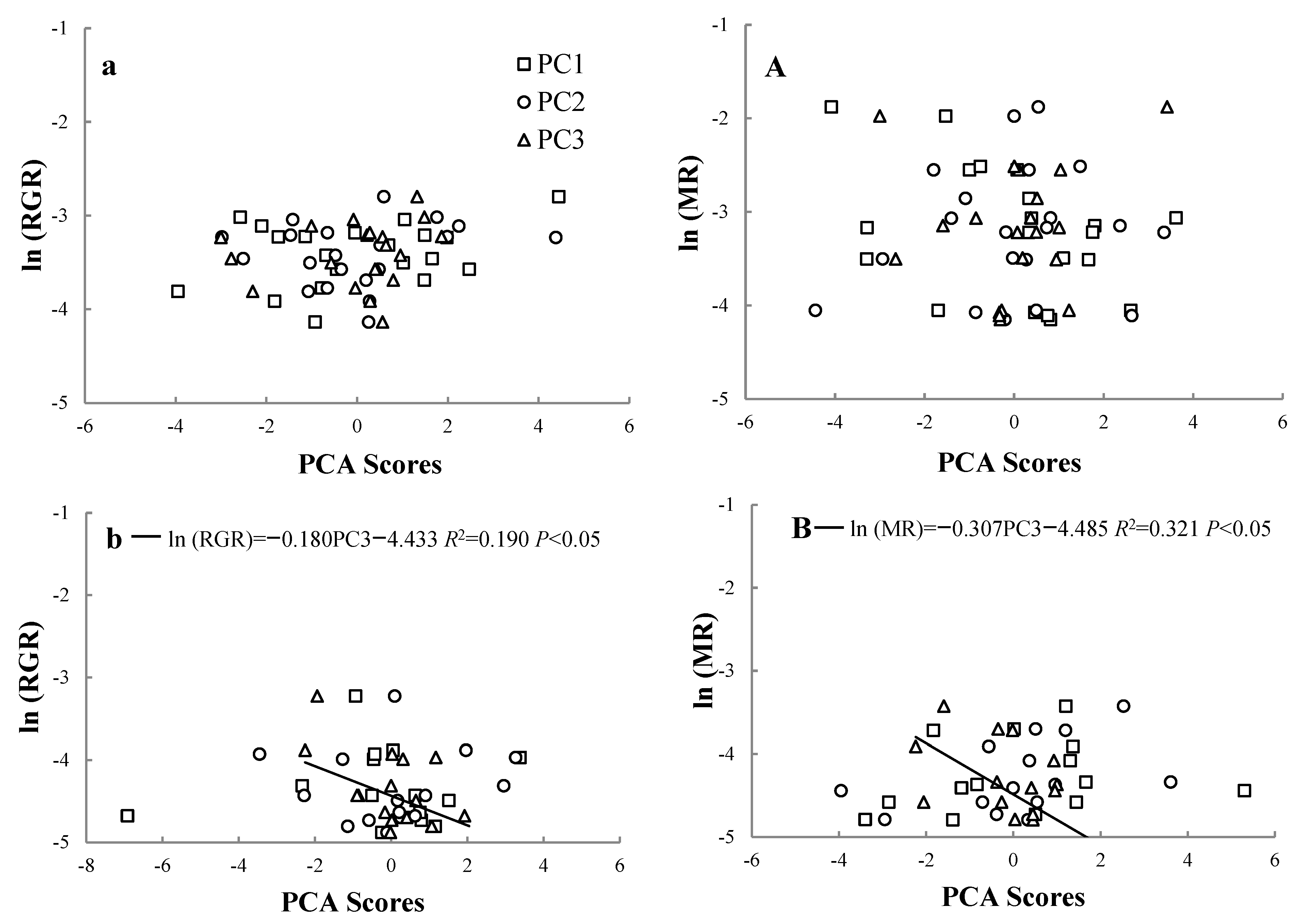
3.1. Relationships between RGR and MR at Different Life Stages
3.2. Relationships between Environmental Factors and RGR
3.3. Relationships between Environmental Factors and MR
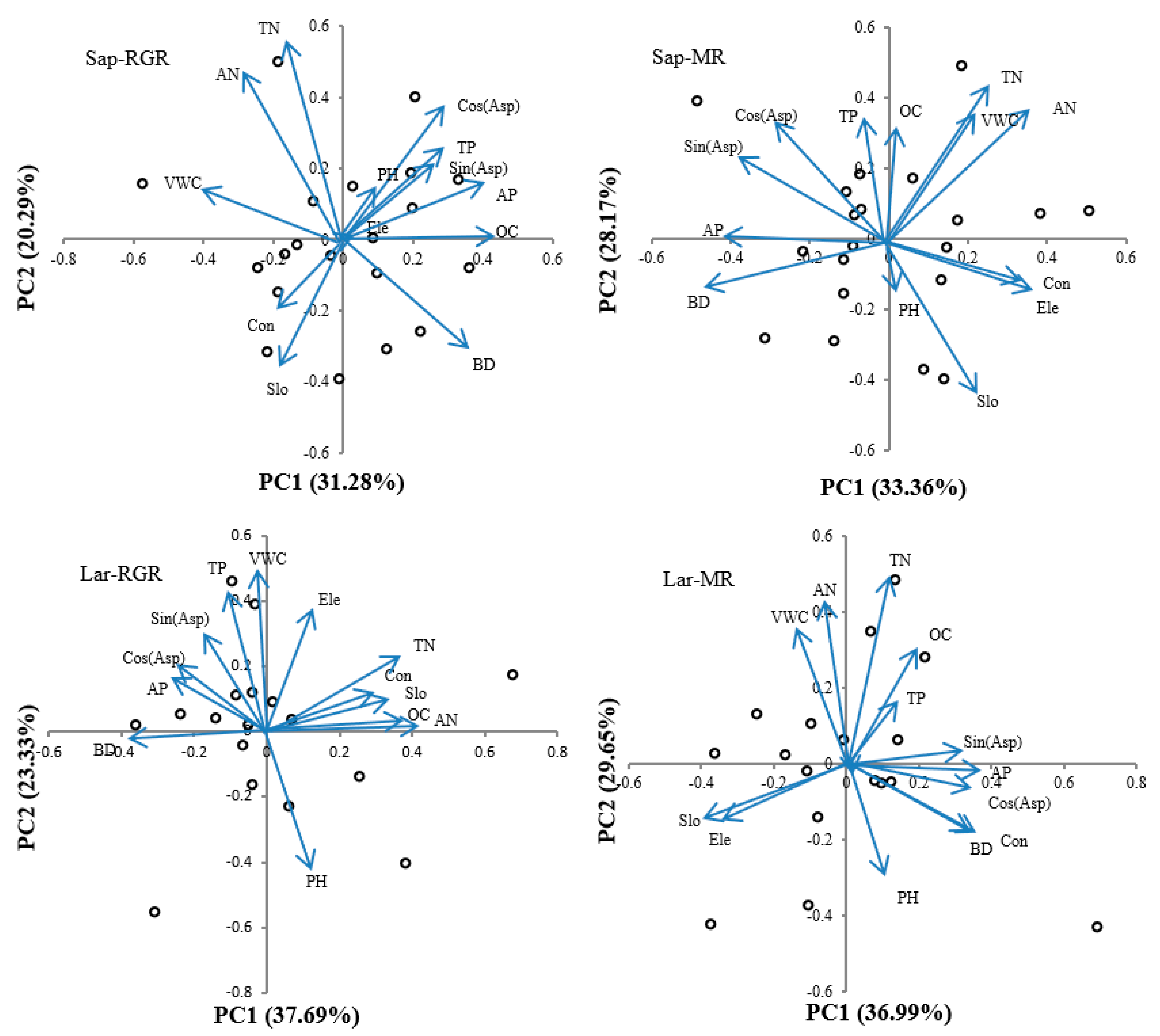
3.4. Results of Topographical and Soil-Based Factors
3.5. Effect of Environmental Factors on RGR and MR on Trees at Different Life Stages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poorter, L.; Wright, S.J.; Paz, H.; Ackerly, D.D.; Condit, R.; Ibarra-Manriquez, G.; Harms, K.E.; Licona, J.C.; Martinez-Ramos, M.; Mazer, S.J.; et al. Are functional traits good predictors of demographic rates? Evidence from five Neotropical forests. Ecology 2008, 89, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.B. Functional traits and the growth-mortality trade-off in tropical trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Lee, S.K.; Suh, G.U.; Park, Y.; Park, P.S. Wind and topography influence the crown growth of Picea jezoensis in a subalpine forest on Mt. Deogyu, Korea. Agric. For. Meteorol. 2012, 166, 207–214. [Google Scholar] [CrossRef]
- Condit, R.; Hubbell, S.P.; Foster, R.B. Mortality rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecol. Monogr. 1995, 65, 419–439. [Google Scholar] [CrossRef]
- Martinez-Garza, C.; Campo, J.; Ricker, M.; Tobon, W. Effect of initial soil properties on six-year growth of 15 tree species in tropical restoration plantings. Ecol. Evol. 2016, 6, 8686–8694. [Google Scholar] [CrossRef] [PubMed]
- Sterck, F.; Markesteijn, L.; Schieving, F.; Poorter, L. Functional traits determine trade-offs and niches in a tropical forest community. Proc. Natl. Acad. Sci. USA 2011, 108, 20627–20632. [Google Scholar] [CrossRef] [Green Version]
- Nakashizuka, T. Species coexistence in temperate, mixed deciduous forests. Trends Ecol. Evol. 2001, 16, 205–210. [Google Scholar] [CrossRef]
- Wychoff, P.H.; Clark, J.S. The relationship between growth and mortality for seven cooccurring tree species in the southern Appalachian Mountains. J. Ecol. 2002, 90, 604–615. [Google Scholar] [CrossRef] [Green Version]
- Perez-Ramos, I.M.; Ourcival, J.M.; Limousin, J.M. Mast seeding under increasing drought: Results from a long-term data set and from a rainfall exclusion experiment. Ecology 2010, 91, 3057–3068. [Google Scholar] [CrossRef] [Green Version]
