Abstract
The home-field advantage (HFA) effect has been reported to occur in coarse woody debris (CWD) and litter. It is thought that the HFA effect may be due to the specialization of decomposers in their original habitats. However, the relative contribution of microorganisms, particularly fungi and bacteria, to deadwood decomposition is unclear because of differences in their functional at-tributes and carbon requirements, and the microorganisms that drive the HFA effect of deadwood are also unclear. Here, we analysed a dataset of microbial PLFA and substrate properties collected from the soil and CWD of two subtropical trees, Cryptomeria japonica and Platycarya strobilacea, from forests dominated by one or the other of the two species, with both species present in the forests. Our results showed that habitat and tree types all significantly affected CWD respiration rates, the CWD respiration rates were significantly higher in the deciduous broadleaf forests (DBF) than in the coniferous forest (CF) regardless of tree types, but no a large HFA of CWD decomposition found (HFA index was 4.75). Most biomarkers indicated bacteria and fungi were more abundant in the DBF than in the CF, and the concentration of microbial PLFAs was higher in Platycarya strobilacea than in Cryptomeria japonica. In addition, the relative abundance of fungi and soil B/F were remarkably positively correlated with CWD respiration, indicating that fungi may be the primary decomposers of CWD. In conclusion, our work highlights the importance of interactions between the three primary drivers (environment, substrate quality and microbes) on CWD decomposition.
1. Introduction
Coarse woody debris (CWD) is structurally and functionally important to forest ecosystems because it contains a large amount of carbon (C) and many nutrients and affects soil fertility and the carbon cycle through its decay [1,2,3]. It is estimated that global deadwood contains approximately 116 Pg of C [4]. The carbon of deadwood accounts for 5–18% of the total carbon in forest ecosystems [5], and over time, the majority of deadwood C is lost through respiration [6]. Therefore, quantifying the rates of both vegetation production and plant material decomposition and accurately assessing CO2 fluxes from CWD decomposition is critical for assessing the contribution of CWD to the current and long-term C balance in forest ecosystems.
Among the studies investigating the variability in decomposition rates of plant material, some have reported that tree types significantly affect the decomposition of deadwood [2]. However, a recent study found that the decomposition of beech deadwood was significantly faster in beech-dominated forests than in Norway spruce-dominated forests [7]. It has increasingly been demonstrated that plant material tends to decompose more rapidly in its habitat of origin (i.e., home) than in other habitats (i.e., away), a phenomenon known as ‘home-field advantage’ (HFA) that has been more extensively studied in litter than in CWD [8,9,10,11,12]. Thus, CWD quality may be relative to the habitat to which CWD is subjected, rather than being predictably by its chemical composition. Furthermore, Ayres et al. [9] had speculated that larger disparities in litter quality may contribute to higher HFAs in litter decomposition. Thus, we need to better understand the drivers of HFA to address this realistic problem of how we predict decomposition accurately.
HFA may result from long-term ecological interactions between decomposer communities and the litter they encounter most frequently. Tree type can significantly influence the distribution of microbial communities in forest soils and plant debris [13,14,15]. Additionally, tree genotypes support the divergence of specific decomposer communities with different soil enzyme activities [16,17]. Specific decomposers of certain tree species have been observed to be better adapted to the conditions of the plant material of these trees (so-called “home”) than to those at any other location (so-called “away”), so decomposer organisms may specialize in decomposing this plant material above them [9,18].
Despite the growing awareness that HFA depends on the functional attributes of microbial communities, little is known about the relative contribution of microbial communities to HFA [19]. In particular, the roles of fungi and bacteria in deadwood decomposition may differ due to differences in their functional attributes and carbon requirements. In comparison to bacteria, fungi are generally considered to possess a stronger ability to decompose recalcitrant plant material, while bacteria are thought to be more efficient in utilizing more labile C compounds [20,21,22]. However, to date, only a small number of studies have focused on both microbial communities when investigating this issue. Furthermore, determining which microorganisms are the main contributors to HFA in CWD has been given only minimal attention.
In this study, we investigated the HFA hypothesis for CWD decomposition in the subtropics by intertransplanting two CWD types, Cryptomeria japonica and Platycarya strobilacea, in the two woodlands, coniferous and deciduous broadleaf forests. We analysed the microbial communities of both CWD types and the soil, as well as the substrate quality and soil properties, in coniferous and deciduous broadleaf forests. The main objective of our study was to first confirm if a large HFA of CWD decomposition would be observed during this transplantation as a result of the large differences between the two CWD types we chose, as predicted for big differences in litter quality [9]. And then to determine if decomposer organisms (especially fungi, considering their important role in lignin decomposition) were responsible for the HFA of CWD decomposition. We would expect to see decomposer community composition to be habitat-specific, and less influenced by CWD types, if it is true that decomposer communities do specialize in decomposing CWD from the plants above them. We test the following hypotheses: (H1) there would be a large, position HFA index if HFA effects exist in CWD decomposition, (H2) microbial community composition would be driven more strongly by habitat than by CWD types, and the biomass or abundance of fungi would follow an HFA pattern similar to that of the CWD itself because the decomposer (especially fungi) communities that are specialized to decomposing CWD is a suspected mechanism driving the HFA of CWD decomposition.
2. Materials and Methods
2.1. Study Area
This study was conducted in a coniferous forest (CF) and deciduous broadleaf forests (DBF) at Mount Lushan in Jiangxi Province, subtropical China (29°31′~29°41′ N, 115°51′~116°07′ E). Lushan is an isolated mountain with an area of approximately 300 km and 30 to 1474 m above sea level. The region has a subtropical monsoon climate with well-defined seasons, and the mean annual precipitation and a temperature range from 1308 to 2068 mm and 17.1 to 11.6 °C, respectively [23], and the soil type was haplic alisol. The coniferous forests dominated by Cryptomeria japonica in the mid-elevation area of Lushan Mountain were established approximately 50 years ago. Deciduous broadleaf forests grow at middle altitudes (600–1000 m) and are dominated by some deciduous woodland species and shrubs, including Platycarya strobilacea and Acer davidii [23].
2.2. Sampling Design
In December 2014, three randomly established sample plots were established in the coniferous forest (CF) and deciduous broadleaf forest (DBF), and the two study areas were at least 800 m apart. The two study areas were similar in elevation, slope, and slope position (see Table 1). The six sample plots were separated by at least 50 m to ensure independence. We selected CWD from two species, Cryptomeria japonica (C. japonica) and Platycarya strobilacea (P. strobilacea) and placed them in situ (home field) and plots of other forest types (visiting field). For C. japonica, the plots in the coniferous forest were the home field, while the plots in the deciduous broad-leaf forest were the visiting field. The opposite was true for P. strobilacea. All selected CWD materials were fresh wood samples (bark intact and sapwood intact) specifically cut from the live trees [10] that were each approximately 1.5 m long and 16 cm in diameter (see Table 1), and they were placed on the forest floor and touch the soil. Each sample area contained 1 CWD of each species. Thus, a total of 12 CWDs were studied (6 plots × 2 CWD segments). The initial chemical properties of the CWD associated with each species are shown in Table 2. In addition, we used a neutral sealant to attach breathing rings (polyvinyl chloride rings, 2 mm thick × 10 cm diameter × 5 cm high) to the surface of each CWD in each sample plot for later determination of RCWD. The respiration rings were mounted approximately 50 cm from one end and 100 cm from the other end of the CWD.

