Transient Flooding and Soil Covering Interfere with Decomposition Dynamics of Populus euphratica Leaf Litter: Changes of Mass Loss and Stoichiometry of C, N, P, and K
Abstract
:1. Introduction
2. Materials and Methods
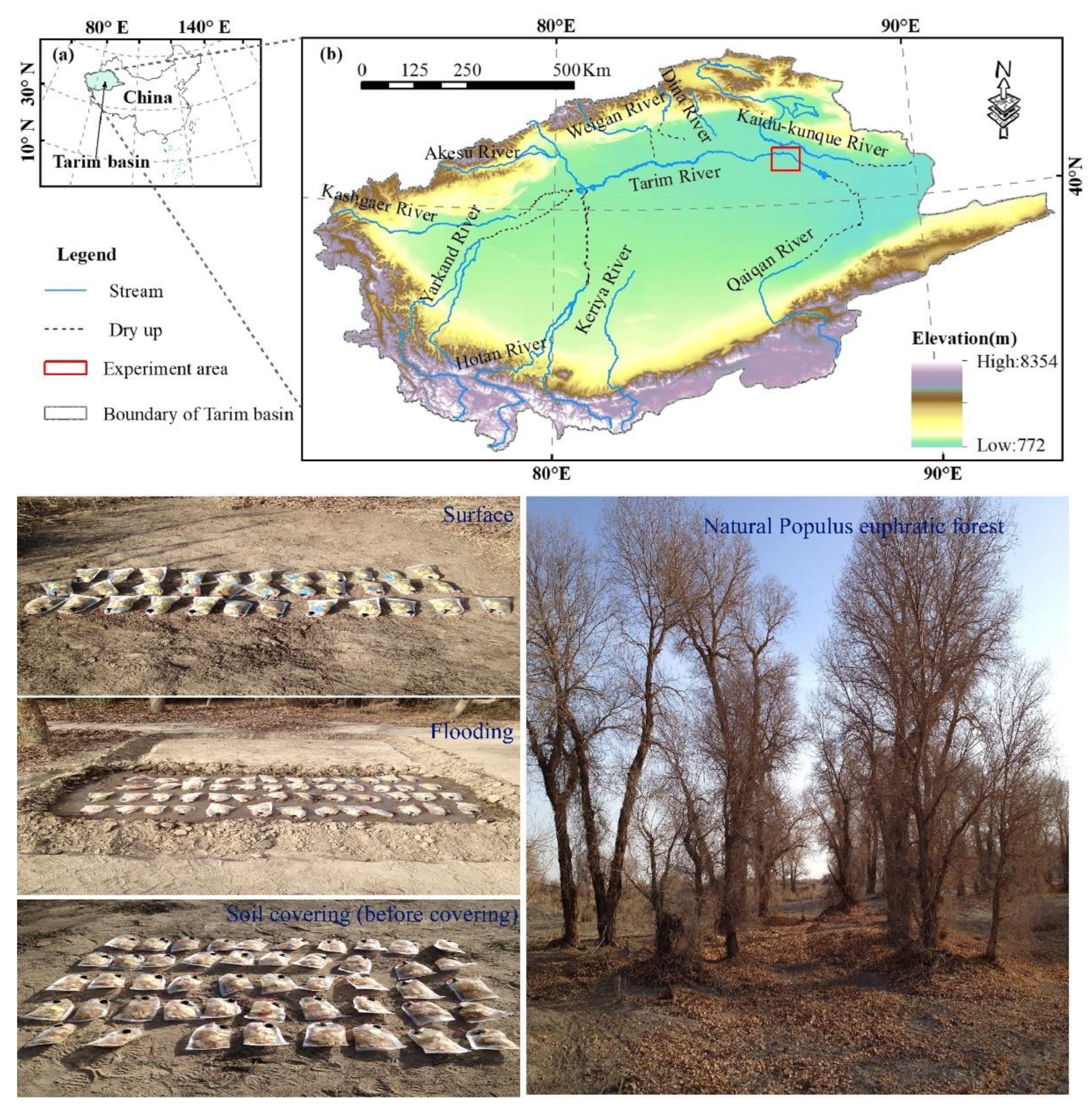
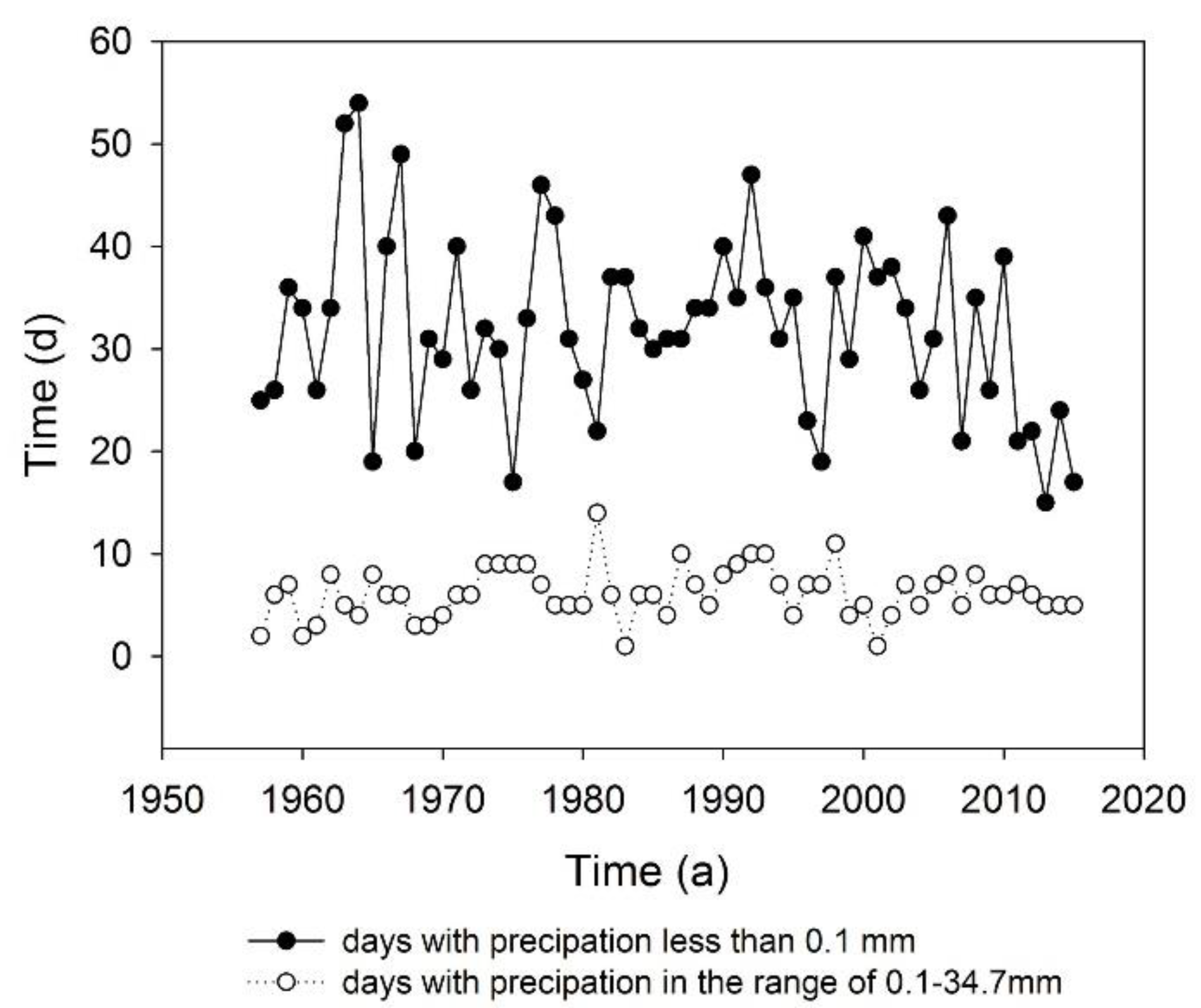
2.1. Research Site
2.2. Sample Preparation and Decomposition Experiment
2.3. Assessment of Mass Loss
2.4. Nutrient Chemical Analysis
2.5. Data Analysis
3. Results
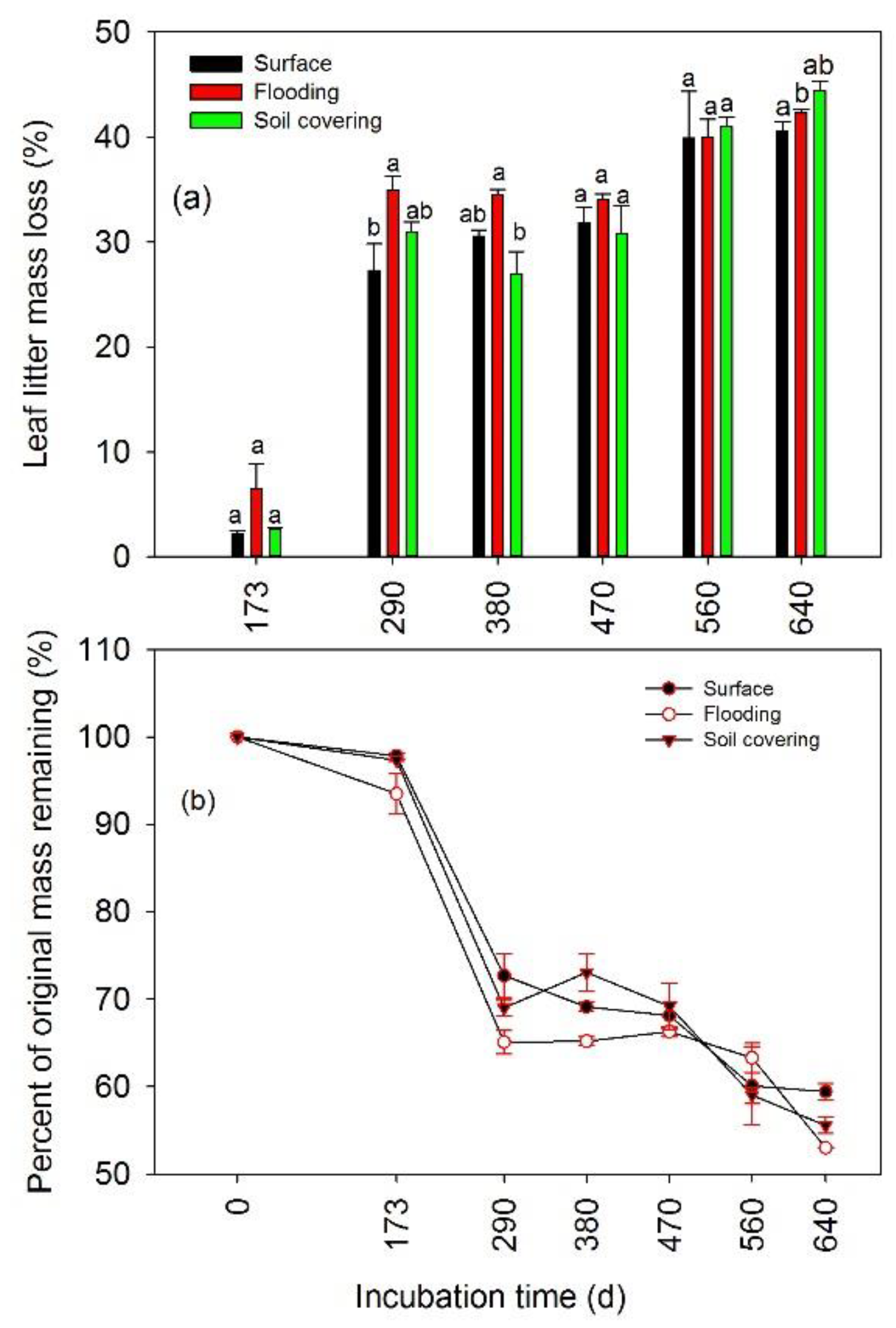
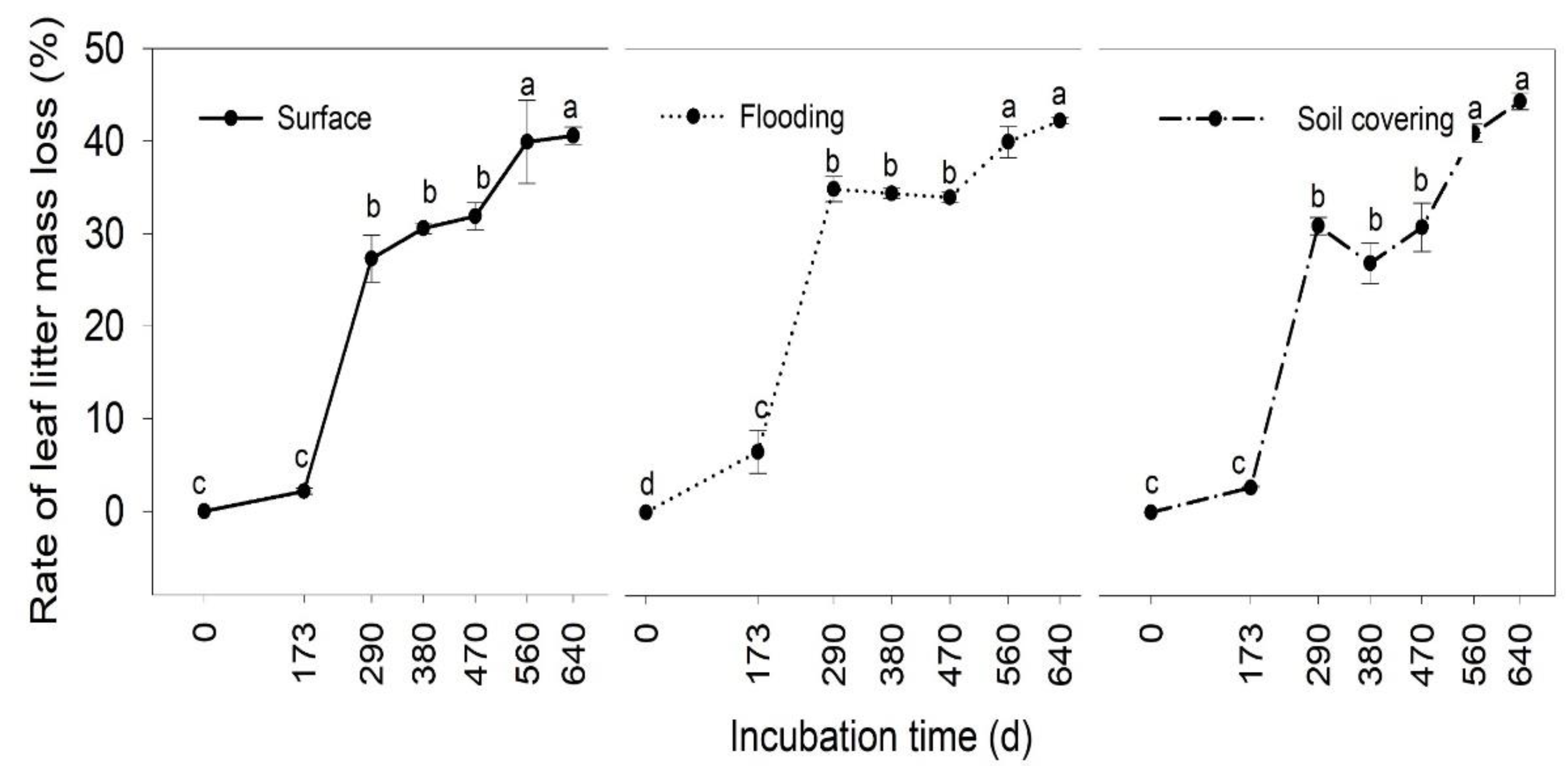
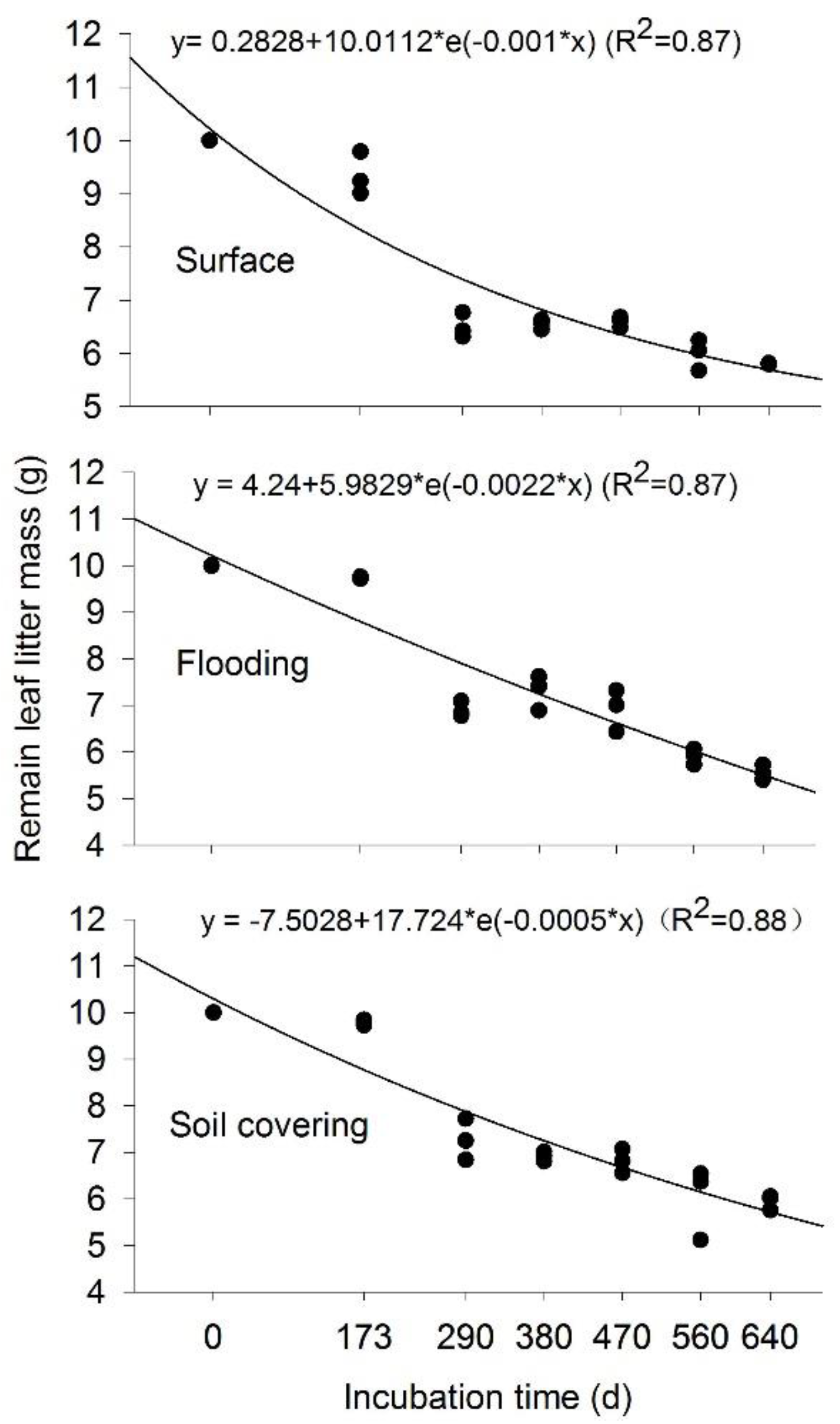
3.1. Temporal Changes of Leaf Litter Mass
3.2. Effects of Transient Flooding and Soil Covering on Leaf Litter Mass
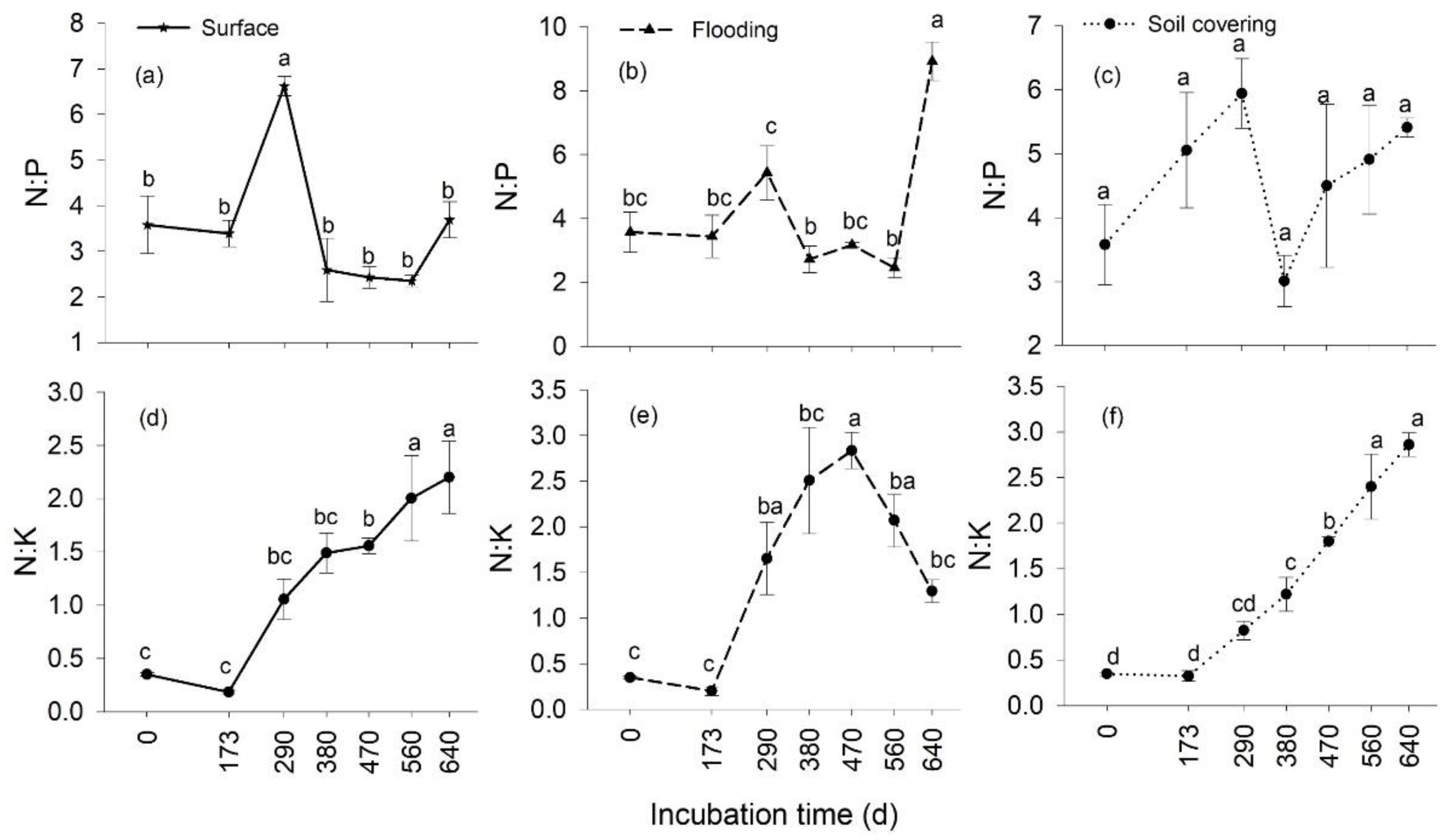
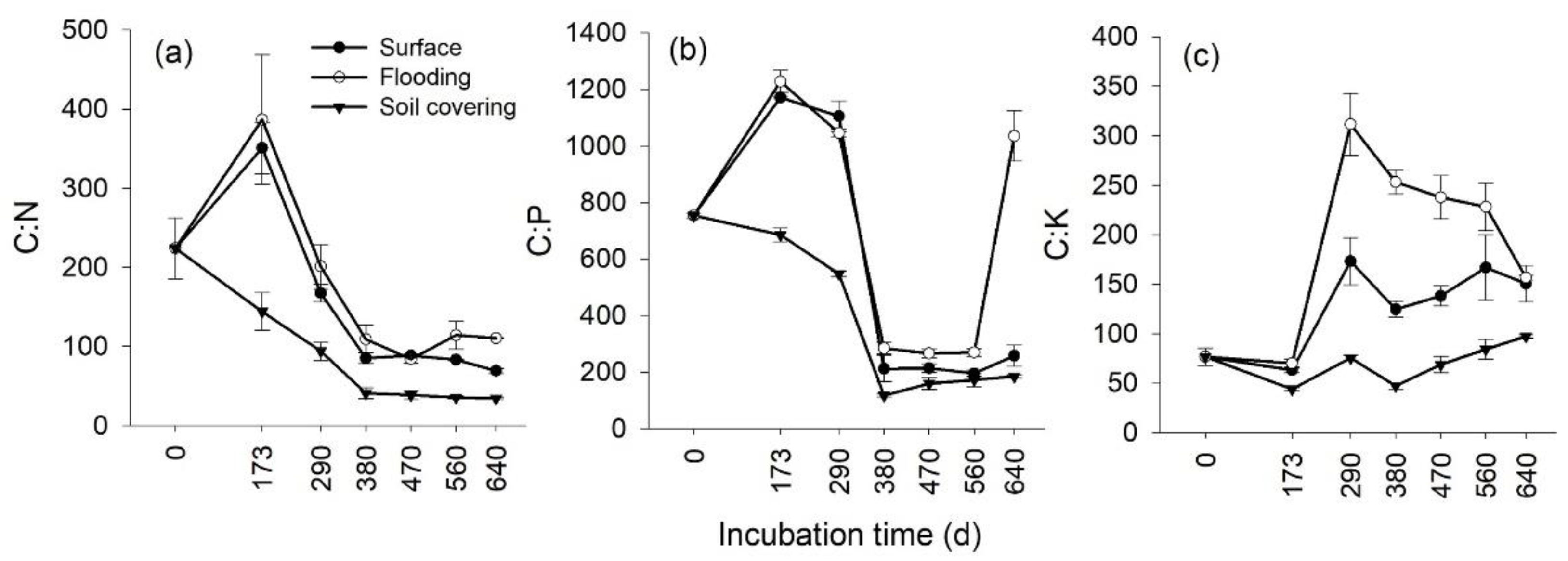
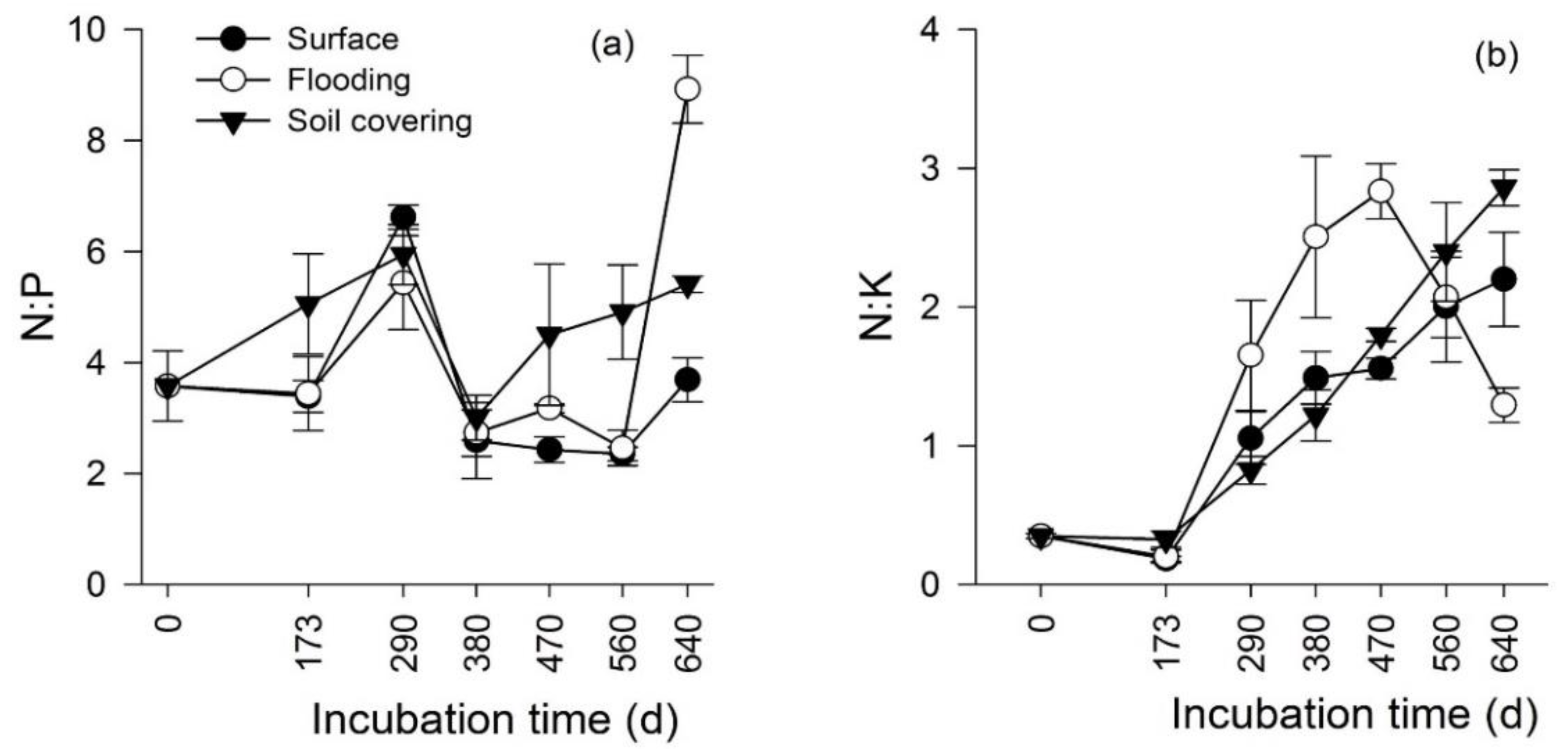
3.3. Temporal Changes in the Stoichiometry of Leaf Litter
3.4. Effects of Transient Flooding and Soil Covering on the Stoichiometry of Leaf Litter
4. Discussion
4.1. Effecst of Transient Flooding and Soil Covering on Leaf Litter Mass Loss
4.2. Effects of Transient Flooding and Soil Covering on the Stoichiometry of Leaf Litter
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magin, A.H.; Aber, J.D. Dissolved organic carbon and nitrogen relationships in forest litter as affected by nitrogen deposition. Soil Biol. Biochem. 2000, 32, 603–613. [Google Scholar]
- Freschet, G.T.; Weedon, J.T.; Aerts, R.; Van Hal, J.R.; Cornelissen, J.H.C. Interspecific differences in wood decay rates: Insights from a new short-term method to study long-term wood decomposition. J. Ecol. 2012, 100, 161–170. [Google Scholar] [CrossRef]
- Angst, Š.; Harantová, L.; Baldrian, P.; Angst, G.; Cajthaml, T.; Straková; Blahut, J.; Veselá; Frouz, J. Tree species identity alters decomposition of understory litter and associated microbial communities: A case study. Biol. Fert. Soils 2019, 55, 525–538. [Google Scholar] [CrossRef] [Green Version]
- Hobbie, B.; Hoorens, H.; Kurokawa, N.; Péreze Harguindeguy, H.M.; Quested, L.S.; Santiago, D.A.; Wardle, I.J.; Wright, R.; Aerts, S.D.; Allison, P.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar]