- Iida, Y.; Kohyama, T.S.; Swenson, N.G.; Su, S.H.; Chen, C.T.; Chiang, J.M.; Sun, I.F. Linking functional traits and demographic rates in a subtropical tree community: The importance of size dependency. J. Ecol. 2014, 102, 641–650. [Google Scholar] [CrossRef]
- Webb, C.O.; Peart, D.R. Habitat associations of trees and seedlings in a Bornean rain forest. J. Ecol. 2000, 88, 464–478. [Google Scholar] [CrossRef]
- Comita, L.; Condit, S.R.; Hubbell, S.P. Developmental changes in habitat associations of tropical trees. J. Ecol. 2007, 95, 482–492. [Google Scholar] [CrossRef]
- Ji, M.; Jin, G.; Liu, Z. Effects of ontogenetic stage and leaf age on leaf functional traits and the relationships between traits in Pinus koraiensis. J. For. Res. 2021, 32, 13–25. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Tanner, E.V.J. The influence of topography on tree growth, mortality, and recruitment in a tropical montane forest. Biotropica 2000, 32, 378–384. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G.; Ravit, B.; Elgersma, K. Feedback in the plant-soil system. Annu. Rev. Environ. Resour. 2005, 30, 75–115. [Google Scholar] [CrossRef] [Green Version]
- Toledo, J.J.; Magnusson, W.E.; Castilho, C.V.; Nascimento, H.E.M. Tree mode of death in Central Amazonia: Effects of soil and topography on tree mortality associated with storm disturbances. For. Ecol. Manag. 2012, 263, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Xiong, G.M.; Chen, Z.G.; Fan, D.Y.; Xie, Z.Q. The topography heterogeneity of Fagus engleriana-Cyclobalanopsis oxyodon community in Shennongjia region. Acta Ecol. Sin. 2004, 24, 2686–2692. [Google Scholar]
- Bader, M.Y.; Ruijten, J.J.A. A topography-based model of forest cover at the alpine tree line in the tropical Andes. J. Biogeogr. 2008, 35, 711–723. [Google Scholar] [CrossRef]
- Zare, S.; Jafari, M.; Tavili, A.; Abbasi, H.; Rostampour, M. Relationship between environmental factors and plant distribution in arid and semiarid area (case study: Shahriyar Rangelands, Iran) American-Eurasian. J. Agric. Environ. Sci. 2001, 10, 97–105. [Google Scholar]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Costa, F.R.C.; Magnusson, W.E.; Luizao, R.C. Mesoscale distribution patterns of Amazonian understorey herbs in relation to topography, soil and watersheds. J. Ecol. 2005, 93, 863–878. [Google Scholar] [CrossRef]
- Piao, T.F.; Comita, L.S.; Jin, G.Z.; Kim, J.H. Density dependence across multiple life stages in a temperate old-growth forest of northeast China. Oecologia 2013, 172, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.Y.; Li, F.R.; Jin, G.Z. Spatial patterns and associations of four species in an old-growth temperate forest. J. Plant Interact. 2014, 9, 745–753. [Google Scholar] [CrossRef]
