Revealing the Cryptic Diversity of Wood-Inhabiting Auricularia (Auriculariales, Basidiomycota) in Europe
Abstract
:1. Introduction
2. Materials and Methods
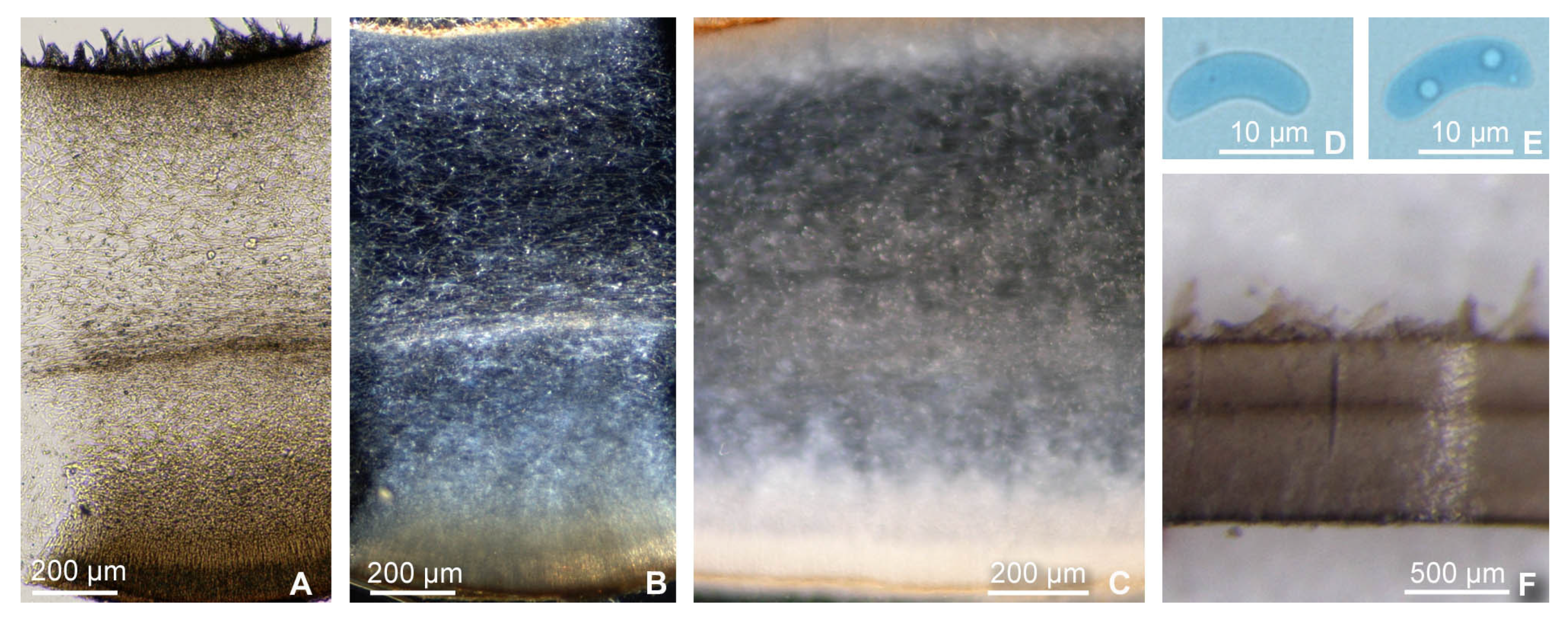
2.1. Collection of Specimens, Morphological Methods
2.2. Molecular Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hibbett, D.S. A phylogenetic overview of the Agaricomycotina. Mycologia 2006, 98, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms; Ten Speed Press: Berkeley, CA, USA, 1993; pp. 395–400. [Google Scholar]
- Yoon, S.J.; Yu, M.A.; Pyun, Y.R.; Hwang, J.K.; Chu, D.C.; Juneja, L.R.; Mourão, P.A.S. The nontoxic mushroom Auricularia auricula contains a polysaccharide with anticoagulant activity mediated by antithrombin. Thromb. Res. 2003, 112, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Lowy, B. A morphological basis for classifying the species of Auricularia. Mycologia 1951, 43, 351–358. [Google Scholar] [CrossRef]
- Lowy, B. The genus Auricularia. Mycologia 1952, 44, 656–693. [Google Scholar] [CrossRef]
- Kobayasi, Y. The genus Auricularia. Bull. Natl. Sci. Mus. Tokyo B 1981, 7, 41–67. [Google Scholar]
- Montoya-Alvarez, A.F.; Hayakawa, H.; Minamya, Y.; Fukuda, T. Phylogenetic relationships and review of the species of Auricularia (Fungi: Basidiomycetes) in Colombia. Caldasia 2011, 33, 55–66. [Google Scholar]
- Weiß, M.; Oberwinkler, F. Phylogenetic relationships in Auriculariales and related groups—hypotheses derived from nuclear ribosomal DNA sequences. Mycol. Res. 2001, 105, 403–415. [Google Scholar] [CrossRef]
- Yan, P.S.; Jiang, J.H.; Wang, D.C.; Luo, X.C.; Zhou, Q. Molecular taxonomic relationships of Auricularia species inferred from RAPD markers. Mycosystema 2002, 21, 47–52. [Google Scholar]
- Looney, B.; Birkebak, J.; Matheny, P.B. Systematics of the genus Auricularia with an emphasis on species from the southeastern United States. N. Am. Fungi 2013, 8, 1–25. [Google Scholar] [CrossRef]
