Abstract
Knowledge regarding changes in soil microbial communities with forest succession is vital to understand soil microbial community shifts under global change scenarios. The composition and diversity of soil microbial communities across a subalpine forest successional series were therefore investigated in the Wanglang National Nature Reserve on the eastern Qinghai-Tibet Plateau, China. The calculated diversity indices of soil bacteria (8.598 to 9.791 for Shannon-Wiener, 0.997 to 0.974 for Simpson, 4131 to 4974 for abundance-based coverage estimator (ACE) and 3007 to 3511 for Species richness indices), and ACE (1323 to 921) and Species richness (1251 to 879) indices of soil fungi decreased from initial to terminal succession stages, but Shannon-Wiener and Simpson of soil fungi indices varied slightly with forest succession. Meanwhile, the composition and structure of soil microbial communities varied markedly with forest succession. The relative abundance of the dominant bacterial phyla (Acidobacteria, Firmicutes and Actinobacteria) and fungal taxa (Mortierellomycota, Rozellomycota and unassigned phylum clade GS01) varied considerably with forest succession. However, regardless of successional stage, Proteobacteria and Acidobacteria dominated soil bacterial communities and Ascomycota and Basidiomycota dominated soil fungal communities. Moreover, the changes in soil microbial diversity with forest succession were significantly affected by soil pH, soil organic carbon, soil temperature, altitude, and non-woody debris stock. Importantly, soil pH was the dominant driver of soil microbial community shift with forest succession. In conclusion, the forests at different succession stages not only conserve same microbial populations, but also nurse unique microbial diversity across the forest succession series; and the biodiversity of soil bacterial and fungal communities has differential responses to forest succession.
1. Introduction
Soil microbial communities play irreplaceable roles in driving the biogeochemical cycles of carbon and nutrients and maintaining site productivity and energy flow [1,2,3]. As a result, investigations on the changes in soil microbial community structure and diversity with environmental factors can provide key evidence for understanding the mechanisms driving bioelement cycles, managing forest ecosystems and conserving soil biodiversity. The present consensus is that the composition, structure and diversity of soil microbial communities can be driven by combinations of biotic and abiotic factors [4,5,6], making the responses of soil microbial communities to environmental changes relatively complex. In particular, the changes in forest community structure could simultaneously alter soil properties [7], microclimate [8], and the input and qualities of above- and below plant biomass [9,10]; these changes could then lead to soil microbial community shifts. For instance, Yan et al. [11] have concluded that more complex forest community structure can provide more diverse food sources for soil microbes and increase the heterogeneity of the soil environment, thereby increasing the biodiversity of the soil microbiota. Proteobacteria, Actinobacteria and Acidobacteria were important members of soil bacterial community, Proteobacteria represent the most metabolically diverse group of anoxygenic chlorophototrophs [12]. The vast majority of Actinobacteria are important saprophytes capable of breaking down a wide range of plant and animal debris in the process of decomposition [13]. Acidobacteria is generally acidophilic, oligotrophic and difficult to cultivate [14]. Forests with abundant plant species diversity have relatively higher amounts of root exudates and leaf litter, and alter soil properties such as nutrient regimes, temperature and moisture dynamics and pH, leading to an increase in the substrate quality and quantity for soil microbes and thus affecting the structure and diversity of soil microbial communities [15]. However, soil microbial community shifts across the forest successional series have not been fully investigated.
Forest succession is an ecological process in which one forest community evolves into another due to the forces of nature or humans [16,17,18]. Global change-driven forest succession [19,20] may modify key soil processes and, in particular, have important impacts on the structure and functioning of soil microbial communities [21]. As a result, full investigation on the changes in soil microbial communities with forest succession is vital to understand the shifts in soil microbial communities under global change scenarios. In theory, forest succession could influence the shifts in soil microbial communities in at least the following ways. First, the quality and quantity of woody and non-woody debris on the forest floor vary greatly with forest succession [22], and the related changes in substrate quality and quantity alter the composition and diversity of soil microbial communities [23]. Second, soil temperature and moisture can vary greatly with forest succession owing to the changes in canopy structure and understory cover [24,25]; both soil temperature and moisture are key factors in determining the composition and structure of soil microbial communities [15,26]. Third, the changes in soil microbial community structure can be driven by soil properties (e.g., pH, nutrient concentration and ecological stoichiometry) [5,27,28], which are sensitive to forest succession [29,30]. Although changes in soil microbial communities with forest types have been widely reported [31,32,33], the shifts in soil microbial communities in response to changes in soil properties, plant debris stock, and soil temperature and moisture across the forest successional series remain unknown.
The subalpine forests distributed on the eastern Qinghai-Tibet Plateau and in the upper reaches of Yangtze River play paramount roles in conserving water and soil, supporting biodiversity, responding to climate change, and participating in the global carbon cycle [34]. As affected by long-term natural disturbances such as earthquakes, debris flows and snowslides, and commercial logging of natural forests, subalpine forest communities of different successional stages have been widely observed in the subalpine forest region [34,35]. To date, changes in woody and non-woody debris stocks across a subalpine forest successional series [22] and changes in soil microbial diversity with gap size [36] and along environmental gradients [37] in the subalpine forest region have been documented. Nevertheless, little information is available on soil microbial community shifts across the subalpine forest successional series.
Based on the importance of forest succession on soil microbial community diversity and composition and little information on successional series in the subalpine forest region, we proposed the following research questions: (1) would soil microbial diversity change with forest succession? (2) would the composition of soil microbial community be richer with succession? (3) what role would forest and soil variables play in the effect of succession on microbial community change? To answer these questions, we investigated the shifts in soil microbial community composition and diversity across the subalpine forest successional series and the key drivers of those shifts. We hypothesized that (1) soil microbial diversity would increase from initial to terminal stages of forest succession; (2) the relative abundance of dominant taxa in soil bacterial and fungal communities would vary markedly among different succession stages, and soil bacterial and fungal communities would respond differentially to forest succession; and (3) non-woody debris stock would dominate the composition and diversity of soil microbial communities across the forest successional series. The objectives of this study were to elucidate the important function of forest succession on the diversity of soil microbial community and explore the key forest variables driver factors of those shifts. Those results help us better understand that maintaining different succession stages of forest communities through moderate disturbances is beneficial to the conservation of soil biodiversity at the forest region level.
2. Material and Methods
2.1. Field Description
The study region is located in Wanglang National Nature Reserve (32°49′–33°02′ N, 103°55′–104°10′ E; 2300–4983 m a.s.l.), Pingwu County, Sichuan, southwestern China, which is distributed on the eastern Qinghai-Tibet Plateau and in the upper reaches of Yangtze River. The area has a semihumid climate typical of the Danba-Songpan region. The annual precipitation is 859.9 mm, and the annual mean temperature is 2.9 °C, with maximum and minimum mean temperatures of 12.7 °C (in July) and −6.1 °C (in January), respectively [35]. The subalpine forest consists of abundant plant species, including mosses, herbs, shrubs, deciduous broadleaved trees and coniferous trees.
2.2. The Method of Selection of Study Plots and Their Characteristics
Wanglang subalpine coniferous region seriously damaged by long-term the commercial logging of natural forests below 2700 m since the 1950s and natural disturbance such as the 1976 Songpan-Pingwu earthquake [38,39]. Forest logging in the area ceased in 1962. Therefore, the Wanglang Nature Reserve has formed many secondary forests in different stages of succession and primary forests that have never been destroyed [35,38,39,40]. We considered their distribution based on forest disturbance history and natural recovery process and interviews with local supervisors, We defined the forests of S1 to S5 succession stages by referring to the succession division of Zhang et al. [35], and defined the primary coniferous forests which have never been destroyed to S6 succession stage. According to Zhang et al. [35], the S1 successional stage was dominated by shrubs (e.g., Salix spp., Ulmus pumila L. and Hippophae rhamnoides L.); the S2 successional stage was dominated by the deciduous broadleaved species Betula spp. and Populus spp.; the S3 successional stage was characterized by mixed forests dominated by deciduous broadleaved species (e.g., Betula spp.) and coniferous species (e.g., Abies faxoniana Rehd.); the S4 successional stage was dominated by middle-age coniferous species with a diameter at breast height (DBH) less than 20 cm (e.g., Larix gmelinii (Rupr.) Kuzen. and Sabina saltuaria Rehd.); the S5 successional stage was dominated by mature coniferous plants with a DBH less than 40 cm and more than 20 cm (e.g., A. faxoniana Rehd.); and the S6 succession stage was characterized as primary coniferous forest dominated by Picea purpurea Mast (Table S1). The traits of the six successional stages, including the coordinates, altitudes, directions, canopy covers, soil names and dominant species are shown in Table S1.
2.3. Soil Sampling Method
Soils across the subalpine forest successional series were sampled in August 2019. Replicate plots (10 m × 20 m) were established in each successional stage as follows: 12 plots in S1, 9 plots in S2, 9 plots in S3, 6 plots in S4, 6 plots in S5 and 6 plots in S6. After removing the litter layers, nine soil samples in each plot were randomly collected from the top 20 cm of the soil profiles by a stainless steel corer (5-cm diameter) and mixed. Moreover, the topsoil temperature (T) of each plot was measured by a portable soil thermometer (CEM, DT-131, Shenzhen, China). The roots and stones were removed from the soil samples, which were then divided into two subsamples. One subsample was stored in a bag on ice, immediately transported to a laboratory, and stored at −80 °C for high-throughput sequencing for microbial community diversity analysis [31]. The other subsample was transported to the laboratory and air-dried for soil property analysis.
2.4. Soil Agrochemical and Chemical Properties Analyses
After being passed through a 1-mm sieve, the air-dried soil samples were used for chemical analyses. Soil pH was measured at a soil: water ratio of 1:2.5 (m:v) with a digital pH meter (FE20K, Mettler-Toledo, Greifensee, Switzerland). The concentrations of total nitrogen (TN) in the soils were determined from a milled sample by combustion at 950 °C using an elemental analyzer (Vario EL Ш, Elementar, Langenselbold, Germany). Soil organic carbon (SOC) was measured by the potassium dichromate oxidation method [41]. The C:N ratio (C:N) was calculated as the mass ratio of SOC and TN [42].
