Decay Resistance of Nano-Zinc Oxide, and PEG 6000, and Thermally Modified Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Beech Wood
2.2. Chemical Treatment of Beech Wood with Nano-ZnO and/or PEG 6000
2.3. Thermal Modification of Beech Wood
2.4. Resistance of Beech Wood to Decaying Fungi
3. Results and Discussion
3.1. Weight Percentage Gain of Chemicals
3.2. Mass Loss at Thermal Modification
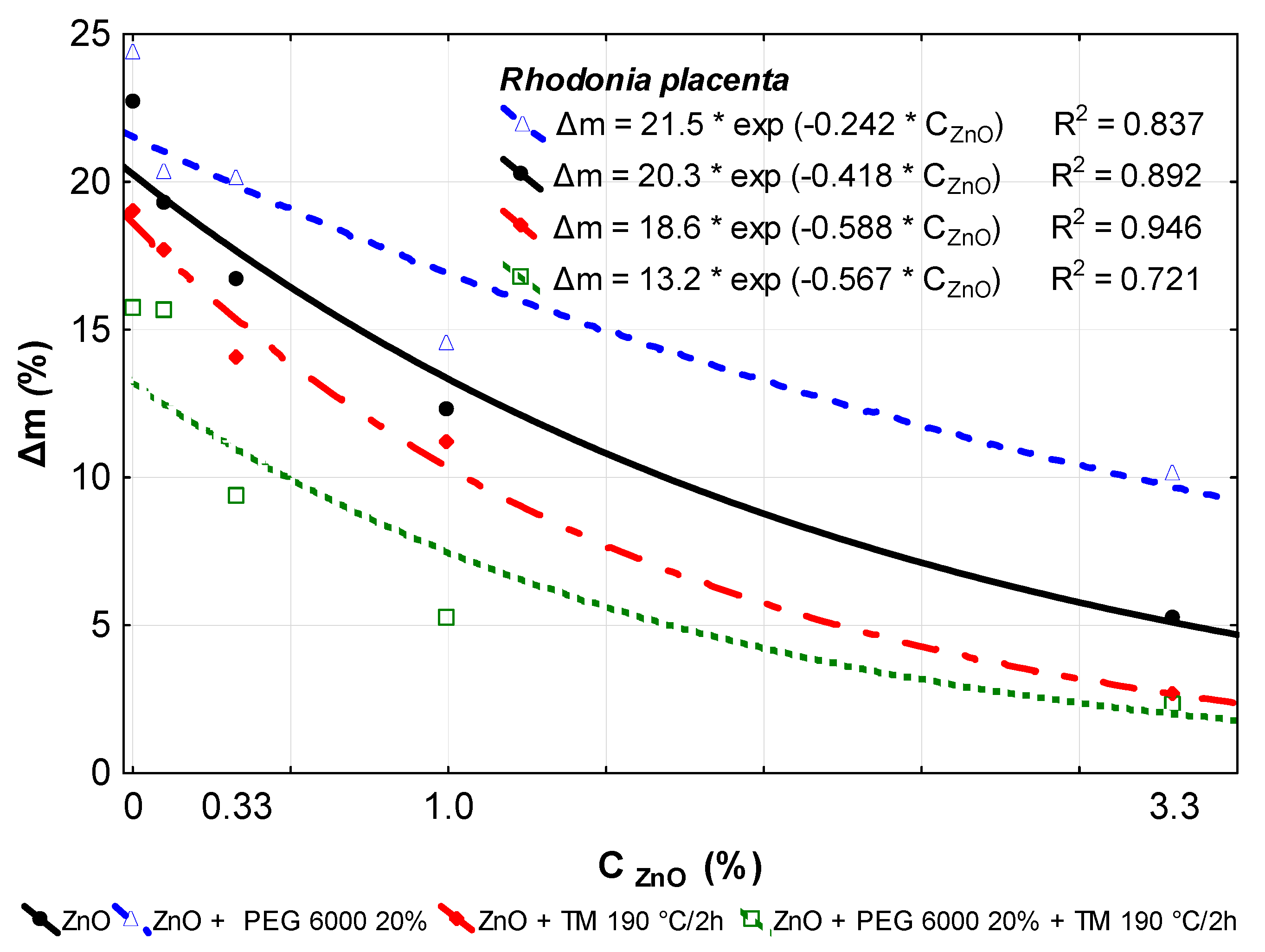
3.3. Decay Resistance
4. Conclusions
- Biologically active nano-ZnO, applied as 0.1 to 3.3 wt.% water system into beech wood using an immersion techniqueof, significantly increased the decay resistance of this nondurable tree species.
