Differential Response of Soil Respiration and Total Belowground Carbon Allocation to Simulated Nitrogen and Phosphorus Deposition in Moso Bamboo Forests
Abstract
1. Introduction
2. Materials and Methodology
2.1. Study Site
2.2. Experimental Design
2.3. Partitioning of RA and RH
2.4. RS Measurement
2.5. Vegetation Production
2.6. Data Analysis
2.7. Carbon Balance Calculations
2.8. TBCA
3. Results
3.1. Monthly Variabilities of ST and SM
3.2. Monthly Variabilities of Soil Carbon Fluxes
3.3. Correlation between Soil Carbon Fluxes and ST/SM
3.4. Annual Cumulative Carbon Fluxes
4. Discussion
4.1. Monthly Dynamics of Carbon Fluxes and Their Correlations with ST and SM
4.2. Effects of N and P deposition on RS and NEP
4.3. Effects of N and P Deposition on TBCA
4.4. Limitation and Uncertainty
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Law, B.E.; Ryan, M.G.; Anthoni, P.M. Seasonal and annual respiration of a ponderosa pine ecosystem. Glob. Chang. Biol. 1999, 5, 169–182. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J. Rhizospheric and heterotrophic components of soil respiration in six Chinese temperate forests. Glob. Chang. Biol. 2007, 13, 123–131. [Google Scholar] [CrossRef]
- Subke, J.-A.; Inglima, I.; Francesca Cotrufo, M. Trends and methodological impacts in soil CO2 efflux partitioning: A metaanalytical review. Glob. Chang. Biol. 2006, 12, 921–943. [Google Scholar] [CrossRef]
- Tremblay, S.L.; D’Orangeville, L.; Lambert, M.-C.; Houle, D. Transplanting boreal soils to a warmer region increases soil heterotrophic respiration as well as its temperature sensitivity. Soil Biol. Biochem. 2018, 116, 203–212. [Google Scholar] [CrossRef]
- Tang, X.; Fan, S.; Qi, L.; Guan, F.; Liu, G.; Du, M. Effects of understory removal on root production, turnover and total belowground carbon allocation in Moso bamboo forests. iForest—Biogeosci. For. 2016, 9, 187–194. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Y.; Liu, L.; Li, X.; Zhao, Y.; Yuan, Y. Research review on total belowground carbon allocation in forest ecosystems. J. Subtrop. Resour. Environ. 2007, 2, 34–42, (In Chinese with English abstract). [Google Scholar]
- Ryan, M.G.; Lavigne, M.B.; Gower, S.T. Annual carbon cost of autotrophic respiration in boreal forest ecosystems in relation to species and climate. J. Geophys. Res. Atmos. 1997, 102, 28871–28883. [Google Scholar] [CrossRef]
- Giardina, C.P.; Ryan, M.G. Total belowground carbon allocation in a fast-growing Eucalyptus plantation estimated using a carbon balance approach. Ecosystems 2002, 5, 487–499. [Google Scholar] [CrossRef]
- Raich, J.W.; Nadelhoffer, K.J. Belowground carbon allocation in forest ecosystems: Global trends. Ecology 1989, 70, 1346–1354. [Google Scholar] [CrossRef]
- Yu, G.; Jia, Y.; He, N.; Zhu, J.; Chen, Z.; Wang, Q.; Piao, S.; Liu, X.; He, H.; Guo, X.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Schwede, D.B.; Simpson, D.; Tan, J.; Fu, J.S.; Dentener, F.; Du, E.; de Vries, W. Spatial variation of modelled total, dry and wet nitrogen deposition to forests at global scale. Environ. Pollut. 2018, 243, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chen, G.; Du, E.; Tian, D.; Xing, A.; Shen, H.; Ji, C.; Zheng, C.; Zhu, J.; Zhu, J.; et al. Effects of nitrogen addition on microbial residues and their contribution to soil organic carbon in China’s forests from tropical to boreal zone. Environ. Pollut. 2021, 268, 115941. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Y.P.; Baldock, J.A.; Jiang, J.; Mo, J.; Zhou, G.; Yan, J. Divergent responses of soil organic carbon accumulation to 14 years of nitrogen addition in two typical subtropical forests. Sci. Total Environ. 2020, 707, 136104. [Google Scholar] [CrossRef]
- Xu, X.; Yan, L.; Xia, J. A threefold difference in plant growth response to nitrogen addition between the laboratory and field experiments. Ecosphere 2019, 10, e02572. [Google Scholar] [CrossRef]
