Carbon and Nitrogen Availability Drives Seasonal Variation in Soil Microbial Communities along an Elevation Gradient
Abstract
1. Introduction
2. Materials and Methods
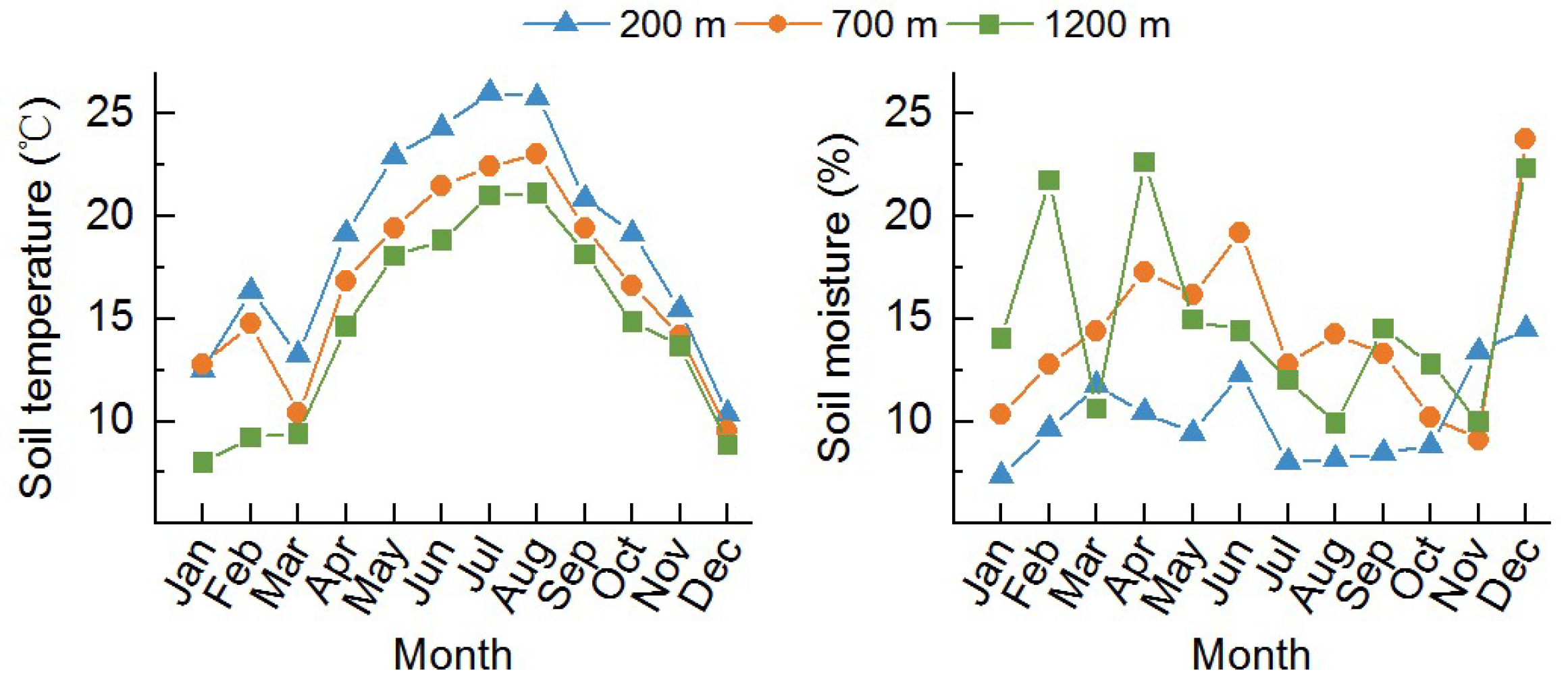
2.1. Site Description
2.2. Sample Collection and Preparation
2.3. Soil Chemical Analysis
2.4. Biomass and Composition of Microbial Communities
2.5. Soil Enzymes Activities and Microbial Carbon Use Efficiency
SC:N = (1/EEAC:N) (BC:N/LC:N)
2.6. Statistical Analysis
3. Results
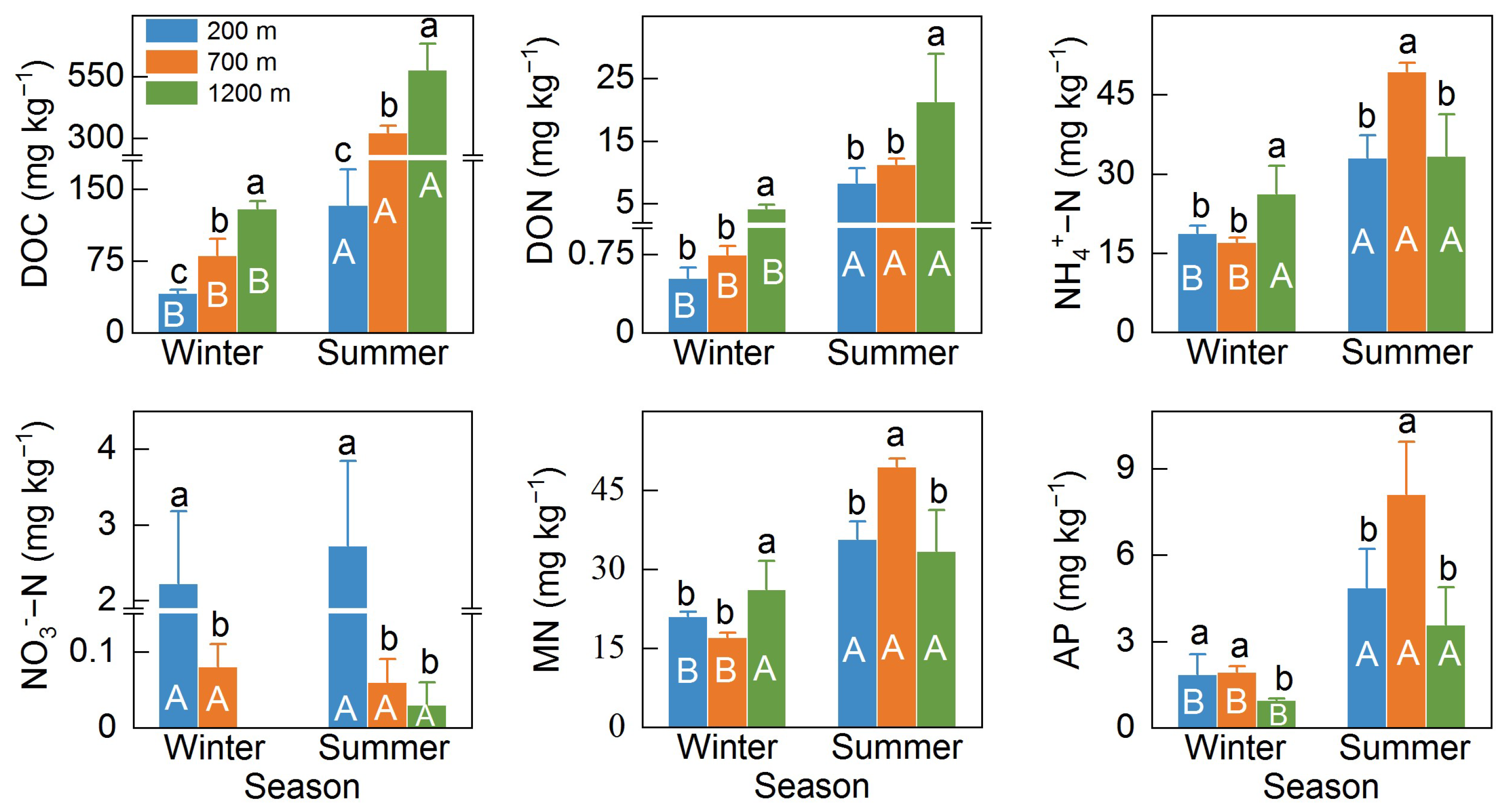
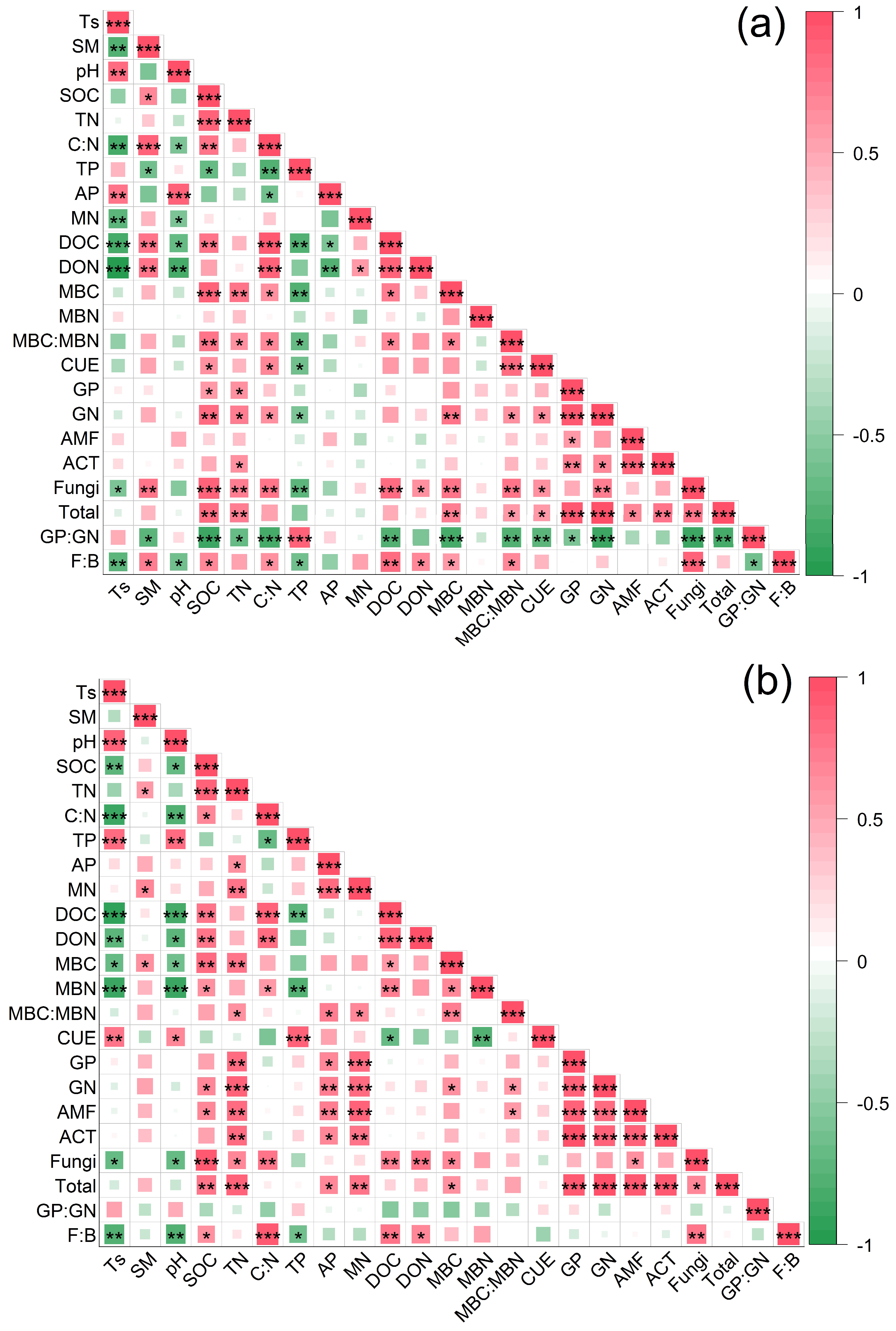
3.1. Soil Properties at Different Elevations
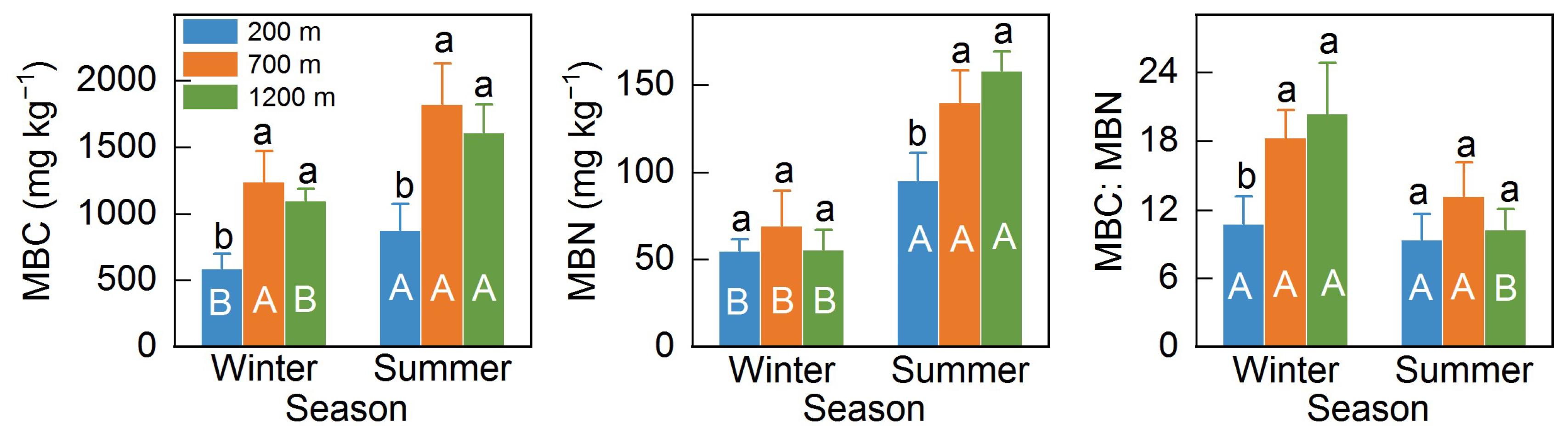
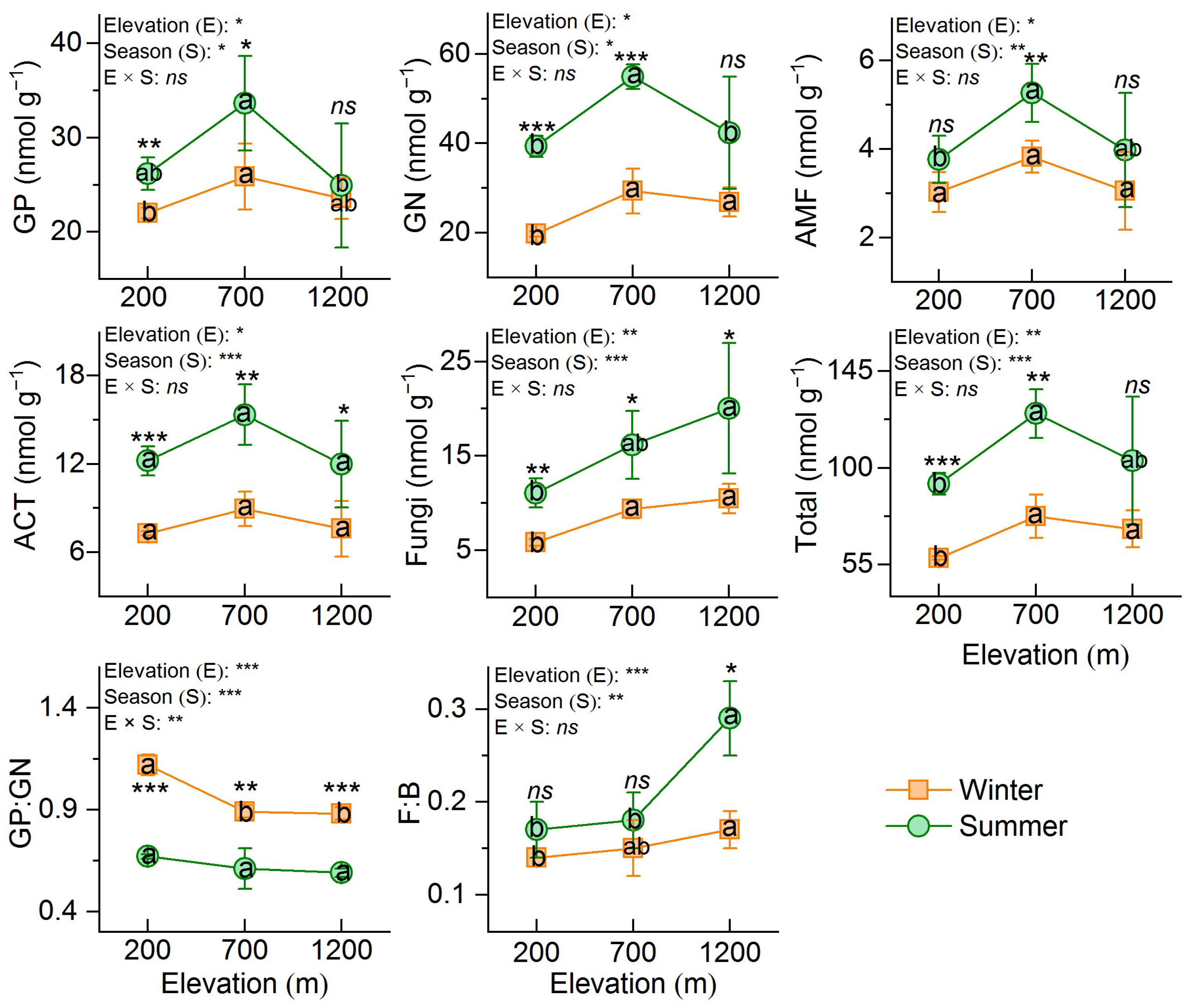
3.2. Microbial Biomass and Community Structure
3.3. CUE and Soil Enzyme Activity
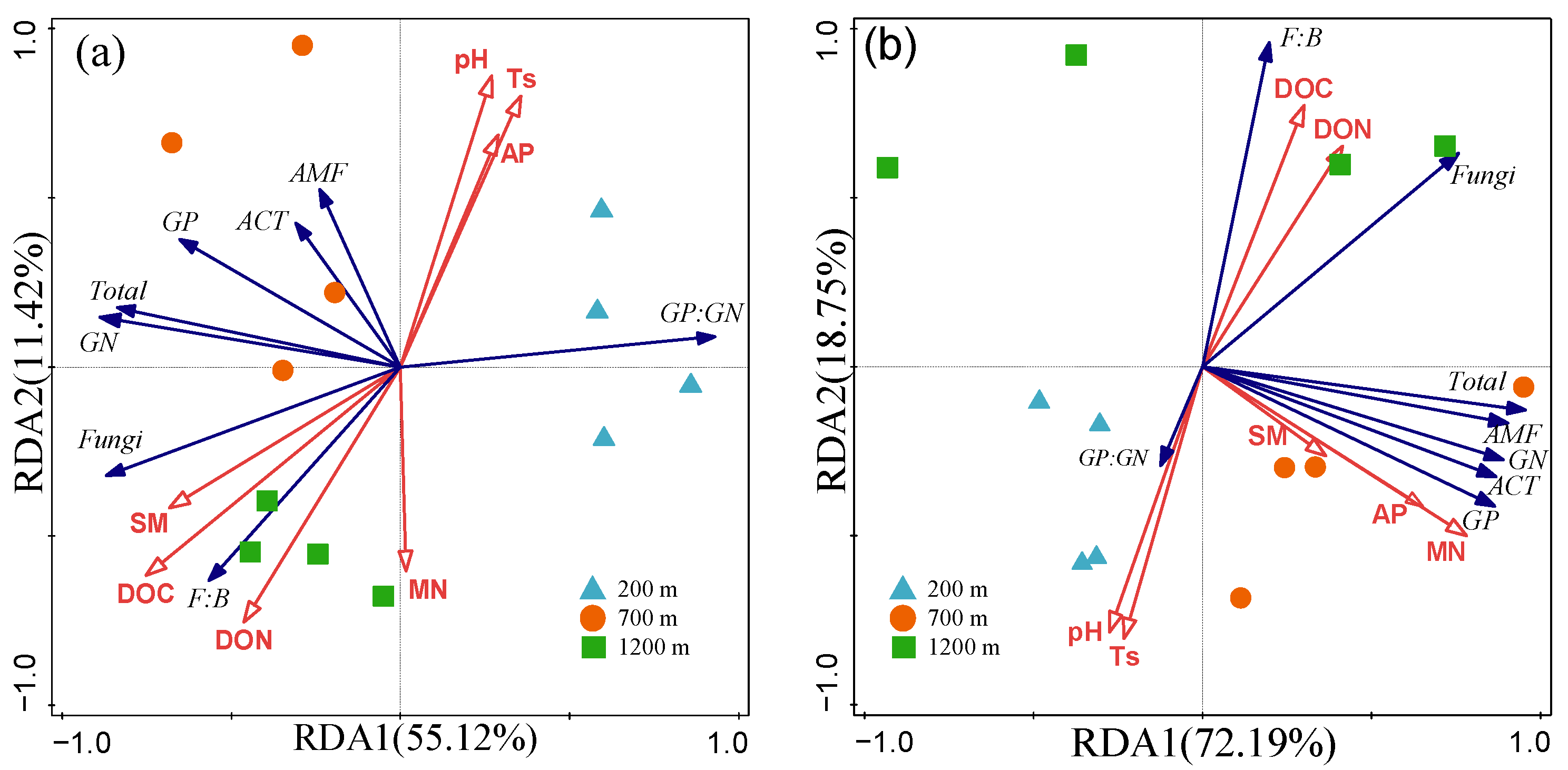
3.4. Factors Drive Seasonal Variation in Soil Microbial Community Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Environment Parameters | Explains % | Pseudo-F | p |
|---|---|---|---|
| Winter | |||
| DOC | 35.5 | 5.5 | 0.020 |
| Ts | 15.9 | 3.0 | 0.096 |
| SM | 8.6 | 1.7 | 0.218 |
| Summer | |||
| MN | 48.9 | 9.6 | 0.002 |
| pH | 24.3 | 8.2 | 0.006 |
| SM | 12.4 | 6.9 | 0.002 |
| Ts | 3.7 | 2.5 | 0.100 |
| DON | 2.4 | 1.7 | 0.176 |
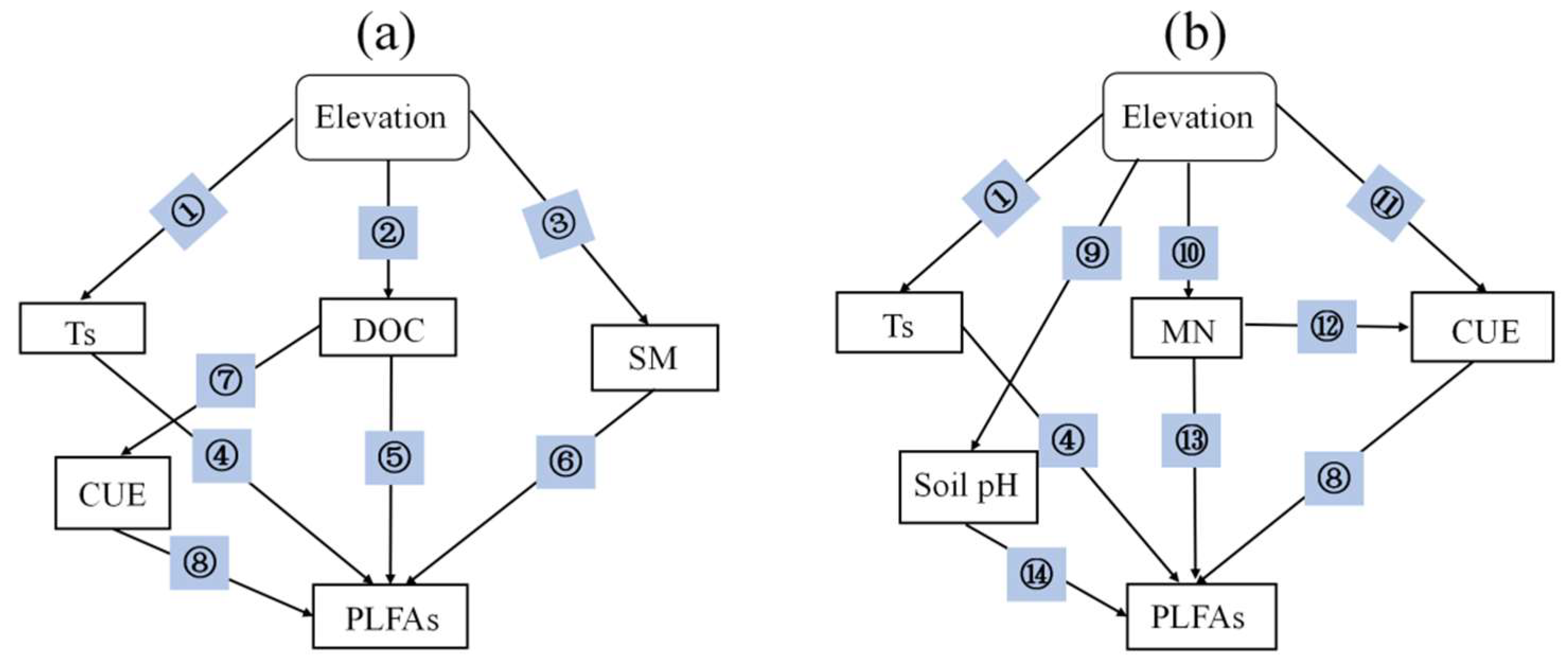
| # | Rationale | References |
|---|---|---|
| 1, 3 | Changes in elevation affect soil temperature and moisture. | [16,82] |
| 2, 10 | Soil nutrient availability and spatial heterogeneity were significantly affected by elevation. | [14] |
| 4 | Warming will change the composition of the soil microbial community. | [83,84] |
| 5, 13 | Soil DOC is an important carbon source for microbial growth. Soil N availability affects the microbial community structure. | [66,85] |
| 6 | Soil moisture is the major factor influencing microbial community structure. | [86] |
| 7, 12 | Soil microbial CUE will increase with nutrient availability. | [87] |
| 8 | The growth of soil microorganisms directly depends on microbial CUE. | [65] |
| 9 | Elevation has a significant effect on soil pH. | [81] |
| 11 | Soil microbial carbon use efficiency was significantly different at different altitudes. | [88] |
| 14 | It is well-known that soil pH is an important factor affecting microbial community composition. | [79] |
References
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Grosso, F.; Iovieno, P.; Alfani, A.; Nicola, F.D. Structure and activity of soil microbial communities in three Mediterranean forests. Appl. Soil Ecol. 2018, 130, 280–287. [Google Scholar] [CrossRef]
- Fan, S.Y.; Sun, H.; Yang, J.Y.; Qin, J.H.; Shen, D.J.; Chen, Y.X. Variations in soil enzyme activities and microbial communities along an altitudinal gradient on the eastern Qinghai-Tibetan plateau. Forests 2021, 12, 681. [Google Scholar] [CrossRef]