Table 1.
General information (mean ± SE) of the CWDs of the two tree species at the two study areas in Lushan Mountain of subtropical China. CWD: Coarse woody debris; CF: Coniferous forest; DBF: Deciduous broad-leaved forest. Note: experiment material was all of similar decay stage.

Table 2.
The substrate quality of CWD (n = 3. means ± 1 se) of the two tree types (C. japonica vs. P. strobilacea) in the two habitats (CF vs. DBF) after decomposition. Different lowercase letters indicate significant differences (p < 0.05). CWD: coarse woody debris; CF: coniferous forest; DBF: deciduous broadleaf forest; Cel: cellulose; Lig: lignin.
2.3. Sample Collection and Processing
In June 2018, within each sample plot, a small 5 cm disc (including bark) was collected from the CWD with a chainsaw, and after removing moss, soil, roots, and other attachments, it was stored in a sterile bag at low temperature and brought back to the laboratory for preprocessing and analysis as soon as possible. The CWD samples collected in the field were milled at low temperature and divided into 2 parts: one part was dried and used to determine substrate quality (C, N, lignin, cellulose, see Table 2), and the other part was stored in the refrigerator at −80 °C for the determination of microbial PLFAs. In addition, three soil samples were collected in the 0~20 cm soil layer (removed litter layer) under each CWD in each plot. All soil samples were placed in sealed bags and brought back to the laboratory. The soil samples were passed through a 1 mm sieve, and visible leaves, twigs, roots, and stones were discarded. The soil under the same CWD of the same plot was mixed and divided into two parts, one for chemical property analysis (C, N, P, and ratios, see Table 3) and the other for microbial PLFA determination in a −80 °C refrigerator.

Table 3.
The soil properties (n = 3. means ± 1 se) under the CWD of the two tree types (C. japonica vs. P. strobilacea) in the two habitats (CF vs. DBF) after decomposition. Different lowercase letters indicate significant differences (p < 0.05). CF: coniferous forest; DBF: deciduous broadleaf forest.
2.4. Measurements of RCWD
In 2018, the breathing rings were rebuilt, and respiration of CWD was measured once in June, just prior to the collection of the CWD and soil samples, using the soil CO2 efflux chamber in the LI-COR portable photosynthesis system (LI-6400-09, LI-COR, Inc., Lincoln, NE, USA) [8].
2.5. Determination of Sample C, N, P, Cellulose, Lignin Content, and PLFA
The CWD and soil samples were passed through a 0.1 mm sieve for grinding. Total organic carbon (C) was determined using the K2Cr2O7-H2SO4 digestion method [24]. Total nitrogen (N) and total phosphorus (P) were determined using an automated chemical analyser (SmartChem 200, Westco, Rome, Italy). The digestion of litter and the soil samples was performed using the H2SO4-H2O2 digestion method with HClO4 added to soil samples prior to analysis of N and P on the automated analyser. Cellulose and lignin contents were measured by the acid detergent lignin method. Samples were stored at −80 °C for analysis of microbial PLFAs. Samples were analysed by leaching, separation, esterification, and extraction [25] followed by gas chromatography (Hewlett-Packard 6890 series GC, FID) using the MIDI software system (MIDI, Inc., Newark, DE, USA) for the fatty acid components. The concentration of each PLFA was calculated as an internal standard concentration of 19:0. The individual PLFAs were summed to estimate the total biomass of the microorganisms (nmol g−1 dry soil or nmol g−1 dry wood).
The fatty acids detected were classified into the following different microbial taxonomic groups: ester-linked branched-chain fatty acids (i14:0, a15:0, i15:0, i16:0, a17:0, and i17:0), representing Gram-positive bacteria (GP) [26]; ester-linked monounsaturated fatty acids (15:1ω8c and 18:1ω7c); ester-linked hydroxy fatty acids (15:0 3OH, 16:0 2OH, and 18:0 2OH); and ester-linked cyclopropane fatty acids (cy17:0 and cy19:0ω8c), representing Gram-negative bacteria (GN) [26,27]. PLFA 14:0, 15:0 and 17:0 were added as indicators of other bacteria [28]. PLFA 18:1ω9c and 18:2ω6c represented fungi [29,30], and 10Me-fatty acids (10Me16:0, 10Me17:0, 10Me18:0) represented actinomycetes [31]. The ratio of all bacterial PLFAs to all fungal PLFAs was used to represent the ratio of bacterial to fungal biomass (B/F) [32]. The ratio of Gram-positive to Gram-negative bacteria (GP/GN) was also calculated.
2.6. Data Analysis
To determine the overall HFA effect, we calculated the HFA index for each pair of reciprocal CWD transplants (following Ayres et al., 2009 [9]). The relative C loss ARMLa was first calculated using Equation (1).
where ARMLa denotes the relative respiration of the CWD of species A at site a, and Aa and Ba denote the respiration percentages of the two species of CWD decomposed at site a, respectively. The relative respiration measurement of CWD was used to calculate the HFA index in Equation (2).
Note that this equation specifically tests for the presence of the HFA effect, not the individual HFA effect of each species in the analysis. Calculating the HFA effect of a single species requires three or more species [9], which is beyond the scope of our study.
Two-way ANOVA was used to examine the effects of habitat (CF vs. DBF), tree types (C. japonica vs. P. strobilacea) and their interactions on the respiration of CWD, matrix quality of CWD (C, N, C/N, cellulose, lignin, and lignin/N), soil chemistry (C, N, P, C/N, C/P, and N/P), and sample microbial PLFAs (total, actinomycetes, GP, GN, bacteria, fungi, GP/GN, and bacteria/fungi). Post hoc tests (Fisher LSD) were performed to check the differences between the means. All of the above analyses were performed by SPSS 20.0 (SPSS Inc., Chicago, IL, USA). In addition, principal component analysis (PCA) was performed using CANOCO 5.0 software (Ithaca, NY, USA) to analyze the differences in microbial community composition under different factors (habitat and tree species). And to assess the significance of habitat, tree species, and interactions affecting the microbial community of CWD and soil, we performed a permutation multivariate analysis of variance (PERMANOVA) using Primer 6.0 software (Plymouth, MA, USA). To determine whether the fluctuations in the microbial composition of CWD and soil could be explained by environmental variables, a redundancy analysis (RDA) was performed using CANOCO 5.0 software (Ithaca, NY, USA), where environmental variables with a VIF < 10 were selected to ensure that covariates did not degrade analysis quality. Differences in PLFA biomarkers between the two tree types or two habitats were assessed using White’s nonparametric t-test (p < 0.05) in STAMP (Statistical Analysis of Metagenomic Profiles) software (version 2.1.3). Column and regression charts were produced by Origin 9.85 (OriginLab Inc., Massachusetts, MA, USA).
3. Results
3.1. RCWD, Substrate Quality of CWD, and Soil Properties
The initial substrate quality of CWD also differed significantly between the two species (p < 0.05, Table 2). Meanwhile, the initial soil properties differed between habitats (CF and DBF). soil C, N, and P contents were higher in DBF than in CF, especially soil C (p < 0.05, Table 3).