- Martin, A.R.; Thomas, S.C.; Zhao, Y. Size-dependent changes in wood chemical traits: A comparison of neotropical saplings and large trees. AoB Plants 2013, 5, plt039. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Manokaran, N.; Lafrankie, J.V.; Hubbell, S.P.; Foster, R.B. Dynamics of the forest communities at Pasoh and Barro Colorado: Comparing two 50-ha plots. Philos. Trans. R. Soc. B Biol. Sci. 1999, 354, 1739–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legendre, P.; Mi, X.C.; Ren, H.B.; Ma, K.P.; Yu, M.J.; Sun, I.F.; He, F.L. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 2009, 90, 663–674. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.J.; Stralberg, D.; Gong, G.Q.; Huang, Z.L.; Ye, W.H.; Wu, L.F. Separating the effects of environment and space on tree species distribution: From population to community. PLoS ONE 2013, 8, e56171. [Google Scholar] [CrossRef] [Green Version]
- Pacala, S.W.; Canham, C.D.; Saponara, J.; Silander, J.A.; Kobe, R.K.; Ribbens, E. Forest models defined by field measurements: Estimation, error analysis and dynamics. Ecol. Monogr. 1996, 66, 1–43. [Google Scholar] [CrossRef]
- Wright, S.J.; Mullerlandau, H.C.; Condit, R.; Hubbell, S.P. Gap dependent recruitment, realized vital rates, and size distributions of tropical trees. Ecology 2003, 84, 3174–3185. [Google Scholar] [CrossRef]
- Kitajima, K.; Poorter, L. Functional basis for resource niche partitioning by tropical trees. Trop. For. Community Ecol. 2008, 10, 160–181. [Google Scholar]
- Kim, C.; Apel, K. Arabidopsis light-dependent NADPH: Protochlorophyllide oxidoreductase A (PORA) is essential for normal plant growth and development: An addendum. Plant Mol. Biol. 2012, 78, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.Y.; Han, Y.C.; Mao, S.C.; Wang, G.P.; Feng, L.; Yang, B.F.; Fan, Z.Y.; Du, W.; Lu, J.H.; Li, Y.B. Light spatial distribution in the canopy and crop development in cotton. PLoS ONE 2014, 9, e113409. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.A.; Alves, L.C.; Freitas, M.C.; Os, B.V.; Wolterbeek, H.T. Lichens (Parmelia sulcata) time response model to environmental elemental availability. Sci. Total Environ. 1999, 232, 105–115. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef]