- On the contrary, PEG 6000 did not increase the decay resistance of beech wood.
- Beech wood that was thermally modified at 190 °C/2 h significantly increased its resistance to the white rot fungus Trametes versicolor by 51.9%, but only slightly to the brown-rot fungus Rhodonia placenta, at 16.5%.
- The additional thermal modification of beech wood treated first with nano-ZnO, or with nano-ZnO and PEG 6000, further increased its decay resistance to T. versicolor—maximally by 99.2%, and to R. placenta maximally by 89.9%.
- Conversely, the good fungicidal efficiency of nano-ZnO was, in several cases, reduced in the case of combination with PEG 6000. This was explained by the steric-mechanical barrier of the bio-inert polyethylene glycol macromolecules—created between the biocidal active nanoparticles of ZnO, and the mycelia of the wood-decaying fungi or their enzymes.
- Nano-ZnO introduced into beech wood in all combinations—alone, before PEG 6000, or before thermal modification—was more effective against the white-rot fungus T. versicolor than against the brown-rot fungus R. placenta.
- Taking into account the need for drying wood that has been chemically treated with aqueous systems of nano-ZnO and PEG, a combined chemical–thermal modification of wood, realized in one technological step, e.g., in the melt of paraffin + nano-ZnO or PEG + nano-ZnO, could be more suitable in practice, as outlined our previous experiments with thermal treatments of wood in the melts of pure paraffin or PEG [33,34].
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture and Rural Development of the Slovak Republic. Report on the Forestry Sector of the Slovak Republic 2020—Green Report; Ministry of Agriculture and Rural Development of the Slovak Republic: Bratislava, Slovakia, 2021; p. 69. Available online: https://www.mpsr.sk/en/index.php?navID=17&id=77 (accessed on 4 April 2022).
- Kúdela, J.; Čunderlík, I. Bukové Drevo—Štruktúra, Vlastnosti, Použitie (“Beech Wood—Structure, Properties, Usage”); Technical University in Zvolen: Zvolen, Slovakia, 2012; p. 152. ISBN 978-80-228-2318-0. [Google Scholar]
- Klement, I.; Réh, R.; Detvaj, J. Základné Charakteristiky Lesných Drevín; NLC Zvolen: Zvolen, Slovakia, 2010; p. 82. [Google Scholar]
- EN 350; Durability of Wood and Wood-Based Products—Testing and Classification of the Durability to Biological Agents of Wood and Wood-Based Materials. European Committee for Standardization: Brussels, Belgium, 2016.
- EN 335; Durability of Wood and Wood-Based Products—Use Classes: Definitions, Application to Solid Wood and Wood-Based Products. European Committee for Standardization: Brussels, Belgium, 2013.
- Teng, T.-J.; Arip, M.N.M.; Sudesh, K.; Nemoikina, A.; Jalaludin, Z.; Ng, E.-P.; Lee, H.L. Conventional technology and nanotechnology in wood preservation: A review. BioResources 2018, 13, 9220–9252. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, A.N.; Bikiaris, D.N.; Mitropoulos, A.C.; Kyzas, G.Z. Nanomaterials and Chemical Modifications for Enhanced Key Wood Properties: A Review. Nanomaterials 2019, 9, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasmini, L.; Rusli, R.; Khadiran, T.; Jalil, R.; Adnan, S. Application of nanotechnology in wood-based products industry: A review. Nanoscale Res. Lett. 2020, 15, 207. [Google Scholar] [CrossRef]
- Clausen, C.A.; Yand, V.W.; Arango, R.A.; Green, F., III. Feasibility of nanozinc oxide as a wood preservative. In Proceedings of the One Hundred Fifth Annual Meeting of the American Wood Protection Association, San Antonio, TX, USA, 19–21 April 2009; pp. 255–260. [Google Scholar]
- Marzbani, P.; Afrouzi, M.Y.; Omidvar, A. The effect of nano-zinc oxide on particleboard decay resistance. Maderas-Cienc. Technol. 2015, 17, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Lykidis, C.; Bak, M.; Mantanis, G.; Németh, R. Biological resistance of pine wood treated with nano-sized zinc oxide and zinc borate against brown-rot fungi. Eur. J. Wood Prod. 2016, 74, 909–911. [Google Scholar] [CrossRef] [Green Version]
- Borges, C.C.; Tonoli, G.H.D.; Cruz, T.M.; Duarte, P.J.; Junqueira, T.A. Nanoparticles-based wood preservatives: The next generation of wood protection? CERNE 2018, 24, 397–407. [Google Scholar] [CrossRef]
- Reinprecht, L.; Vidholdová, Z.; Kožienka, M. Decay inhibition of lime wood with zinc oxide nanoparticles used in combination with acrylic resin. Acta Fac. Xylol. Zvolen 2015, 57, 43–52. [Google Scholar]