2.5. Soil DNA Extraction, PCR Amplification and High-Throughput Sequencing
Total soil DNA was extracted from 0.5 g soil samples using the FastDNA Spin Kit for Soil (MP Biomedicals, Santa Ana, CA, USA) according to the instructions of the manufacturer. The V4 hypervariable region of the 16S rDNA [43] and the internal transcribed spacer (ITS1 and ITS2) region of rDNA [44] were selected as sequencing targets for bacteria and fungi, respectively. The library was built using the NEBNext® Ultra™ DNA Library Prep Kit for Illumina (NEB, Ipswich, MA, USA) at Novogene Company (Beijing, China). Sequencing was performed using a paired_end sequencing strategy with barcode connectors based on the Illumina MiSeq PE250 platform (Illumina Inc., San Diego, CA, USA). The barcode sequences were spliced with FLASH (v 1.2.7) software to obtain the raw tags [45]. The clean tags were obtained by quality filtering the raw tags using QIIME (v1.9.0) software [46,47]. Chimeric sequences were removed using the UCHIME algorithm [48]. Operational taxonomic unit (OTU) clustering for all the effective tags with 97% identity was performed by UPARSE (v 7.0.1001) software after discarding singletons [49]. The SSUrRNA database of SILVA132 [50] and Mothur software, and the UNIT (v 7.2) database [51] and QIIME (v 1.9.0) [52] software were selected as species annotation analysis for 16S rDNA and ITS, respectively.
2.6. Statistical Analyses
Alpha diversity indices of soil microbial communities (Shannon-Wiener, Simpson, abundance-based coverage estimator (ACE) and species richness) were calculated using QIIME (v 1.9.0) software [53]. To identify soil bacterial and fungal taxa differentially represented among the six forest successional stages, differentially abundant taxa were selected using LEfSe (v 1.0) software at a linear discriminant analysis (LDA) score of 4 [54]. Analysis of similarities (ANOSIM) was performed based on the Bray–Curtis distance algorithm [55] to identify the significance of the differences in the microbial communities among different succession stages. A redundancy analysis (RDA) was performed to screen the environmental factors that influence species differences at the phylum level among different stages of succession. Differential the abundance of soil microbial phyla composition, alpha diversity indices, and the concentrations of soil SOC, TN, C:N, temperature and pH, the stocks of woody debris (WD) and non-woody debris (NWD) among successional stages were tested by Kruskal-Wallis test and Wilcoxon rank-sum test. These tests were completed with IBM SPSS Statistics v. 20 (IBM Corporation, New York, NY, USA). Differences were considered significant at the 0.05 significance level. The metrics of the ANOSIM and RDA were determined using the vegan package [56] in R (v 2.15.3) software [57]. The R2 and p value of the RDA, indicating the effects of each environmental factor on species distribution, were calculated using the Envfit function of the vegan package [56].
3. Results
3.1. Changes in Soil Properties and Stocks of Woody Debris and Non-Woody Debris with Succession