- Wu, Y.P.; Ma, B.; Zhou, L.; Wang, H.Z.; Xu, J.M.; Kemmitt, S.; Brookes, P.C. Changes in the soil microbial community structure with latitude in eastern China, based on phospholipid fatty acid analysis. Appl. Soil Ecol. 2009, 43, 234–240. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. The soil microbiome-from metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef]
- Männistö, M.; Ganzert, L.; Tiirola, M.; Häggblom, M.M.; Stark, S. Do shifts in life strategies explain microbial community responses to increasing nitrogen in tundra soil? Soil Biol. Biochem. 2016, 96, 216–228. [Google Scholar] [CrossRef]
- Mellado-Vázquez, P.G.; Lange, M.; Gleixner, G. Soil microbial communities and their carbon assimilation are affected by soil properties and season but not by plants differing in their photosynthetic pathways (C3 vs. C4). Biogeochemistry 2019, 142, 175–187. [Google Scholar] [CrossRef]
- Fu, D.G.; Wu, X.N.; Qiu, Q.T.; Duan, C.Q.; Jones, D.L. Seasonal variations in soil microbial communities under different land restoration types in a subtropical mountains region, Southwest China. Appl. Soil Ecol. 2020, 153, 103634. [Google Scholar] [CrossRef]
- Baldrian, P.; Šnajdr, J.; Merhautová, V.; Dobiášová, P.; Cajthaml, T.; Valášková, V. Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol. Biochem. 2013, 56, 60–68. [Google Scholar] [CrossRef]
- Regan, K.M.; Nunan, N.; Boeddinghaus, R.S.; Baumgartner, V.; Berner, D.; Boch, S.; Oelmann, Y.; Overmann, J.; Prati, D.; Marhan, S. Seasonal controls on grassland microbial biogeography: Are they governed by plants, abiotic properties or both? Soil Biol. Biochem. 2014, 71, 21–30. [Google Scholar] [CrossRef]
- Dong, W.Y.; Zhang, X.Y.; Dai, X.Q.; Fu, X.L.; Yang, F.T.; Liu, X.Y.; Sun, X.M.; Wen, X.F.; Schaeffer, S. Changes in soil microbial community composition in response to fertilization of paddy soils in subtropical China. Appl. Soil Ecol. 2014, 84, 140–147. [Google Scholar] [CrossRef]
- Lejon, D.P.H.; Sebastia, J.; Lamy, I.; Chaussod, R.; Ranjard, L. Relationships between soil organic status and microbial community density and genetic structure in two agricultural soils submitted to various types of organic management. Microb. Ecol. 2007, 53, 650–663. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bowman, W.D.; Kaufmann, R.; Schmidt, S.K. A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 2005, 20, 634–641. [Google Scholar] [CrossRef]
- Siles, J.A.; Cajthaml, T.; Filipova, A.; Minerbi, S.; Margesin, R. Altitudinal, seasonal and interannual shifts in microbial communities and chemical composition of soil organic matter in Alpine forest soils. Soil Biol. Biochem. 2017, 112, 1–13. [Google Scholar] [CrossRef]