- Petraglia, A.; Cacciatori, C.; Chelli, S.; Fenu, G.; Calderisi, G.; Gargano, D.; Abeli, T.; Orsenigo, S.; Carbognani, M. Litter decomposition: Effects of temperature driven by soil moisture and vegetation type. Plant Soil 2019, 435, 187–200. [Google Scholar] [CrossRef]
- Zhong, Y.Q.W.; Yan, W.M.; Wang, R.W.; Shangguan, Z.P. Differential responses of litter decomposition to nutrient addition and soil water availability with long-term vegetation recovery. Biol. Fertile Soils 2017, 53, 939–949. [Google Scholar] [CrossRef]
- Horodecki, P.; Jagodziński, A.M. Tree species effects on litter decomposition in pure stands on afforested post-mining sites. For. Ecolo. Manag. 2017, 406, 1–11. [Google Scholar] [CrossRef]
- Purahong, W.; Krüger, D.; Buscot, F.; Wubet, T. Correlations between the composition of modular fungal communities and litter decomposition-associated ecosystem functions. Fungal Ecol. 2016, 22, 106–114. [Google Scholar] [CrossRef]
- Zhang, T.A.; Luo, Y.Q.; Chen, H.Y.H.; Ruan, H.H. Responses of litter decomposition and nutrient release to N addition: A meta-analysis of terrestrial ecosystems. Appl. Soil Ecol. 2018, 128, 35–42. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.Y.; Li, D.W.; Li, Z.X.; Song, L.; Chen, K.; Yun, F. Slower rates of litter decomposition of dominant epiphytes in the canopy than on the forest floor in a subtropical montane forest, southwest China. Soil Biol. Biochem. 2014, 70, 211–220. [Google Scholar] [CrossRef]
- Alhamd, L.; Arakaki, S.; Hagihara, A. Decomposition of leaf litter of four tree species in a subtropical evergreen broad-leaved forest, Okinawa Island, Japan. For. Ecol. Manag. 2004, 202, 1–11. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Reich, P.B.; Oleksyn, J.; Ogdahl, M.; Żytkowiak, R.; Hale, C.; Karolewski, P. Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 2006, 87, 2288–2297. [Google Scholar] [CrossRef]