- Schulte-Uebbing, L.; de Vries, W. Global-scale impacts of nitrogen deposition on tree carbon sequestration in tropical, temperate, and boreal forests: A meta-analysis. Glob. Chang. Biol. 2018, 24, e416–e431. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef]
- Penuelas, J.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Sardans, J. Anthropogenic global shifts in biospheric N and P concentrations and ratios and their impacts on biodiversity, ecosystem productivity, food security, and human health. Glob. Chang. Biol. 2020, 26, 1962–1985. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, T.X.; Zhang, J.; Li, X.W.; Hu, H.L.; Liu, L.; Xiao, Y.L. Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol. Biochem. 2013, 58, 255–264. [Google Scholar] [CrossRef]
- Wei, S.; Tie, L.; Liao, J.; Liu, X.; Du, M.; Lan, S.; Li, X.; Li, C.; Zhan, H.; Huang, C. Nitrogen and phosphorus co-addition stimulates soil respiration in a subtropical evergreen broad-leaved forest. Plant Soil 2020, 450, 171–182. [Google Scholar] [CrossRef]
- Cao, Y.S.; Lin, Y.B.; Rao, X.Q.; Fu, S.L. Effects of artificial nitrogen and phosphorus depositions on soil respiration in two plantations in Southern China. J. Trop. For. Sci. 2011, 23, 110–116. [Google Scholar]
- Zhang, J.; Li, Y.; Wang, J.; Chen, W.; Tian, D.; Niu, S. Different responses of soil respiration and its components to nitrogen and phosphorus addition in a subtropical secondary forest. For. Ecosyst. 2021, 8, 37. [Google Scholar] [CrossRef]
- Song, X.; Peng, C.; Zhou, G.; Gu, H.; Li, Q.; Zhang, C. Dynamic allocation and transfer of non-structural carbohydrates, a possible mechanism for the explosive growth of Moso bamboo (Phyllostachys heterocycla). Sci. Rep. 2016, 6, 25908. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Yang, Z.; Liu, L.; Lai, Y.; Lei, J.; Fan, S.; Tang, X. Consistent Effects of Canopy vs. Understory Nitrogen Addition on Soil Respiration and Net Ecosystem Production in Moso Bamboo Forests. Forests 2021, 12, 1427. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, J.; Wang, X.; Xu, J.; Li, Z. Report for Chinese Forest Resource—The 7th National Forest Inventory; China Forestry Publishing House: Beijing, China, 2009; p. 60. (In Chinese) [Google Scholar]
- Li, Y.; Feng, P. Bamboo resources in China based on the ninth national forest inventory data. World Bamboo Ratt. 2019, 17, 45–48, (In Chinese with English Abstract). [Google Scholar]
- Song, X.; Chen, X.; Zhou, G.; Jiang, H.; Peng, C. Observed high and persistent carbon uptake by Moso bamboo forests and its response to environmental drivers. Agric. For. Meteorol. 2017, 247, 467–475. [Google Scholar] [CrossRef]
- Tang, X.; Fan, S.; Qi, L.; Guan, F.; Du, M.; Zhang, H. Soil respiration and net ecosystem production in relation to intensive management in Moso bamboo forests. Catena 2016, 137, 219–228. [Google Scholar] [CrossRef]
- Li, Q.; Song, X.; Chang, S.X.; Peng, C.; Xiao, W.; Zhang, J.; Xiang, W.; Li, Y.; Wang, W. Nitrogen depositions increase soil respiration and decrease temperature sensitivity in a Moso bamboo forest. Agric. For. Meteorol. 2019, 268, 48–54. [Google Scholar] [CrossRef]
- Zhang, J.B.; Li, Q.; Lv, J.H.; Peng, C.H.; Gu, Z.K.; Qi, L.H.; Song, X.Z.; Song, X.Z. Management scheme influence and nitrogen addition effects on soil CO2, CH4, and N2O fluxes in a Moso bamboo plantation. For. Ecosyst. 2021, 8, 6. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Gurmesa, G.A.; Yu, G.; Li, L.; Zhang, W.; Fang, H.; Mo, J. Effects of nitrogen deposition on carbon cycle in terrestrial ecosystems of China: A meta-analysis. Environ. Pollut. 2015, 206, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, X.; Xu, K. Effect of chronic nitrogen fertilization on soil CO2 flux in a temperate forest in North China: A 5-year nitrogen addition experiment. J. Soils Sed. 2018, 18, 506–516. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Marschner, P. Soil respiration, microbial biomass and nutrient availability in soil after addition of residues with adjusted N and P concentrations. Pedosphere 2017, 27, 76–85. [Google Scholar] [CrossRef]