- Espeleta, J.F.; Clark, D.A. Multi-scale variation in fine-root biomass in a tropical rain forest: A seven-year study. Ecol. Monogr. 2007, 77, 377–404. [Google Scholar] [CrossRef] [Green Version]
- Makhalanyane, T.P.; Valverde, A.; Velázquez, D.; Gunnigle, E.; Gorthem, M.W.V.; Quesada, A.; Cowan, D.A. Ecology and biogeochemistry of cyanobacteria in soils, permafrost, aquatic and cryptic polar habitats. Biodivers. Conserv. 2015, 24, 819–840. [Google Scholar] [CrossRef]
- Jones, M.M.; Tuomisto, H.; Borcard, D.; Legendre, P.; Clark, D.B.; Olivas, P.C. Explaining variation in tropical plant community composition: Influence of environmental and spatial data quality. Oecologia 2008, 155, 593–604. [Google Scholar] [CrossRef]
- Shen, Y.; Santiago, L.S.; Shen, H.; Ma, L.; Lian, J.; Cao, H.; Lu, H.; Ye, W. Determinants of change in subtropical tree diameter growth with ontogenetic stage. Oecologia 2014, 175, 1315–1324. [Google Scholar] [CrossRef] [Green Version]
- Garciaoliva, F.; Maass, J.M.; Galicia, L. Rainstorm analysis and rainfall erosivity of a seasonal tropical region with a strong cyclonic influence on the Pacific Coast of mexico. J. Appl. Meteorol. 1995, 34, 2491–2498. [Google Scholar] [CrossRef] [Green Version]
- Dina, O.; Jin, G. Species-habitat association affects demographic variation across different life stages in an old-growth temperate forest. Perspect. Pant Ecol. Evol. Syst. 2019, 40, 125482. [Google Scholar]
- Verhoeven, A.S.; Demmigadams, B.; Iii, W.W.A. Enhanced employment of the xanthophyll cycle and thermal energy dissipation in spinach exposed to high light and N stress. Plant Physiol. 1997, 113, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Gundersen, P.; Emmett, B.A.; Kjønaas, O.J.; Koopmans, C.J.; Tietema, A. Impact of nitrogen deposition on nitrogen cycling in forests: A synthesis of NITREX data. For. Ecol. Manag. 1998, 101, 37–55. [Google Scholar] [CrossRef]
- Myers, J.A.; Chase, J.M.; Jimenez, I.; Jorgensen, P.M.; Araujo-Murakami, A.; Paniagua-Zambrana, N.; Seidel, R. Beta-diversity in temperate and tropical forests reflects dissimilar mechanisms of community assembly. Ecol. Lett. 2013, 16, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, G.; Andrade, J.L.; Meinzer, F.C.; Holbrook, N.M.; Cavelier, J.; Jackson, P.; Celis, A. Stem water storage and diurnal patterns of water use in tropical forest canopy trees. Plant Cell Environ. 1998, 21, 397–406. [Google Scholar] [CrossRef]

| Tree Species | Saplings | Tree Species | Large Trees | ||
|---|---|---|---|---|---|