- Thomas, K.; Jijeesh, C.M.; Seethalakshmi, K.K. Litter production, decomposition and nutrient mineralization dynamics of Ochlandra setigera: A rare bamboo species of Nilgiri Biospher Reserve, India. J. For. Res. 2014, 25, 579–584. [Google Scholar] [CrossRef]
- Ge, J.L.; Xie, Z.Q. Leaf litter carbon, nitrogen, and phosphorus stoichiometric patterns as related to climatic factors and leaf habits across Chinese broad-leaved tree species. Plant Ecol. 2017, 218, 1063–1076. [Google Scholar] [CrossRef]
- Berg, B.; Liu, C.J.; Laskowski, R.; Davey, M. Relationships between nitrogen, acid-unhydrolyzable residue, and climate among tree foliar litters. Can. J. For. Res. 2013, 43, 103–107. [Google Scholar] [CrossRef]
- Erickson, H.E.; Helmer, E.; Brandeis, T.J.; Lugo, A.E. Controls on fallen leaf chemistry and forest floor element masses in native and novel forests across a tropical island. Ecosphere 2014, 5, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.S.; Joergensen, R.G. Stoichiometry of the soil microbial biomass in response to amendments with varying C/N/P/S ratios. Biol. Fertile Soils 2019, 55, 265–274. [Google Scholar] [CrossRef]
- Marklein, A.R.; Winbourne, J.B.; Enders, S.K.; Gonzalez, D.J.; Huysen, T.V.; Izquierdo, J.G.; Light, D.R.; Carolina, M.R.; Antonio, B.M. Leaf chemistry of woody species in the Brazilian cerrado and seasonal forest: Response to soil and taxonomy and effects on decomposition rates. Plant Ecol. 2016, 217, 1467–1479. [Google Scholar]
- Ren, C.J.; Chen, J.; Deng, J.; Zhao, F.Z.; Han, X.H.; Yang, G.H.; Tong, X.G.; Feng, Y.Z.; Shelton, S.; Ren, G.X. Response of microbial diversity to C:N:P stoichiometry in fine root and microbial biomass following afforestation. Biol. Fert. Soils 2017, 53, 457–468. [Google Scholar] [CrossRef]
- Ling, H.B.; Zhang, P.; Xu, H.L.; Zhao, X.F. How to regenerate and protect desert riparian Populus euphratica forest in arid areas. Sci. Rep. 2015, 5, 15418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, V.D.; Tatiana, M.; Chen, Y.N.; Svetlana, S.; Victor, A.C.; Saglara, M. A review on salinity adaptation mechanism and characteristics of Populus euphratica, a boon for arid ecosystems. Acta Ecol. Sin. 2016, 36, 497–503. [Google Scholar] [CrossRef]