- Bae, K.; Fahey, T.; Yanai, R.; Fisk, M. Soil nitrogen availability affects belowground carbon allocation and soil respiration in Northern Hardwood Forests of New Hampshire. Ecosystems 2015, 18, 1179–1191. [Google Scholar] [CrossRef]
- Tian, J.; Dungait, J.A.J.; Lu, X.; Yang, Y.; Hartley, I.P.; Zhang, W.; Mo, J.; Yu, G.; Zhou, J.; Kuzyakov, Y. Long-term nitrogen addition modifies microbial composition and functions for slow carbon cycling and increased sequestration in tropical forest soil. Glob. Chang. Biol. 2019, 25, 3267–3281. [Google Scholar] [CrossRef]
- Du, Y.; Guo, P.; Liu, J.; Wang, C.; Yang, N.; Jiao, Z. Different types of nitrogen deposition show variable effects on the soil carbon cycle process of temperate forests. Glob. Chang. Biol. 2014, 20, 3222–3228. [Google Scholar] [CrossRef]
- Gao, Q.; Dai, B.; Luo, C.; Liu, L.; Ma, D.; Zhang, C. Spatial heterogeneity of soil physical properties in Phyllostachys heterocycla cv pubescens forest, South Sichuan Bamboo Sea. Acta Eco. Sin. 2016, 36, 2255–2263, (In Chinese with English abstract). [Google Scholar]
- Tian, H.; Yang, J.; Lu, C.; Xu, R.; Canadell, J.G.; Jackson, R.B.; Arneth, A.; Chang, J.; Chen, G.; Ciais, P.; et al. The Global N2O Model Intercomparison Project. Bull. Am. Meteorol. Soc. 2018, 99, 1231–1251. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Jian, J.; Bahn, M.; Wang, C.; Bailey, V.L.; Bond-Lamberty, B. Prediction of annual soil respiration from its flux at mean annual temperature. Agric. For. Meteorol. 2020, 287, 107961. [Google Scholar] [CrossRef]
- Tang, X.; Fan, S.; Qi, L.; Liu, G.; Guan, F.; Du, M.; Shen, C. Effect of different managements on carbon storage and carbon allocation in Moso Bamboo Forest ( Phyllostachys pubescen). Acta Agric. Univ. Jiangxiensis 2012, 34, 736–742, (In Chinese with English abstract). [Google Scholar]
- Tang, X.; Fan, S.; Qi, L.; Guan, F.; Su, W.; Du, M. A comparison of soil respiration, carbon balance and root carbon use efficiency in two managed Moso bamboo forests in subtropical China. Ann. For. Res. 2016, 59, 3–20. [Google Scholar] [CrossRef]
- Tang, X.; Fan, S.; Qi, L.; Guan, F.; Cai, C.; Du, M. Soil respiration and carbon balance in a Moso bamboo (Phyllostachys heterocycla (Carr.) Mitford cv. Pubescens) forest in subtropical China. Iforest 2015, 8, 606–614. [Google Scholar] [CrossRef]
- Jian, J.; Steele, M.K.; Day, S.D.; Quinn Thomas, R.; Hodges, S.C. Measurement strategies to account for soil respiration temporal heterogeneity across diverse regions. Soil Biol. Biochem. 2018, 125, 167–177. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhuang, S.Y.; Sun, B.; Ji, H.B.; Li, C.M.; Zhou, S. Estimation of biomass and carbon storage of moso bamboo (Phyllostachys pubescens Mazel ex Houz.) in southern China using a diameter-age bivariate distribution model. Forestry 2014, 87, 674–682. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, P.; Xu, Q. (Eds.) Carbon Sequestration and Transform in Bamboo Ecosystem; Science Press: Beijing, China, 2010; pp. 105–137. (In Chinese) [Google Scholar]
- Brunner, I.; Bakker, M.R.; Björk, R.G.; Hirano, Y.; Lukac, M.; Aranda, X.; Børja, I.; Eldhuset, T.D.; Helmisaari, H.S.; Jourdan, C.; et al. Fine-root turnover rates of European forests revisited: An analysis of data from sequential coring and ingrowth cores. Plant Soil 2013, 362, 357–372. [Google Scholar] [CrossRef]
- McClaugherty, C.A.; Aber, J.D.; Melillo, J.M. The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 1982, 63, 1481–1490. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, P. Density, storage and spatial distributbion of carbon in Phyllostachy pubescens forest. Sci. Silvae Sin. 2004, 40, 20–24, (In Chinese with English abstract). [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 11 June 2019).