The stock of woody debris (WD), soil concentrations of SOC, TN and C:N showed a significant tendency of increase from the S1 to S6 succession stages (all p < 0.05). On the contrary, the stock of non-woody debris (NWD), soil temperature (T) and pH were observed significantly higher in initial than terminal stages of succession (all p < 0.05; Table 1). Especially, the S4 succession stage had the lowest soil concentrations of SOC and TN and the highest soil C:N and soil pH (Table 1).

Table 1.
Soil physicochemical properties and the stocks of woody debris and non-woody debris among different succession stages.
3.2. Changes in the Sequence Data and Soil Microbial Alpha Diversity
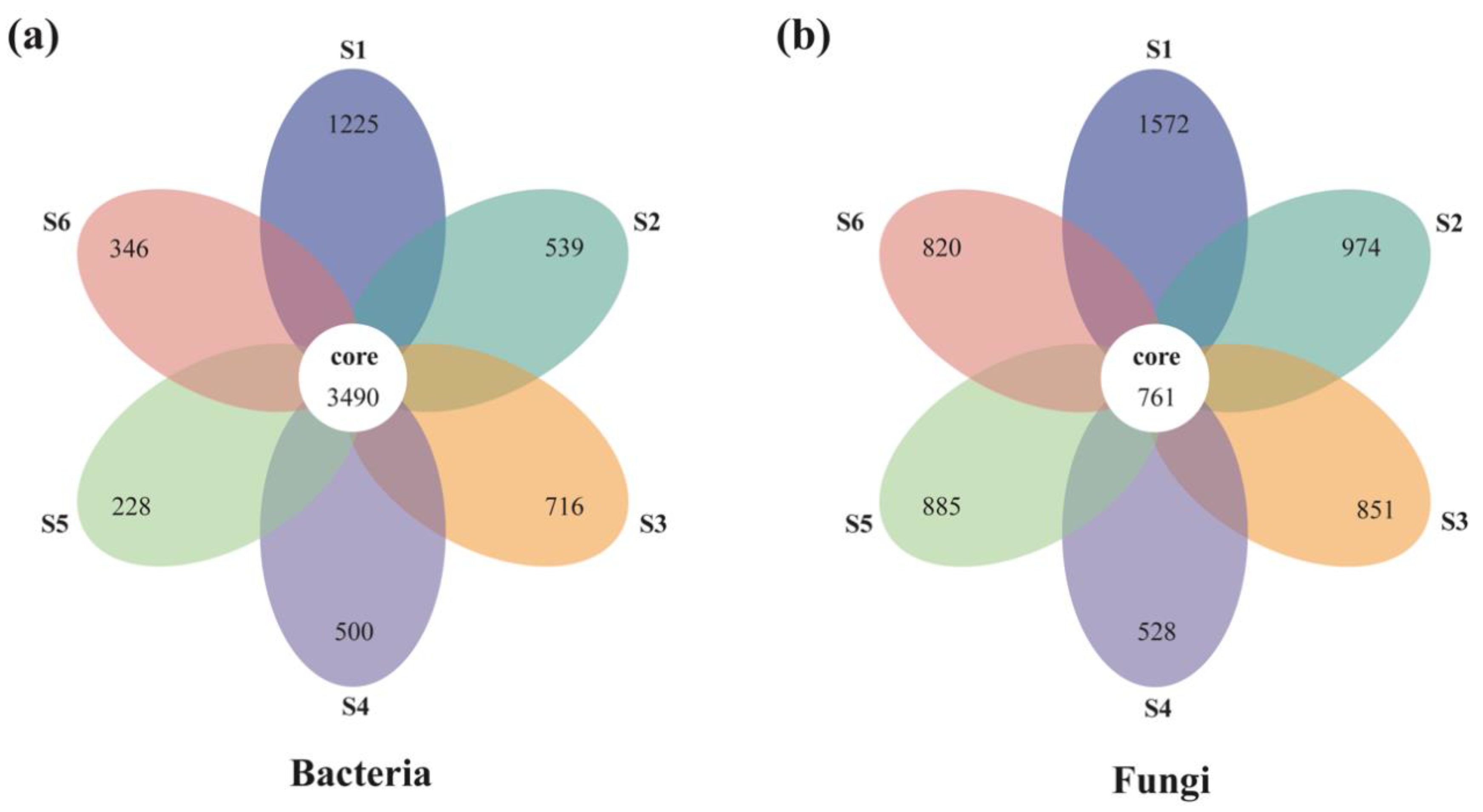
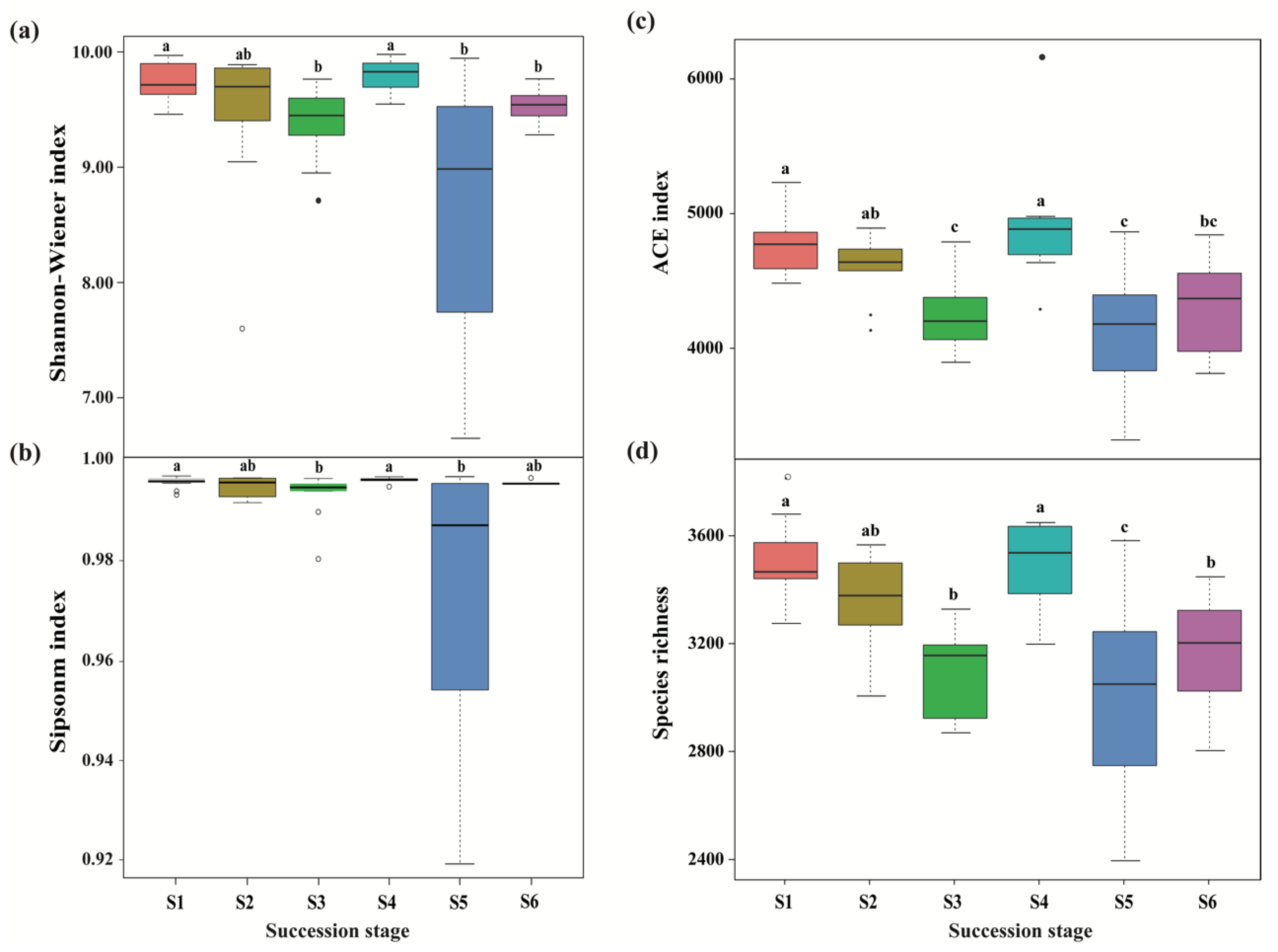
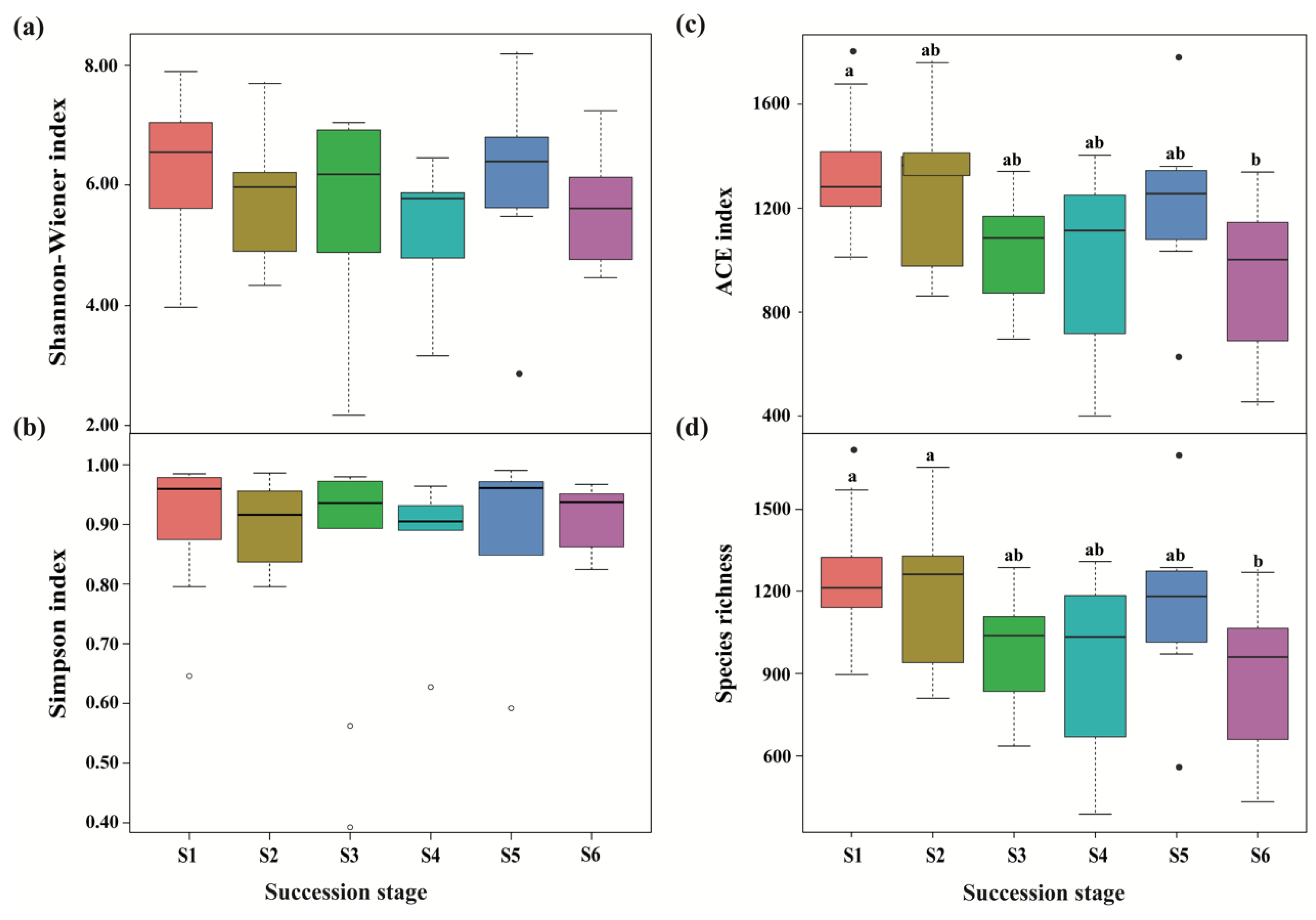
A total of 3,004,539 and 2,845,500 high-quality effective sequences of bacteria and fungi were acquired across the subalpine forest successional series; the sequences were clustered with 97% identity into 13,684 and 12,233 OTUs, respectively. Among these OTUs, 3490 bacterial and 761 fungal OTUs were common in soil microbial communities at six successional stages (Figure 1). Soil bacterial and fungal communities at the initial succession stage (S1) had the most independent OTUs (1225 and 1572, respectively) (Figure 1). The alpha diversity of soil bacterial community at the initial successional stage (S1) was significantly higher than that at the terminal successional stages (S5 and S6) (p < 0.05; Figure 2), although there was not a remarkable difference in fungal alpha diversity among the six forest successional stages according to these two indices (p > 0.05; Figure 3). However, ACE and Species richness indices in fungal communities were significantly higher at the S1 succession stage than at the S6 succession stage (p < 0.05; Figure 2 and Figure 3).
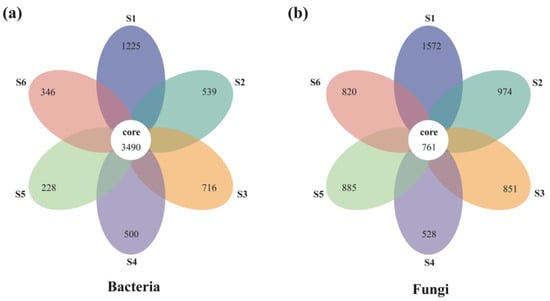
Figure 1.
Flower diagram of the common and unique bacterial (a) and fungal (b) OTUs across a subalpine forest succession series. S1 to S6 represent successional stages from 1 to 6, respectively.
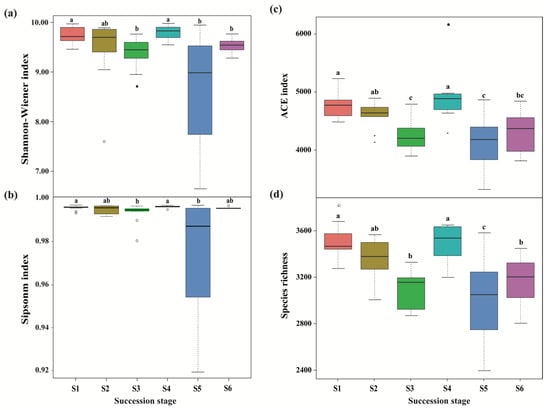
Figure 2.
Differences in bacterial alpha diversity across a subalpine forest succession series. Calculations based on the OTUs at 97% sequence identity. Shannon-Wiener index (a), Simpson index (b), ACE index (c) and Species richness (d) represent the indices of alpha diversity. Values followed by different lowercase letters mean significant difference (p < 0.05) among six successional series based on Wilcoxon rank-sum test. S1 to S6 represent successional stages from 1 to 6, respectively.
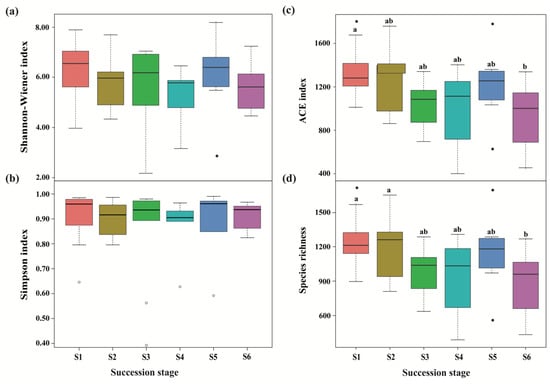
Figure 3.
Differences in fungal alpha diversity across a subalpine forest succession series. Calculations based on the OTUs at 97% sequence identity. Shannon-Wiener index (a), Simpson index (b), ACE index (c) and Species richness (d) represent the indices of alpha diversity. Values followed by different lowercase letters mean significant difference (p < 0.05) among six successional series based on Wilcoxon rank-sum test. S1 to S6 represent successional stages from 1 to 6, respectively.