- Corenblit, D.; Steiger, J.; Gurnell, A.M.; Naiman, R.J. Plants intertwine fluvial landform dynamics with ecological succession and natural selection: A niche construction perspective for riparian systems. Glob. Ecol. Biogeogr. 2009, 18, 507–520. [Google Scholar] [CrossRef]
- Stromberg, J.C.; Tluczek, M.G.F.; Hazelton, A.F.; Hoori, A. A century of riparian forest expansion following extreme disturbance: Spatiotemporal change in Populus/Salix/Tamarix forests along the Upper San Pedro River, Arizona, USA. For. Ecol. Manag. 2010, 259, 1181–1189. [Google Scholar] [CrossRef]
- Yang, Y.H.; Chen, Y.N.; Li, W.H.; Zhu, C.G.; Waqas, M. Inoculation of Funneliformis mosseae to enhance desiccation tolerance of Populus euphratica seedling in Hyper-arid region. Int. J. Agric. Biol. 2017, 19, 983–991. [Google Scholar] [CrossRef]
- Wang, S.J. The Status, Conservation and Recovery of Global Resources of Populus euphratica. World For. Res. 1996, 6, 37–44. [Google Scholar]
- Chen, Y.J.; Li, W.H.; Liu, J.Z.; Yang, Y.H. Effects of water conveyance embankments on riparian forest communities at the middle reaches of the Tarim River. Ecohydrology 2013, 6, 937–948. [Google Scholar] [CrossRef]
- Langhans, S.D.; Tockner, K. The role of timing, duration, and frequency of inundation in controlling leaf litter decomposition in a river-floodplain ecosystem (Tagliamento, northeastern Italy). Oecologia 2006, 147, 501–509. [Google Scholar] [CrossRef]
- Li, C.D.; Li, X.Y.; Sun, H.J.; Li, L.; Lin, L.S. Decomposition characteristics of Karelinia caspia, Alhagi sparsifolia and Populus euphratica leaves in extremely arid areas. J. Desert Res. 2019, 39, 193–201. [Google Scholar]
- Almagro, M.; Martínez-López, J.; Maestre, F.T.; Rey, A. The contribution of photodegradation to litter decomposition in semiarid Mediterranean grasslands depends on its interaction with local humidity conditions, litter quality and position. Ecosystems 2017, 20, 527–542. [Google Scholar] [CrossRef]