- Wu, F.T.; Peng, C.H.; Liu, W.G.; Liu, Z.H.; Wang, H.; Chen, D.X.; Li, Y.D. Effects of nitrogen additions on soil respiration in an Asian tropical montane rainforest. Forests 2021, 12, 802. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J. On the temperature dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Yuste, J.C.; Janssens, I.A.; Carrara, A.; Meiresonne, L.; Ceulemans, R. Interactive effects of temperature and precipitation on soil respiration in a temperate maritime pine forest. Tree Phys. 2003, 23, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Nakane, K.; Nakatsubo, T.; Koizumi, H. Seasonal changes in the contribution of root respiration to total soil respiration in a cool-temperate deciduous forest. Plant Soil 2003, 255, 311–318. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Y.; Qian, W.; Gao, R.; Niu, Z.; Han, Y. Total belowground carbon allocation in Castanopsis kawakamii and Chinese fir plantations in subtropical area of China. Acta Ecol. Sin. 2005, 25, 2824–2829, (In Chinese with English abstract). [Google Scholar]
- Chen, G.; Yang, Y.; Gao, R.; Xie, J.; Yang, Z.; Mao, Y. Changes in belowground carbon allocation in a Chinese fir chronosequence in Fujian Province. J. Plant Ecol. (Chin. Version) 2008, 32, 1285–1293. [Google Scholar] [CrossRef]
- Han, H.; Du, Y.; Hui, D.; Jiang, L.; Zhong, M.; Wan, S. Long-term antagonistic effect of increased precipitation and nitrogen addition on soil respiration in a semiarid steppe. Ecol. Evol. 2017, 7, 10804–10814. [Google Scholar] [CrossRef]
- Wan, S.; Norby, R.J.; Ledford, J.; Weltzin, J.F. Responses of soil respiration to elevated CO2, air warming, and changing soil water availability in a model old-field grassland. Glob. Chang. Biol. 2007, 13, 2411–2424. [Google Scholar] [CrossRef]
- Zhang, J.; Ru, J.; Song, J.; Li, H.; Li, X.; Ma, Y.; Li, Z.; Hao, Y.; Chi, Z.; Hui, D.; et al. Increased precipitation and nitrogen addition accelerate the temporal increase in soil respiration during 8-year old-field grassland succession. Glob. Chang. Biol. 2022, 28, 3944–3959. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Gavrichkova, O. Time lag between photosynthesis and carbon dioxide efflux from soil: A review of mechanisms and controls. Glob. Chang. Biol. 2010, 16, 3386–3406. [Google Scholar] [CrossRef]