3.3. Composition and Structure of Soil Microbial Community
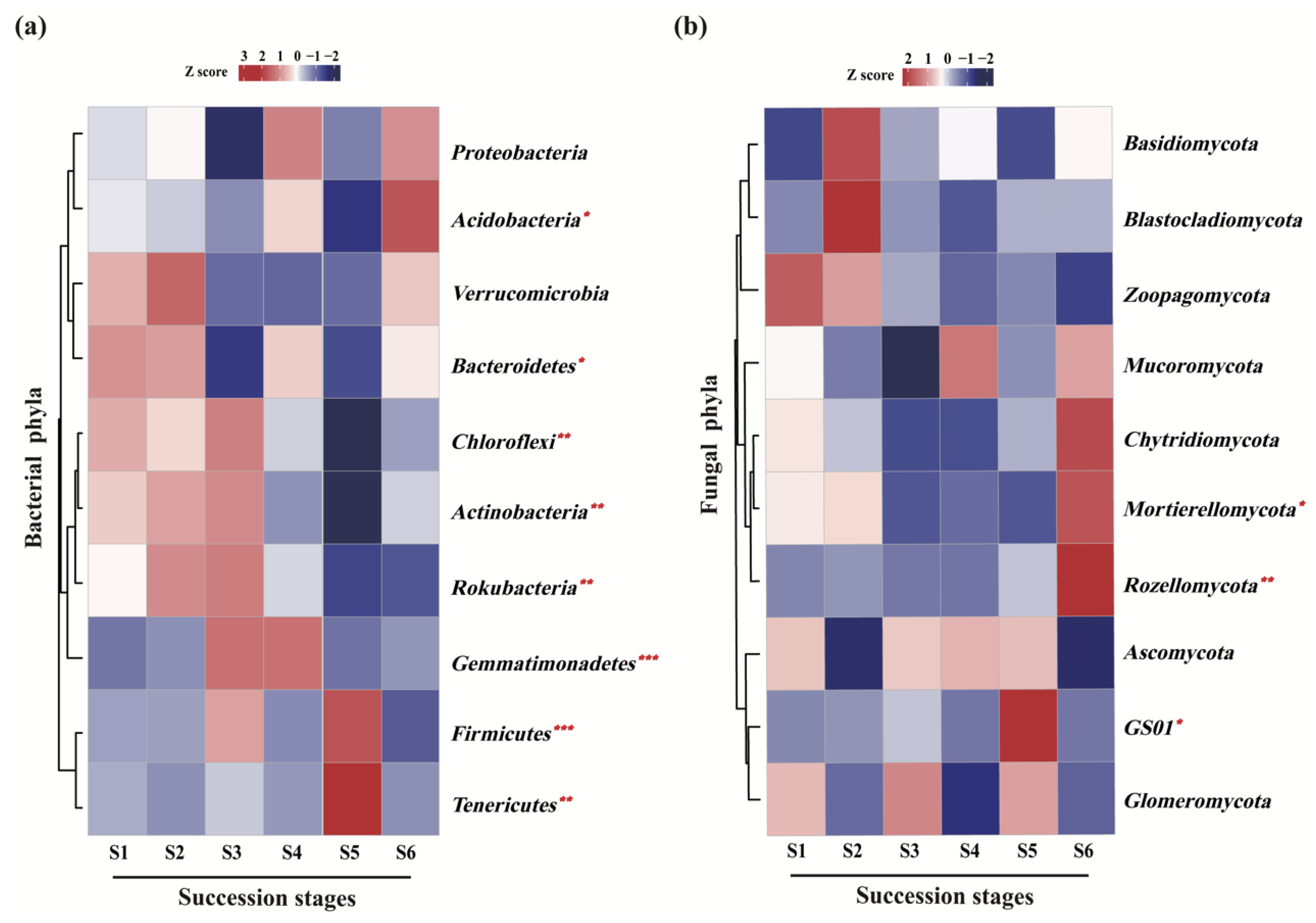
At the phylum level, soil bacterial communities at the six successional stages were dominated by Proteobacteria (37.7–45.2%), Acidobacteria (15.3–27.5%) and Actinobacteria (5.0–10.4%) (Table 2). The relative abundance of eight of the 10 most abundant phyla varied markedly (p < 0.05) with forest succession, except for that of Proteobacteria and Verrucomicrobia (Figure 4a). In particular, higher relative abundance of Firmicutes (p < 0.001), Tenericutes (p < 0.01) and Acidobacteria (p < 0.05) were observed at terminal succession stages (S5 or S6) (Figure 4b; Table S2). In contrast, the relative abundance of Chloroflexi (p < 0.01), Actinobacteria (p < 0.01) and Rokubacteria (p < 0.01) were markedly higher at the initial successional stages (S1, S2 and S3) (Figure 4a; Table S2). Soil fungal communities across the subalpine forest successional series were dominated by Ascomycota (27.7–46.4%), Basidiomycota (13.5–31.8%) and Mortierellomycota (2.0–12.5%) (Table 3). The relative abundance of the other phyla totaled less than 2% (Table 3). Among the fungal phyla, clade GS01 was not enriched in the S4 and S6 successional stages, and Blastocladiom was not enriched in the S4 successional stage (Table 3). Furthermore, the significantly higher relative abundance of Mortierellomycota (p < 0.05), Rozellomycota (p < 0.01) and clade GS01 (p < 0.05) were observed at the terminal succession stage (S5 or S6) (Figure 4b; Table S2).

Table 2.
Percentage composition of the dominant phyla of soil bacterial community among different succession stages.
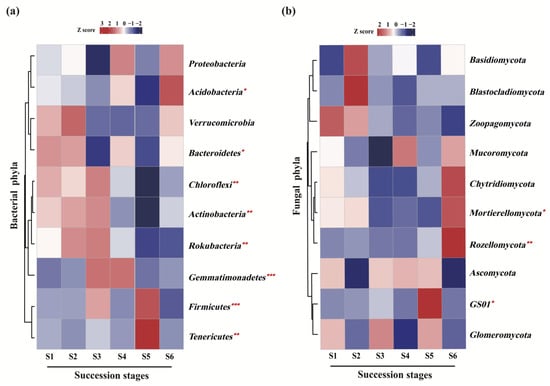
Figure 4.
Relative abundance and significance analyses of the dominant soil bacterial (a) and fungal (b) communities at phylum level across a subalpine forest succession series. Significant effect: * p < 0.05, ** p < 0.01, *** p < 0.001. Kruskal-Wallis test. S1 to S6 represent successional stages from 1 to 6, respectively.

Table 3.
Percentage composition of the dominant phyla of soil fungal community among different succession stages.
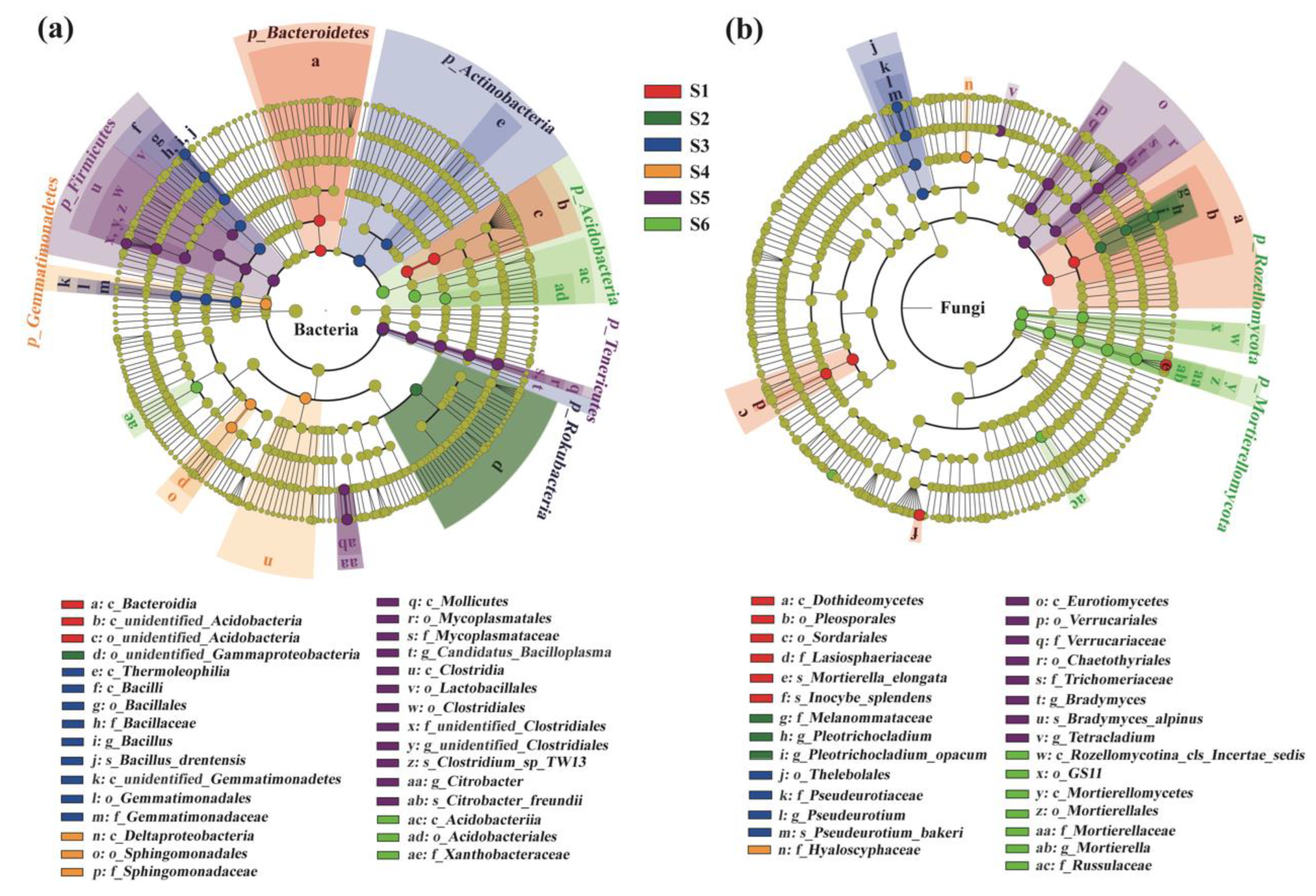
Furthermore, based on an LDA score of 4, linear discriminant analysis effect size (LEfSe) indicated that there were a large number of significantly different taxa (biomarkers) among different succession stages (Figure 5). In total, 38 and 31 taxa (including the phylum, class, order, family, genus and species levels) were selected as biomarkers for soil bacterial and fungal communities, respectively, along the succession gradients (Figure 5).