- Malysheva, V.F.; Bulakh, E.M. Contribution to the study of the genus Auricularia (Auriculariales, Basidiomycota) in Russia. Nov. Sist. Nizsh. Rast. 2014, 48, 164–180. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, Y.; Malysheva, V.F.; Du, P.; Dai, Y.C. Species clarification of the most important and cultivated Auricularia mushroom “Heimuer”: Evidence from morphological and molecular data. Phytotaxa 2014, 186, 241–253. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, Y.; He, S.H.; Bandara, A.R.; Hyde, K.D.; Malysheva, V.F.; Li, D.W.; Dai, Y.C. Global diversity and taxonomy of the Auricularia auricula-judae complex (Auriculariales, Basidiomycota). Mycol. Prog. 2015, 14, 1–16. [Google Scholar] [CrossRef]
- Wu, F.; Tohtirjap, A.; Fan, L.F.; Zhou, L.W.; Alvarenga, R.L.M.; Gibertoni, T.B.; Dai, Y.C. Global diversity and updated phylogeny of Auricularia (Auriculariales, Basidiomycota). J. Fungi 2021, 7, 933. [Google Scholar] [CrossRef] [PubMed]
- Jülich, W. Die Nichtblätterpilze, Gallertpilze und Bauchpilze (Aphyllophorales, Heterobasidiomycetes, Gastromycetes); Gustav Fischer Verlag: Jena, Germany, 1984; p. 392. [Google Scholar]
- Læssøe, T.; Petersen, J.H. Fungi of Temperate Europe; Princeton University Press: Princeton, NJ, USA, 2019; p. 1182. [Google Scholar]
- Pilát, A. Přehled evropských Auriculariales a Tremellales se zvláštním zřetelem k československým druhům. Acta Mus. Nat. Pragae 1957, 13B, 115–210. [Google Scholar]
- Gange, A.C.; Gange, E.G.; Mohammad, A.B.; Boddy, L. Host shift in fungi caused by climate change? Fungal. Ecol. 2011, 4, 184–190. [Google Scholar] [CrossRef]
- Duncan, E.G.; Macdonald, J.A. Micro-evolution in Auricularia auricula. Mycologia 1967, 59, 803–818. [Google Scholar] [CrossRef]
- Cho, S.E.; Kwag, Y.N.; Lee, D.H.; Han, J.G.; Kim, C.S. Current taxonomical status of Korean Auricularia species. Kor. J. Mycol. 2021, 49, 21–31. [Google Scholar] [CrossRef]
- Wu, F.; Dai, Y.C. Notes on the nomenclature of the Auricularia auricula-judae complex. Mycosystema 2015, 34, 604–611. [Google Scholar] [CrossRef]
- Shirouzu, T.; Inaba, S.; Ushijima, S.; Okuda, Y.; Nagasawa, E. Taxonomic study of Japanese “Auricularia auricula-judae” and “A. polytricha” based on molecular phylogeny and morphological comparison. Jpn. J. Mycol. 2018, 59, 7–20. [Google Scholar] [CrossRef]
- Index Herbariorum. Available online: http://sweetgum.nybg.org/ih/ (accessed on 11 February 2022).