- Song, X.; Yuan, H.; Kimberley, M.O.; Jiang, H.; Zhou, G.; Wang, H. Soil CO2 flux dynamics in the two main plantation forest types in subtropical China. Sci. Total Environ. 2013, 444, 363–368. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Hu, J.; Tang, C.; Zhang, S.; Fu, S.; Jiang, P.; Ge, T.; Luo, Y.; Song, X.; et al. Rates of soil respiration components in response to inorganic and organic fertilizers in an intensively-managed Moso bamboo forest. Geoderma 2021, 403, 115212. [Google Scholar] [CrossRef]
- Luan, J.W.; Liu, S.R.; Wang, J.X.; Zhu, X.L.; Shi, Z.M. Rhizospheric and heterotrophic respiration of a warm-temperate oak chronosequence in China. Soil Biol. Biochem. 2011, 43, 503–512. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, P.; Wang, H.; Zhou, G.; Wu, J.; Yang, F.; Qian, X. Seasonal soil CO2 efflux dynamics after land use change from a natural forest to Moso bamboo plantations in subtropical China. For. Ecol. Manag. 2011, 262, 1131–1137. [Google Scholar] [CrossRef]
- Zhou, X.; Sherry, R.A.; An, Y.; Wallace, L.L.; Luo, Y. Main and interactive effects of warming, clipping, and doubled precipitation on soil CO2 efflux in a grassland ecosystem. Glob. Biogeochem. Cycles 2006, 20, GB1003. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Z.J.; Chen, G.S.; Fan, Y.X.; Liu, Q.; Tian, H. Characteristics of soil respiration in Phyllostachys edulis forest in Wanmulin Natural Reserve and related affecting factors. Chin. J. Appl. Ecol. 2011, 22, 1212–1218. (In Chinese) [Google Scholar]
- Song, X.Z.; Peng, C.H.; Zhao, Z.Y.; Zhang, Z.T.; Guo, B.H.; Wang, W.F.; Jiang, H.; Zhu, Q.A. Quantification of soil respiration in forest ecosystems across China. Atmos. Environ. 2014, 94, 546–551. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, G.; Bai, S.H.; Song, J.; Shang, Y.; He, M.; Wang, X.; Zheng, Z. Differential response of soil respiration to nitrogen and phosphorus addition in a highly phosphorus-limited subtropical forest, China. For. Ecol. Manag. 2019, 448, 499–508. [Google Scholar] [CrossRef]
- Feng, J.G.; Zhu, B. A global meta-analysis of soil respiration and its components in response to phosphorus addition. Soil Biol. Biochem. 2019, 135, 38–47. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Bond-Lamberty, B.; Bailey, V.L.; Chen, M.; Gough, C.M.; Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 2018, 560, 80–83. [Google Scholar] [CrossRef]