- Sun, H.Y.; Wu, Y.H.; Zhou, J.; Bing, H.J.; Zhu, H. Climate influences the alpine soil bacterial communities by regulating the vegetation and the soil properties along an altitudinal gradient in SW China. Catena 2020, 195, 104727. [Google Scholar] [CrossRef]
- Cao, R.; Yang, W.Q.; Chang, C.C.; Wang, Z.; Wang, Q.; Li, H.; Tan, B. Differential seasonal changes in soil enzyme activity along an altitudinal gradient in an alpine-gorge region. Appl. Soil Ecol. 2021, 166, 104078. [Google Scholar] [CrossRef]
- Bhople, P.; Samad, A.; Šišić, A.; Antonielli, L.; Sessitsch, A.; Keiblinger, K.; Djukic, I.; Zehetner, F.; Zechmeister-Boltenstern, S.; Joergensen, R.G.; et al. Variations in fungal community structure along elevation gradients in contrasting Austrian Alpine ecosystems. Appl. Soil Ecol. 2022, 177, 104508. [Google Scholar] [CrossRef]
- Donhauser, J.; Frey, B. Alpine soil microbial ecology in a changing world. FEMS Microbiol. Ecol. 2018, 94, fiy099. [Google Scholar] [CrossRef]
- Praeg, N.; Pauli, H.; Illmer, P. Microbial diversity in bulk and rhizosphere soil of Ranunculus glacialis along a high-alpine altitudinal gradient. Front. Microbiol. 2019, 10, 1429. [Google Scholar] [CrossRef]
- Shigyo, N.; Umeki, K.; Hirao, T. Plant functional diversity and soil properties control elevational diversity gradients of soil bacteria. FEMS Microbiol. Ecol. 2019, 95, fiz025. [Google Scholar] [CrossRef]
- Ogwu, M.C.; Takahashi, K.; Dong, K.; Song, H.K.; Moroenyane, I.; Waldman, B.; Adams, J.M. Fungal elevational Rapoport pattern from a High Mountain in Japan. Sci. Rep. UK 2019, 9, 6570. [Google Scholar] [CrossRef]
- Gai, J.P.; Tian, H.; Yang, F.Y.; Christie, P.; Li, X.L.; Klironomos, J.N. Arbuscular mycorrhizal fungal diversity along a Tibetan elevation gradient. Pedobiologia 2012, 55, 145–151. [Google Scholar] [CrossRef]
- Sheng, Y.Y.; Cong, W.; Yang, L.S.; Liu, Q.; Zhang, Y.G. Forest soil fungal community elevational distribution pattern and their ecological assembly processes. Front. Microbiol. 2019, 10, 2226. [Google Scholar] [CrossRef]
- Wang, J.T.; Zheng, Y.M.; Hu, H.W.; Zhang, L.M.; Li, J.; He, J.Z. Soil pH determines the alpha diversity but not beta diversity of soil fungal community along altitude in a typical Tibetan forest ecosystem. J. Soil Sediment 2015, 15, 1224–1232. [Google Scholar] [CrossRef]
- Liu, D.; Liu, G.H.; Chen, L.; Wang, J.T.; Zhang, L.M. Soil pH determines fungal diversity along an elevation gradient in Southwestern China. Sci. China Life Sci. 2018, 61, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, J.N.; Read, Q.D.; Henning, J.A.; Sanders, N.J.; Classen, A.T. Consistently inconsistent drivers of microbial diversity and abundance at macroecological scales. Ecology 2017, 98, 1757–1763. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Voříšková, J.; Větrovský, T.; Baldrian, P. The bacterial community inhabiting temperate deciduous forests is vertically stratified and undergoes seasonal dynamics. Soil Biol. Biochem. 2015, 87, 43–50. [Google Scholar] [CrossRef]