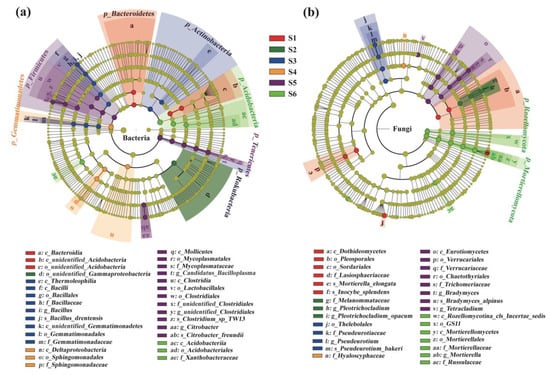
Figure 5.
Cladograms generated by linear discriminant analysis effect size (LEfSe) indicating differences in the bacterial (a) and fungal (b) taxa across a subalpine forest succession series. Different colored bars indicate taxa were enrichment in the corresponding succession stages. S1 to S6 represent successional stages from 1 to 6, respectively.
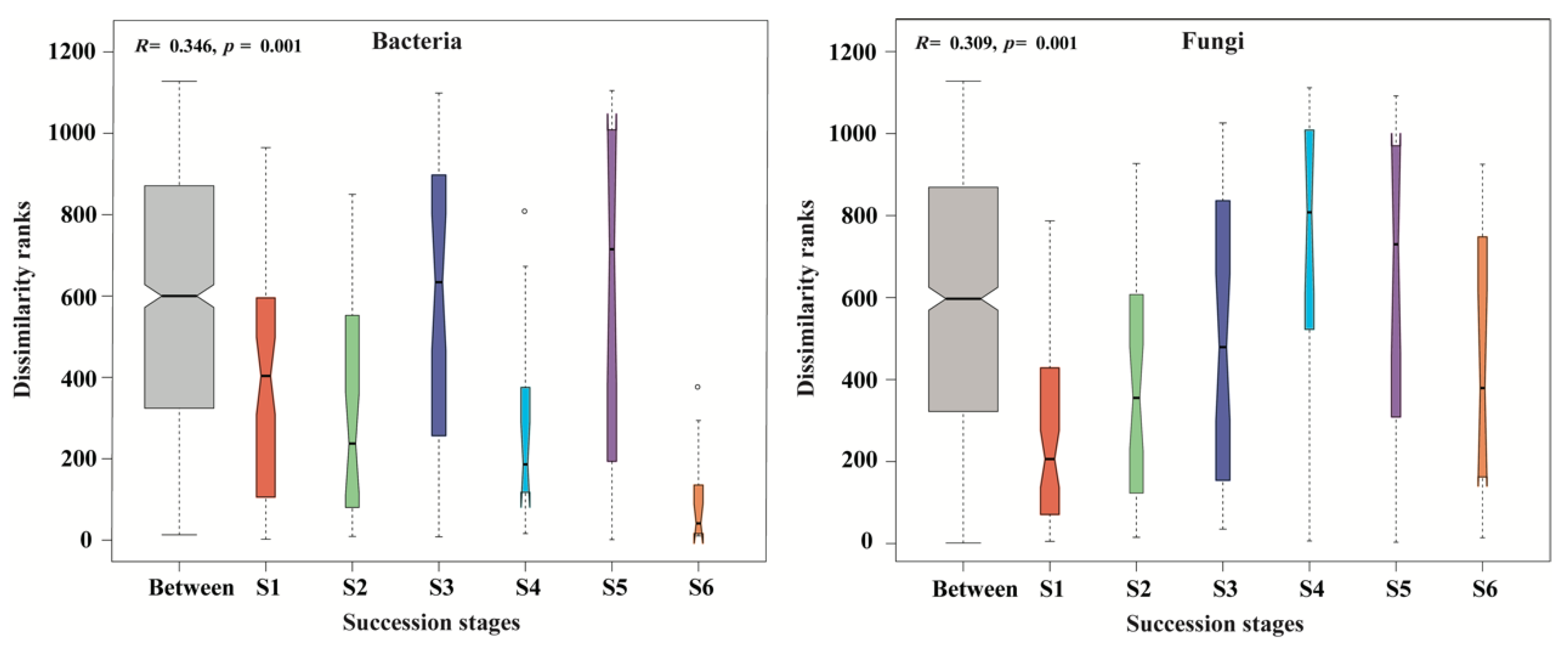
ANOSIM test verified that the composition of soil bacterial and fungal communities was substantially distinct among the six successional stages (all p = 0.001; Figure 6). Specifically, ANOSIM showed that there were significant differences in soil bacterial community structure among different succession stages, except between S1 and S2, between S1 and S4, and between S3 and S4. Slight differences in soil fungal community composition were observed between S1 and S2, between S3 and S5 and between S4 and S5, but significant differences were found between the remaining successional stages (Table S3).
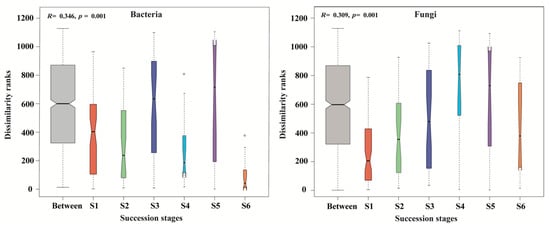
Figure 6.
Boxplot of analysis of similarities (ANOSIM) based on the Bray–Curtis distances of samples for the dissimilarities of bacterial and fungal communities across a subalpine forest succession series. S1 to S6 represent successional stages from 1 to 6, respectively.
3.4. Relationships of Soil Microbial Diversity with Forest Variables
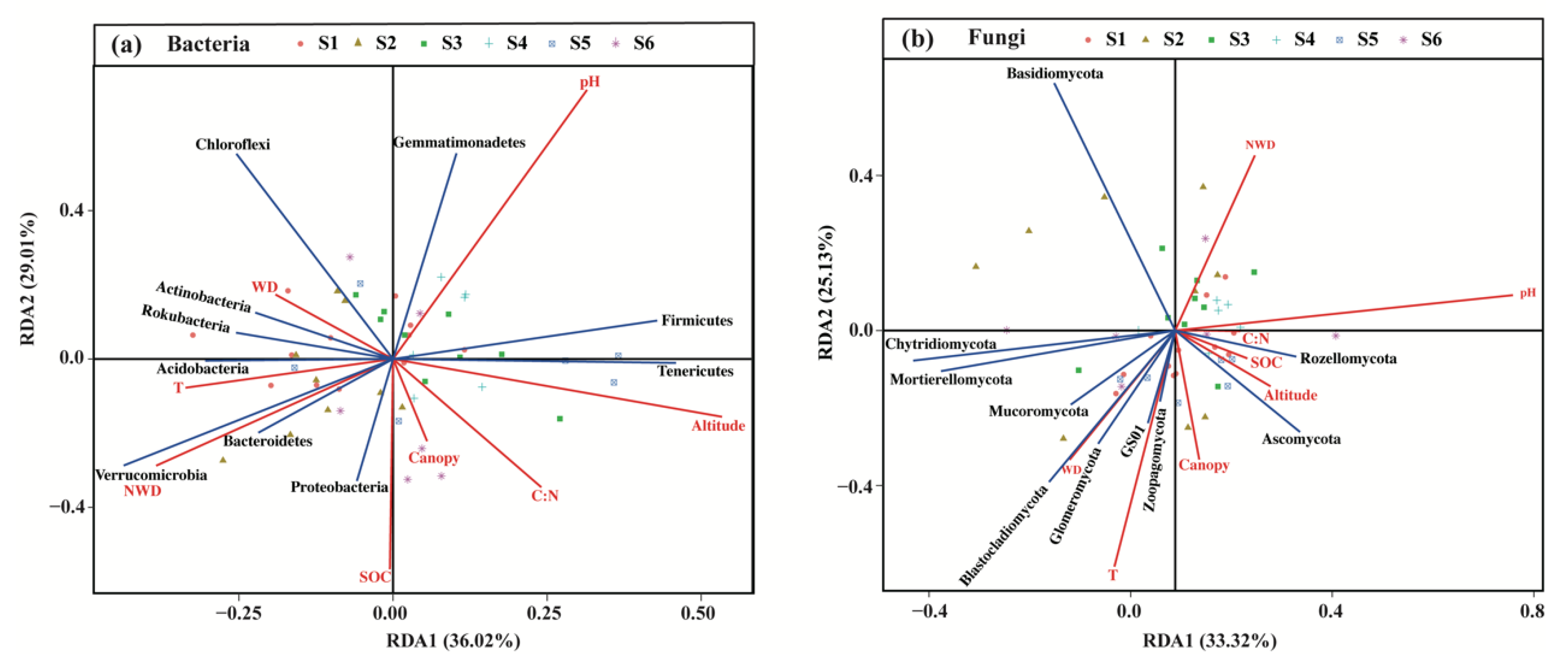
According to the analysis of detrended correspondence analysis (DCA) (Table S4), RDA was selected to analyze the relationships of soil microbial diversity with second forest variables based on the analysis of variance inflation factor (VIF) (Table S5). RDA confirmed that the variations in soil bacterial and fungal communities across the successional series were associated with forest variables (Figure 7). Among the forest variables, soil pH had an extremely significant effect on soil bacterial (p < 0.01) and fungal community composition (p < 0.001; Table 4 and Table 5). Meanwhile, soil bacterial diversity was also significantly affected by SOC, soil T, the stock of non-woody debris (NWD) and altitude (p < 0.05; Table 4), while soil fungal community composition was also significantly influenced by soil temperature (T) (p < 0.05; Table 5). In particular, among dominant bacterial phyla, soil pH was significantly and positively correlated with the relative abundance of Gemmatimonadetes and Firmicutes, but markedly and negatively correlated with the abundance of Proteobacteria, Bacteroidetes and Verrucomicrobia (Figure 7a). The stock of NWD and soil temperature (T) was prominently and positively correlated with the abundance of most of the bacterial phyla, but observably and negatively correlated with the abundance of Gemmatimonadetes, Firmicutes and Tenericutes. On the contrary, the correlation of altitude and most of soil bacterial community composition was negative (Figure 7a). In addition, the relative abundance of Proteobacteria and Bacteroidetes were also significantly and positively correlated with the concentration of SOC (Figure 7a). Among dominant fungal phyla, soil pH was notably positively correlated with the abundance of Rozellomycota and Ascomycota not the other dominant phyla (Figure 7b). In contrast, soil temperature (T) was significantly and positively correlated with the abundance of the most abundant phyla, except for Rozellomycota and Basidiomycota (Figure 7b).