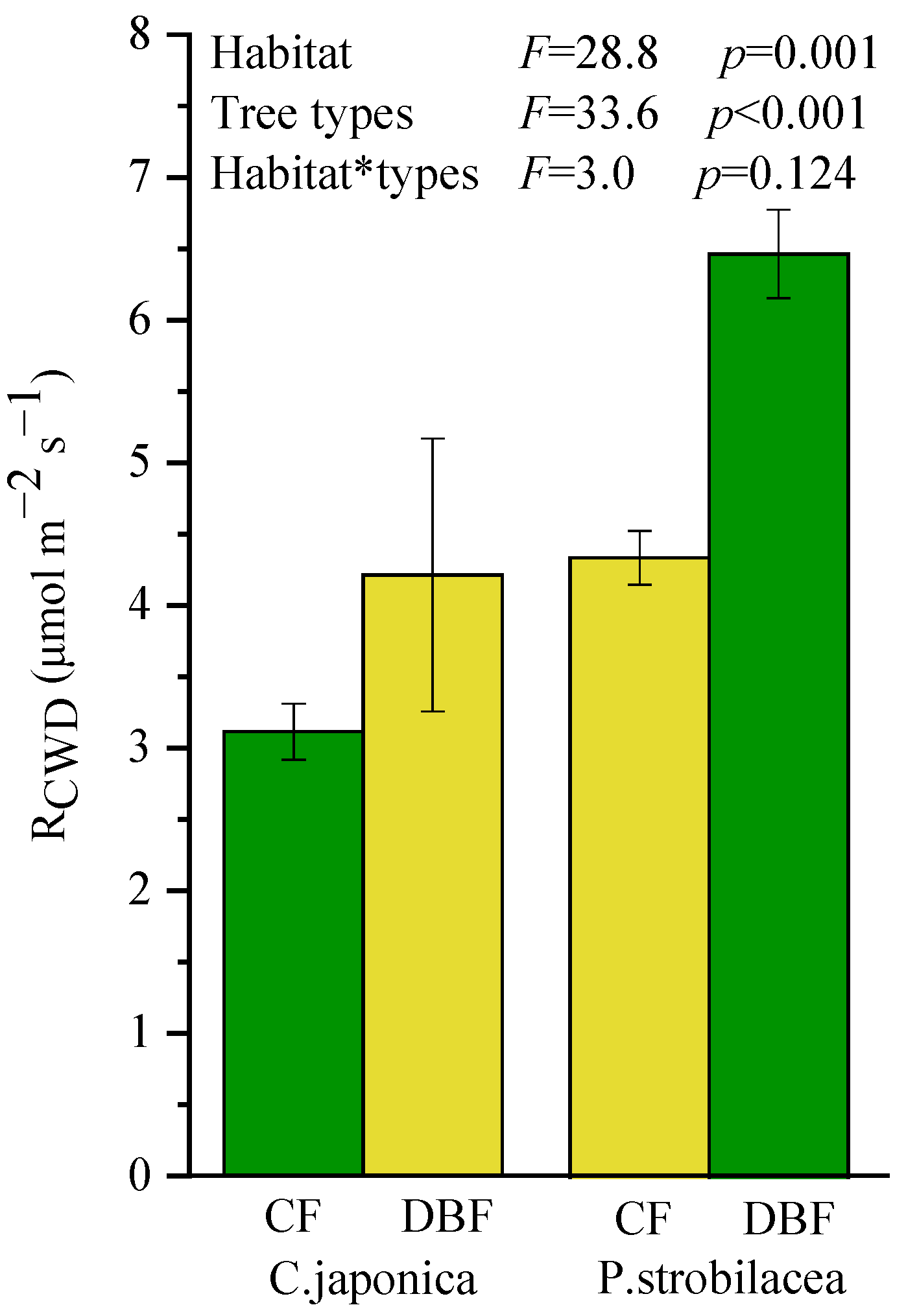
Both habitat (p = 0.001) and tree types (p < 0.001) significantly affected the respiration rates of CWD (RCWD), but there was no significant interaction (p = 0.124; Figure 1). The RCWD was significantly higher in P. strobilacea than in C. japonica, and it was statistically higher in the DBF than in the CF regardless of tree types. Thus, the CWD of P. strobilacea decomposed more rapidly in “home” (deciduous broadleaf forest), while the CWD of C. japonica decomposed more slowly in “home” (coniferous forest); no large HFA of CWD decomposition was found (HFA index was 4.75). Only habitat considerably affected CWD quality (Table 2), but habitat and tree types all significantly affected soil P (Table 3). The soil P content was significantly higher in the DBF than in the CF.
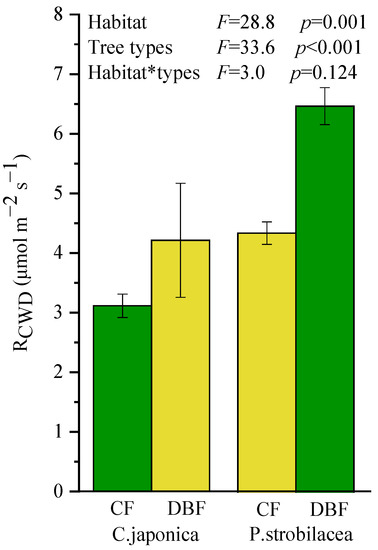
Figure 1.
The respiration rate of CWD (RCWD) of two types (C. japonica vs. P. strobilacea) either at “home” (green bars) or “away” (yellow bars). CWD: coarse woody debris; CF: coniferous forest; DBF: deciduous broadleaf forest. Values are mean ± standard error (n = 3).