- Frey, S.D.; Ollinger, S.; Nadelhoffer, K.; Bowden, R.; Brzostek, E.; Burton, A.; Caldwell, B.A.; Crow, S.; Goodale, C.L.; Grandy, A.S.; et al. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 2014, 121, 305–316. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. A global analysis of fine root production as affected by soil nitrogen and phosphorus. Proc. R. Soc. B 2012, 279, 3796–3802. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Fan, S.; Liu, G.; Guan, F.; Guo, B.; Tang, X. Stoichiometric characteristics of carbon, nitrogen and phosphorus in Phyllostachys pubescens forests of China. Chin. J. Plant Ecol. 2016, 40, 760–774, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Luo, M.; Moorhead, D.L.; Ochoa-Hueso, R.; Mueller, C.W.; Ying, S.C.; Chen, J. Nitrogen loading enhances phosphorus limitation in terrestrial ecosystems with implications for soil carbon cycling. Funct. Ecol. 2022, in press. [CrossRef]
- Capek, P.; Manzoni, S.; Kastovska, E.; Wild, B.; Diakova, K.; Barta, J.; Schnecker, J.; Biasi, C.; Martikainen, P.J.; Alves, R.J.E.; et al. A plant-microbe interaction framework explaining nutrient effects on primary production. Nat. Ecol. Evol. 2018, 2, 1588–1596. [Google Scholar] [CrossRef]
- Amthor, J.S. The McCree–de Wit–Penning de Vries–Thornley Respiration Paradigms: 30 Years Later. Ann. Bot. 2000, 86, 1–20. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Z.; Piao, S.; Peng, C.; Ciais, P.; Wang, Q.; Li, X.; Zhu, X. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef]
- Fernandez-Martinez, M.; Vicca, S.; Janssens, I.A.; Luyssaert, S.; Campioli, M.; Sardans, J.; Estiarte, M.; Penuelas, J. Spatial variability and controls over biomass stocks, carbon fluxes, and resource-use efficiencies across forest ecosystems. Trees Struct. Funct. 2014, 28, 597–611. [Google Scholar] [CrossRef]
- Davidson, E.A.; Savage, K.; Bolstad, P.; Clark, D.A.; Curtis, P.S.; Ellsworth, D.S.; Hanson, P.J.; Law, B.E.; Luo, Y.; Pregitzer, K.S.; et al. Belowground carbon allocation in forests estimated from litterfall and IRGA-based soil respiration measurements. Agric. For. Meteorol. 2002, 113, 39–51. [Google Scholar] [CrossRef]
- McDowell, N.G.; Balster, N.J.; Marshall, J.D. Belowground carbon allocation of Rocky Mountain Douglas-fir. Can. J. For. Res. 2001, 31, 1425–1436. [Google Scholar] [CrossRef]