- Lyu, M.K.; Li, X.J.; Xie, J.S.; Homyak, P.M.; Ukonmaanaho, L.; Yang, Z.J.; Liu, X.F.; Ruan, C.Y.; Yang, Y.S. Root-microbial interaction accelerates soil nitrogen depletion but not soil carbon after increasing litter inputs to a coniferous forest. Plant Soil 2019, 444, 153–164. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S.C. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Xu, G.; Chen, J.; Berninger, F.; Pumpanen, J.; Bai, J.W.; Yu, L.; Duan, B.L. Labile, recalcitrant, microbial carbon and nitrogen and the microbial community composition at two Abies faxoniana forest elevations under elevated temperatures. Soil Biol. Biochem. 2015, 91, 1–13. [Google Scholar] [CrossRef]
- White, D.C.; Davis, W.M.; Nickels, J.S.; King, J.D.; Bobbie, R.J. Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 1979, 40, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Hobbs, P.J.; Frostegård, Å. Changes in soil fungal: Bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol. Fertil. Soils 1996, 22, 261–264. [Google Scholar] [CrossRef]
- Tunlid, A.; Hoitink, H.A.J.; Low, C.; White, D.C. Characterization of bacteria that suppress Rhizoctonia damping-off in bark compost media by analysis of fatty acid biomarkers. Appl. Environ. Microbiol. 1989, 55, 1368–1374. [Google Scholar] [CrossRef]
- Denef, K.; Roobroeck, D.; Wadu, M.C.M.; Lootens, P.; Boeckx, P. Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biol. Biochem. 2009, 41, 144–153. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Ushio, M.; Balser, T.C.; Kitayama, K. Effects of condensed tannins in conifer leaves on the composition and activity of the soil microbial community in a tropical montane forest. Plant Soil 2013, 365, 157–170. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E.; Tunlio, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Swallow, M.; Quideau, S.A.; MacKenzie, M.D.; Kishchuk, B.E. Microbial community structure and function: The effect of silvicultural burning and topographic variability in northern Alberta. Soil Biol. Biochem. 2009, 41, 70–777. [Google Scholar] [CrossRef]
- Kimura, M.; Asakawa, S. Comparison of community structures of microbiota at main habitats in rice field ecosystems based on phospholipid fatty acid analysis. Biol. Fertil. Soils 2006, 43, 20–29. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Turner, B.L.; Talbot, J.M.; Waring, B.G.; Powers, J.S.; Kuske, C.R.; Moorhead, D.L.; Follstad Shah, J.J. Stoichiometry of microbial carbon use efficiency in soils. Ecol. Monogr. 2016, 8, 172–189. [Google Scholar] [CrossRef]
- Batten, K.M.; Scow, K.M.; Davies, K.F.; Harrison, S.P. Two invasive plants alter soil microbial community composition in serpentine grasslands. Biol. Invasions 2006, 8, 217–230. [Google Scholar] [CrossRef]