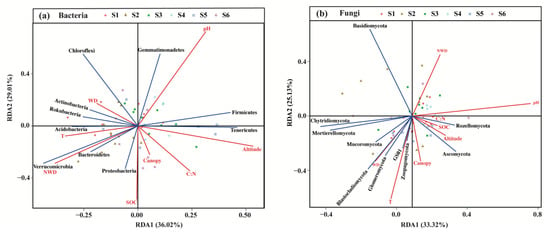
Figure 7.
Redundancy analysis (RDA) about the effect of forest variables on the differences in the compositions of soil bacterial (a) and fungal (b) communities across a subalpine forest succession series. SOC: the concentrations of soil organic carbon; C:N: the mass ratio of SOC and soil total nitrogen; T: soil temperature; pH: soil pH; WD: the stock of woody debris; NWD: the stock of non-woody debris. S1 to S6 represent successional stages from 1 to 6, respectively.

Table 4.
Significance of the effects of forest variables on bacterial community composition among different succession stages.

Table 5.
Significance of the effects of forest variables on fungal community composition among different succession stages.
4. Discussion
The composition and diversity of soil bacterial and fungal communities varied significantly with forest succession. The alpha diversity of soil bacteria and fungi decreased from initial to terminal succession stages except for Shannon-Wiener and Simpson indices of soil fungal community, which was in contrast to our first hypothesis. Proteobacteria and Acidobacteria dominated soil bacterial communities, and Ascomycota and Basidiomycota dominated soil fungal communities in all six successional stages. However, a significant difference was observed in the relative abundance of dominant soil microbial phyla among the six successional stages. Meanwhile, the ANOSIM results demonstrated that soil microbial community structure was substantially different among the six successional stages. These results were consistent with our second hypothesis. RDA showed that soil pH had the greater effect on the differences in the composition of soil communities across the forest successional series, while non-woody debris stock was correlated with only the composition of soil bacterial community, partly supporting our third hypothesis. Generally, these results confirm that forests in different successional stages not only nursed the same dominant microbial communities but also support unique biomarkers and that the biodiversity in soil bacterial and fungal communities differed greatly in response to forest vegetation succession.
4.1. Changes in the Diversity of Soil Microbial Community with Forest Succession
Soil microbial diversity demonstrated a global tendency to decrease from initial to terminal succession stages; an exception to this finding was demonstrated by the Shannon-Wiener and Simpson indices for the fungal communities, which was supported by the findings of several studies [58,59]. However, the changes in soil bacterial and fungal alpha diversity varied greatly with forest succession from those reported previously (Table 6). A considerable increase in bacterial alpha diversity and an increase followed by a decrease in fungal diversity were found with forest succession in different regions [25,30,32]. Meanwhile, Zhang et al. [31] and Liu et al. [25] observed a trend of decreasing first and then increasing. In addition, the alpha diversity of soil bacterial and fungal communities did not show significant changes with succession [30,42,59]. As succession progressed, the aboveground plant community changed significantly. However, at middle and late successional stages, the changes in plant species and the intensification of competition led to a loss of soil nutrients and a decrease in the diversity of soil microbial communities [25]. Moreover, the decreased alpha diversity index may be due to the different growth strategies of microbes; in particular, at initial succession stages, the soil microbial population lives in harsh and unpredictable conditions and has a high reproductive rate but a low survival rate, resulting in high diversity but low abundance. At terminal succession stages, the microbial population lives in a favorable and predictable environment, with a low reproductive rate but a high survival rate, resulting in increased competitiveness and a stable population number [31]. In addition, the differences in soil bacterial and fungal alpha diversity may have occurred since soil microorganisms have differing nutrient preferences [60].

Table 6.
Comparisons of forest soil microbial community alpha diversity index across forest succession series in different regions.
4.2. Changes in Soil Microbial Community Composition with Forest Succession
Proteobacteria, Acidobacteria and Actinobacteria were the dominant bacterial phyla in the six forest successional stages, but their relative abundance differed significantly, which was consistent with the findings of a growing number of studies in different ecosystems [62,63,64]. Fierer et al. [65] analyzed the extent to which these phyla were dominant in many soil bacterial communities based on phylogenetic analysis. The abundance of Proteobacteria increased with increasing vegetation successional stage, and this result was generally consistent with findings in New Zealand [66]. These findings suggested that Proteobacteria likely play a functional role in the restoration of soil [67]. Many soil Proteobacteria are copiotrophic and become abundant when labile substrates are available during secondary forest succession [65]. However, a higher abundance of Actinobacteria was observed at initial successional stages in our study since Acidobacteria are oligotrophic and prefer nutrient-poor environments. Notably, Planctomycetes, the dominant phylum in other forest succession ecosystems [31,68,69], was not dominant in these subalpine successional forests. This phenomenon suggested that serious water and soil erosion during subalpine forest succession might limit habitat availability for Planctomycetes, which is mainly present in oceans, marine sediments, freshwater lakes and wastewater [70].
Ascomycota and Basidiomycota were two dominant phyla in soil fungal communities among the six successional stages, but slight changes were observed in their abundance among the stages. This result was consistent with the findings of previous studies [25,71] but was contradictory to the observation in a Pinus yunnanensis forest [42]. Meanwhile, the abundance of Mortierellomycota, Rozellomycota and clade GS01 at terminal succession stages (S5 and S6) were higher than those in the other stages. Generally, the members of Ascomycota are common in extreme environments, while Mortierellomycota, Rozellomycota or clade GS01 members may have a strong preference for host plants in infertile soil, as these fungi exhibited environmental selection pressure and dispersal limitation with forest succession. Therefore, the abundance of these taxa in soil fungal communities increased with forest succession, indicating the increased accumulation of soil nutrients and maturation of the ecosystem.
The structure of soil bacterial and fungal communities differed substantially among the six successional stages based on ANOSIM analyses. This result was also observed in previous research [32]. However, other observations showed a lack of significant difference of community structure among successional stages [72]. These results suggested that the development pattern is consistent between plant and microbial communities and showed that above- and underground communities follow a synchronous succession process [73]. This further verified the importance of plant succession in microbial community structure.
4.3. The Roles of Forest Variables in Microbial Community Composition and Forest Succession
Our observations showed that the composition of soil microbial communities across the successional series was significantly affected by forest variables (e.g., soil pH, soil organic carbon, non-woody debris stock, altitude and soil temperature), which was consistent with the findings of previous studies [61,74,75]. Soil pH is a critical factor that affects the structure of soil bacterial and fungal communities [76,77,78]. Landesman et al. [27] have confirmed that the changes in tree species caused by secondary succession can lead to the acidification of forest soil, which was in line with our first result. Meanwhile, the members of Acidobacteria, Mortierellomycota and Rozellomycota [79,80], can physiologically tolerate acidic pH levels [81,82]. Therefore, the increase in the number of these microbial communities at terminal succession stages could be explained by the acidification of soil pH in this period. In addition to soil pH, soil temperature was also strongly correlated with the characteristics of soil microbial communities [83,84]. Soil temperature can not only directly accelerate microbial metabolic rates and biochemical processes [85,86,87] but also indirectly affect activity levels by changing the temperature dependency of the community [88]. In addition, plant successional processes, including nutrient and water uptake, as well as the initiation, branching, and orientation of root growth, also result in changes in soil temperature [89]. These factors could result in microbial communities performing variably over the succession period in response to soil temperatures.
Our results also found that the composition of soil bacterial communities, but not the composition of soil fungal communities, was significantly correlated with SOC and non-woody debris stock across the forest successional series, which was inconsistent with the findings of some previous studies [90,91]. Soil bacterial and fungal communities utilize soil organic carbon at different rates [92,93]. Variations in NWD among successional stage and vegetation type evoke different microbial responses to the quantitative and qualitative differences in plant compounds [94]. Meanwhile, our results showed that the soil concentration of SOC increased with plant community succession, which may be due to the decomposition of plant NWD. The concentration of soil organic matter strongly affects the structure and function of soil microbial communities [95,96]. Our results further demonstrated that distinct soil and vegetation properties could induce different soil microbial composition across forest succession series.
5. Conclusions
The diversity and composition of soil microbial communities varied greatly with forest succession. We summarized the following points:
- (1)
- The alpha diversity of soil bacteria, and ACE and Species richness indices of fungi significantly decreased with plant community succession, while there were slight changes in Shannon-Wiener and Simpson indices of soil fungi, indicating that soil bacterial and fungal communities had differential responses to forest succession.
- (2)
- The relative abundance of the most dominant phyla exhibited prominent differences among the six successional stages, although Proteobacteria and Acidobacteria, and Ascomycota and Basidiomycota were the dominant phyla of bacteria and fungi, respectively, regardless of succession level. The structure of soil microbial communities showed an obvious separation across the forest successional series.
- (3)
- Soil pH significantly affected the composition of soil microbial communities, while the NWD stock was correlated with only the change in soil bacterial community composition with forest succession, suggesting that the factors driving the changes in the structure and diversity of soil bacterial and fungal communities with forest succession differed greatly in the subalpine forest ecosystem.