- Johnson, D.; Campbell, C.D.; Lee, J.A.; Callaghan, T.V. Arctic microorganisms respond more to elevated UV-B radiation than CO2. Nature 2002, 416, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.T.; Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Uselman, S.M.; Snyder, K.A.; Blank, R.R.; Jones, T.J. UVB exposure does not accelerate rates of litter decomposition in a semi-arid riparian ecosystem. Soil Biol. Biochem. 2011, 43, 1254–1265. [Google Scholar] [CrossRef]
- Barnes, P.W.; Throop, H.L.; Hewins, D.B.; Abbene, M.L.; Archer, S.R. Soil coverage reduces photodegradation and promotes the development of soil-microbial films on dryland leaf litter. Ecosystems 2012, 15, 311–321. [Google Scholar] [CrossRef]
- Han, L.; Xi, L.Q.; Wang, J.Q.; Wang, H.Z.; Yu, Z.R. Life history characteristics and spatial distribution of Populus pruinosa population at the upper reaches of Tarim River. Acta Ecol. Sin. 2013, 33, 6181–6190. [Google Scholar] [CrossRef]
- Liu, G.F.; Cornwell, W.K.; Pan, X.; Ye, D.; Liu, F.H.; Huang, Z.Y.; Dong, M.; Cornelissen, J.H.C. Decomposition of 51 semidesert species from wide-ranging phylogeny is faster in standing and sand-buried than in surface leaf litters: Implications for carbon and nutrient dynamics. Plant Soil 2015, 396, 175–187. [Google Scholar] [CrossRef]
- Xu, Y. Effects of different temperatures and different times on the drying quality of herbaceous plants. Bot. Res. 2016, 5, 125–131. [Google Scholar]
- Yang, Y.H.; Zhou, H.H.; Ye, Z.X.; Li, W.H.; Zhu, C.G.; Chen, Y.N. Effects of transient flooding on leaf litter decomposition: A case study of Populus euphratica leaf in an arid area. Int. J. Agric. Biol. 2019, 21, 1385–1392. [Google Scholar]
- Lu, R.K. Analytical Methods for Soil and Agrochemistry; Agricultural Science Technology Press: Beijing, China, 2000. [Google Scholar]