3.2. PLFA Analysis
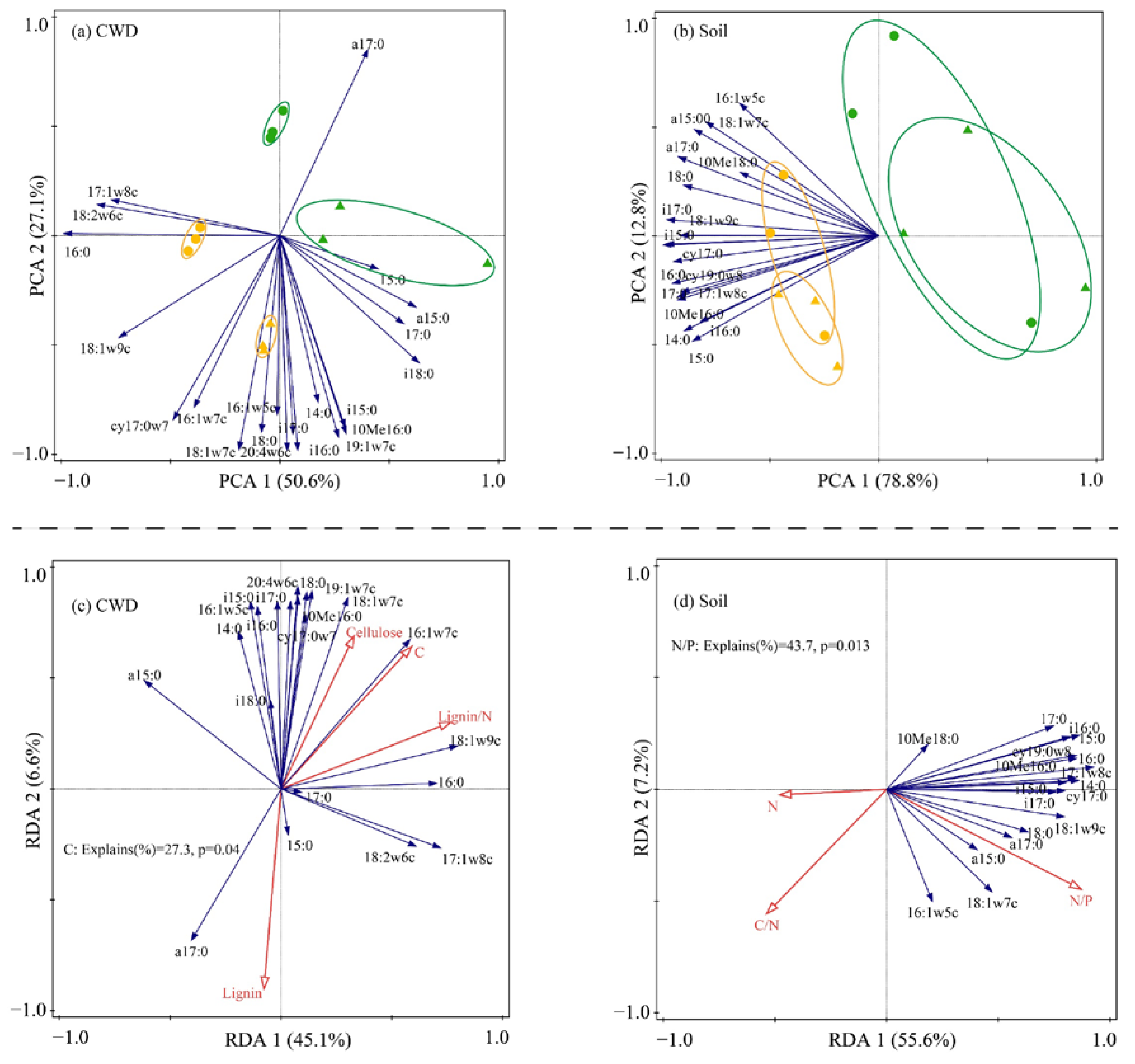
Habitat had a significant effect on the microbial community composition of CWD and soil (Table 4; Figure 2a,b). In addition, habitat also had a significant effect on the microbial PLFAs of CWD and soil (Table 5 and Table 6), with higher values observed in the CF than in the DBF. In contrast, tree types only had a significant effect on the microbial PLFAs of CWD (Figure 2a), and the total PLFAs of P. strobilacea were significantly higher only in CF, the fungal PLFAs of P. strobilacea were significantly higher than those of C. japonica regardless of the habitat (Table 5). In addition, the concentrations of total, bacterial and fungal PLFAs in CWD were significantly positively correlated with the concentrations of soil total, bacterial and fungal PLFAs (Table 7).

Table 4.
The effect of habitat, tree types and their interactions on the microbial community composition of CWD and soil was tested using PERMANOVA. Significant effects are presented in bold. % = percentage explained variation.
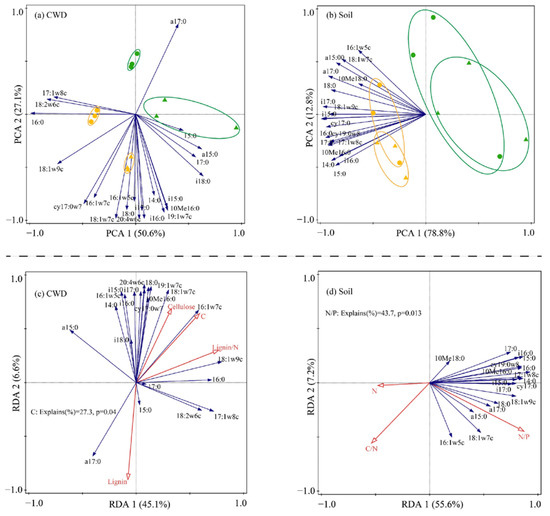
Figure 2.
Principal component analysis (PCA) depicting CWD (a) and soil (b) microbial community composition for C. japonica (triangle) and P. strobilacea (round) which were incubated in coniferous (yellow colour) and deciduous broadleaf forest (green colour); and redundancy analysis (RDA) of relationships between CWD (c) and soil (d) microbial community attributes and properties of CWD and soil. CWD: coarse woody debris.

Table 5.
The microbial PLFAs (nmol g−1) and relative abundance (%) of CWD (n = 3. means ± 1 se) of the two tree types (C. japonica vs. P. strobilacea) in the two habitats (CF vs. DBF) after decomposition. Different lowercase letters indicate significant differences (p < 0.05). CWD: coarse woody debris; CF: coniferous forest; DBF: deciduous broadleaf forest. Total: total PLFA; Actino: actinomycete; B/F: the ratio of bacteria to fungi; GP/GN: the ratio of GP to GN.

Table 6.
The soil microbial PLFAs (nmol g−1) and relative abundance (%) (n = 3. means ± 1 se) under the CWD of two tree types (C. japonica vs. P. strobilacea) in the two habitats (CF vs. DBF) after decomposition. Different lowercase letters indicate significant differences (p < 0.05). CWD: coarse woody debris; CF: coniferous forest; DBF: deciduous broadleaf forest. Total: total PLFA; Actino: actinomycete; B/F: the ratio of bacteria to fungi; GP/GN: the ratio of GP to GN.

Table 7.
The Pearson correlations between the microbial PLFAs (nmol g−1) of CWD and soil. CWD: coarse woody debris; Total: total PLFA; Actino: actinomycete; B/F: the ratio of bacteria to fungi; GP/GN: the ratio of GP to GN. * p < 0.05, ** p < 0.01.
The microbial communities of the CWD and soil were closely related to the chemical properties of the substrate and soil. RDA revealed that the C content and C/N of CWD explained 20.1% and 18.7% of the variation in the microbial community structure of CWD, respectively (Figure 3c). The content of soil P and soil C were significantly associated with the soil microbial community of CWD, respectively explained 30.2% and 29.4% of the variation in the soil microbial community structure (Figure 2d).
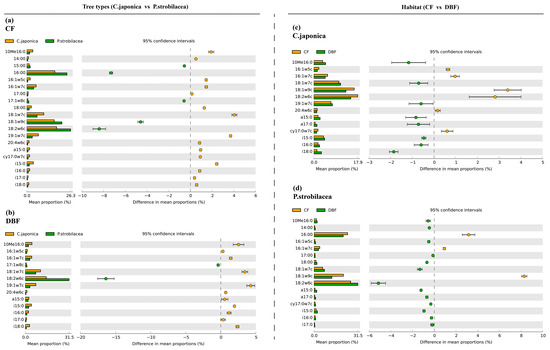
Figure 3.