| RGR | MR | RGR | MR | ||
| Abies nephrdepis | 0.049 | 0.015 | Abies nephrdepis | 0.008 | 0.012 |
| Acer pictum subsp. mono | 0.025 | 0.017 | Acer pictum subsp. mono | 0.007 | 0.008 |
| Acer tegmentosum | 0.047 | 0.030 | Acer tegmentosum | 0.020 | 0.024 |
| Acer ukurunduense | 0.040 | 0.029 | Acer ukurunduense | 0.013 | 0.013 |
| Aralia elata | 0.061 | 0.078 | Betula costata | 0.011 | 0.012 |
| Betula costata | 0.016 | 0.078 | Fraxinus mandschurica | 0.018 | 0.002 |
| Fraxinus mandschurica | 0.023 | 0.081 | Phellodendron amurensis | 0.019 | 0.010 |
| Maackia amurensis | 0.044 | 0.030 | Picea jezoensis | 0.005 | 0.024 |
| Phellodendron amurensis | 0.022 | 0.138 | Picea koraiensis | 0.008 | 0.016 |
| Picea jezoensis | 0.039 | 0.017 | Pinus koraiensis | 0.005 | 0.005 |
| Picea koraiensis | 0.039 | 0.016 | Populus davidiara | 0.011 | 0.020 |
| Pinus koraiensis | 0.041 | 0.042 | Populus ussuriensis | 0.039 | 0.011 |
| Populus ussuriensis | 0.020 | 0.153 | Prunus padus | 0.009 | 0.032 |
| Prunus padus | 0.030 | 0.040 | Sorbus pohuashanens | 0.018 | 0.010 |
| Quercus mongolica | 0.039 | 0.017 | Tilia amurensis | 0.009 | 0.006 |
| Sorbus pohuashanens | 0.032 | 0.046 | Tilia mandshurica | 0.011 | 0.008 |
| Tilia amurensis | 0.028 | 0.046 | Ulmus laciniata | 0.009 | 0.008 |
| Tilia mandshurica | 0.036 | 0.040 | |||
| Ulmus japonica | 0.031 | 0.042 | |||
| Ulmus laciniata | 0.028 | 0.057 | |||
| Model | Saplings | Large Trees | ||
|---|---|---|---|---|
| ln (RGR) | ln (MR) | ln (RGR) | ln (MR) | |
| Ele | 0.518 | −0.277 | −0.320 | −0.264 |
| Slo | 0.274 | −0.187 | −0.250 | 0.121 |
| Sin (Asp) | −0.296 | 0.146 | 0.613 | −0.414 |
| Cos (Asp) | −0.276 | 0.076 | 0.481 | −0.485 * |
| Con | 0.088 | −0.242 | 0.078 | 0.039 |
| BD | 0.125 | 0.469 * | −0.012 | −0.031 |
| TN | 0.053 | −0.312 | −0.195 | 0.269 |
| AN | 0.008 | −0.33 | 0.094 | 0.107 |
| TP | 0.347 * | −0.038 | 0.183 | 0.409 * |
| AP | 0.126 | 0.436 * | −0.208 | 0.138 |
| OC | 0.325 * | 0.078 | −0.324 | 0.517 * |
| pH | 0.024 | 0.209 | −0.274 | −0.169 |
| VWC | −0.278 | 0.049 | −0.548 * | −0.155 |
| Model | Principal Component | Eigenvalue | Variance Contribution (%) |
|---|---|---|---|
| Sap-RGR | PC1 | 4.066 | 31.28 |
| PC2 | 2.637 | 20.29 | |
| PC3 | 2.211 | 17.01 | |
| Sap-MR | PC1 | 4.337 | 33.36 |
| PC2 | 3.662 | 28.17 | |
| PC3 | 2.065 | 15.88 | |
| Lar-RGR | PC1 | 4.900 | 37.69 |
| PC2 | 3.033 | 23.33 | |
| PC3 | 1.926 | 14.82 | |
| Lar-MR | PC1 | 4.808 | 36.99 |
| PC2 | 3.855 | 29.65 | |