- Wang, J.; Song, B.; Ma, F.; Tian, D.; Li, Y.; Yan, T.; Quan, Q.; Zhang, F.; Li, Z.; Wang, B.; et al. Nitrogen addition reduces soil respiration but increases the relative contribution of heterotrophic component in an alpine meadow. Funct. Ecol. 2019, 33, 2239–2253. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, W.; Zhu, S.; Wan, S.; Luo, Y.; Yan, J.; Wang, K.; Lei, L.; Dai, H.; Li, P. Can canopy addition of nitrogen better illustrate the effect of atmospheric nitrogen deposition on forest ecosystem? Sci. Rep. 2015, 5, 11245. [Google Scholar] [CrossRef] [PubMed]
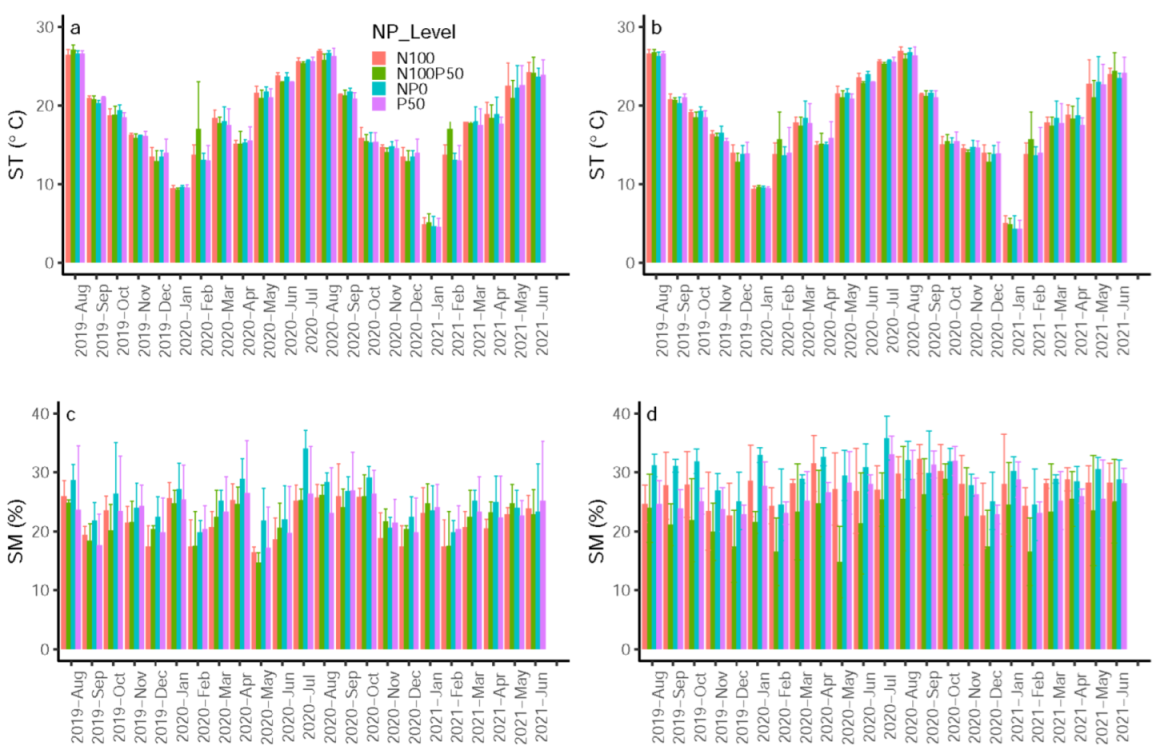
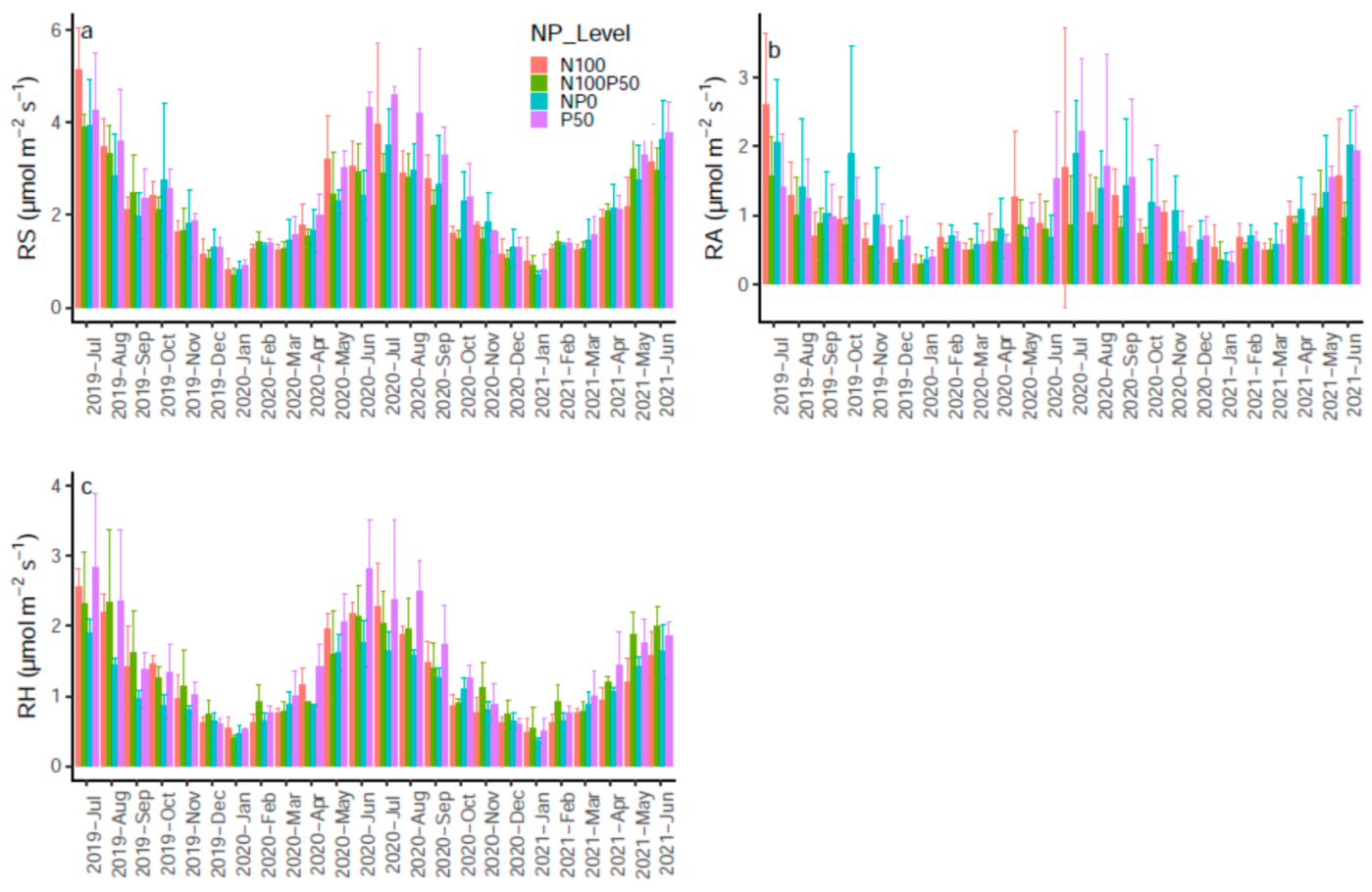
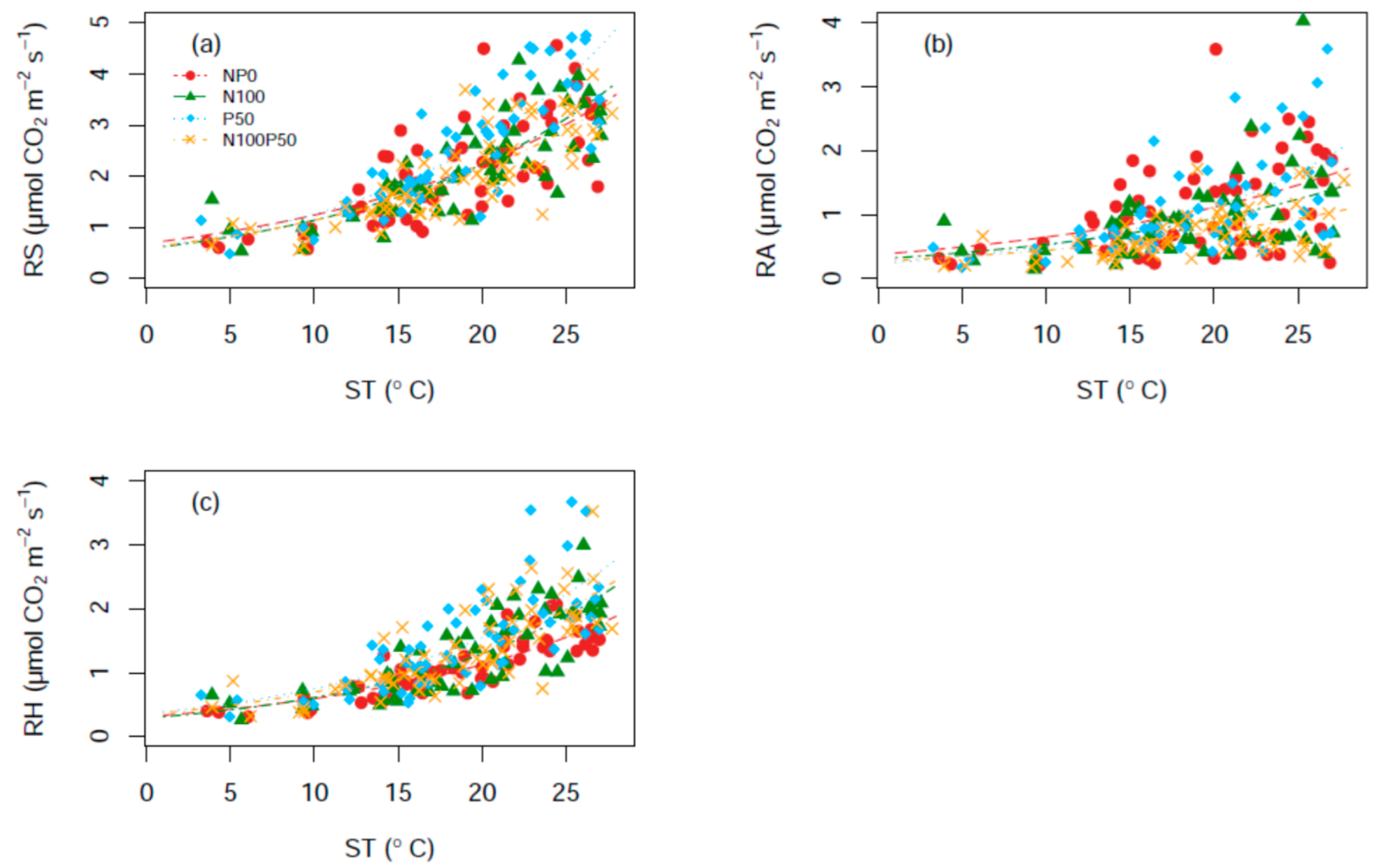
| Factor | ST | SM | RS | RA | RH | ||
|---|---|---|---|---|---|---|---|
| Untrenched | Trenched | Untrenched | Trenched | ||||
| N | 0.835 | 0.606 | 0.241 | 0.007 | 0.460 | 0.316 | 0.558 |
| P | 0.927 | 0.891 | 0.997 | 0.621 | 0.841 | 0.492 | 0.112 |
| N × P | 0.646 | 0.686 | 0.347 | 0.261 | 0.130 | 0.567 | 0.078 |
| Month | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Month × N | 0.673 | 0.897 | 0.646 | 0.312 | 0.400 | 0.258 | 0.997 |
| Month × P | 0.729 | 0.828 | 0.052 | 0.968 | 0.319 | 0.277 | 0.064 |
| Month × N × P | 0.770 | 0.988 | 0.169 | 0.915 | 0.242 | 0.630 | 0.337 |
| Respiration Components | Treatment | c | d | R2 | Q10 |
|---|---|---|---|---|---|
| RS | NP0 | 0.554 (0.422, 0.947) | 0.067 (0.042, 0.077) | 0.547 | 1.96 |
| N100 | 0.580 (0.364, 0.796) | 0.067 (0.051, 0.084) | 0.718 | 1.96 | |
| P50 | 0.593 (0.405, 0.782) | 0.075 (0.061, 0.089) | 0.752 | 2.12 | |
| N100P50 | 0.605 (0.415, 0.795) | 0.063 (0.049, 0.077) | 0.698 | 1.88 | |
| RA | NP0 | 0.375 (0.109, 0.641) | 0.054 (0.022, 0.087) | 0.302 | 1.72 |
| N100 | 0.303 (0.084, 0.525) | 0.056 (0.023, 0.090) | 0.446 | 1.76 | |
| P50 | 0.232 (0.070, 0.395) | 0.078 (0.048, 0.110) | 0.400 | 2.20 | |
| N100P50 | 0.266 (0.127, 0.404) | 0.050 (0.026, 0.074) | 0.463 | 1.65 | |
| RH | NP0 | 0.312 (0.231, 0.393) | 0.064 (0.053, 0.076) | 0.771 | 1.90 |
| N100 | 0.287 (0.179, 0.394) | 0.075 (0.059, 0.092) | 0.713 | 2.12 | |
| P50 | 0.362 (0.204, 0.519) | 0.073 (0.053, 0.092) | 0.637 | 2.07 | |
| N100P50 | 0.346 (0.209, 0.483) | 0.070 (0.052, 0.088) | 0.634 | 2.01 |