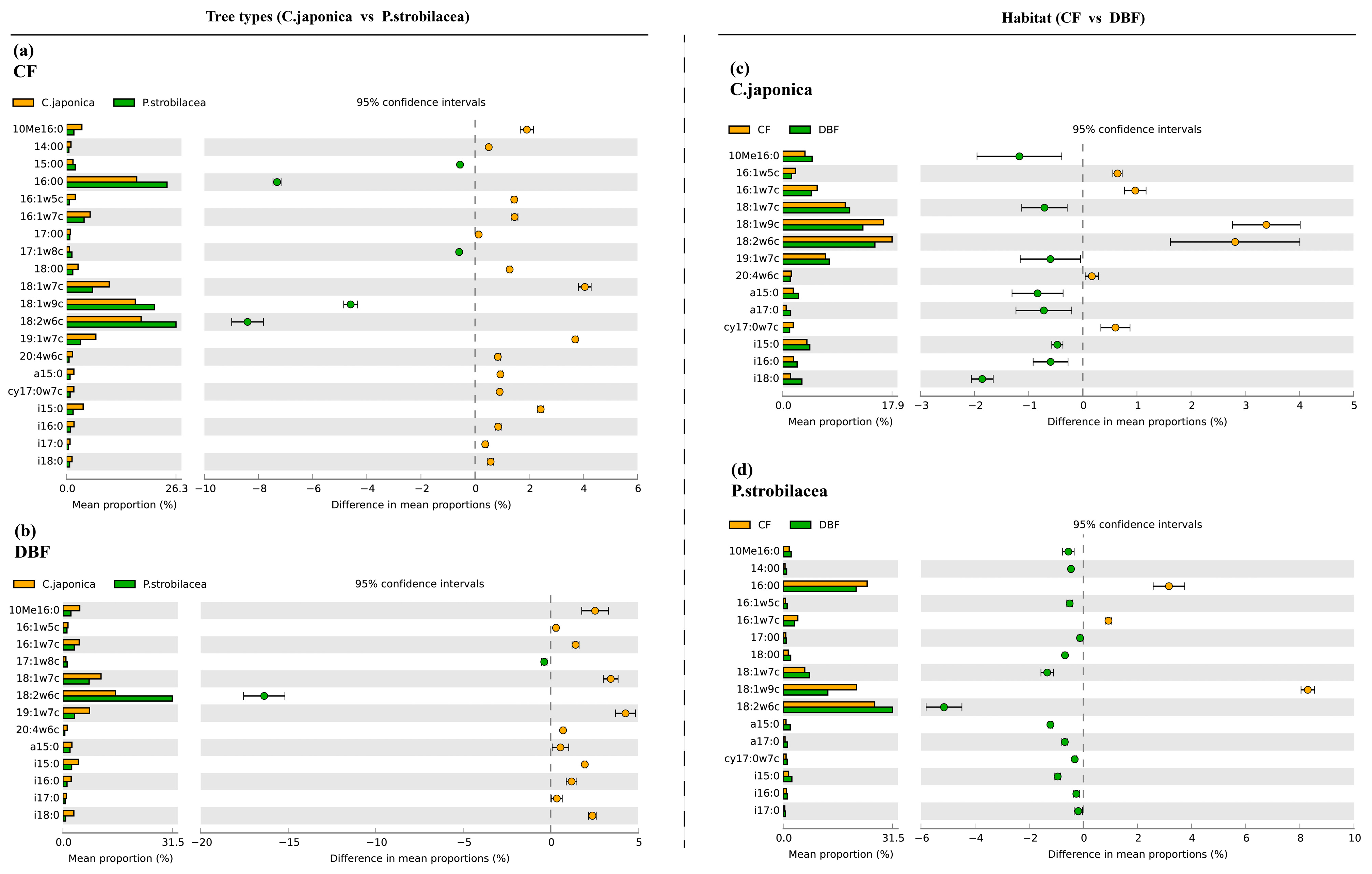
The differentially abundant PLFA biomarkers of CWD between the two tree types (C. japonica vs. P. strobilacea) (a,b) and the two habitats (CF vs. DBF) (c,d). CWD: coarse woody debris; CF: coniferous forest; DBF: deciduous broadleaf forest.
White’s nonparametric t-test in STAMP software was used to better understand the differences in the microbial composition of the CWD between the two habitats (CF vs. DBF) or the two tree types (C. japonica vs. P. strobilacea). The PLFA biomarkers 18:2ω6c were more abundant in P. strobilacea than in C. japonica, regardless of habitat (Figure 4a,b), but the changes of 18:1ω7c are oppositive to that of it. The PLFA biomarkers a15:0, i16:0, 18:1ω7c, 10Me16:0, cy17:0ω7c and 18:2ω6c were more abundant in the DBF than in the CF (Figure 4c,d).
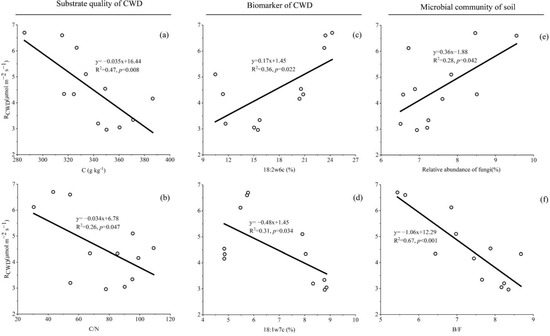
Figure 4.
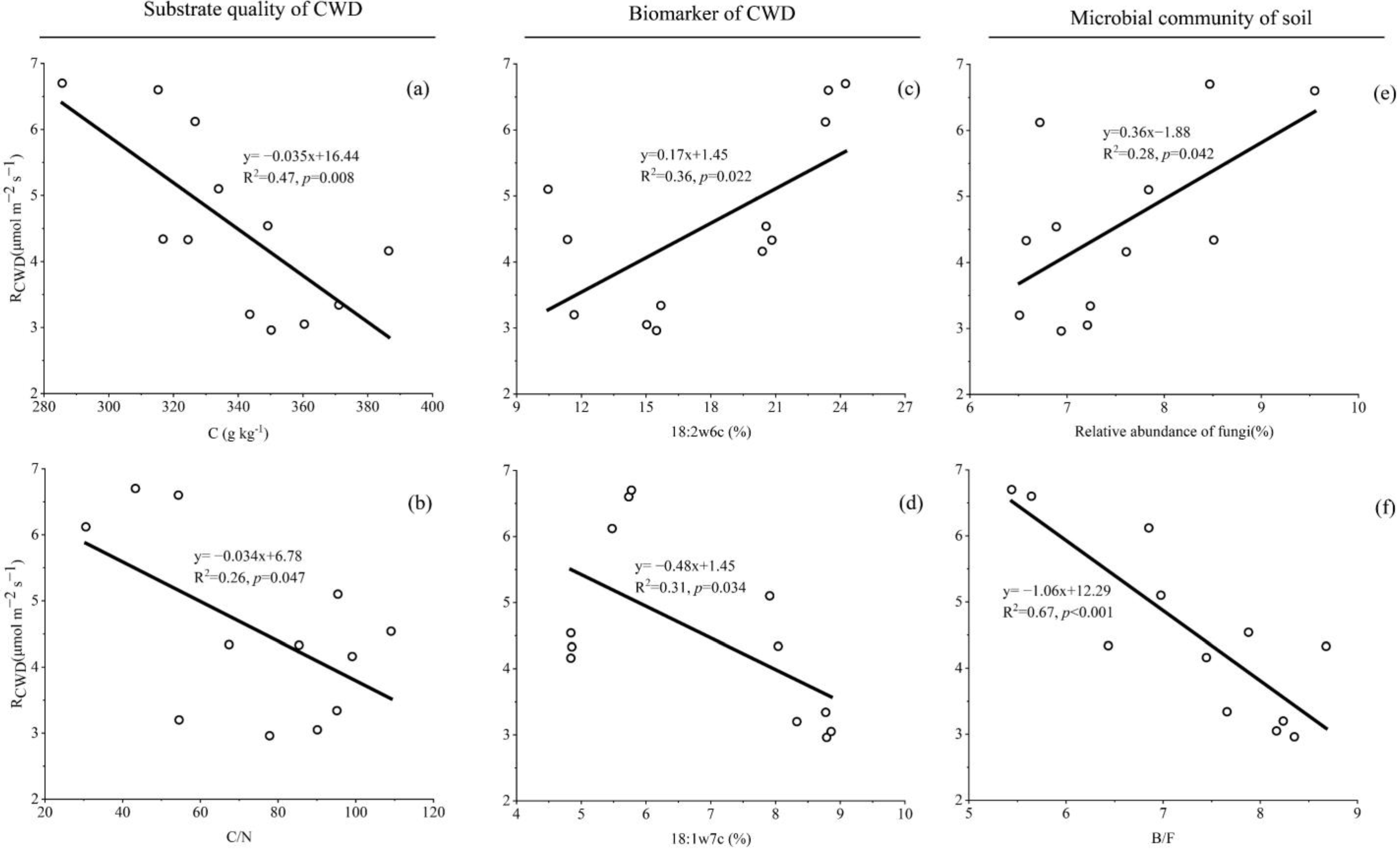
Univariate linear regression of RCWD and substrate quality of CWD (a,b), relative abundance of the biomarker of CWD (c,d), relative abundance of soil fungi (e) and soil B/F (f). RCWD: respiration rate of CWD; CWD: coarse woody debris.