- Clémençon, H. Zwei verbesserte Präparierlösungen für die microskopische Untersuchung von Pilze. Zeitschr. F. Pilzk. 1972, 38, 49–53. [Google Scholar]
- Wong, G.J.; Wells, K. Comparative morphology, compatibility, and interfertility of Auricularia cornea, A. polytricha, and A. tenuis. Mycologia 1987, 79, 847–856. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlasák, J.; Kout, J.; Chen, Q.; Dai, Y.C. Fuscoporia caymanensis sp. nov. (Basidiomycota, Hymenochaetaceae), a new species from tropical America. Phytotaxa 2020, 472, 135–146. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; Mark, P.; Avres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes3.2: Efficient Bayesian phylogenetic inference and model choice, across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Yuan, Y.; Rivoire, B.; Dai, Y.C. Phylogeny and diversity of the Auricularia mesenterica (Auriculariales, Basidiomycota) complex. Mycol. Progress. 2015, 14, 1–9. [Google Scholar] [CrossRef]


| Species | Sample | ITS number |
|---|---|---|
| Auricularia africana | T3 | MH213349 |
| A. africana | Ryvarden 44929 | MH213350 |
| A. americana | Dai 13476 | KM396766 |
| A. americana | LE 296428 | KJ698429 |
| A. americana | HHB 11370 | KM396766 |
| A. angiospermarum | TJV-93-12-SP | KT152096 |
| A. angiospermarum | Cui 12360 | KT152097 |
| A. angiospermarum | HHB 11037 | KT152098 |
| A. asiatica | Dai 16149 | KX022010 |
| A. asiatica | OM 13932 | MZ618931 |
| A. asiatica | BBH 895 | KX621160 |
| A. auricula-judae | MW 446 | AF291268 |
| A. auricula-judae | Dai 13210 | KM396769 |
| A. auricula-judae | MT 7 | KM396771 |
| A. auricula-judae | JK 1110 | OM747869 |
| A. auricula-judae | TFCMic 00056 | OM747870 |
| A. australiana | HN 3213 | MZ647504 |
| A. australiana | HT 190 | MZ647503 |
| A. brasiliana | CRSL 886 | KP729274 |
| A. brasiliana | AN-MA 42 | KP729275 |
| A. brasiliana | RSC 359 | KP729276 |
| A. cerrina sp. nov. | HK 677 | OM747871 |
| A. cerrina sp. nov. | HK 1510 | OM747872 |
| A. conferta | Dai 18825 | MZ647500 |
| A. conferta | Dai 18826 | MZ647505 |
| A. cornea | Dai 12587 | KX022012 |
| A. cornea | Dai 15336 | KX022014 |
| A. cornea | YG-DR 1 | MH213353 |
| A. cornea | MJ 1708 | OM747873 |
| A. fibrillifera | Dai 13598A | KP765615 |
| A. fibrillifera | F 234519 | KP765610 |
| A. fibrillifera | Cui 6704 | KP765613 |
| A. fuscosuccinea | FP-102573 | KX022027 |
| A. fuscosuccinea | Dai 17451 | MH213368 |
| A. fuscosuccinea | Dai 17422 | MH213367 |
| A. heimuer | Dai 13503 | KM396789 |
| A. heimuer | Dai 13765 | KM396793 |
| A. heimuer | Dai 2291 | KM396785 |
| A. lateralis | Dai 16416 | KX022023 |
| A. lateralis | Dai 16420 | KX022025 |
| A. lateralis | Dai 15670 | KX022022 |
| A. mesenterica | LYBR 5353 | KM396801 |
| A. mesenterica | Kytovuori-89-333 | KP729284 |