- Wieder, R.K.; Lang, G.E. A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 1982, 63, 1636–1642. [Google Scholar] [CrossRef] [Green Version]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Sun, Z.G.; Mou, X.J.; Liu, J.S. Effects of flooding regimes on the decomposition and nutrient dynamics of Calamagrostis angustifolia litter in the Sanjiang Plain of China. Environ. Earth Sci. 2012, 66, 2235–2246. [Google Scholar] [CrossRef] [Green Version]
- Cusack, D.F.; Chou, W.W.; Yang, W.; Harmon, M.E.; Silver, W.L. Controls on long-term root and leaf litter decomposition in neotropical forests. Glob. Ecol. Biogeogr. 2009, 15, 1339–1355. [Google Scholar] [CrossRef]
- Okin, G.S.; Parsons, A.J.; Wainwright, J.; Herrick, J.E.; Bestelmeyer, B.T.; Peters, D.C.; Fredrickson, E.L. Do changes in connectivity explain desertification? Bioscience 2009, 59, 237–244. [Google Scholar] [CrossRef]
- Yang, L.; Jennifer, Y.K. Effects of UV exposure and litter position on decomposition in a Californian grassland. Ecosystems 2014, 17, 158–168. [Google Scholar]
- Bocock, K.L.; Gilbert, O.J.W. The disappearance of leaf litter under different woodland conditions. Plant Soil 1957, 9, 179–185. [Google Scholar] [CrossRef]
- Trap, J.; Akpa-Vinceslas, M.; Margerie, P.; Boudsocq, S.; Richard, F.; Decaens, T.; Aubert, M. Slow decompostion of leaf litter from mature Fagussylvatica trees promotes offspring nitrogen acquisition by interacting with ectomycorrhizal fungi. J. Ecol. 2017, 105, 528–539. [Google Scholar] [CrossRef]
- Barnard, R.L.; Osborne, C.A.; Firestone, M.K. Changing precipitation pattern alters soil microbial community response to wet-up under a Mediterranean-type climate. ISME J. 2015, 9, 946–957. [Google Scholar] [CrossRef] [Green Version]
- Daniela, W.; Nico, E.; Simone, C. Plant species richness does not attenuate responses of soil microbial and nematode communities to a flood event. Soil Biol. Biochem. 2015, 89, 135–149. [Google Scholar]
- Judith Sarneel, J.M.; Ciska Veen, G.F. Legacy effects of altered flooding regimes on decomposition in a boreal floodplain. Plant Soil 2017, 421, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.Q.; Wang, Y.Z.; Chen, C.R.; Jiang, Q.; Han, S.J.; Xu, Z.H. Effects of inundation and stranding on leaf decomposition and chemical transformation. Aquat. Sci. 2018, 80, 9. [Google Scholar] [CrossRef]