To sum up, forest communities at different successional stages not only share dominant soil bacterial and fungal taxa but also support significantly different dominant groups of soil bacteria and fungi with community succession. The results imply that maintaining different succession stages of forest communities through moderate disturbances is beneficial to the conservation of soil biodiversity at the forest region level.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13020289/s1, Table S1: Site characteristics across a successional gradient in the subalpine forest on eastern Qinghai-Tibet Plateau; Table S2: Discrepancy of relative abundance of the dominant phyla of soil bacterial and fungal community composition among different forest succession stages; Table S3: The analysis of similarities (ANOSIM) among different forest succession stages based on the distance algorithm of Bray–Curtis; Table S4: The detrended correspondence analysis (DCA) based on based on the OTUs data at 97% sequence identity; Table S5: The detection of collinearity of forest variables by the analysis of Variance Inflation Factor (VIF).
Author Contributions
W.Y.: Conceptualization, funding acquisition, project administration, resources, supervision, validation and writing—review and editing. Z.W.: conceptualization, data curation, investigation, methodology and writing—original draft. F.L.: funding acquisition and investigation. Z.W., Y.B., J.H., F.L., X.L., Y.D. and Y.J.: investigation. Z.W., R.C. and H.W.: methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 32071554, 32001139).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Data in Table 6 is available through Zhang et al. 2016, Chai et al. 2019, Zhao et al. 2020, Li et al. 2020, Liu et al. 2020 (25), Liu et al. 2020 (62), Shang et al., 2021, Wang et al. 2021.
Acknowledgments
The authors of this study appreciate the field assistance of Wanglang National Nature Reserve involved in the initial sampling assignments.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bodelier, P. Toward Understanding, Managing, and Protecting Microbial Ecosystems. Front. Microbiol. 2011, 2, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yu, Z.; Huang, Z.; Davis, M.; Yang, Y. Litter decomposition, residue chemistry and microbial community structure under two subtropical forest plantations: A reciprocal litter transplant study. Appl. Soil Ecol. 2016, 101, 84–92. [Google Scholar] [CrossRef]
- Lamarche, J.; Bradley, R.L.; Hooper, E.; Shipley, B.; Beaunoir, A.-M.S.; Beaulieu, C. Forest Floor Bacterial Community Composition and Catabolic Profiles in Relation to Landscape Features in Québec’s Southern Boreal Forest. Microb. Ecol. 2007, 54, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Change Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Demenois, J.; Rey, F.; Ibanez, T.; Stokes, A.; Carriconde, F. Linkages between root traits, soil fungi and aggregate stability in tropical plant communities along a successional vegetation gradient. Plant Soil 2018, 424, 319–334. [Google Scholar] [CrossRef]
- Behera, S.K.; Mishra, A.K.; Sahu, N.; Kumar, A.; Singh, N.; Kumar, A.; Bajpai, O.; Chaudhary, L.B.; Khare, P.B.; Tuli, R. The study of microclimate in response to different plant community association in tropical moist deciduous forest from northern India. Biodivers. Conserv. 2012, 21, 1159–1176. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Garcia-Palacios, P.; Milla, R.; Gallardo, A.; Maestre, F.T. Soil characteristics determine soil carbon and nitrogen availability during leaf litter decomposition regardless of litter quality. Soil Biol. Biochem. 2015, 81, 134–142. [Google Scholar] [CrossRef]
- Wang, G.; Liu, G.; Xu, M. Above- and belowground dynamics of plant community succession following abandonment of farmland on the Loess Plateau, China. Plant Soil 2009, 316, 227–239. [Google Scholar] [CrossRef]
- Yan, H.; Gu, X.; Shen, H. Microbial decomposition of forest litter: A review. Chin. J. Ecol. 2010, 29, 1827–1835. [Google Scholar]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; Van Veen, J.A.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brockett, B.F.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Finegan, B. Forest succession. Nature 1984, 312, 109–114. [Google Scholar] [CrossRef]
- Walker, L.R.; Walker, J.; Hobbs, R.J. Linking Restoration and Ecological Succession; Springer: Berlin, Germamy, 2007. [Google Scholar]
- Powers, J.S.; Marín-Spiotta, E. Ecosystem Processes and Biogeochemical Cycles in Secondary Tropical Forest Succession. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 497–519. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Camarero, J.J.; Gutiérrez, E. Pace and Pattern of Recent Treeline Dynamics: Response of Ecotones to Climatic Variability in the Spanish Pyrenees. Clim. Chang. 2004, 63, 181–200. [Google Scholar] [CrossRef]
- Fernández-Alonso, M.J.; Yuste, J.C.; Kitzler, B.; Ortiz, C.; Rubio, A. Changes in litter chemistry associated with global change-driven forest succession resulted in time-decoupled responses of soil carbon and nitrogen cycles. Soil Biol. Biochem. 2018, 120, 200–211. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, L.; Bai, Y.; Li, F.; Hou, J.; Li, X.; Jiang, Y.; Deng, Y.; Zheng, B.; Yang, W. Changes in plant debris and carbon stocks across a subalpine forest successional series. For. Ecosyst. 2021, 8, 40. [Google Scholar] [CrossRef]
- Knapp, B.A.; Rief, A.; Seeber, J. Microbial communities on litter of managed and abandoned alpine pastureland. Biol. Fertil. Soils 2011, 47, 845–851. [Google Scholar] [CrossRef]
- He, L.; Ivanov, V.Y.; Bohrer, G.; Thomsen, J.E.; Vogel, C.S.; Moghaddam, M. Temporal dynamics of soil moisture in a northern temperate mixed successional forest after a prescribed intermediate disturbance. Agric. For. Meteorol. 2013, 180, 22–33. [Google Scholar] [CrossRef]
- Liu, J.; Jia, X.; Yan, W.; Zhong, Y.; Shangguan, Z. Changes in soil microbial community structure during long-term secondary succession. Land Degrad. Dev. 2020, 31, 1151–1166. [Google Scholar] [CrossRef]
- Bell, C.W.; Acosta-Martinez, V.; McIntyre, N.E.; Cox, S.; Tissue, D.T.; Zak, J.C. Linking Microbial Community Structure and Function to Seasonal Differences in Soil Moisture and Temperature in a Chihuahuan Desert Grassland. Microb. Ecol. 2009, 58, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Landesman, W.J.; Nelson, D.M.; Fitzpatrick, M.C. Soil properties and tree species drive ß-diversity of soil bacterial communities. Soil Biol. Biochem. 2014, 76, 201–209. [Google Scholar] [CrossRef]
- Zhang, J.; Elser, J.J. Carbon:Nitrogen:Phosphorus Stoichiometry in Fungi: A Meta-Analysis. Front. Microbiol. 2017, 8, 1281. [Google Scholar] [CrossRef]
- Ouyang, S.; Xiang, W.; Gou, M.; Lei, P.; Chen, L.; Deng, X.; Zhao, Z. Variations in soil carbon, nitrogen, phosphorus and stoichiometry along forest succession in southern China. Biogeosciences Discuss. 2017, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Shang, R.; Li, S.; Huang, X.; Liu, W.; Lang, X.; Su, J. Effects of Soil Properties and Plant Diversity on Soil Microbial Community Composition and Diversity during Secondary Succession. Forests 2021, 12, 805. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Chai, Y.; Cao, Y.; Yue, M.; Tian, T.; Yin, Q.; Dang, H.; Quan, J.; Zhang, R.; Wang, M. Soil Abiotic Properties and Plant Functional Traits Mediate Associations Between Soil Microbial and Plant Communities During a Secondary Forest Succession on the Loess Plateau. Front. Microbiol. 2019, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Banning, N.C.; Gleeson, D.; Grigg, A.H.; Grant, C.D.; Andersen, G.; Brodie, E.L.; Murphy, D.V. Soil Microbial Community Successional Patterns during Forest Ecosystem Restoration. Appl. Environ. Microbiol. 2011, 77, 6158–6164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Wu, F. The Ecosystem Processes and Management of the Subalpine Coniferous Forest in the Upper Reaches of Yangtze River; Science Press: Beijing, China, 2021. [Google Scholar]
- Zhang, Y.; Duan, B.; Xian, J.; Korpelainen, H.; Li, C. Links between plant diversity, carbon stocks and environmental factors along a successional gradient in a subalpine coniferous forest in Southwest China. For. Ecol. Manag. 2011, 262, 361–369. [Google Scholar] [CrossRef]
- Yang, B.; Pang, X.; Hu, B.; Bao, W.; Tian, G. Does thinning-induced gap size result in altered soil microbial community in pine plantation in eastern Tibetan Plateau? Ecol. Evol. 2017, 7, 2986–2993. [Google Scholar] [CrossRef]
- Zhao, P.; Bao, J.; Wang, X.; Liu, Y.; Li, C.; Chai, B. Deterministic processes dominate soil microbial community assembly in subalpine coniferous forests on the Loess Plateau. PeerJ 2019, 7, e6746. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.H.; Zisheng, Q.; Jie, L. Structure and dynamics of subalpine forests in the Wang Lang Natural Reserve, Sichuan, China. Vegetatio 1996, 124, 25–38. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Li, J.; Kang, D. Species diversity of primary and secondary forests in Wanglang Nature Reserve. Glob. Ecol. Conserv. 2020, 22, e01022. [Google Scholar] [CrossRef]
- Kang, D.; Lv, J.; Li, S.; Chen, X.; Wang, X.; Li, J. Community change characteristics of Abies faxoniana: A case study in the Wanglang Nature Reserve. Ecol. Indic. 2019, 107, 105594. [Google Scholar] [CrossRef]
- Bao, S. Agricultural and Chemistry Analysis of Soil; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Li, S.; Huang, X.; Shen, J.; Xu, F.; Su, J. Effects of plant diversity and soil properties on soil fungal community structure with secondary succession in the Pinus yunnanensis forest. Geoderma 2020, 379, 114646. [Google Scholar] [CrossRef]
- Carini, P.; Marsden, P.J.; Leff, J.W.; Morgan, E.E.; Strickland, M.S.; Fierer, N. Relic DNA is abundant in soil and obscures estimates of soil microbial diversity. Nat. Microbiol. 2016, 2, 16242. [Google Scholar] [CrossRef]
- Yang, T.; Adams, J.M.; Shi, Y.; Sun, H.; Cheng, L.; Zhang, Y.; Chu, H. Fungal community assemblages in a high elevation desert environment: Absence of dispersal limitation and edaphic effects in surface soil. Soil Biol. Biochem. 2017, 115, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 21, 5271–5277. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘vegan’. In Community Ecology Package; Version 2.4.5; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2011; Available online: https://www.R-project.org/ (accessed on 26 December 2021).