- Reinprecht, L.; Vidholdová, Z.; Gašpar, F. Decay inhibition of maple wood with nano-zinc oxide used in combination with essential oils. Acta Fac. Xylol. Zvolen 2016, 58, 51–58. [Google Scholar] [CrossRef]
- Reinprecht, L.; Iždinský, J.; Vidholdová, Z. Biological resistance and application properties of particleboards containing nano-zinc oxide. Adv. Mater. Sci. Eng. 2018, 2018, 2680121. [Google Scholar] [CrossRef] [Green Version]
- Terzi, E.; Köse, C.; Kartal, S.N. Mold resistance of nano and micronized particles-treated wood after artificial weathering process. J. Anatol. Environ. Animal Sci. 2019, 4, 643–646. [Google Scholar] [CrossRef]
- Bak, M.; Németh, R. Effect of different nanoparticle treatments on the decay resistance of wood. BioResources 2018, 13, 7886–7899. [Google Scholar] [CrossRef] [Green Version]
- Hocker, E.; Almkvist, G.; Sahlstedt, M. The Vasa experience with polyethylene glycol: A conservator’s perspective. J. Cult. Herit. 2012, 13, 175–182. [Google Scholar] [CrossRef]
- Majka, J.; Zborowska, M.; Fejter, M.; Waliszewska, B.; Olek, W. Dimensional stability and hygroscopic properties of PEG treated irregularly degraded waterlogged Scots pine wood. J. Cult. Herit. 2018, 31, 133–140. [Google Scholar] [CrossRef]
- Broda, M. Biological effectiveness of archaeological oak wood treated with methyltrimethoxysilane and PEG against brown-rot fungi and moulds. Int. Biodeterior. Biodegrad. 2018, 134, 110–116. [Google Scholar] [CrossRef]
- Almkvist, G.; Norbakhsh, S.; Bjurhager, I.; Varmuza, K. Prediction of tensile strength in iron-contaminated archaeological wood by FT-IR spectroscopy—A study of degradation in recent oak and Vasa oak. Holzforschung 2016, 70, 855–865. [Google Scholar] [CrossRef]
- Tjeerdsma, B.F.; Boonstra, M.; Pizzi, A.; Tekely, P.; Militz, H. Characterisation of thermally modified wood: Molecular reasons for wood performance improvement. Holz Als Roh-Und Werkst. 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Srinivas, K.; Pandey, K.K. Effect of heat treatment on color changes, dimensional stability, and mechanical properties of wood. J. Wood Chem. Technol. 2012, 32, 304–316. [Google Scholar] [CrossRef]
- Sandberg, D.; Kutnar, A.; Mantanis, G. Wood modification technologies—A review. iForest 2017, 10, 895–908. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Antikainen, J.; Luostarinen, K.; Mononen, K.; Heräjärvi, H. Wetting-induced changes on the surface of thermally modified Scots pine and Norway spruce wood. Wood Sci. Technol. 2018, 52, 1181–1193. [Google Scholar] [CrossRef]
- Lunguleasa, A.; Ayrilmis, N.; Spirchez, C.; Özdemir, F. Investigation of the effects of heat treatment applied to beech plywood. Drvna Industrija 2018, 69, 349–355. [Google Scholar] [CrossRef]
- Taşdelen, M.; Can, A.; Sivrikaya, H. Some physical and mechanical properties of maritime pine and poplar exposed to oil-heat treatment. Turk. J. For. 2019, 20, 254–260. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification—Chemical, Thermal and Other Processes; John Wiley & Sons: Chichester, UK, 2006; p. 260. [Google Scholar]
- Esteves, B.; Pereira, H. Wood modification by heat treatment: A review. BioResources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Kocaefe, D.; Huang, X.; Kocaefe, Y. Dimensional Stabilization of Wood. Curr. For. Rep. 2015, 1, 151–161. [Google Scholar] [CrossRef]
- Kamperidou, V. The biological durability of thermally- and chemically-modified black pine and poplar wood against basidiomycetes and mold action. Forests 2019, 10, 1111. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Cao, J.; Wang, X. Properties of PEG/thermally modified wood flour/polypropylene (PP) composites. For. Stud. China 2012, 14, 307–314. [Google Scholar] [CrossRef]
- Reinprecht, L.; Repák, M. The impact of paraffin-thermal modification of beech wood on its biological, physical and mechanical properties. Forests 2019, 10, 1102. [Google Scholar] [CrossRef] [Green Version]
- Reinprecht, L.; Repák, M. Beech wood thermally modified in the melt of polyethylene glycol. BioResources 2022, 17, 652–672. [Google Scholar] [CrossRef]
- Van Acker, J.; Stevens, M.; Carey, J.; Sierra-Alvarez, R.; Militz, H.; Le Bayon, I.; Kleist, G.; Peek, R.D. Biological durability of wood in relation to end-use. Holz Als Roh-Und Werkst. 2003, 61, 35–45. [Google Scholar] [CrossRef]
- EN 113-2; Durability of Wood and Wood-Based Products—Test Method against Wood Destroying Basidiomycetes—Part 2: Assessment of Inherent or Enhanced Durability. European Committee for Standardization: Brussels, Belgium, 2020.