3.3. Relationships between Respiration of CWD and Influencing Factors
The result shows that the factor with a significant linear relationship with RCWD is a relative abundance of 18:2ω6c and 18:1ω7c of CWD, a relative abundance of soil fungi and soil B/F (Figure 4c–f). The CWD respiration was positively correlated with a relative abundance of 18:2ω6c of CWD (p = 0.022) and soil fungi (p = 0.042), while it was negatively correlated with a relative abundance of 18:1ω7c of CWD (p = 0.034) and soil B/F (p < 0.001). In addition, the content of carbon in CWD after decomposition was negatively correlated with RCWD, this shows that the decomposition will cause the loss of carbon in CWD.
4. Discussion
4.1. Home-Field Advantage of CWD Decomposition
In this study, RCWD was significantly affected by habitat and CWD types (Figure 1), but we found no evidence for our hypothesis of a large HFA of CWD decomposition (H1) despite a significant difference in quality for the CWDs. The CWD of both tree types decomposed faster in the DBF than in the CF indicates that CWD of P. strobilacea decomposes more rapidly at “home” than at “away”, while the CWD decomposition trend of C. japonica contradicts this. Although previous studies have demonstrated that wood decomposition rates are accelerated at “home” in comparison to at “away” [7], the changes were more pronounced in the broadleaf trees than in the conifers. Our study did not observe consistent results but rather suggested that CWD decomposition is more habitat-dependent, thus seems to show a different HFA of CWD decomposition (positive for P. strobilacea and negative for C. japonica). However, the previously noted study [7] did not use a true reciprocal deadwood transfer experiment but still met the definition of “home-field advantage” [9]. Therefore, the HFA hypothesis of CWD decomposition is controversial and needs to be proven in more field studies.
In addition, tree types explained the largest part of the variation in RCWD (Figure 1) and were a very important factor in CWD decomposition; the CWD of P. strobilacea decomposed faster than the CWD of C. japonica. Several previous studies have stated that the decay period of deadwood varies greatly among tree species [2,33], which may be explained by the difference in chemical properties between different tree species, especially initial substrate quality. In general, the lignin, N, and P in wood are good determinants of decay [34,35,36]. In our study, there was a significant difference in the initial substrate quality between the two types of CWD, and this difference seems to explain the variation in CWD decomposition among the different tree types. Lignin is difficult to decompose due to its large and complex structure, so wood with higher decomposition rates tends to have a lower content of lignin [33]. But our study did not observe the above results. Considering the decomposers, decomposition should not be simply assumed to be positively correlated with CWD quality (P. strobilacea > C. japonica) [37].
4.2. Decomposer Community Composition
We found almost support amongst the microbial decomposers for our hypothesis that decomposer community composition would be driven more by habitat than by litter types (H2). The microbial community composition of CWD is influenced by both habitat and litter types (Table 4; Figure 2a), whereas the soil microbial community composition is influenced only by habitat (Table 4; Figure 2b). This suggests that the microorganisms play a critical role in the decomposition of plant debris [2,38], and CWD may be more important than soil microorganisms in driving CWD decomposition, but only a few studies have paid attention to it [8]. The CWD decomposed faster in the DBF where inversely signally lower concentrations of microbial PLFAs of soil and CWD were observed compared to coniferous forests. Previous studies reported broadleaf forests generally have a higher abundance of soil microbes than those in coniferous forests due to the nutrient-rich soils of broadleaf forests [39,40]. We also observed higher soil nutrients in the DBF than in the CF regardless of decay phases (before or after decomposition), thus indicating that the soil microbial community is not only influenced by soil nutrients, but also interacts more with the wood-inhabiting microbial communities at any CWD decay phases [7,41]. Microorganisms inhabiting CWD after decomposition were closely related to their C content (Figure 2c), and CWD with higher C content was observed to have higher microbial PLFA concentrations (Table 5). Since soil microbes are closely associated with CWD-inhabiting microbes (Table 7), an increase in microbial PLFA concentrations of CWD may lead to an increase in soil microbial PLFA concentrations. Furthermore, the soil N/P was generally low in our study (Table 3), and Güsewell et al. [42] assumed that microorganisms are not limited by soil P under these conditions and that a higher P content may instead inhibit microbial growth [43], which explains the significant negative correlation between soil P and soil microbial communities (Figure 2d).
Tree types significantly affected CWD decomposition rates and CWD-inhabiting microbial communities (Figure 1; Table 4) but did not change the soil microbial communities (Table 4). Thus when soil organisms are habitat-specific and perform at the level regulated by the quality of their resources, CWD decomposition is positively correlated with its quality [37]. In this study, two types of CWD were decomposed in the same habitat, its quality rather than soil microorganisms primarily regulated the decomposition no matter which habitat (CF or DBF). Moreover, high-quality CWD may inhabit more microbes compared to low-quality CWD, and the C, N and C/N of CWD may be better predictors.
4.3. Fungi Drive CWD Decomposition
We observed no significant HFA for CWD decomposition, and thus found no support for our hypothesis that the biomass or abundance of fungi would follow an HFA pattern similar to that of the CWD decomposition. However, we observed that the changes in the relative abundance of the biomarker (18:2ω6c) were almost similar to the changes in RCWD (Figure 3), and the relative abundance of the biomarker and soil fungi had a significantly positive correlation with RCWD (Figure 4c,e). Thus, this indicates that the relative abundance of fungi may be an important player in CWD decomposition. Similarly, some previous studies have reported that fungi play a decisive role in the decomposition of detritus [41,44,45]. Purahong et al. [7] also emphasized the importance of fungi in the decomposition of deadwood, which is accelerated by the enrichment of fungi. Fungi are considered to be some of the most important organisms in wood decomposition because they directly produce the enzymes responsible for decomposing woody substrates [46].