- Oliver, R.; Nadine, G.; Derek, P.; Martin, K.; Mortize, M.; Jens, B.; Dominik, B. Flooding duration affects the structure of terrestrial and aquatic microbial Eukaryotic communities. Microb. Ecol. 2018, 75, 875–887. [Google Scholar]
- Lewis, W.M.; Hamilton, S.K.; Rodrgiuez, M.A.M.; Sauders, J.F. Ecological determinism on the Orinoco floodplain. Bioscience 2000, 50, 681. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.Y.; Song, L.; Wang, X.; Li, Y.W.; Zhang, Y.P.; Sha, L.Q. Ecological stoichiometry characteristics of the litterfall-humus-soil continuum systems under different successional stages of the subtropical forest in SW China. Mt. Res. 2017, 35, 274–282, (In Chinese with English abstract). [Google Scholar]
- Moore, T.R.; Trofymow, J.A.; Prescott, C.E.; Fyles, J.; Titus, B.D.; CIDET Working Group. Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests. Ecosystems 2006, 9, 46–62. [Google Scholar] [CrossRef]
- Limpens, J.; Berendse, F. How litter quality affects mass loss and N loss from decomposing Sphagnum. Oikos 2003, 103, 537–547. [Google Scholar] [CrossRef]
- Hirabayashi, Y.; Mahendran, R.; Koirala, S.; Konoshima, L.; Yamazaki, D.; Watanabe, S.; Kim, H.; Kanae, S. Global flood risk under climate change. Nat. Clim. Chang. 2013, 3, 816–821. [Google Scholar] [CrossRef]
- Ladwig, L.M.; Sinsabaugh, R.L.; Collins, S.L.; Thomey, M.L. Soil enzyme responses to varying rainfall regimes in Chihuahuan desert soils. Ecosphere 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and flow regulation of the world’s large river systems. Science 2005, 30, 405–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.T.; Smith, L.M. The effects of flooding regimes on decomposition of Polygonum pensylvanicum in playa wetlands (Southern Great Plains, USA). Aquat. Bot. 2002, 74, 97–108. [Google Scholar] [CrossRef]




| Parameter | Source | DF | Sum of Squares | F Ratio | Sig. |
|---|---|---|---|---|---|
| Leaf litter mass loss (%) | DT | 2 | 107.651 | 5.886 | <0.0001 |