- Knorr, W.; Prentice, I.C.; House, J.I.; Holland, E.A. Long-term sensitivity of soil carbon turnover to warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef]
- Lyu, M.K.; Nie, Y.Y.; Giardina, C.P.; Vadeboncoeur, M.A.; Ren, Y.B.; Fu, Z.Q.; Wang, M.H.; Jin, C.S.; Liu, X.M.; Xie, J.S. Litter quality and site characteristics interact to affect the response of priming effect to temperature in subtropical forests. Funct. Ecol. 2019, 33, 2226–2238. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Cotrufo, M.F.; Sun, L.F.; Jiang, P.; Liu, Z.P.; Bai, E. Elevated temperature increases the accumulation of microbial necromass nitrogen in soil via increasing microbial turnover. Glob. Chang. Biol. 2020, 26, 5277–5289. [Google Scholar] [CrossRef]
- Lovell, R.D.; Jarvis, S.C.; Bardgett, R.D. Soil microbial biomass and activity in long-term grassland: Effects of management changes. Soil Biol. Biochem. 1995, 27, 969–975. [Google Scholar] [CrossRef]
- Feng, W.T.; Zou, X.M.; Schaefer, D. Above-and belowground carbon inputs affect seasonal variations of soil microbial biomass in a subtropical monsoon forest of southwest China. Soil Biol. Biochem. 2009, 41, 978–983. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Trivedi, P.; Osanai, Y.; Liu, Y.R.; Hamonts, K.; Jeffries, T.C.; Singh, B.K. Carbon content and climate variability drive global soil bacterial diversity patterns. Ecol. Monogr. 2016, 86, 373–390. [Google Scholar] [CrossRef]
- Kemmitt, S.J.; Wright, D.; Goulding, K.W.; Jones, D.L. pH regulation of carbon and nitrogen dynamics in two agricultural soils. Soil Biol. Biochem. 2006, 38, 898–911. [Google Scholar] [CrossRef]
- Salamanca, E.F.; Raubuch, M.; Joergensen, R.G. Microbial reaction of secondary tropical forest soils to the addition of leaf litter. Appl. Soil. Ecol. 2006, 31, 53–61. [Google Scholar] [CrossRef]
- Berger, T.W.; Berger, P. Greater accumulation of litter in spruce (Picea abies) compared to beech (Fagus sylvatica) stands is not a consequence of the inherent recalcitrance of needles. Plant Soil 2012, 358, 349–369. [Google Scholar] [CrossRef]
- Kaiser, C.; Franklin, O.; Dieckmann, U.; Richter, A. Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol. Lett. 2014, 17, 680–690. [Google Scholar] [CrossRef]
- Khan, K.S.; Mack, R.; Castillo, X.; Kaiser, M.; Joergensen, R.G. Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 2016, 271, 115–123. [Google Scholar] [CrossRef]
- Ni, Y.Y.; Yang, T.; Zhang, K.P.; Shen, C.C.; Chu, H.Y. Fungal communities along a small-scale elevational gradient in an alpine tundra are determined by soil carbon nitrogen ratios. Front. Microbiol. 2018, 9, 1815. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Jia, X.; Han, L.; Liu, Z.; Kang, S.Z.; Zhao, Y.H. Fungal community diversity in soils along an elevation gradient in a Quercus aliena var. acuteserrata forest in Qinling Mountains, China. Appl. Soil Ecol. 2021, 167, 104104. [Google Scholar] [CrossRef]