- Webb, J.S.; Nixon, M.; Eastwood, I.M.; Greenhalgh, M.; Robson, G.D.; Handley, P.S. Fungal colonization and biodeterioration of plasticized polyvinyl chloride. Appl. Environ. Microbiol. 2000, 66, 3194–3200. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.D. Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. Int. Biodeterior. Biodegrad. 2003, 52, 69–91. [Google Scholar] [CrossRef]
- Tiralová, Z.; Reinprecht, L. Fungal decay of acrylate treated wood. In Proceedings of the 35th International Research Group on Wood Preservation Annual Meeting: Proceedings (Doc. No. IRG/WP, 04-30357), Ljubljana, Slovenia, 6–10 June 2004; p. 7. [Google Scholar]
- Cappitelli, F.; Sorlini, C. Microorganisms attack synthetic polymers in items representing our cultural heritage. Appl. Environ. Microbiol. 2008, 74, 564–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harandi, D.; Ahmadi, H.; Achachluei, M.M. Comparison of TiO2 and ZnO nanoparticles for the improvement of consolidated wood with polyvinyl butyral against white rot. Int. Biodeterior. Biodegrad. 2016, 108, 142–148. [Google Scholar] [CrossRef]
- Kartal, S.N.; Green, F., III; Clausen, C.A. Do the unique properties of nanometals affect leachability or efficacy against fungi and termites? Int. Biodeterior. Biodegrad. 2009, 63, 490–495. [Google Scholar] [CrossRef]
- Mantanis, G.I.; Terziev, E.; Kartal, S.N.; Papadopoulos, A.N. Evaluation of mold, decay and termite resistance of pine wood treated with zinc- and copper-based nanocompounds. Int. Biodeterior. Biodegrad. 2014, 90, 140–144. [Google Scholar] [CrossRef]
- Soltani, M.; Najafi, A.; Yousefian, S.; Naji, H.R.; Bakar, E.S. Water repellent effect and dimension stability of beech wood impregnated with nano-zinc oxide. BioResources 2013, 8, 6280–6287. [Google Scholar] [CrossRef] [Green Version]
- Green, F., III; Clausen, C.A. Copper tolerance of brown-rot fungi: Time course of oxalic acid production. Int. Biodeterior. Biodegrad. 2003, 51, 145–149. [Google Scholar] [CrossRef]
- Németh, R.; Bak, M.; Yimmou, B.M.; Csupor, K.; Molnár, S.; Csóka, L. Nano-zink as an agent against wood destroying fungi. In Proceedings of the Annual IAWS Meeting, 5th International Symposium on the Interaction of Wood with Various Forms of Energy, Technical University in Zvolen, Zvolen, Slovakia, 26–28 September 2012. [Google Scholar]
- Bak, M.; Yimmou, B.M.; Csupor, K.; Németh, R.; Csóka, L. Enhancing the durability of wood against wood destroying fungi using nano-zink. In Proceedings of the International Scientific Conference on Sustainable Development & Ecological Footprint, Sopron, Hungary, 26–27 March 2012. [Google Scholar]