| IT | 5 | 8591.536 | 187.907 | <0.0001 | |
| DTxIT | 10 | 140.042 | 1.531 | 0.168 |
| Parameter | Source | DF | Sum of Squares | F Ratio | Sig. |
|---|---|---|---|---|---|
| C:N | DT | 2 | 87,710.168 | 21.133 | 0.000 |
| IT | 6 | 433,920.533 | 34.849 | 0.000 | |
| DTxIT | 12 | 62,589.602 | 2.514 | 0.014 | |
| C:P | DT | 2 | 1,104,381.04 | 276.372 | 0.000 |
| IT | 6 | 6,675,927.884 | 556.885 | 0.000 | |
| DTxIT | 12 | 1,400,480.984 | 58.412 | 0.000 | |
| C:K | DT | 2 | 151,570.791 | 109.838 | 0.000 |
| IT | 6 | 112,204.409 | 27.103 | 0.000 | |
| DTxIT | 12 | 80,105.034 | 9.675 | 0.000 | |
| N:P | DT | 2 | 13.664 | 6.559 | 0.003 |
| IT | 6 | 100.136 | 16.022 | 0.000 | |
| DTxIT | 12 | 63.957 | 5.117 | 0.000 | |
| N:K | DT | 2 | 2.013 | 6.201 | 0.004 |
| IT | 6 | 39.769 | 40.834 | 0.000 | |
| DTxIT | 12 | 10.123 | 5.197 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhou, H.; Wang, W.; Zhu, C.; Cui, D.; Ye, Z. Transient Flooding and Soil Covering Interfere with Decomposition Dynamics of Populus euphratica Leaf Litter: Changes of Mass Loss and Stoichiometry of C, N, P, and K. Forests 2022, 13, 476. https://doi.org/10.3390/f13030476
Yang Y, Zhou H, Wang W, Zhu C, Cui D, Ye Z. Transient Flooding and Soil Covering Interfere with Decomposition Dynamics of Populus euphratica Leaf Litter: Changes of Mass Loss and Stoichiometry of C, N, P, and K. Forests. 2022; 13(3):476. https://doi.org/10.3390/f13030476
Chicago/Turabian StyleYang, Yuhai, Honghua Zhou, Wanrui Wang, Chenggang Zhu, Dong Cui, and Zhaoxia Ye. 2022. "Transient Flooding and Soil Covering Interfere with Decomposition Dynamics of Populus euphratica Leaf Litter: Changes of Mass Loss and Stoichiometry of C, N, P, and K" Forests 13, no. 3: 476. https://doi.org/10.3390/f13030476
APA StyleYang, Y., Zhou, H., Wang, W., Zhu, C., Cui, D., & Ye, Z. (2022). Transient Flooding and Soil Covering Interfere with Decomposition Dynamics of Populus euphratica Leaf Litter: Changes of Mass Loss and Stoichiometry of C, N, P, and K. Forests, 13(3), 476. https://doi.org/10.3390/f13030476