- Zhong, Z.; Zhang, X.; Wang, X.; Fu, S.; Wu, S.; Lu, X.; Ren, C.; Han, X.; Yang, G. Soil bacteria and fungi respond differently to plant diversity and plant family composition during the secondary succession of abandoned farmland on the Loess Plateau, China. Plant Soil 2020, 448, 183–200. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Cui, M.; Zhou, Z.; Zhang, Q.; Li, Y.; Ha, W.; Pang, D.; Luo, J.; Zhou, J. Effects of secondary succession on soil fungal and bacterial compositions and diversities in a karst area. Plant Soil 2021, 1–12. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Wang, Y.; Li, P.; Zhang, Y.; Zhang, X. Responses of soil microbial communities to nutrient limitation in the desert-grassland ecological transition zone. Sci. Total Environ. 2018, 642, 45–55. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Bai, L.; Wang, J.Y.; Deng, J.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J. Change in soil bacterial community during secondary succession depend on plant and soil characteristics. Catena 2019, 173, 246–252. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Hai, X.; Li, J.; Shangguan, Z.; Peng, C.; Deng, L. Long-term forest succession improves plant diversity and soil quality but not significantly increase soil microbial diversity: Evidence from the Loess Plateau. Ecol. Eng. 2020, 142, 105631. [Google Scholar] [CrossRef]
- Kolton, M.; Harel, Y.M.; Pasternak, Z.; Graber, E.R.; Elad, Y.; Cytryn, E. Impact of Biochar Application to Soil on the Root-Associated Bacterial Community Structure of Fully Developed Greenhouse Pepper Plants. Appl. Environ. Microbiol. 2011, 77, 4924–4930. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.G.; Jeong, S.-Y.; Cho, K.-S. Functional rigidity of a methane biofilter during the temporal microbial succession. Appl. Microbiol. Biotechnol. 2014, 98, 3275–3286. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Jangid, K.; Whitman, W.B.; Condron, L.M.; Turner, B.L.; Williams, M.A. Soil bacterial community succession during long-term ecosystem development. Mol. Ecol. 2013, 22, 3415–3424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Wallenstein, M.D.; Brodie, E.L. Differential Growth Responses of Soil Bacterial Taxa to Carbon Substrates of Varying Chemical Recalcitrance. Front. Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Fuerst, J.A.; Sagulenko, E. Beyond the bacterium: Planctomycetes challenge our concepts of microbial structure and function. Nat. Rev. Microbiol. 2011, 9, 403–413. [Google Scholar] [CrossRef]
- Brunner, I.; Plötze, M.; Rieder, S.; Zumsteg, A.; Furrer, G.; Frey, B. Pioneering fungi from the Damma glacier forefield in the Swiss Alps can promote granite weathering. Geobiology 2011, 9, 266–279. [Google Scholar] [CrossRef]
- Xu, H.; Du, H.; Zeng, F.; Song, T.; Peng, W. Diminished rhizosphere and bulk soil microbial abundance and diversity across succession stages in Karst area, southwest China. Appl. Soil Ecol. 2021, 158, 103799. [Google Scholar] [CrossRef]
- López-Lozano, N.E.; Heidelberg, K.B.; Nelson, W.C.; García-Oliva, F.; Eguiarte, L.E.; Souza, V. Microbial secondary succession in soil microcosms of a desert oasis in the Cuatro Cienegas Basin, Mexico. PeerJ 2013, 1, e47. [Google Scholar] [CrossRef] [Green Version]
- Montagna, M.; Berruti, A.; Bianciotto, V.; Cremonesi, P.; Giannico, R.; Gusmeroli, F.; Lumini, E.; Pierce, S.; Pizzi, F.; Turri, F.; et al. Differential biodiversity responses between kingdoms (plants, fungi, bacteria and metazoa) along an Alpine succession gradient. Mol. Ecol. 2018, 27, 3671–3685. [Google Scholar] [CrossRef]
- Qiang, W.; He, L.; Zhang, Y.; Liu, B.; Liu, Y.; Liu, Q.; Pang, X. Aboveground vegetation and soil physicochemical properties jointly drive the shift of soil microbial community during subalpine secondary succession in southwest China. Catena 2021, 202, 105251. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Bardgett, R.D.; Vitousek, P.M.; Maestre, F.T.; Williams, M.A.; Eldridge, D.J.; Lambers, H.; Neuhauser, S.; Gallardo, A.; García-Velázquez, L.; et al. Changes in belowground biodiversity during ecosystem development. Proc. Natl. Acad. Sci. USA 2019, 116, 6891–6896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendershot, J.N.; Read, Q.D.; Henning, J.A.; Sanders, N.J.; Classen, A.T. Consistently inconsistent drivers of microbial diversity and abundance at macroecological scales. Ecology 2017, 98, 1757–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Wang, J.; Meng, Z.; Xu, R.; Chen, J.; Zhang, Y.; Hu, T. Fertility-related interplay between fungal guilds underlies plant richness–productivity relationships in natural grasslands. New Phytol. 2020, 226, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Imamura, S.; Taniguchi, T.; Tateno, R. Does conversion from natural forest to plantation affect fungal and bacterial biodiversity, community structure, and co-occurrence networks in the organic horizon and mineral soil? For. Ecol. Manag. 2019, 446, 238–250. [Google Scholar] [CrossRef]
- Cui, J.; Wang, J.; Xu, J.; Xu, C.; Xu, X. Changes in soil bacterial communities in an evergreen broad-leaved forest in east China following 4 years of nitrogen addition. J. Soils Sediments 2017, 17, 2156–2164. [Google Scholar] [CrossRef]
- Colman, D.R.; Poudel, S.; Hamilton, T.L.; Havig, J.R.; Selensky, M.J.; Shock, E.L.; Boyd, E.S. Geobiological feedbacks and the evolution of thermoacidophiles. ISME J. 2018, 12, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, J.F.; Carere, C.R.; Lee, C.K.; Wakerley, G.L.; Evans, D.W.; Button, M.; White, D.; Climo, M.D.; Hinze, A.M.; Morgan, X.C.; et al. Microbial biogeography of 925 geothermal springs in New Zealand. Nat. Commun. 2018, 9, 2876. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Parker, K.M.; Luo, Y.T.; Wan, S.; Wallace, L.L.; Hu, S. Soil microbial responses to experimental warming and clipping in a tallgrass prairie. Glob. Chang. Biol. 2005, 11, 266–277. [Google Scholar] [CrossRef]
- Frey, S.; Drijber, R.; Smith, H.; Melillo, J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol. Biochem. 2008, 40, 2904–2907. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of Size and Temperature on Metabolic Rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suseela, V.; Conant, R.T.; Wallenstein, M.D.; Dukes, J.S. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob. Chang. Biol. 2012, 18, 336–348. [Google Scholar] [CrossRef]
- BÁRCENAS-MORENO, G.; GÓMEZ-BRANDÓN, M.; Rousk, J.; Bååth, E. Adaptation of soil microbial communities to temperature: Comparison of fungi and bacteria in a laboratory experiment. Glob. Chang. Biol. 2009, 15, 2950–2957. [Google Scholar] [CrossRef]
- Kaspar, T.C.; Bland, W.L. Soil temperature and root growth. Soil Sci. 1992, 154, 290–299. [Google Scholar] [CrossRef]
- Li, Q.; Song, A.; Yang, H.; Müller, W.E.G. Impact of Rocky Desertification Control on Soil Bacterial Community in Karst Graben Basin, Southwestern China. Front. Microbiol. 2021, 12, 448. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Bakker, M.G.; Bradeen, J.M.; Kinkel, L.L. Plant community richness and microbial interactions structure bacterial communities in soil. Ecology 2015, 96, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Drenovsky, R.E.; Vo, D.; Graham, K.J.; Scow, K.M. Soil Water Content and Organic Carbon Availability Are Major Determinants of Soil Microbial Community Composition. Microb. Ecol. 2004, 48, 424–430. [Google Scholar] [CrossRef]
- Sun, R.; Dsouza, M.; Gilbert, J.A.; Guo, X.; Wang, D.; Guo, Z.; Ni, Y.; Chu, H. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ. Microbiol. 2016, 18, 5137–5150. [Google Scholar] [CrossRef]
- Sariyildiz, T.; Anderson, J. Interactions between litter quality, decomposition and soil fertility: A laboratory study. Soil Biol. Biochem. 2003, 35, 391–399. [Google Scholar] [CrossRef]
- Grayston, S.; Campbell, C.; Bardgett, R.; Mawdsley, J.; Clegg, C.; Ritz, K.; Griffiths, B.; Rodwell, J.; Edwards, S.; Davies, W.; et al. Assessing shifts in microbial community structure across a range of grasslands of differing management intensity using CLPP, PLFA and community DNA techniques. Appl. Soil Ecol. 2004, 25, 63–84. [Google Scholar] [CrossRef]
- Franklin, R.B.; Mills, A.L. Importance of spatially structured environmental heterogeneity in controlling microbial community composition at small spatial scales in an agricultural field. Soil Biol. Biochem. 2009, 41, 1833–1840. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).