However, we did not observe changes in the content of some biomarkers indicating bacteria consistent with changes in RCWD, these biomarkers were enriched in the CWD of C. japonica compared to that of P. strobilacea, we also no found a significant correlation between bacterial abundance and RCWD. Bacteria are generally considered to be more efficient than fungi in utilizing labile carbon compounds [20,22], so the higher proportion of labile carbon in some litter may stimulate competition among microorganisms, which may explain the higher content of bacterial PLFAs in the CF than in the DBF in this study. At least four bacterial roles in deadwood de-composition have been considered: (1) influencing wood permeability; (2) directly attacking wood; (3) fixing nitrogen; and (4) greatly impacting the overall microbial community composition through antagonistic activities [47,48]. Our work supports the fourth role of bacteria in wood decomposition due to the observed significant negative correlation between RCWD and soil B/F (Figure 4f). In addition, the changes in the content of some biomarkers indicating bacteria (e.g., 18:1ω7c) are opposite to the changes in the content of 18:2ω6c (Figure 3 and Figure 4). Several studies have shown that nitrogen-fixing bacteria may be important in litter decomposition processes, as they can interact directly with fungi that produce exoenzymes [49]. Nitrogen-fixing bacteria provide additional N to wood-inhabiting fungi to produce fruiting bodies [50] or mycelium biomass [51], which may stimulate CWD decomposition. Our results confirm this conclusion only modestly, CWD with a high decomposition rate contains high content of N, but the response of N content to habitat and tree types was not significant.
Finally, the RCWD used in this study is an instantaneous measure of CWD surface decomposition and does not directly reflect the residual mass of the entire wood strip throughout the decay process. Thus, the changes in residual mass during decay, especially in response to field sites, also need to be explored to more accurately assess the role of different microorganisms (bacteria and fungi) in driving changes in CWD decomposition. Furthermore, the PLFA analysis method provides limited classification information [52], and future work could use second-generation sequencing technology due to its ability to provide higher resolution outcomes.
5. Conclusions
We found that habitat and tree types all significantly affected CWD respiration rates, with both CWD respiration rates being significantly higher in the DBF than in the CF, but no large HFA of CWD decomposition was found. In addition, habitat significantly affected the microbial community structure of CWD and soil, microbes of CWD may be more important than soil microbes in driving the CWD decomposition. Fungi may be the major decomposer of CWD due to their abundance being positively correlated with RCWD, and bacteria may influence the CWD decomposition by impacting the overall microbial community composition (e.g., fungi). Our work highlights the importance of interactions between the three primary drivers (environment, substrate quality and microbes) on CWD decomposition.
Author Contributions
Conceptualization, Y.L.; Investigation, H.W. and Y.Z.; Data Curation, H.W.; Writing—Original Draft Preparation, H.W.; Writing—Review & Editing, H.W., L.Z., W.D., J.L., C.W. and Y.L.; Visualization, H.W.; Supervision, Y.L.; Project Administration, Y.L.; Funding Acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (31960303).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
We are grateful to the Lushan Mountain National Forest Ecological Station for providing the study sites. We thank the two anonymous reviewers for their suggestions on improving this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eaton, J.M.; Lawrence, D. Woody debris stocks and fluxes during succession in a dry tropical forest. For. Ecol. Manag. 2006, 232, 46–55. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.; Lattin, J.; Anderson, N.; Cline, S.; Aumen, N.; Sedell, J.R. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar]
- Hicks, W.T.; Harmon, M.E.; Myrold, D.D. Substrate controls on nitrogen fixation and respiration in woody debris from the Pacific Northwest, USA. For. Ecol. Manag. 2003, 176, 25–35. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Chambers, J.Q.; Schimel, J.P.; Nobre, A.D. Respiration from coarse wood litter in central Amazon forests. Biogeochemistry 2001, 52, 115–131. [Google Scholar] [CrossRef]
- Purahong, W.; Kahl, T.; Kruger, D.; Buscot, F.; Hoppe, B. Home-Field Advantage in Wood Decomposition Is Mainly Mediated by Fungal Community Shifts at “Home” Versus “Away”. Microb. Ecol. 2019, 78, 725–736. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Wang, H.; Huang, G.; Shu, C.; Kong, F.; Zhang, Y.; Geoff Wang, G.; Liu, Y. Home-field advantage of CWD decomposition in subtropical forests varied by field sites. For. Ecol. Manag. 2019, 444, 127–137. [Google Scholar] [CrossRef]
- Ayres, E.; Steltzer, H.; Simmons, B.L.; Simpson, R.T.; Steinweg, J.M.; Wallenstein, M.D.; Mellor, N.; Parton, W.J.; Moore, J.C.; Wall, D.H. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol. Biochem. 2009, 41, 606–610. [Google Scholar] [CrossRef]
- Gholz, H.L.; Wedin, D.A.; Smitherman, S.M.; Harmon, M.E.; Parton, W.J. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Glob. Chang. Biol. 2000, 6, 751–765. [Google Scholar] [CrossRef]
- Asplund, J.; Kauserud, H.; Bokhorst, S.; Lie, M.H.; Ohlson, M.; Nybakken, L. Fungal communities influence decomposition rates of plant litter from two dominant tree species. Fungal Ecol. 2018, 32, 1–8. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, S. Leaf litter decomposition in urban forests: Test of the home-field advantage hypothesis. Ann. For. Sci. 2016, 73, 1063–1072. [Google Scholar] [CrossRef]
- Hoppe, B.; Purahong, W.; Wubet, T.; Kahl, T.; Bauhus, J.; Arnstadt, T.; Hofrichter, M.; Buscot, F.; Kruger, D. Linking molecular deadwood-inhabiting fungal diversity and community dynamics to ecosystem functions and processes in Central European forests. Fungal Divers. 2016, 77, 367–379. [Google Scholar] [CrossRef]
- Binkley, D.; Giardina, C. Why do Tree Species Affect Soils? The Warp and Woof of Tree-soil Interactions. Biogeochemistry 1998, 42, 89–106. [Google Scholar] [CrossRef]
- Ushio, M.; Kitayama, K.; Balser, T.C. Tree species effects on soil enzyme activities through effects on soil physicochemical and microbial properties in a tropical montane forest on Mt. Kinabalu, Borneo. Pedobiologia 2010, 53, 227–233. [Google Scholar] [CrossRef]
- Lamit, L.J.; Busby, P.E.; Lau, M.K.; Compson, Z.G.; Wojtowicz, T.; Keith, A.R.; Zinkgraf, M.S.; Schweitzer, J.A.; Shuster, S.M.; Gehring, C.A.; et al. Tree genotype mediates covariance among communities from microbes to lichens and arthropods. J. Ecol. 2015, 103, 840–850. [Google Scholar] [CrossRef] [Green Version]