- Koranda, M.; Kaiser, C.; Fuchslueger, L.; Kitzler, B.; Sessitsch, A.; Zechmeister-Boltenstern, S.; Richter, A. Fungal and bacterial utilization of organic substrates depends on substrate complexity and N availability. FEMS Microbiol. Ecol. 2014, 87, 142–152. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Cao, Y.S.; Fu, S.L.; Zou, X.M.; Cao, H.L.; Shao, Y.H.; Zhou, L.X. Soil microbial community composition under Eucalyptus plantations of different age in subtropical China. Eur. J. Soil Biol. 2010, 46, 128–135. [Google Scholar] [CrossRef]
- Shigyo, N.; Umeki, K.; Hirao, T. Seasonal dynamics of soil fungal and bacterial communities in cool-temperate montane forests. Front. Microbiol. 2019, 10, 1944. [Google Scholar] [CrossRef] [PubMed]
- Adu, J.K.; Oades, J.M. Utilization of organic materials in soil aggregates by bacteria and fungi. Soil Biol. Biochem. 1978, 10, 117–122. [Google Scholar] [CrossRef]
- Keiblinger, K.M.; Hall, E.K.; Wanek, W.; Szukics, U.; Hämmerle, I.; Ellersdorfer, G.; Böck, S.; Strauss, J.; Sterflinger, K.; Richter, A. The effect of resource quantity and resource stoichiometry on microbial carbon-use-efficiency. FEMS Microbiol. Ecol. 2010, 73, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.L.; Bell, J.; Pendall, E.; Ogle, K. Does declining carbon-use efficiency explain thermal acclimation of soil respiration with warming? Glob. Change Biol. 2013, 19, 252–263. [Google Scholar] [CrossRef]
- Kramer, C.; Trumbore, S.; Fröberg, M.; Dozal, L.M.C.; Zhang, D.C.; Xu, X.M.; Santos, G.M.; Hanson, P.J. Recent (<4 year old) leaf litter is not a major source of microbial carbon in a temperate forest mineral soil. Soil Biol. Biochem. 2010, 42, 1028–1037. [Google Scholar]
- Sulman, B.N.; Brzostek, E.R.; Medici, C.; Shevliakova, E.; Menge, D.N.; Phillips, R.P. Feedbacks between plant N demand and rhizosphere priming depend on type of mycorrhizal association. Ecol. Lett. 2017, 20, 1043–1053. [Google Scholar] [CrossRef]
- Suz, L.M.; Bidartondo, M.I.; van der Linde, S.; Kuyper, T.W. Ectomycorrhizas and tipping points in forest ecosystems. New Phytol. 2021, 231, 1700–1707. [Google Scholar] [CrossRef]
- Djukic, I.; Zehetner, F.; Watzinger, A.; Horacek, M.; Gerzabek, M.H. In situ carbon turnover dynamics and the role of soil microorganisms therein: A climate warming study in an Alpine ecosystem. FEMS Microbiol. Ecol. 2013, 83, 112–124. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Jiang, L.F.; Luo, Y.Q. Trends in soil microbial communities during secondary succession. Soil Biol. Biochem. 2017, 115, 92–99. [Google Scholar] [CrossRef]