| Treatment | RS (g C m−2 year−1) | RA(g C m−2 year−1) | RH (g C m−2 year−1) | RH/RS (Unitless) | Litterfall (g C m−2 year−1) | Root NPP (g C m−2 year−1) | Vegetation NPP (g C m−2 year−1) | NEP (g C m−2 year−1) | TBCA (g C m−2 year−1) | TBCA_mba (g C m−2 year−1) | RCUE (Unitless) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NP0 | 810 ± 163 a | 401 ± 158 a | 409 ± 15 b | 0.52 ± 0.11 a | 463 ± 139 a | 192 ± 41 ab | 475 ± 109 a | 529 ± 225 a | 594 ± 174 a | 348 ± 227 ab | 0.34 ± 0.10 b |
| N100 | 881 ± 97 a | 368 ± 130 a | 513 ± 51 ab | 0.59 ± 0.10 a | 479 ± 162 a | 195 ± 56 ab | 364 ± 298 a | 331 ± 341 a | 563 ± 142 a | 401 ± 179 ab | 0.41 ± 0.15 ab |
| P50 | 945 ± 91 a | 393 ± 112 a | 551 ± 107 a | 0.65 ± 0.04 a | 407 ± 102 a | 149 ± 53 b | 342 ± 143 a | 198 ± 104 a | 542 ± 106 a | 538 ± 141 a | 0.27 ± 0.10 b |
| N100P50 | 766 ± 89 a | 265 ± 50 a | 500 ± 60 ab | 0.58 ± 0.10 a | 486 ± 148 a | 245 ± 44 a | 407 ± 217 a | 393 ± 274 a | 510 ± 88 a | 280 ± 121 b | 0.48 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, Z.; Tang, X.; Liu, L.; Lai, Y.; Lei, J.; Zeng, C.; Ma, X.; Du, M.; Cai, C.; et al. Differential Response of Soil Respiration and Total Belowground Carbon Allocation to Simulated Nitrogen and Phosphorus Deposition in Moso Bamboo Forests. Forests 2022, 13, 1860. https://doi.org/10.3390/f13111860
Li J, Yang Z, Tang X, Liu L, Lai Y, Lei J, Zeng C, Ma X, Du M, Cai C, et al. Differential Response of Soil Respiration and Total Belowground Carbon Allocation to Simulated Nitrogen and Phosphorus Deposition in Moso Bamboo Forests. Forests. 2022; 13(11):1860. https://doi.org/10.3390/f13111860
Chicago/Turabian StyleLi, Jingji, Zhihan Yang, Xiaolu Tang, Liang Liu, Yunsen Lai, Junjie Lei, Changli Zeng, Xinshan Ma, Manyi Du, Chunju Cai, and et al. 2022. "Differential Response of Soil Respiration and Total Belowground Carbon Allocation to Simulated Nitrogen and Phosphorus Deposition in Moso Bamboo Forests" Forests 13, no. 11: 1860. https://doi.org/10.3390/f13111860
APA StyleLi, J., Yang, Z., Tang, X., Liu, L., Lai, Y., Lei, J., Zeng, C., Ma, X., Du, M., Cai, C., & Fan, S. (2022). Differential Response of Soil Respiration and Total Belowground Carbon Allocation to Simulated Nitrogen and Phosphorus Deposition in Moso Bamboo Forests. Forests, 13(11), 1860. https://doi.org/10.3390/f13111860