| PC3 | 1.808 | 13.91 |
| Model | Saplings | Large trees | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RGR | MR | RGR | MR | |||||||||
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
| Ele | 0.023 | 0.010 | 0.575 | 0.341 | −0.119 | 0.445 | 0.121 | 0.367 | 0.176 | −0.349 | −0.148 | 0.315 |
| Slo | −0.177 | −0.313 | 0.395 | 0.222 | −0.398 | −0.293 | 0.352 | 0.107 | 0.271 | −0.386 | −0.146 | −0.348 |
| Sin (Asp) | 0.254 | 0.189 | −0.335 | −0.348 | 0.231 | 0.295 | −0.179 | 0.302 | −0.039 | 0.316 | 0.033 | 0.048 |
| Cos (Asp) | 0.276 | 0.346 | −0.258 | −0.261 | 0.321 | 0.260 | −0.244 | 0.206 | −0.049 | 0.331 | −0.065 | 0.054 |
| Con | −0.184 | −0.170 | −0.064 | 0.316 | −0.113 | 0.342 | 0.296 | 0.114 | 0.347 | 0.367 | −0.191 | −0.089 |
| BD | 0.357 | −0.306 | 0.066 | −0.425 | −0.113 | −0.182 | −0.376 | −0.024 | 0.255 | 0.337 | −0.172 | −0.281 |
| TN | −0.156 | 0.541 | 0.205 | 0.239 | 0.419 | −0.158 | 0.370 | 0.209 | 0.189 | 0.109 | 0.497 | 0.030 |
| AN | −0.279 | 0.458 | 0.094 | 0.341 | 0.356 | −0.035 | 0.410 | 0.009 | −0.135 | −0.070 | 0.434 | 0.023 |
| TP | 0.294 | 0.244 | 0.422 | −0.052 | 0.333 | −0.073 | −0.104 | 0.429 | 0.270 | 0.139 | 0.167 | −0.162 |
| AP | 0.395 | 0.150 | 0.075 | −0.373 | 0.001 | 0.264 | −0.260 | 0.158 | 0.358 | 0.371 | −0.017 | 0.066 |
| OC | 0.397 | 0.006 | 0.288 | 0.025 | 0.313 | −0.430 | 0.372 | 0.032 | −0.202 | 0.191 | 0.307 | −0.025 |
| PH | 0.101 | 0.129 | −0.016 | 0.026 | −0.127 | 0.271 | 0.126 | −0.424 | 0.036 | 0.095 | −0.294 | 0.017 |
| VWC | −0.388 | 0.144 | 0.079 | 0.213 | 0.340 | 0.221 | −0.021 | 0.489 | −0.386 | −0.151 | 0.363 | 0.361 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Jin, G. Effects of Habitat Filtering on Tree Growth and Mortality across Life Stages in an Old-Growth Temperate Forest. Forests 2022, 13, 923. https://doi.org/10.3390/f13060923
Han D, Jin G. Effects of Habitat Filtering on Tree Growth and Mortality across Life Stages in an Old-Growth Temperate Forest. Forests. 2022; 13(6):923. https://doi.org/10.3390/f13060923
Chicago/Turabian StyleHan, Daxiao, and Guangze Jin. 2022. "Effects of Habitat Filtering on Tree Growth and Mortality across Life Stages in an Old-Growth Temperate Forest" Forests 13, no. 6: 923. https://doi.org/10.3390/f13060923
APA StyleHan, D., & Jin, G. (2022). Effects of Habitat Filtering on Tree Growth and Mortality across Life Stages in an Old-Growth Temperate Forest. Forests, 13(6), 923. https://doi.org/10.3390/f13060923