- Li, X.J.; Liu, X.F.; Xie, J.S.; Zhang, Q.F.; Yang, Z.J.; Schindlbacher, A.; Yang, Y.S. Contribution of above ground litterfall and roots to the soil CO2 efflux of two sub-tropical Cunninghamia lanceolata and Castanopsis carlesii forests. Agric. For. Meteorol. 2021, 311, 108671. [Google Scholar] [CrossRef]
- Edwards, K.A.; Jefferies, R.L. Inter-annual and seasonal dynamics of soil microbial biomass and nutrients in wet and dry low-Arctic sedge meadows. Soil Biol. Biochem. 2013, 57, 83–90. [Google Scholar] [CrossRef]
- Freppaz, M.; Said-Pullicino, D.; Filippa, G.; Curtaz, F.; Celi, L.; Zanini, E. Winter-spring transition induces changes in nutrients and microbial biomass in mid-alpine forest soils. Soil Biol. Biochem. 2014, 78, 54–57. [Google Scholar] [CrossRef]
- Potila, H.; Sarjala, T. Seasonal fluctuation in microbial biomass and activity along a natural nitrogen gradient in a drained peatland. Soil Biol. Biochem. 2004, 36, 1047–1055. [Google Scholar] [CrossRef]
- Feng, J.Y.; Li, Z.; Hao, Y.F.; Wang, J.; Ru, J.Y.; Song, J.; Wan, S.Q. Litter removal exerts greater effects on soil microbial community than understory removal in a subtropical-warm temperate climate transitional forest. For. Ecol. Manag. 2022, 505, 119867. [Google Scholar] [CrossRef]
- Curtin, D.; Peterson, M.E.; Anderson, C.R. pH-dependence of organic matter solubility: Base type effects on dissolved organic C, N, P, and S in soils with contrasting mineralogy. Geoderma 2016, 271, 161–172. [Google Scholar] [CrossRef]
- Siles, J.A.; Margesin, R. Seasonal soil microbial responses are limited to changes in functionality at two Alpine forest sites differing in altitude and vegetation. Sci. Rep. UK 2017, 7, 2204. [Google Scholar] [CrossRef]
- Ni, Y.Y.; Yang, T.; Ma, Y.Y.; Zhang, K.P.; Soltis, P.S.; Soltis, D.E.; Gilbert, J.A.; Zhao, Y.P.; Fu, C.X.; Chu, H.Y. Soil pH determines bacterial distribution and assembly processes in natural mountain forests of eastern China. Glob. Ecol. Biogeogr. 2021, 30, 2164–2177. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhou, Y.J.; Jia, X.; Han, L.; Liu, L.; Ren, K.; Ye, X.; Pei, Y.J. Soil characteristics and microbial community structure on along elevation gradient in a Pinus armandii forest of the Qinling Mountains, China. For. Ecol. Manag. 2022, 503, 119793. [Google Scholar] [CrossRef]
- He, X.J.; Hou, E.Q.; Liu, Y.; Wen, D.Z. Altitudinal patterns and controls of plant and soil nutrient concentrations and stoichiometry in subtropical China. Sci. Rep. 2016, 6, 24261. [Google Scholar] [CrossRef]
- Zhang, W.; Parker, K.M.; Luo, Y.; Wan, S.; Wallace, L.L.; Hu, S. Soil microbial responses to experimental warming and clipping in a tallgrass prairie. Glob. Change Biol. 2005, 11, 266–277. [Google Scholar] [CrossRef]