- Lykidis, C.; Mantanis, G.; Adamopoulos, S.; Kalafata, K.; Arabatzis, I. Effects of nano-sized zinc oxide and zinc borate impregnation on brown rot resistance of black pine (Pinus nigra L.) wood. Wood Mater. Sci. Eng. 2013, 8, 242–244. [Google Scholar] [CrossRef]


| Treatment of Beech Wood | Nano-ZnO WPG (%) | PEG 6000 WPG (%) |
|---|---|---|
| ZnO 0.1% | 0.051 | - |
| ZnO 0.33% | 0.148 | - |
| ZnO 1.0% | 0.532 | - |
| ZnO 3.3% | 1.548 | - |
| PEG 6000 20% | - | 8.36 |
| ZnO 0.1% + PEG 6000 20% | 0.047 | 9.18 |
| ZnO 0.33% + PEG 6000 20% | 0.161 | 8.14 |
| ZnO 1.0% + PEG 6000 20% | 0.568 | 9.64 |
| ZnO 3.3% + PEG 6000 20% | 1.497 | 9.72 |
| Treatment of Beech Wood | R. placenta Δm (%) | T. versicolor Δm (%) |
|---|---|---|
| Original–Reference | 22.73 (2.86) | 21.81 (4.02) |
| ZnO 0.1% | 19.27 (4.16) d | 15.19 (2.11) c |
| ZnO 0.33% | 16.68 (2.46) b | 4.25 (2.26) a |
| ZnO 1.0% | 12.29 (2.42) a | 3.88 (1.82) a |
| ZnO 3.3% | 5.25 (1.98) a | 0.86 (0.06) a |
| PEG 6000 20% | 24.40 (2.47) d | 21.96 (2.76) d |
| ZnO 0.1% + PEG 6000 20% | 20.32 (4.52) d | 19.98 (2.08) d |
| ZnO 0.33% + PEG 6000 20% | 20.14 (3.14) d | 11.14 (2.74) a |
| ZnO 1.0% + PEG 6000 20% | 14.54 (2.25) a | 5.47 (0.47) a |
| ZnO 3.3% + PEG 6000 20% | 10.13 (4.49) a | 4.65 (0.36) a |
| TM 190 °C/2 h | 18.97 (2.22) c | 10.50 (1.08) a |
| ZnO 0.1% + TM 190 °C/2 h | 17.66 (2.76) b | 11.27 (1.28) a |
| ZnO 0.33% + TM 190 °C/2 h | 14.01 (1.49) a | 8.20 (1.61) a |
| ZnO 1.0% + TM 190 °C/2 h | 11.20 (4.49) a | 3.74 (0.71) a |
| ZnO 3.3% + TM 190 °C/2 h | 2.63 (2.13) a | 0.18 (0.14) a |
| PEG 6000 20% + TM 190 °C/2 h | 15.75 (2.99) a | 8.63 (1.76) a |
| ZnO 0.1% + PEG 6000 20% + TM 190 °C/2 h | 15.62 (2.41) a | 5.39 (1.38) a |
| ZnO 0.33% + PEG 6000 20% + TM 190 °C/2 h | 9.38 (3.65) a | 6.03 (1.28) a |
| ZnO 1.0% + PEG 6000 20% + TM 190 °C/2 h | 5.23 (2.83) a | 2.38 (0.18) a |
| ZnO 3.3% + PEG 6000 20% + TM 190 °C/2 h | 2.29 (0.37) a | 1.73 (0.46) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinprecht, L.; Repák, M.; Iždinský, J.; Vidholdová, Z. Decay Resistance of Nano-Zinc Oxide, and PEG 6000, and Thermally Modified Wood. Forests 2022, 13, 731. https://doi.org/10.3390/f13050731
Reinprecht L, Repák M, Iždinský J, Vidholdová Z. Decay Resistance of Nano-Zinc Oxide, and PEG 6000, and Thermally Modified Wood. Forests. 2022; 13(5):731. https://doi.org/10.3390/f13050731
Chicago/Turabian StyleReinprecht, Ladislav, Miroslav Repák, Ján Iždinský, and Zuzana Vidholdová. 2022. "Decay Resistance of Nano-Zinc Oxide, and PEG 6000, and Thermally Modified Wood" Forests 13, no. 5: 731. https://doi.org/10.3390/f13050731
APA StyleReinprecht, L., Repák, M., Iždinský, J., & Vidholdová, Z. (2022). Decay Resistance of Nano-Zinc Oxide, and PEG 6000, and Thermally Modified Wood. Forests, 13(5), 731. https://doi.org/10.3390/f13050731