| A. mesenterica | Miettinen 12680 | KP729286 |
| A. mesenterica | YG 029 | MZ618938 |
| A. minutissima | Dai 14881 | KT152104 |
| A. minutissima | Dai 15455 | KX022030 |
| A. minutissima | LE 296424 | KJ698434 |
| A. nigricans | Ahti 55718 | MH213372 |
| A. nigricans | TJY 93-242 | KM396803 |
| A. nigricans | Ahti 36234 | KM396802 |
| A. novozealandica | PDD 75110 | KX022032 |
| A. novozealandica | PDD 83897 | KX022034 |
| A. novozealandica | PDD 81195 | KX022033 |
| A. orientalis | Dai 14875 | KP729270 |
| A. orientalis | Dai 1831 | KP729271 |
| A. orientalis | Dai15813 | KX022036 |
| A. pilosa | LWZ20190421-7 | MZ647506 |
| A. pilosa | JMH 45 | KM267731 |
| A. pusio | AK 174 | MH213373 |
| A. pusio | AK 547 | MH213374 |
| A. pusio | Smith 18 | MH213375 |
| A. scissa | DR 777 | KM396804 |
| A. scissa | Ahti 49388 | KM396805 |
| A. scissa | TFB 11193 | JX065160 |
| A. sinodelicata | Cui 8596 | MH213376 |
| A. sinodelicata | Dai 13926 | MH213379 |
| A. sinodelicata | Dai 12242 | MH213381 |
| A. srilankensis | Dai 19522 | MZ647501 |
| A. srilankensis | Dai 19519 | MZ647507 |
| A. srilankensis | Dai 19575 | MZ647502 |
| A. subglabra | Dai 17403 | MH213382 |
| A. subglabra | Dai 17394 | MH213383 |
| A. subglabra | TFB 10046 | JX524199 |
| A. submesenterica | Dai 792 | KX022037 |
| A. submesenterica | Dai 14773 | KX022038 |
| A. submesenterica | Dai 15450 | MH213386 |
| A. thailandica | MFLU 130396 | KR336690 |
| A. thailandica | Dai 15335 | KX022026 |
| A. thailandica | Dai 13655A | KP765619 |
| A. tibetica | Cui 12267 | KT152106 |
| A. tibetica | Cui 12337 | KT152108 |
| A. tibetica | Dai 15604 | MH213388 |
| A. tremellosa | AJS 5896 | JX065162 |
| A. tremellosa | TENN 28734 | JX065159 |
| A. tremellosa | Dai 17419 | MH213391 |
| A. villosula | Dai 13450 | KM396812 |
| A. villosula | LE 296422 | NR137873 |
| A. villosula | MFLU 162128 | KX621164 |
| Elmerina efibulata | Yuan 4525 | MZ618945 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kout, J.; Wu, F. Revealing the Cryptic Diversity of Wood-Inhabiting Auricularia (Auriculariales, Basidiomycota) in Europe. Forests 2022, 13, 532. https://doi.org/10.3390/f13040532
Kout J, Wu F. Revealing the Cryptic Diversity of Wood-Inhabiting Auricularia (Auriculariales, Basidiomycota) in Europe. Forests. 2022; 13(4):532. https://doi.org/10.3390/f13040532
Chicago/Turabian StyleKout, Jiří, and Fang Wu. 2022. "Revealing the Cryptic Diversity of Wood-Inhabiting Auricularia (Auriculariales, Basidiomycota) in Europe" Forests 13, no. 4: 532. https://doi.org/10.3390/f13040532
APA StyleKout, J., & Wu, F. (2022). Revealing the Cryptic Diversity of Wood-Inhabiting Auricularia (Auriculariales, Basidiomycota) in Europe. Forests, 13(4), 532. https://doi.org/10.3390/f13040532