- Yu, H.Y.; Ma, Q.H.; Liu, X.D.; Xu, Z.Z.; Zhou, G.S.; Shi, Y.H. Short-and long-term warming alters soil microbial community and relates to soil traits. Appl. Soil. Ecol. 2018, 131, 22–28. [Google Scholar] [CrossRef]
- Zhang, B.; Chao, L.; He, H.B.; Zhang, X.D. Variations in soil microbial communities and residues along an altitude gradient on the northnern slope of Changbai mountain, China. PLoS ONE 2013, 8, e66184. [Google Scholar]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porparato, A.; Agren, G.I. Enviromental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Mgana, K.Z.; Sietio, Q.M.; Meyer, N.; Poeplau, C.; Adamczyk, S.; Biasi, C.; Kalu, S.; Rasanen, M.; Ambus, P.; Fritze, H.; et al. Microbial carbon use efficiency along an altitudinal gradient. Soil Biol. Biochem. 2022, 173, 108799. [Google Scholar] [CrossRef]


| Elevation (m a.s.l) | 200 | 700 | 1200 |
|---|---|---|---|
| Stand density (Tree hm−2) | 1696 | 1384 | 542 |
| Tree height (m) | 20.2 | 25.2 | 25.0 |
| Diameter at breast height (cm) | 16.7 | 16.4 | 28.8 |
| Annual temperature (°C) | 20.2 | 17.6 | 14.2 |
| Annual precipitation (mm) | 2411 | 2374 | 2481 |
| Soil pH | 5.05 (0.32) a | 4.86 (0.21) a | 4.23 (0.02) b |
| Bulk density (g cm−3) | 1.10 (0.07) a | 0.74 (0.11) b | 0.78 (0.08) b |
| Soil organic carbon (g kg−1) | 26.14 (3.24) b | 50.53 (6.50) a | 53.31 (7.21) a |
| Total nitrogen (g kg−1) | 1.90 (0.29) b | 3.15 (0.34) a | 2.62 (0.38) a |
| Soil C/N | 13.76 (0.82) c | 16.08 (0.39) b | 20.35 (1.51) a |
| Total phosphorus (g kg−1) | 0.39 (0.02) a | 0.32 (0.02) b | 0.28 (0.01) c |
| Soil temperature (°C) | 18.85 (0.08) a | 16.74 (0.07) b | 14.65 (0.12) c |
| Soil moisture (%) | 10.18 (1.79) b | 14.47 (0.97) a | 15.00 (0.54) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Lyu, M.; Deng, C.; Li, X.; Lu, Y.; Lin, W.; Jiang, Y.; Xie, J. Carbon and Nitrogen Availability Drives Seasonal Variation in Soil Microbial Communities along an Elevation Gradient. Forests 2022, 13, 1657. https://doi.org/10.3390/f13101657
Xiong X, Lyu M, Deng C, Li X, Lu Y, Lin W, Jiang Y, Xie J. Carbon and Nitrogen Availability Drives Seasonal Variation in Soil Microbial Communities along an Elevation Gradient. Forests. 2022; 13(10):1657. https://doi.org/10.3390/f13101657
Chicago/Turabian StyleXiong, Xiaoling, Maokui Lyu, Cui Deng, Xiaojie Li, Yuming Lu, Weisheng Lin, Yongmeng Jiang, and Jinsheng Xie. 2022. "Carbon and Nitrogen Availability Drives Seasonal Variation in Soil Microbial Communities along an Elevation Gradient" Forests 13, no. 10: 1657. https://doi.org/10.3390/f13101657
APA StyleXiong, X., Lyu, M., Deng, C., Li, X., Lu, Y., Lin, W., Jiang, Y., & Xie, J. (2022). Carbon and Nitrogen Availability Drives Seasonal Variation in Soil Microbial Communities along an Elevation Gradient. Forests, 13(10), 1657. https://doi.org/10.3390/f13101657