- Purahong, W.; Arnstadt, T.; Kahl, T.; Bauhus, J.; Kellner, H.; Hofrichter, M.; Krüger, D.; Buscot, F.; Hoppe, B. Are correlations between deadwood fungal community structure, wood physico-chemical properties and lignin-modifying enzymes stable across different geographical regions? Fungal Ecol. 2016, 22, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Van der Wal, A.; Ottosson, E.; De Boer, W. Neglected role of fungal community composition in explaining variation in wood decay rates. Ecology 2015, 96, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Fanin, N.; Fromin, N.; Bertrand, I. Functional breadth and home-field advantage generate functional differences among soil microbial decomposers. Ecology 2016, 97, 1023–1037. [Google Scholar] [CrossRef]
- de Graaff, M.A.; Classen, A.T.; Castro, H.F.; Schadt, C.W. Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol. 2010, 188, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Hunt, H.; Coleman, D.; Ingham, E.; Ingham, R.; Elliott, E.; Moore, J.; Rose, S.; Reid, C.; Morley, C.J.B.; Soils, F.O. The detrital food web in a shortgrass prairie. Biol. Fertil. Soils 1987, 3, 57–68. [Google Scholar] [CrossRef]
- Paterson, E.; Osler, G.; Dawson, L.A.; Gebbing, T.; Sim, A.; Ord, B. Labile and recalcitrant plant fractions are utilised by distinct microbial communities in soil: Independent of the presence of roots and mycorrhizal fungi. Soil Biol. Biochem. 2008, 40, 1103–1113. [Google Scholar] [CrossRef]
- Liu, X.Z.; Wang, L. Scientific Survey and Study of Biodiversity on the Lushan Nature Reserve in Jiangxi Province; Science Press: Beijing, China, 2010. [Google Scholar]
- Bao, S.D. Agriculture Chemistry Analysis of Soil; China Agriculture Press: Beijing, China, 1999. [Google Scholar]
- Bossio, D.A.; Scow, K.M. Impacts of Carbon and Flooding on Soil Microbial Communities: Phospholipid Fatty Acid Profiles and Substrate Utilization Patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Pietri, J.C.A.; Brookes, P.C. Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol. Biochem. 2009, 41, 1396–1405. [Google Scholar] [CrossRef]
- Zelles, L.J. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E.; Tunlio, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Olsson, P. A Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.K.; Mori, T.; Mao, Q.G.; Zhou, K.J.; Zhou, G.Y.; Nie, Y.X.; Mo, J.M. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. Annual carbon flux from woody debris for a boreal black spruce fire chronosequence. J. Geophys. Res. 2002, 108, WFX-1. [Google Scholar] [CrossRef]
- Freschet, G.T.; Aerts, R.; Cornelissen, J.H.C. Multiple mechanisms for trait effects on litter decomposition: Moving beyond home-field advantage with a new hypothesis. J. Ecol. 2012, 100, 619–630. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Perez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.S. Extending the leaf economics spectrum to decomposition: Evidence from a tropical forest. Ecology 2007, 88, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Weedon, J.T.; Cornwell, W.K.; Cornelissen, J.H.; Zanne, A.E.; Wirth, C.; Coomes, D.A.J.E.L. Global meta-analysis of wood decomposition rates: A role for trait variation among tree species? Ecol. Lett. 2009, 12, 45–56. [Google Scholar] [CrossRef] [PubMed]
- John, M.G.S.; Orwin, K.H.; Dickie, I.A. No ‘home’ versus ‘away’ effects of decomposition found in a grassland–forest reciprocal litter transplant study. Soil Biol. Biochem. 2011, 43, 1482–1489. [Google Scholar] [CrossRef]
- Luo, D.; Cheng, R.M.; Shi, Z.M.; Wang, W.X. Decomposition of Leaves and Fine Roots in Three Subtropical Plantations in China Affected by Litter Substrate Quality and Soil Microbial Community. Forests 2017, 8, 412. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 2012, 44, 31–38. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Wang, Y.; Sun, S.; Liu, L. Quantifying components of soil respiration and their response to abiotic factors in two typical subtropical forest stands, southwest China. PLoS ONE 2015, 10, e0117490. [Google Scholar] [CrossRef] [Green Version]
- Mäkipää, R.; Rajala, T.; Schigel, D.; Rinne, K.T.; Pennanen, T.; Abrego, N.; Ovaskainen, O. Interactions between soil- and dead wood-inhabiting fungal communities during the decay of Norway spruce logs. ISME J. 2017, 11, 1964–1974. [Google Scholar] [CrossRef] [Green Version]
- Güsewell, S.; Gessner, M.O. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
- Johnston, E.R.; Kim, M.; Hatt, J.K.; Phillips, J.R.; Yao, Q.; Song, Y.; Hazen, T.C.; Mayes, M.A.; Konstantinidis, K.T. Phosphate addition increases tropical forest soil respiration primarily by deconstraining microbial population growth. Soil Biol. Biochem. 2019, 130, 43–54. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Hyodo, F.; Kawakami, S. Foraging association between myxomycetes and fungal communities on coarse woody debris. Soil Biol. Biochem. 2018, 121, 95–102. [Google Scholar] [CrossRef]
- Lin, G.G.; Chen, Z.X.; Zeng, D.H. Presence of Mycorrhizal Fungal Hyphae Rather than Living Roots Retards Root Litter Decomposition. Forests 2019, 10, 502. [Google Scholar] [CrossRef] [Green Version]
- Arnstadt, T.; Hoppe, B.; Kahl, T.; Kellner, H.; Kruger, D.; Bassler, C.; Bauhus, J.; Hofrichter, M. Patterns of laccase and peroxidases in coarse woody debris of Fagus sylvatica, Picea abies and Pinus sylvestris and their relation to different wood parameters. Eur. J. For. Res. 2016, 135, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Greaves, H. The bacterial factor in wood decay. Wood Sci. Technol. 1971, 5, 6–16. [Google Scholar] [CrossRef]
- Kielak, A.M.; Scheublin, T.R.; Mendes, L.W.; van Veen, J.A.; Kuramae, E.E. Bacterial Community Succession in Pine-Wood Decomposition. Front. Microbiol. 2016, 7, 231. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, B.; Kahl, T.; Karasch, P.; Wubet, T.; Bauhus, J.; Buscot, F.; Kruger, D. Network Analysis Reveals Ecological Links between N-Fixing Bacteria and Wood-Decaying Fungi. PLoS ONE 2014, 9, e88141. [Google Scholar] [CrossRef] [Green Version]
- Robie, J.; White, D. Lipid analysis in microbial ecology: Quantitative approaches to the study of microbial communities. Bioscience 1989, 39, 535–541. [Google Scholar] [CrossRef]
- Weißhaupt, P.; Pritzkow, W.; Noll, M. Nitrogen metabolism of wood decomposing basidiomycetes and their interaction with diazotrophs as revealed by IRMS. Int. J. Mass Spectrom. 2011, 307, 225–231. [Google Scholar] [CrossRef]
- Purahong, W.; Stempfhuber, B.; Lentendu, G.; Francioli, D.; Reitz, T.; Buscot, F.; Schloter, M.; Kruger, D. Influence of Commonly Used Primer Systems on Automated Ribosomal Intergenic Spacer Analysis of Bacterial Communities in Environmental Samples. PLoS ONE 2015, 10, e0118967. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).